Abstract
This prospective study investigated the effects of acupuncture alone or combined with analgesics in chronic pain and quality of life assessed by owners for up to 24 weeks in 181 dogs with neurological and musculoskeletal diseases. The scores before and after the onset of treatment were evaluated using the Wilcoxon test and the evolution of success was evaluated by Kaplan-Meier curves. Differences were considered significant at P < 0.05. The success rates for Helsinki chronic pain index (HCPI), quality of life assessment, and visual analog scales (VAS) for pain and locomotion were 79%, 84%, 78%, and 78% of the animals, respectively, when both diseases and groups of treatment were combined. Dogs with musculoskeletal disorders had greater improvement in HCPI (P = 0.003) and VAS locomotion (P = 0.045) than those with neurological disorders. Use of acupuncture alone or in combination with analgesics reduced pain and improved quality of life in dogs with neurological and musculoskeletal diseases.
Résumé
Effet de l’acupuncture dans la douleur et la qualité de vie dans les maladies neurologiques et musculo-squelettiques chez le chiens. Cette étude prospective a étudié les effets de l’acupuncture (AP) seul ou combinée avec des analgésiques pour traiter la douleur chronique et de la qualité de vie évaluée par les propriétaires pendant 24 semaines à 181 chiens atteints de maladies neurologiques et musculo-squelettiques. Les scores des animaux ont été évalués avant et après le début du traitement au moyen du test de Wilcoxon et l’évolution du succès par des courbes de Kaplan-Meier. Les différences ont été considérées comme significatives lorsque P < 0,05. Le taux de réussite pour l’indice de la douleur chronique de Helsinki (IDCH), évaluation de la qualité de vie et des échelles visuelles analogiques (EVA) pour la douleur et la locomotion étaient respectivement de 79 %, 84 %, 78 %, et 78 %. des animaux, respectivement, lorsque les deux types de maladies, et les deux groupes de traitement ont été combinés. Les chiens souffrant de maladies musculo-squelettiques ont une plus grande amélioration de IDCH (P = 0,003) et EVA locomotion (P = 0,045) scores que ceux souffrant de maladies neurologiques. Utilisation d’AP seul ou associé à traitements analgésiques réduite la douleur et meilleure qualité de vie chez les chiens atteints de maladies neurologiques et musculo-squelettiques.
(Traduit par les auteurs)
Introduction
Although the assessment and management of acute pain in animals has advanced considerably, chronic pain is still underestimated and, therefore, undertreated, because the diagnosis is complex and treatment is limited (1). Validated tools have been developed for evaluation of acute (2) and chronic pain (3–6), and although acute pain is well-evaluated by veterinarians (2), the owner is more apt to identify chronic pain based on changes in the pet’s attitude and behavior (7). Assessment of quality of life is important to measure treatment efficacy in chronic cases (3,7). Chronic pain and quality of life have been assessed by validated questionnaires in dogs with joint diseases (4,6,8), cancer (3), spinal cord injuries (9), and quality of life in general (7).
Due to the complex multi-dimension of chronic pain and the limitation of pharmacological treatment, acupuncture (AP) may be a good option in a multimodal approach (10,11). Meta-analyses of high-quality, randomized and well-controlled studies demonstrated that the analgesic effect of AP was better than sham AP or no treatment in 29 clinical studies with nearly 18 000 human patients with osteoarthritis (OA) and chronic headache and shoulder, back, and neck pain (12). Another meta-analysis including 14 studies with about 4000 human patients with OA of the knee showed that AP provided better pain relief than sham AP or standard care treatment (13).
Acupuncture is a traditional Eastern medicine therapy performed by introducing needles in acupoints. This technique embraces several clinical applications due to its wide mechanism of action involving the neuroendocrine system (14). Several techniques may be used alone or in combination to stimulate the acupoints, such as single needle, electroacupuncture (EA), laserpuncture (LASER), and ozone therapy. Electroacupuncture is the most used technique for relieving central and peripheral pain, as it combines mechanical and electrical effects (14). Laserpuncture involves the use of laser in acupoints (15) and is recommended for animals that do not tolerate needling. Ozone therapy is used in acupoints (ozonepuncture) or by intra-rectal insufflation (16).
There are few high-quality veterinary medical studies that have evaluated the effects of AP in treating pain and improving quality of life in dogs. Gold implants in acupoints reduced pain scores by 65% in dogs with hip dysplasia and improved mobility and decreased pain signals in 83% of these animals (17). Recovery of ambulation and deep pain sensation was faster in dogs with thoracolumbar intervertebral disk disease (IVDD) treated with EA, combined with standard medical treatment, such as corticosteroids and/or opioids, than with conventional treatment alone (10). Electroacupuncture was more effective (79%) than delayed decompressive surgery (40%), as measured by return of ambulation and decreased neurologic deficits, in dogs with grades IV and V thoracolumbar IVDD (11).
Based on the hypothesis that AP reduces pain and improves the dog’s quality of life either when used alone or in combination with conventional and adjuvant analgesia, and that dogs affected by musculoskeletal diseases would improve faster than dogs with neurological diseases, the aim of this study was to evaluate the efficacy of AP and related techniques alone or in combination with analgesics in chronic pain and quality of life of dogs with neurological and musculoskeletal diseases, using pre-validated scales answered by owners.
