Abstract
A miniature schnauzer dog presenting with hyphema and glaucoma of the right eye had a retinal neoplasm. Neoplastic cells stained positively for glial fibrillary acidic protein, vimentin, and S-100 and largely negatively for oligodendrocyte transcription factor 2 by immunohistochemistry. The clinical and histopathological features of canine retinal astrocytomas are discussed.
Résumé
Astrocytome rétinal chez un chien. Un chien Schnauzer miniature a été présenté avec de l’hyphéma et du glaucome dans l’oeil droit et avait un néoplasme rétinal. Les cellules néoplastiques ont donné un résultat positif par immunohistochimie pour la protéine fibrillaire gliale acide, la vimentine et S-100 et les résultats étaient en grande partie négatifs pour le facteur de transcription 2 des oligodendrocytes. Les caractéristiques cliniques et histopathologiques des astrocytomes rétinaux canins sont discutés.
(Traduit par Isabelle Vallières)
Case description
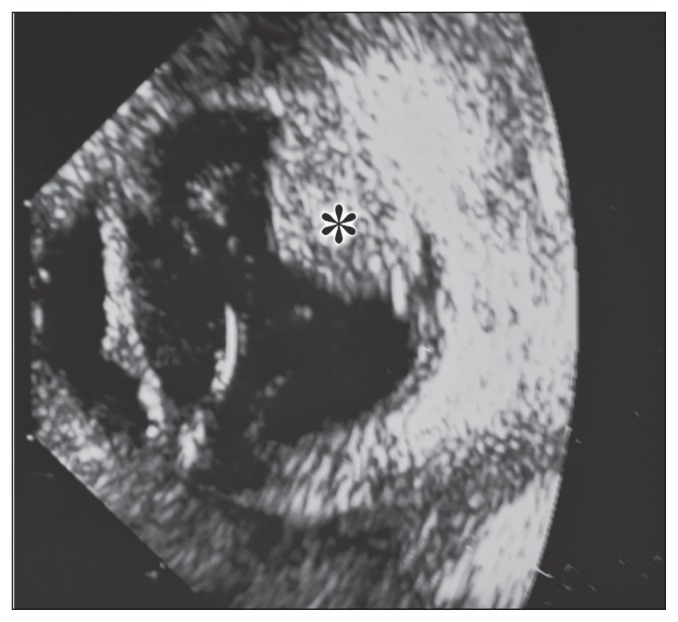
A 9.5-year-old, spayed female miniature schnauzer dog was referred to the Midwest Veterinary Referral Center because of a 1-week history of increased redness and cloudiness to the right eye (OD) noted by a general veterinary practitioner. An ophthalmic examination of the right eye by a board-certified veterinary ophthalmologist (NK) revealed an absent menace response, absent dazzle reflex, absent direct pupillary light reflex, moderate episcleral injection, mild corneal edema, high intraocular pressure (60 mmHg), dense hyphema in the ventral 1/3 of the anterior chamber, and some blood clots adhered to the axial anterior lens capsule. No abnormalities were noted in the left eye (OS). The dog was otherwise healthy and no abnormalities were recognized by complete blood (cell) count, blood chemistry profile, and chest radiographs conducted by the general veterinary practitioner and a physical examination and a neurologic examination performed by one of the authors (NK). B-mode ultrasonographic examination OD using a 10 MHz probe (Acuson Sequoia 512; Siemens Healthineers, Malvern, Pennsylvania, USA) performed by one of the authors (NK) showed a mushroom-shaped, relatively homogenously hyperechoic mass arising from the mid-dorsal chorioretinal region (Figure 1). Enucleation of the affected eye was performed and the globe was placed in 10% neutral buffered formalin and submitted for gross pathologic and histopathologic examination.
Figure 1.
B-mode, 10 MHz ocular ultrasound examination OD shows a relatively homogenously hyperechoic mass (asterisk) contiguous to the mid-dorsal chorioretinal region.