Materials and methods
Animals
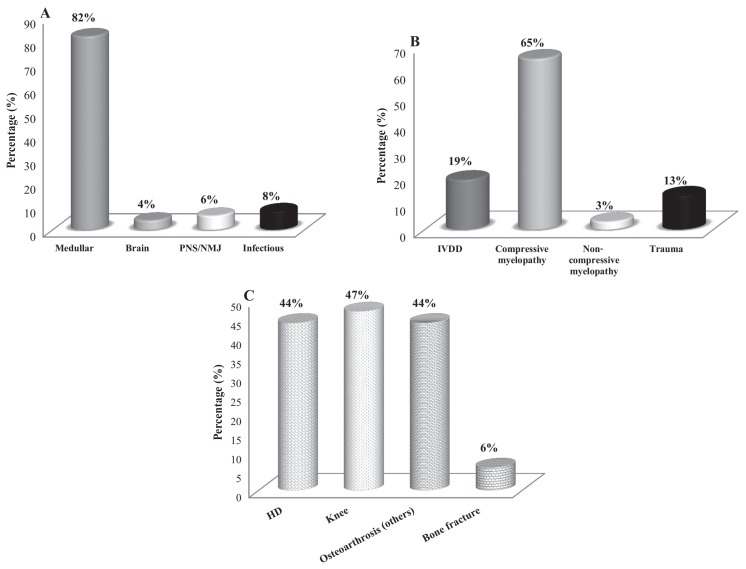
This prospective study involved dogs with 2 main causes of chronic pain: neurological (n = 145) and musculoskeletal (n = 36) diseases. The selection of animals was based on physical, neurological, and orthopedic examination and complementary diagnostics: radiography, computed tomography, magnetic resonance imaging (MRI), electroneuromyography, and clinical pathology tests. Intervertebral disk disease (IVDD) and non-compressive myelopathy were diagnosed by MRI and compressive myelopathy and trauma by radiographic image. All musculoskeletal diseases were diagnosed by radiography. According to these assessments, each clinical case was classified and included in the main types of specific conditions of neurological or musculoskeletal diseases which are discriminated in the results (Figure 1).
Figure 1.
A — Prevalence (%) of the types of neurological diseases (n = 145) (PNS — peripheral nervous system; NMJ — neuromuscular junction); B — spinal cord injury (n = 119) (IVDD — intervertebral disc disease); and C — musculoskeletal diseases (HD — hip dysplasia) in dogs treated with acupuncture and related techniques (ALG) and acupuncture and analgesic therapy (AAG), for up to 24 wk (n = 36).
Initially all animals were subjected to once-weekly sessions of AP, for about 20 min and the maximum trial period was 24 wk. Once the animals improved, the AP sessions were conducted every other week. Sucess of treatment was analyzed by comparing data before, during, and after the proposed therapy. The dogs were discharged when treatment was successful before 24 wk. Cancer patients were excluded, as well as animals with comorbidities or when treatments were modified since the first assessment carried out before any treatment at the beginning of the study.
This study was approved by the Institutional Ethical Committee on Animal Use for Research (protocol number 150/2014). The owners were informed about the study and signed a consent form.
Evaluation
Dog owners answered at each treatment session, every wk or at least every other wk, 2 validated questionnaires, the HCPI (8) and health-related quality of life scale for dogs with signs of pain secondary to cancer (QLA) (3) and the VAS for pain and locomotion (5). The HCPI is a multifactorial and descriptive questionnaire to assess chronic pain in dogs and consists of 11 questions, with a minimum score of 0 and a maximum score of 44, representing maximum intensity of chronic pain (8). The QLA consists of 12 questions; “0” and “36” are considered the worst and best quality of life, respectively (3). The VAS is a line of 100 mm length; for pain (VASpain) or locomotion (VASloc), “zero” is the best and “100” is the worst possible condition (5). The HCPI was translated to the native language of the owners and was in process of validation during the study; QLA was already validated in the native language of owners.
Successful treatment was based on a composite score of ≤ 22 in the HCPI, which corresponds to 50% of the total score, ≥ 24 for QLA, which is the score empirically indicative of good quality of life (66% of the total score) and ≤ 33 mm for both VAS, which is a score empirically used for intervention analgesia in acute pain. Success was considered for each individual scale; therefore, some cases were included as success in one scale but not in another. All baselines scores were taken at the first AP session before the begining of treatment. Dogs that had baseline scores below the success cut-off scores for HCPI, QLA, and VAS and worsened after treatment were classified as treatment failures or unsuccessful and were included in the analysis. Dogs that were already scored as success at the beginning of treatment and remained as such, were not included in the survival curves.
Treatment
Animals were treated as follows: alternative medicine (ALG, n = 50), which included AP and related techniques [EA, LASER and ozone and/or less frequently pharmacopuncture (injection of microdoses of drugs into acupoints) or moxibustion (the stimulation of an acupoint by burning a cylinder of moxa placed close to the acupoint)] and alternative medicine associated with conventional analgesics and adjuvant analgesics (AAG, n = 131). In this group, analgesics [nonsteroidal (NSAIDs) and steroidal (SAIDs) anti-inflammatory drugs, opioids, amitriptyline, amantadine, gabapentin] and adjuvant analgesics [nutraceuticals, transcutaneous electrical nerve stimulation (TENS), magnetic and antalgic physical therapy] were used alone or in combination, and the protocols were discontinued or modified according to the individual clinical response.
Animals that were not under treatment before or were not treated with analgesics or adjuvant analgesics after the beginning of AP, were included in the ALG group. Animals that were under any treatment before or were treated with analgesics or adjuvant analgesics after the beginning of AP were included in the AAG group. In most animals of the AAG group, the use of analgesics, especially NSAIDs and SAIDs, was already in progress. When the clinical condition improved after the AAG treatment, drugs were discontinued, AP was continued but the dogs remained in the same group (AAG).
Statistical analysis
Non-parametric data were analyzed by Wilcoxon test to compare the scores of each scale and type of disease (neurological versus musculoskeletal) before and after the onset of treatments. Kaplan-Meier curves were used to evaluate the percentage of success during AAG and ALG treatments. The dogs were only discharged when treatment was successful before 24 wk. Differences were considered significant when P < 0.05.