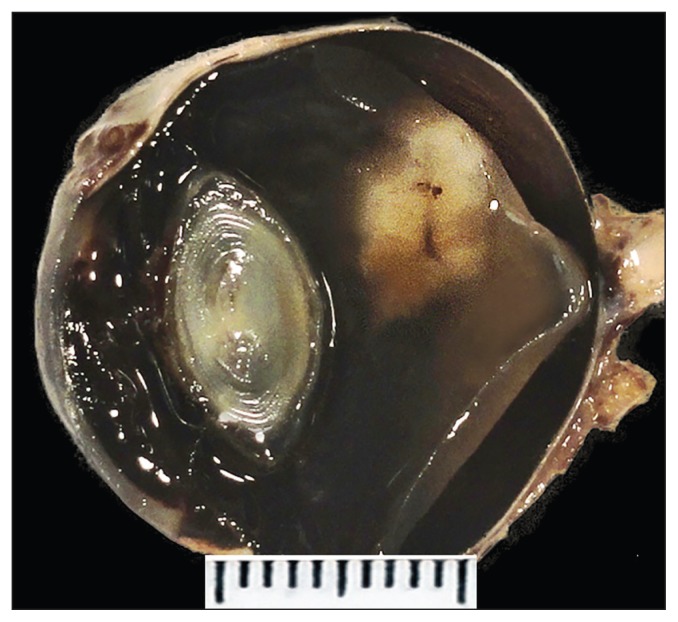
Grossly there was an approximately 5-mm sized, white to tan, solitary nodule arising from the detached dorsal retina (Figure 2). Based on the ultrasonographic and macroscopic findings, differential diagnoses included primary and secondary chorioretinal neoplastic lesions such as melanocytic tumors, gliomas, primitive neuroectodermal tumors and lymphomas, and chronic inflammatory lesions such as mycotic granulomatous inflammation.
Figure 2.
Grossly there is an approximately 5-mm sized white to tan, solitary nodule associated with the detached dorsal retina.
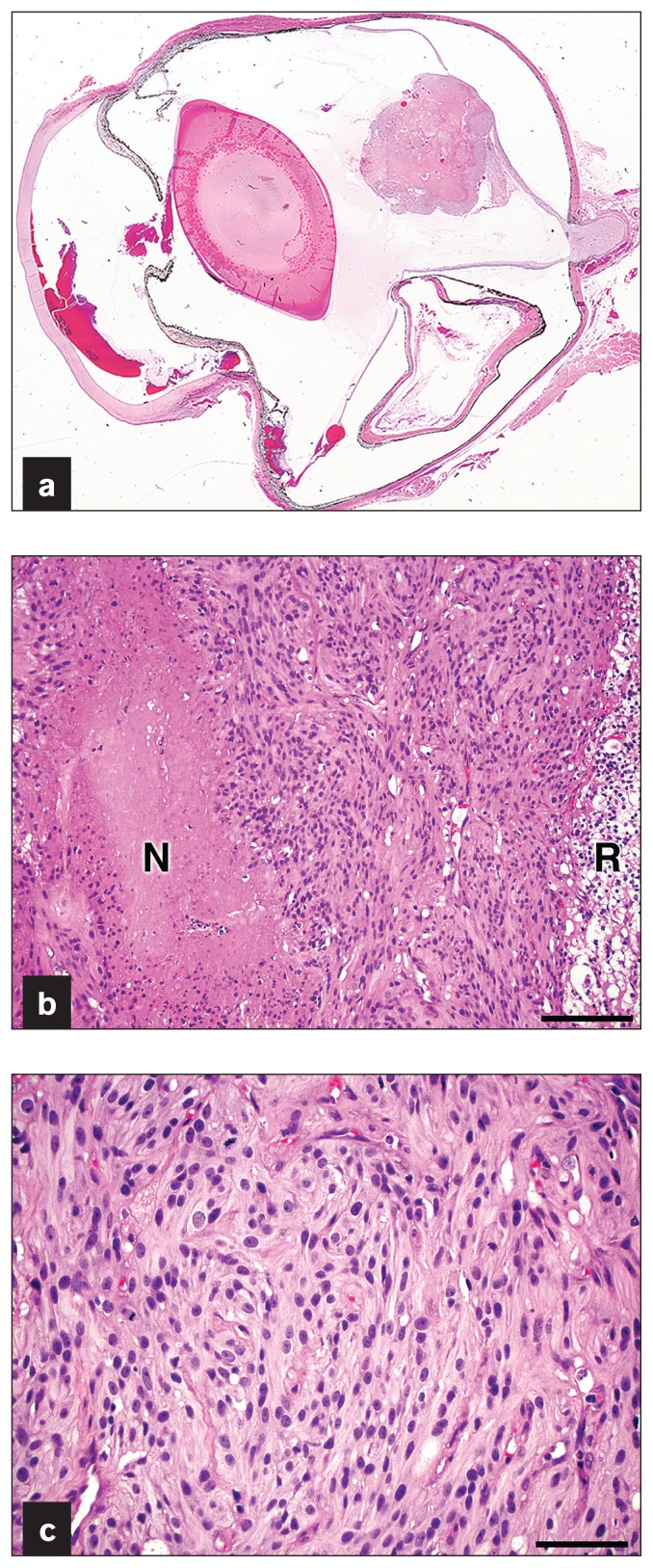
Microscopically the specimen was characterized by an unen-capsulated, well-circumscribed, highly cellular nodular mass that continued to the posterior aspect of the detached dorsal retina (Figure 3a). The mass was composed of highly cellular anaplastic spindle cells arranged in interlacing fascicles and had scattered frequent small caliber vessels and necrotic foci with pseudopalisading of tumor cells (Figure 3b). Approximately 50% of the tumor area was necrotic. Neoplastic cells had variably distinct cell borders, abundant pale eosinophilic fibrillar cytoplasm, and oval nuclei with finely stippled chromatin and 1 to 2 variably distinct nucleoli (Figure 3c). There were 8 mitoses observed in 10 high power fields. The retina contiguous to the mass showed blending of the 2 nuclear layers with vacuolar changes (Figure 3b) or atrophy with loss of ganglion cells and nuclei of the inner and outer nuclear layers. Serial sections of the paraffin-embedded specimen confirmed that the neoplasm was confined to the retina and there was no microscopic evidence of optic nerve invasion. The retina was detached from the underlying hypertrophic retinal pigmented epithelium. Other microscopic findings included hyphema, formation of a pre-iridal fibrovascular membrane, and peripheral anterior synechia. The histopathologic findings of the mass were consistent with a retinal glioma.
Figure 3.
a — Subgrossly there is a well-circumscribed nodular mass arising from the detached dorsal retina. b — The mass is highly cellular and composed of spindle cells arranged in interlacing fascicles with frequent small caliber vessels and foci of necrosis (“N”) with pseudopalisading of tumor cells. Note that the retina (“R”) contiguous to the neoplasm was degenerate with vacuolar changes. H&E stain. Bar = 100 μm. c — Neoplastic cells have variably distinct cell borders, abundant fibrillar cytoplasm, and oval nuclei with finely stippled chromatin and 1 to 2 variably distinct nucleoli. Mitoses are observed. H&E stain. Bar = 50 μm.