Results
We selected 181 of 254 evaluated animals based on the inclusion and exclusion criteria. Demographic data included: 61% female and 39% male dogs; 29% crossbred, 16% dachshund, 9% poodle, 8% Labrador, 3% Rottweiller, and 35% other breeds; 8% were < 1-year-old, 55% between 1- and 10-years-old, and 37% > 10-years-old; 31% were < 10 kg, 43% weighed between 10 and 25 kg, and 26% weighed > 25 kg.
Statistical power of all analyses was > 95%. In about 15% of the cases it was not always the same owner who filled out the questionnaires from 1 session to another. The distribution of each specific type of neurological and musculoskeletal disease is expressed in Figure 1.
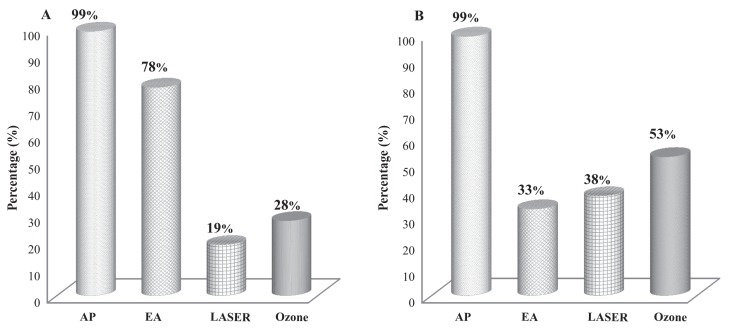
The frequency of use of different techniques associated with AP to treat neurological and musculoskeletal cases is described in Figure 2. From the dogs that received EA, 6% also received laser and ozone in acupoints.
Figure 2.
Frequency (%) of use of different techniques associated with acupuncture (AP) in A — dogs with neurological disease (n = 145) and B — dogs with musculoskeletal disease (n = 36) treated for up to 24 wk (EA — electroacupuncture).
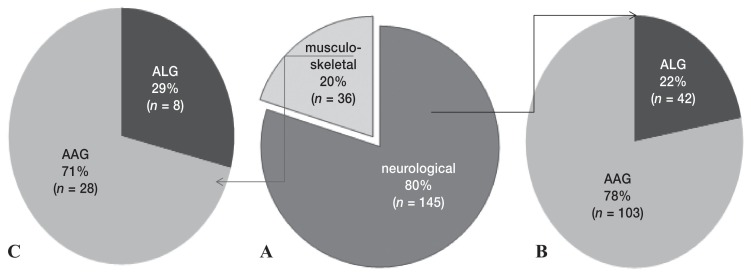
The AAG treatment was predominant in both neurological and musculoskeletal cases (Figure 3).
Figure 3.
A — Prevalence (%) of neurological and musculoskeletal diseases in 181 dogs and distribution of dogs treated with acupuncture and related techniques (ALG) or acupuncture and analgesic therapy (AAG), for up to 24 wk in B — neurological (n = 145), and in C — musculoskeletal disease cases (n = 36).
Scale evaluation
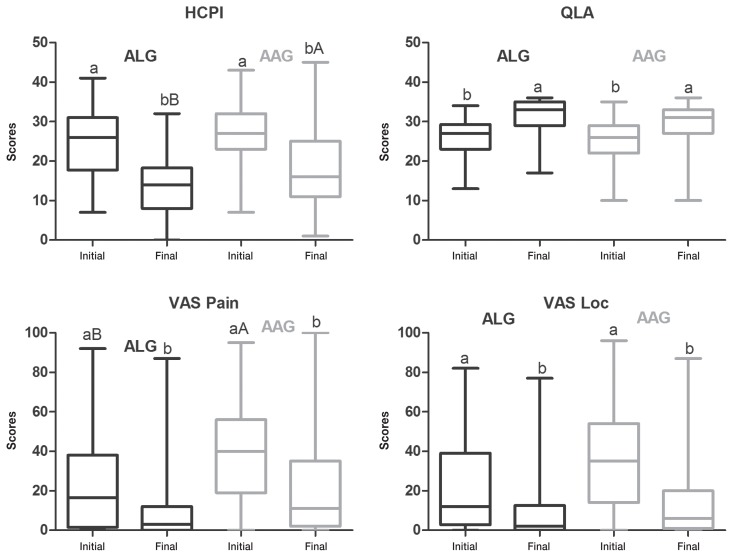
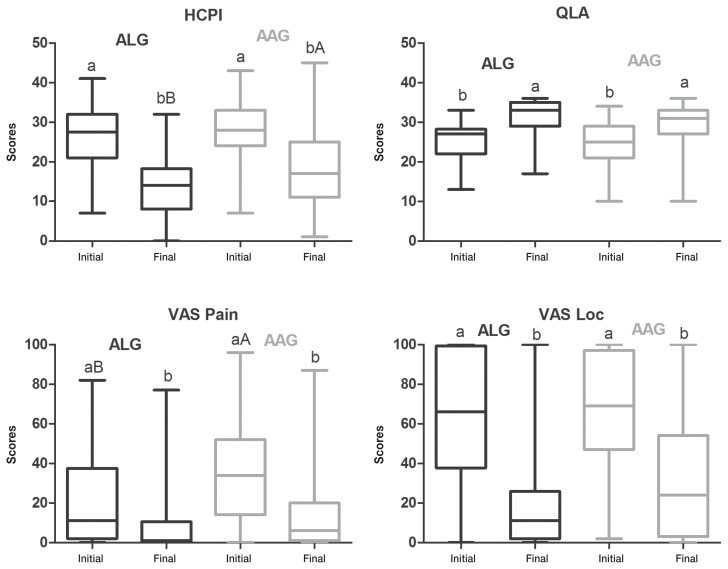
Before treatments all animals scored outside the success range in at least 1 of the scales and most dogs scored outside the success score range for all 4 scales. At the beginning of treatment, there was no difference between ALG and AAG for HCPI, QLA, and VASloc, when both neurological and musculoskeletal disorders were grouped (n = 181) (Figure 4) or in neurological patients only (n = 145) (Figure 5). Success was achieved in QLA and VASloc at the end of both treatments, with no difference between them; however, HCPI scores were lower in ALG than in AAG (P = 0.01).
Figure 4.
Box-and-whisker plots of Helsinki chronic pain index (HCPI), quality of life assessment (QLA), visual analog scale for pain (VASpain) and locomotion (VASloc) for dogs with neurological (n = 145) and musculoskeletal (n = 36) diseases grouped, before and after treatment with acupuncture and related techniques (ALG, n = 50) or acupuncture and analgesic therapy (AAG, n = 131), either when success was achieved before 24 wk or, when this was not the case, at 24 wk of treatment. The top and bottom box lines represent the interquartile range (25% to 75%), the line within the box represents the median and the extremes of the whiskers represent the minimum and maximum values. Different capital letters indicate significant differences between groups at each time, with A > B; different small letters indicate significant differences between baseline and end of trial in the same group, with a > b.
Figure 5.
Box-and-whisker plots of Helsinki chronic pain index (HCPI), quality of life assessment (QLA), visual analog scale for pain (VASpain) and locomotion (VASloc) for dogs with neurological diseases (n = 145), before and after treatment with acupuncture and related techniques (ALG, n = 42) or acupuncture and analgesic therapy (AAG, n = 103), either when success was achieved before 24 wk or, when this was not the case, at 24 wk of treatment. The top and bottom box lines represent the interquartile range (25% to 75%), the line within the box represents the median and the extremes of the whiskers represent the minimum and maximum values. Different capital letters indicate significant differences between groups at each time, with A > B; different small letters indicate significant differences between baseline and end of trial in the same group, with a > b.
The initial VASpain scores for the AAG group were higher than the scores for the ALG group in all animals (Figure 4) and in neurological patients (Figure 5). VASpain scores were reduced in both groups at the end of the trial.
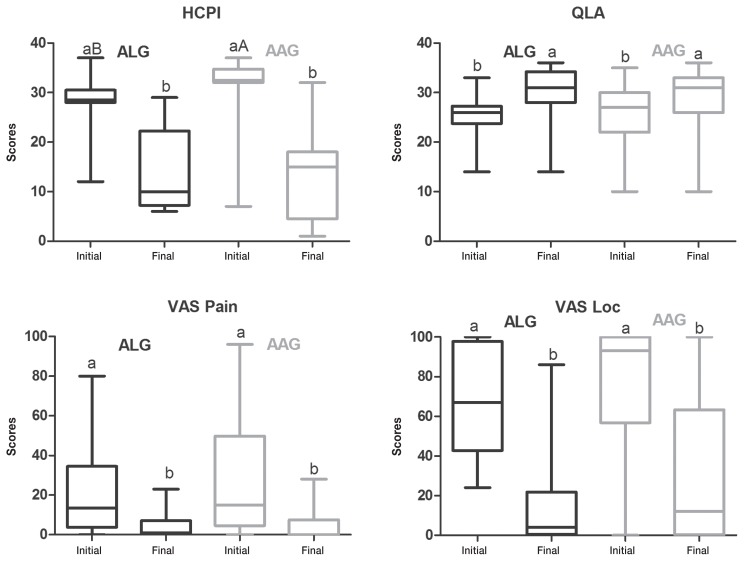
There was no difference between groups for QLA and both VAS scores in musculoskeletal patients (n = 36), at the beginning and at the end of the trial period. These scores were reduced after both treatments (Figure 6). Initial HCPI scores for the ALG group were lower than for the AAG group (P = 0.01), with no difference between groups at the end of treatment. The HCPI scores were reduced in both groups (Figure 6).
Figure 6.
Box-and-whisker plots of Helsinki chronic pain index (HCPI), quality of life assessment (QLA), visual analog scale for pain (VASpain) and locomotion (VASloc) for dogs with musculoskeletal diseases (n = 36), before and after treatment with acupuncture and related techniques (ALG, n = 8) or acupuncture and analgesic therapy (AAG, n = 28), either when success was achieved before 24 wk or, when this was not the case, at 24 wk of treatment for up to 24 wk. The top and bottom box lines represent the interquartile range (25% to 75%), the line within the box represents the median and the extremes of the whiskers represent the minimum and maximum values. Different capital letters indicate significant differences between groups at each time, with A > B; different small letters indicate significant differences between baseline and end of trial in the same group, with a > b.
Survival curves
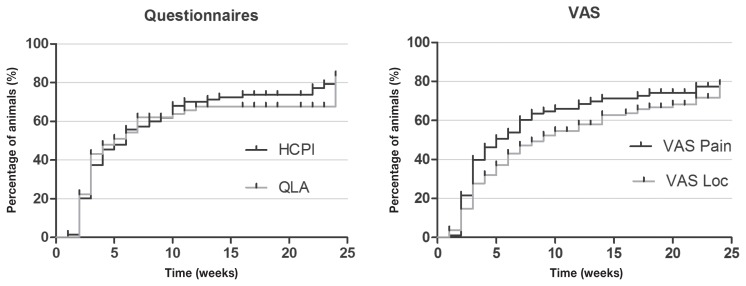
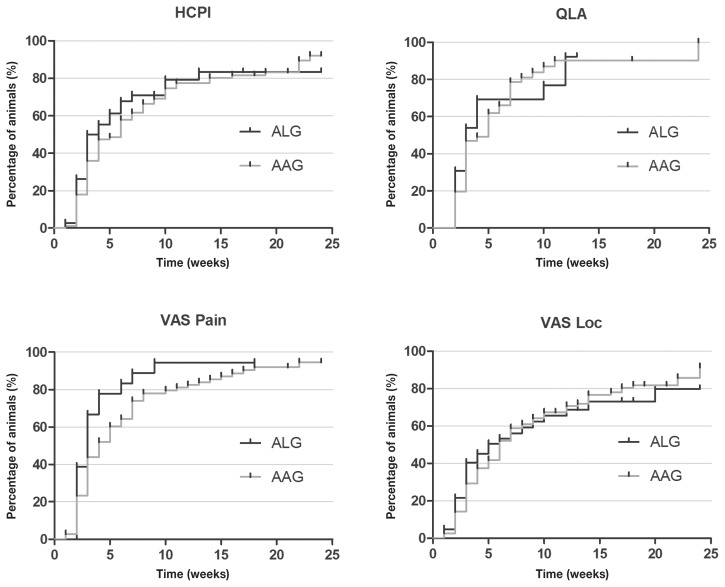
According to the analysis of Kaplan-Meier curves, at the end of 24 wk, success was achieved in 79%, 84%, 78% and 78% of animals suffering from neurological and musculoskeletal diseases combined (submitted to both treatments), according to HCPI, QLA and both VAS, respectively (Figure 7).