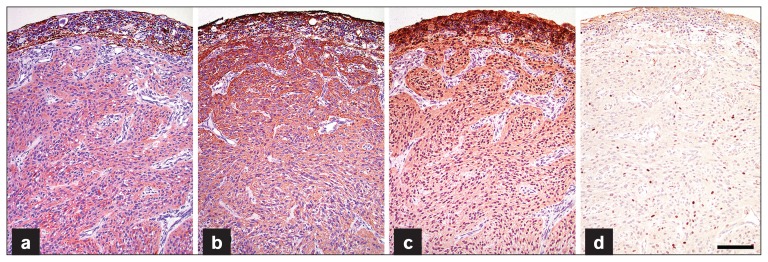
Unstained paraffin-embedded tissue sections (5 μm) were prepared and immunohistochemical staining was carried out for further characterization of the neoplastic cells. Antibodies used were glial fibrillary acidic protein (GFAP) (Dako, Carpentaria, California, USA diluted 1:2000), a marker for glial cells; vimentin (Dako, M70311, diluted 1:200), a marker for mesenchymal cells; S-100 (Dako, Z0311, diluted 1:600), a marker for glial cells; oligodendrocyte transcription factor 2 (Olig2) (Millipore; Billerica, Massachusetts, USA. AB9610, diluted 1:750), a marker for oligodendrocytes, and melan A (Dako, M7196, diluted 1:100), a marker for melanocytes. Horseradish peroxidase-based EnVision system with AEC chromogen kit (Dako) was used to visualize the sites recognized by primary antibodies. Canine cerebrum sections (for GFAP and Olig2), intestinal sections (for vimentin) and cutaneous melanoma (for S-100 and melan A) served as positive control samples. Non-immune serum from the same species of animals as the primary antibody was used as a negative control. Neoplastic cells stained diffusely positive for GFAP, vimentin, and S-100 by immunohistochemistry (Figures 4a, 4b, 4c). Neoplastic cells’ nuclei stained largely negative for Olig2 by immunohistochemistry and less than 10% of the cells’ nuclei among the neoplasm showed positive nuclear immunoreactivity for Olig2, a transcription factor of oligodendrocytes (Figure 4d). Neoplastic cells stained negatively for melan A by immunohistochemistry (data not shown). Based on the histopathologic and immunohistochemical characteristics, the tumor was diagnosed as a retinal astrocytoma, most likely high-grade (glioblastoma). Telephone conversation with the owner 40 mo after enucleation revealed that the dog was alive and well. Following enucleation, the dog did not receive any ancillary treatment and chest radiographs were not taken to evaluate pulmonary metastasis.
Figure 4.
Neoplastic cells stain diffusely positive for GFAP (a), vimentin (b), and S-100 (c) by immunohistochemistry. Only a small number of cells’ nuclei within the tumor exhibit positive nuclear immunostaining for Olig2 (d). Bar = 100 μm.
Discussion
In this case, a retinal glioma, particularly an astrocytoma, was strongly suspected by the histomorphologic findings (1–4). Positive immunohistochemistry staining for GFAP, vimentin, and S-100 confirmed the diagnosis of an astrocytoma. It has been reported that astrocytomas exhibit positive immunohistochemical staining for GFAP, vimentin, and S-100 (1–3,5–9). Olig2 is a marker of oligodendrocytes and oligodendrogliomas. Studies in humans have shown that some astrocytomas stain positively for Olig2 by immunohistochemistry and hence distinction between oligodendrogliomas and astrocytomas cannot be made by Olig2 immunohistochemistry alone (10,11). Even though it is not specific for oligodendrogliomas, Olig2 immunohistochemistry can help differentiate oligodendrogliomas from other gliomas (10,11). The immunohistochemistry finding of only occasional positive nuclear staining for Olig2 in this case was suggestive of an astrocytoma. In addition, the microscopic findings of the neoplasm were not consistent with oligodendrogliomas that demonstrate a so-called “fried-egg” or “honeycomb” appearance due to distinct cell membranes, cytoplasmic clearing and round hyperchromatic nuclei (4). The distinction between oligodendrogliomas and astrocytomas can be important since it has been reported that oligodendrogliomas are more chemosensitive and have a better outcome than astrocytomas in humans (12). No large studies have been reported concerning tumor behavior in dogs with brain gliomas. Reported survival time based on case reports of canine brain astrocytomas has ranged from 3 to 8 mo with chemotherapy (3).