Figure 7.
Kaplan-Meier curve for Helsinki chronic pain index (HCPI), quality of life assessment (QLA), visual analog scale for pain (VASpain) and locomotion (VASloc) for dogs with neurological (n = 145) and musculoskeletal (n = 36) diseases, during 24 wk of treatment with (ALG, n = 50) or acupuncture and analgesic therapy (AAG, n = 131). Each rung represents the percentage of animals which obtained scores of ≤ 22 at HCPI, ≥ 24 at QLA and ≤ 33 in both VAS over time. The small vertical lines in each graph represent the week in which treatment was successful in at least 1 patient. The vertical axis represents the percentage success achieved at each week of treatment until 24 wk.
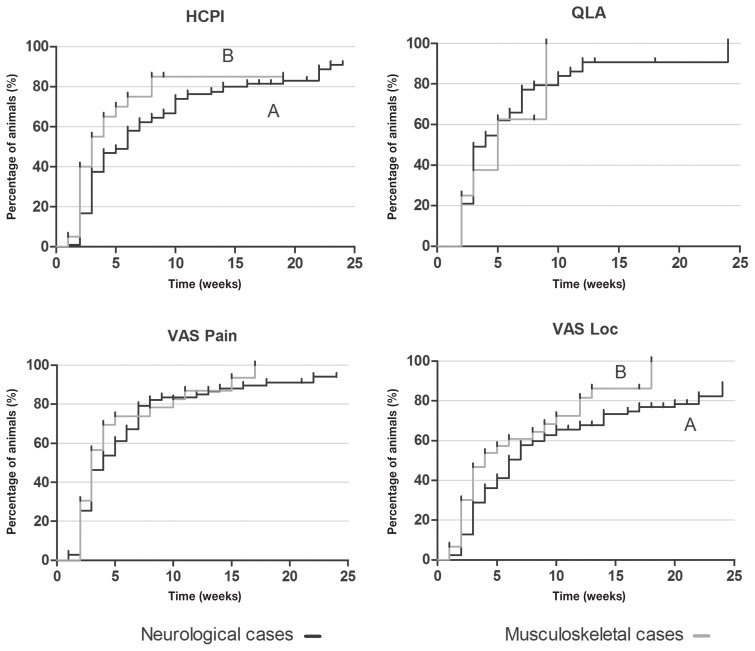
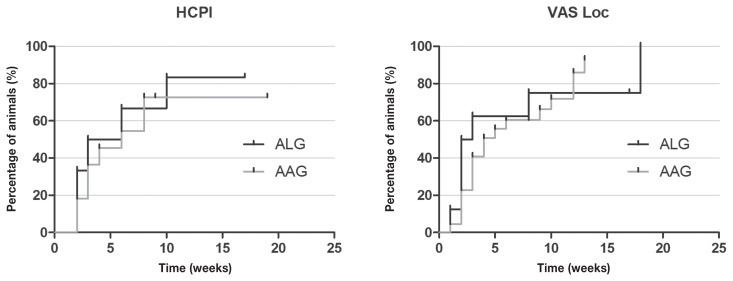
Dogs with musculoskeletal diseases improved faster than those with neurological diseases according to HCPI and VASloc scores (P = 0.003 and P = 0.045, respectively) (Figure 8). Although there was no difference for QLA and VASpain scores between the 2 types of diseases, QLA scores also improved faster in dogs with musculoskeletal disorders (Figure 8).
Figure 8.
Kaplan-Meier curve for Helsinki chronic pain index (HCPI), quality of life assessment (QLA), visual analog scale for pain (VASpain) and locomotion (VASloc) for dogs with neurological (n = 145) and musculoskeletal (n = 36) diseases grouped, during 24 wk of treatment with (ALG, n = 50) or acupuncture and analgesic therapy (AAG, n = 131). Each rung represents the percentage of animals which obtained scores of ≤ 22 at HCPI, ≥ 24 at QLA and ≤ 33 in both VAS over time. Different capital letters express differences between groups during 24 wk, with A > B. The small vertical lines in each graph represent the week in which treatment was successful in at least 1 patient. The vertical axis represents the percentages success achieved at each week of treatment until 24 wk.
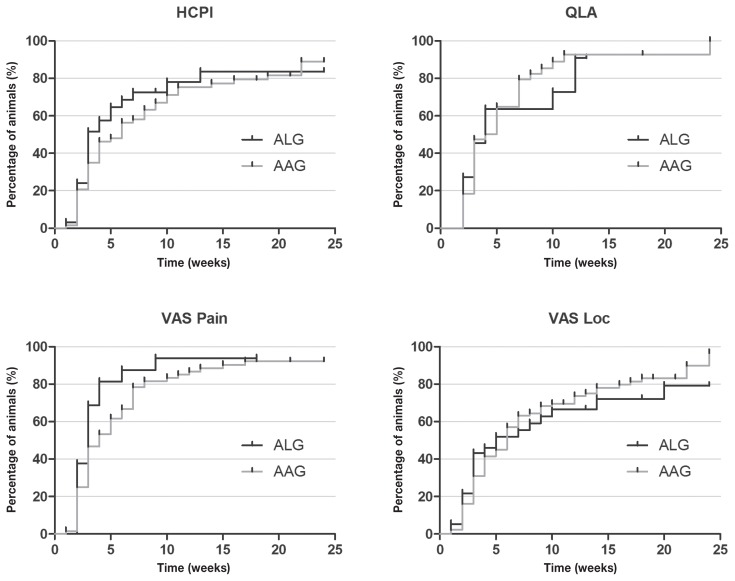
There was no difference between treatments when both diseases were grouped or when each disease was analyzed separately (Figures 9–11).
Figure 9.
Kaplan-Meier curve for Helsinki chronic pain index (HCPI), quality of life assessment (QLA), visual analog scale for pain (VASpain) and locomotion (VASloc) for dogs with neurological (n = 145) and musculoskeletal (n = 36) diseases grouped, during 24 wk of treatment with acupuncture and related techniques (ALG, n = 50) or acupuncture and analgesic therapy (AAG, n = 131). Each rung represents the percentage of animals which obtained scores of ≤ 22 at HCPI, ≥ 24 at QLA and ≤ 33 in both VAS over time. The small vertical lines in each graph represent the week in which treatment was successful in at least 1 patient. The vertical axis represents the percentage success achieved at each week of treatment until 24 wk.
Figure 10.
Kaplan-Meier curve for Helsinki chronic pain index (HCPI), quality of life assessment (QLA), visual analog scale for pain (VASpain) and locomotion (VASloc) for dogs with neurological diseases (n = 145), during 24 wk of treatment with acupuncture and related techniques (ALG, n = 42) or acupuncture and analgesic therapy (AAG, n = 103). Each rung represents the percentage of animals which obtained scores of ≤ 22 at HCPI, ≥ 24 at QLA and ≤ 33 in both VAS over time. The small vertical lines in each graph represent the week in which treatment was successful in at least one patient. The vertical axis represents the percentage success achieved at each week of treatment until 24 wk.
Figure 11.
Kaplan-Meier curve for Helsinki chronic pain index (HCPI) and visual analogue scale for locomotion (VASloc) of 36 dogs with musculoskeletal diseases, during 24 wk of treatment with acupuncture and related techniques (ALG, n = 8) or acupuncture and analgesic therapy (AAG, n = 28). Each rung represents the percentage of animals which obtained scores of ≤ 22 at HCPI, ≥ 24 and ≤ 33 in VASloc over time. The small vertical lines in each graph represent the week in which treatment was successful in at least 1 patient. The vertical axis represents the percentage success achieved at each week of treatment until 24 wk.
Discussion
Acupuncture and related techniques or the combination of AP and other analgesic modalities reduce chronic pain and improve quality of life in dogs suffering from neurological and musculoskeletal diseases (10,11,17). Conditions such as spinal cord injuries and hip-joint arthritis usually show moderate clinical benefits after conventional or surgical treatment (10,11,18).
The analgesic effect produced by AP and related techniques in this study has been previously shown in both acute (16,19,20) and chronic pain (10,11,17,21). Possible local and systemic mechanisms behind the AP effect are based on the release of several neurotransmitters (such as endorphins, enkephalins, dynorphins, serotonin, norepinephrine, dopamine, and acetyl-choline), changes in cell signaling, modulation of the N-methyl-D-aspartate receptor and reduction of hyperalgesia and allodynia in patients suffering from chronic and neuropathic pain. These mechanisms have been reviewed elsewhere (22,23). Acupuncture also regulates inflammation processes and growth factors by increasing blood circulation in affected areas (24).
Electroacupunture is the most common AP-related technique used for analgesia in previous studies (10,11,18) and here. This technique combines the mechanical effect of the needle and electrical stimulation. The quality and intensity of the hypoalgesic effect depends on the choice of acupoints and frequency of electrical stimulation used, which determine the class of neuropeptides released in the central nervous system (18).
When OA is considered specifically, AP reduces metalloproteinases and increases their tissue inhibitors, suggesting a chondroprotective effect in rats (25). The combination of other techniques with AP in the ALG group also likely contributed to the analgesic effect observed here. Both laserpuncture (15) and intra-rectal ozone insufflation and ozoniopuncture (16) were effective in avoiding or minimizing postoperative pain in dogs undergoing ovariohysterectomy. Ozone therapy has anti-inflammatory and analgesic properties, and by promoting a very short acting oxidative stress, stimulates the endogenous defence mechanisms and mediates an immune response (16).
In this study, the treatment with AP (ALG) or AP combined with analgesics (AAG) was chosen according to specific requirements of each case and, therefore, there were no defined criteria by which to assign animals to each group and it is not possible to make any comparison about efficacy between these different treatments. This is a limitation of the study as one would expect that other analgesic regimes were included in AAG, because animals were suffering more intense pain, as shown by the greater pre-treatment VAS pain scores in the AAG group. The inclusion of analgesics could contribute to a better and quicker recovery in this group. However, according to all assessed instruments, both treatments improved neurological and musculoskeletal signs. There is some controversy regarding the benefit of combining AP and conventional medicine. A previous report suggested that the use of NSAIDs and SAIDs combined with AP, provided better results than the use of AP alone in dogs with thoracolumbar IVDD (26). However, in our study, the neurological patients treated with AP only showed a better recovery compared to those in which AP was combined with conventional medicine but this result may be biased by different disease severity between groups. In humans suffering from chronic low back pain, AP was more effective for pain relief and functional improvement compared with no treatment or AP associated with conventional therapy (27).