Primary intraocular tumors in domestic animals are uncommon; however, melanocytic tumors and iridociliary adenomas are the 2 most common types of these tumors in dogs (13,14). The distribution of choroidal melanomas can be similar to the case herein; however, melanoma was ruled out in this case based on the histomorphologic findings and negative immunostaining for melan A. As the name implies, iridociliary adenomas arise from the iris and/or ciliary body. The distribution of the present neoplasm and its histological appearance were not compatible with an iridociliary adenoma. Primitive neuroectodermal tumors of the retina have been reported in dogs (15). Among the primary intraocular primitive neuroectodermal tumors that occur in dogs, retinoblastomas are found in younger dogs (≤ 2 y of age), whereas medulloepitheliomas tend to occur in older dogs (> 7 y of age) (15). A primitive neuroectodermal tumor was unlikely in this case as rosettes and other histologic features typical of primitive neuroectodermal tumors were not observed. In addition, GFAP positive immunoreactivity in this tumor was not consistent with primitive neuroectodermal tumors (15,16).
Gliomas are common primary tumors of the central nervous system in dogs. They arise from glial cells and can be classified into astrocytoma, oligodendroglioma, oligoastrocytoma, and ependymoma according to their cellular lineage (1). Astrocytomas are the most common glioma in dogs and histologically subclassified as low-grade astrocytoma (well-differentiated), medium-grade astrocytoma (anaplastic), and high-grade astrocytoma (glioblastoma) (1–4). Low-grade astrocytomas are composed of well-differentiated astrocytic cells without cytological atypia or mitotic activity, medium-grade astrocytomas are composed of highly cellular anaplastic astrocytic cells with mitotic activity and high-grade astrocytomas additionally have vascular proliferation and/or necrosis (1–4). In this case, histopathologic findings of cellular and nuclear pleomorphism, mitoses, vascular proliferation, and necrosis supported a diagnosis of high-grade astrocytoma (glioblastoma). In humans, necrosis is a central feature of high-grade gliomas and no histological feature is reported to be more powerful in predicting poor prognosis in human brain gliomas (17). Although the prognosis of canine brain astrocytomas based on the histopathologic classification has not been established, similar to human cases, high-grade astrocytomas most likely involve a poor prognosis in dogs (9). Naranjo et al (5) reported that necrosis was observed in all 17 canine ocular astrocytomas in their retrospective study of 18 ocular gliomas regardless of their histopathologic grade. The presence of necrosis was also noted in other canine cases of ocular astrocytomas (6,7). Unlike brain or spinal cord astrocytomas, necrosis appears to be a common pathologic change in ocular astrocytomas with limited value for the grading of histological malignancy. Naranjo et al (5) suggested that the most reliable positive prognostic indicator for canine ocular gliomas was the absence of tumor invasion of the optic nerve at surgery. In accordance with this suggestion enucleation alone was effective in this case despite the histological features suggestive of a high-grade astrocytoma. Although a thorough follow-up including chest radiographs was not performed after enucleation in this case, no clinical signs of recurrence or metastasis were evident to the owner 40 mo after the surgery.