Our results suggest, like previous ones, that when used alone or in combination with analgesics or adjuvant analgesics, AP is a relevant conservative treatment to alleviate pain and improve quality of life in dogs with neurological and/or musculoskeletal diseases. The use of a multimodal therapeutic approach may reduce doses of conventional analgesics and therefore their adverse effects (14).
Although AP and related techniques (ALG) produced similar results to the combination of AP with conventional analgesics (AAG) when success curves of the neurological diseases were compared, in contrast, especially for HCPI, musculoskeletal patients showed a better outcome after AP associated with other analgesic modalities, as reported in some human OA studies (28).
Previous studies reported that both AP and carprofen reduced pain in dogs with hip dysplasia, but only AP maintained the effect for 2 wk after the end of treatment (29). Other types of local stimulation, such as gold implant, without the need for weekly AP sessions, reduced pain for of up to 2 y in dogs with hip dysplasia (17). In humans AP was more effective than NSAID to treat carpal tunnel syndrome (30), thus it is important to highlight the additional value of this technique to minimize chronic pain. Our study included a wide range of diseases and analgesics, strengthening the importance of a multimodal treatment in dogs with chronic pain.
Osteoarthritis can cause hyperalgesia and evolve into neuropathic pain, given the fact that, for example, 80% of afferent neurons in the knee joint are nociceptors (31). Therefore use of analgesic adjuvants, such as gabapentin and amitriptyline may be required, combined with AP, as in this study, because treatment with NSAID or SAID is limited (1).
The promising results of the association of AP with conventional medicine strengthen the concept that AP may be used in addition to conventional therapy to treat chronic pain. Medicine should be integrative, wherein all possible therapies should be used to improve the patient’s condition.
Animals with musculoskeletal diseases achieved better results for HCPI and VASloc than those with neurological disorders. This may be biased by the fact that some dogs with neurological problems were suffering from mild to moderate OA, which may have hindered their recovery. In contrast, musculoskeletal cases were exclusively orthopedic, with no neurological deficits.
There is no validated test to assess outcome in canine neurological diseases. The HCPI has only been validated for OA, and not for neurological cases; however, it was necessary to use the same scales to compare both neurological and musculoskeletal diseases. Since most of the HCPI assessment involves locomotion, which is also compromised in neurological cases, the authors considered that this instrument might be used for the neurological cases as well in this study.
Although the QLA questionnaire was designed for dogs with cancer-related pain, we considered it useful to evaluate the quality of life in dogs with neurological and musculoskeletal diseases in this study, as by the time the study was started, to our knowledge, there was no other tool to assess quality of life in dogs (3). A new tool to assess quality of life in various diseases was published after the beginning of this study (7).
A study investigating reliability and validity of VAS to measure chronic pain in dogs with OA (5) concluded that there was a significant correlation between the VAS score and the quality-of-life scale and HCPI scores. Use of VAS was valid and reliable, but face validity was only observed once the owners perceived that pain had abated and returned after initiating and withdrawing NSAID treatment, respectively. Considering that VAS is a poor pain assessment tool for untrained owners (5), we included 2 other validated questionnaires in the study.
This study had some limitations. For ethical reasons a placebo was not included as a negative control group, once the owner had requested AP to treat their pet. However a previous study showed that the analgesic effect of inserting needles into sham points was not significant (19). A temporal and/or placebo improvement effect cannot be disregarded, but HCPI has been validated to compensate for this effect (8). In a recent blinded controlled study from our group comparing AP versus carprofen versus placebo to treat hip dysplasia in dogs, by using the same measurement tools, the placebo effect was negligible (29).
Another limiting factor was the difficulty that some owners felt in interpreting some questions, but the owners were not given any guidance on how to complete the questionnaires. In about 15% cases it was not always the same owner who filled out the questionnaires from one session to another, but as questionnaires were filled every week or every other week, this would reduce a possible variation of the overall results based on evaluation by different owners.
As we used patients from the routine practice, some variables could not be controlled. A limitation, inherent to any clinical experiment, is the heterogeneity of the population and epidemiological data, with different severity of diseases and associations among them. Therefore the authors did not use the same treatment protocol or same acupoints and treatments were adjusted individually according to the specific need of each animal.
We conclude that, when used alone or combined with analgesics, AP and related techniques reduced pain and improved quality of life in dogs with neurological and musculoskeletal diseases. Dogs with musculoskeletal disorders had a better improvement in chronic pain and locomotion than those with neurological disorders. The clinical relevance of our findings is that AP is an important conservative therapeutic tool to be included in the multimodal treatment protocols of neurological and musculoskeletal diseases in dogs.