This report presents the diagnosis of a retinal astrocytoma in a dog and provides further insights into the clinical and histopathological aspects of this rare intraocular neoplasm in dogs. Although rare, an astrocytoma should be considered as a differential diagnosis for a solitary retinal mass in dogs. Immunohistochemistry for GFAP, vimentin, S-100 and Olig2 is useful for confirming the diagnosis of an astrocytoma. Unlike brain/spinal cord astrocytomas, necrosis appears to be a common pathology finding in retinal astrocytomas regardless of their histolopathologic grade. The prognosis of a retinal astrocytoma following enucleation alone appears to be good when the lesion is confined to the retina. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Koestner A, Higgins RJ. Tumors of the nervous system. In: Meuten DJ, editor. Tumors in Domestic Animals. 4th ed. Ames, Iowa: Iowa State University Press; 2002. pp. 697–738. [Google Scholar]
- 2.Cantile C, Youssef S. Nervous system. In: Maxie MG, editor. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 1. St. Louis, Missouri: Elsevier; 2016. pp. 250–406. [Google Scholar]
- 3.Stoica G, Kim HT, Hall DG, Coates JR. Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet Pathol. 2004;41:10–19. doi: 10.1354/vp.41-1-10. [DOI] [PubMed] [Google Scholar]
- 4.Koestner A, Bilzer T, Fatzer R, Schulman FY, Summers BA, Van Winkle TJ. Histologic Classification of Tumors of the Nervous System of Domestic Animals. Vol. 5. Washington, DC: Armed Forces Institute of Pathology; 2002. (Second series). [Google Scholar]
- 5.Naranjo C, Schobert C, Dubielzig RR. Canine ocular gliomas: A retrospective study. Vet Ophthalmol. 2008;11:356–362. doi: 10.1111/j.1463-5224.2008.00658.x. [DOI] [PubMed] [Google Scholar]
- 6.Meyerholz DK, Haynes JS. Solitary retinal astrocytoma in a dog. Vet Pathol. 2004;41:177–178. doi: 10.1354/vp.41-2-177. [DOI] [PubMed] [Google Scholar]
- 7.Caswell J, Curtis C, Gibbs B. Astrocytoma arising at the optic disc in a dog. Can Vet J. 1999;40:427–428. [PMC free article] [PubMed] [Google Scholar]
- 8.Herpers MJ, Ramaekers FC, Aldeweireldt J, Moesker O, Slooff J. Co-expression of glial fibrillary acidic protein- and vimentin-type intermediate filaments in human astrocytomas. Acta Neuropathol. 1986;70:333–339. doi: 10.1007/BF00686093. [DOI] [PubMed] [Google Scholar]
- 9.Stoica G, Levine J, Wolff J, Murphy K. Canine astrocytic tumors: A comparative review. Vet Pathol. 2011;48:266–275. doi: 10.1177/0300985810389543. [DOI] [PubMed] [Google Scholar]
- 10.Yokoo H, Nobusawa S, Takebayashi H, et al. Anti-human Olig2 antibody as a useful immunohistochemical marker of normal oligodendrocytes and gliomas. Am J Pathol. 2004;164:1717–1725. doi: 10.1016/S0002-9440(10)63730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohnishi A, Sawa H, Tsuda M, et al. Expression of the oligodendroglial lineage-associated markers Olig1 and Olig2 in different types of human gliomas. J Neuropathol Exp Neurol. 2003;62:1052–1059. doi: 10.1093/jnen/62.10.1052. [DOI] [PubMed] [Google Scholar]
- 12.Fortin D, Cairncross GJ, Hammond RR. Oligodendroglioma: An appraisal of recent data pertaining to diagnosis and treatment. Neurosurgery. 1999;45:1279–1291. doi: 10.1097/00006123-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Dubielzig RR. Tumor of the eye. In: Meuten DJ, editor. Tumors in Domestic Animals. 4th ed. Ames, Iowa: Iowa State University Press; 2002. pp. 739–754. [Google Scholar]
- 14.Miller PR, Dubielzig RR. Ocular tumor. In: Withrow SJ, Vail DM, Page RL, editors. Small Animal Clinical Oncology. 5th ed. St. Louis, Missouri: Elsevier; 2013. pp. 597–607. [Google Scholar]
- 15.Regan DP, Dubielzig RR, Zeiss CJ, Charles B, Hoy SS, Ehrhart EJ. Primary primitive neuroectodermal tumors of the retina and ciliary body in dogs. Vet Ophthalmol. 2013;16:87–93. doi: 10.1111/vop.12056. [DOI] [PubMed] [Google Scholar]
- 16.Jensen OA, Kaarrsholm S, Prause JU, Heegaard S. Neuroepithelial tumor of the retina in a dog. Vet Ophthalmol. 2003;6:57–60. doi: 10.1046/j.1463-5224.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- 17.Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]