Acknowledgment
We are grateful to FAPESP (São Paulo Research Foundation) for the provision of a masters scholarship (Process 2013/02462-1). CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Mathews K, Kronen PW, Lascelles D, et al. (WSAVA) Guidelines for recognition, assessment and treatment of pain. J Small Anim Pract. 2014;55:10–68. doi: 10.1111/jsap.12200. [DOI] [PubMed] [Google Scholar]
- 2.Reid J, Nolan AM, Hughes JML, Lascelles D, Pawson P, Scott EM. Development of the short-form Glasgow Composite Measure Pain Scale (CMPS-SF) and derivation of an analgesic intervention score. Animal Welfare. 2007;16:97–104. [Google Scholar]
- 3.Yazbek KVB, Fantoni DT. Validity of a health-related quality-of-life scale for dogs with signs of pain secondary to cancer. J Am Vet Med Assoc. 2005;226:1354–1358. doi: 10.2460/javma.2005.226.1354. [DOI] [PubMed] [Google Scholar]
- 4.Brown DC, Boston RC, Coyne JC, Farrar JT. Ability of the Canine Brief Pain Inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc. 2008;233:1278–1283. doi: 10.2460/javma.233.8.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hielm-Björkman AK, Kapatkin AS, Rita JH. Reliability and validity of a visual analogue scale used by owners to measure chronic pain attributable to osteoarthritis in their dogs. Am J Vet Res. 2011;72:601–607. doi: 10.2460/ajvr.72.5.601. [DOI] [PubMed] [Google Scholar]
- 6.Walton MB, Cowderoy E, Lascelles D, Innes JF. Evaluation of construct and criterion validity for the ‘Liverpool Osteoarthritis in Dogs’ (LOAD) clinical metrology instrument and comparison to two other instruments. PLoS One. 2013 doi: 10.1371/journal.pone.0058125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid J, Wiseman ML, Scott EM, Nolan AM. Development, validation and reliability of a web-based questionnaire to measure health-related quality of life in dogs. J Small Anim Pract. 2013;54:227–233. doi: 10.1111/jsap.12059. [DOI] [PubMed] [Google Scholar]
- 8.Hielm-Björkman AK, Rita JH, Tulamo RM. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish owners of dogs with chronic signs of pain caused by osteoarthritis. Am J Vet Res. 2009;70:727–734. doi: 10.2460/ajvr.70.6.727. [DOI] [PubMed] [Google Scholar]
- 9.Levine JM, Budke CM, Levine GJ, Kerwin SC, Hettlich BF, Slater MR. Owner-perceived, weighted quality-of-life assessments in dogs with spinal cord injuries. J Am Vet Med Assoc. 2008;233:931–935. doi: 10.2460/javma.233.6.931. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi AM, Matera JM, Pinto AC. Evaluation of electroacupuncture treatment for thoracolumbar intervertebral disk disease in dogs. J Am Vet Med Assoc. 2007;231:913–918. doi: 10.2460/javma.231.6.913. [DOI] [PubMed] [Google Scholar]
- 11.Joaquim JGF, Brondani JT, Luna SPL, Torelli SR, Rahal CR, Freitas FP. Comparison of decompressive surgery, electroacupuncture, and decompressive surgery followed by electroacupuncture for the treatment of dogs with intervertebral disk disease with long-standing severe neurologic deficits. J Am Vet Med Assoc. 2010;236:1225–1229. doi: 10.2460/javma.236.11.1225. [DOI] [PubMed] [Google Scholar]
- 12.MacPherson H, Maschino AC, Lewith G, Foster NE, Witt CM, Vickers AJ Acupuncture Trialists’ Collaboration. Characteristics of acupuncture treatment associated with outcome: An individual patient meta-analysis of 17,922 patients with chronic pain in randomised controlled trials. PLoS One. 2013 doi: 10.1371/journal.pone.0077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao L, Zhang XL, Gao YS, Jiang Y. Needle acupuncture for osteoarthritis of the knee. A systematic review and updated meta-analysis. Saudi Med. 2012;33:526–532. [PubMed] [Google Scholar]
- 14.Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482–503. doi: 10.1097/ALN.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taffarel MO, Cardoso GS, Luna SPL, et al. Preemptive analgesia with laserpuncture in dogs undergoing ovariohysterectomy. Vet Anaesth Analg. 2013;40:4. [Google Scholar]
- 16.Teixeira LR, Luna SPL, Taffarel MO, et al. Comparison of intrarectal ozone, ozone administered in acupoints and meloxicam for postoperative analgesia in bitches undergoing ovariohysterectomy. Vet J. 2013;197:794–799. doi: 10.1016/j.tvjl.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Jaeger GT, Larsen S, Søli N, Moe L. Two years follow-up study of the pain-relieving effect of gold bead implantation in dogs with hip-joint arthritis. Acta Vet Scand. 2007;49:9. doi: 10.1186/1751-0147-49-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang JW, Jeong SM, Seo KM, Nam TC. Effects of corticosteroid and electroacupuncture on experimental spinal cord injury in dogs. J Vet Sci. 2003;4:97–101. [PubMed] [Google Scholar]
- 19.Cassu RN, Luna SPL, Clark RM, Kronka SN. Electroacupuncture analgesia in dogs: Is there a difference between uni or bilateral stimulation? Vet Anaesth Analg. 2008;35:52–61. doi: 10.1111/j.1467-2995.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 20.Luna SPL, Martino ID, Lorena SER, et al. Acupuncture and pharmacopuncture are as effective as morphine or carprofen for postoperative analgesia in bitches undergoing ovariohysterectomy. Acta Cirurgica Brasileira. 2015;30:831–837. doi: 10.1590/S0102-865020150120000007. [DOI] [PubMed] [Google Scholar]
- 21.Cho JH, Nam DH, Kim KT, Lee JH. Acupuncture with non-steroidal anti-inflammatory drugs (NSAIDs) versus acupuncture or NSAIDs alone for the treatment of chronic neck pain: An assessor-blinded randomised controlled pilot study. Acupunct Med. 2014;32:17–23. doi: 10.1136/acupmed-2013-010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Fry LM, Neary S, Sharrock J, Rychel JK. Acupuncture for analgesia in veterinary medicine. Top Companion Anim Med. 2014;29:35–42. doi: 10.1053/j.tcam.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Park SI, Sunwoo YY, Jung YJ, et al. Therapeutic effects of acupuncture through enhancement of functional angiogenesis and granulogenesis in rat wound healing. Evid Based Complement Alternat Med. 2012 doi: 10.1155/2012/464586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bao F, Sun H, Wu ZH, Wang DH, Zhang YX. Effect of acupuncture on expression of matrix metalloproteinase and tissue inhibitor in cartilage of rats with knee osteoarthritis. Zhongguo Zhen Jiu. 2011;31:241–246. [PubMed] [Google Scholar]
- 26.Still J. Analgesic effects of acupuncture in thoracolumbar disc disease in dogs. J Small Anim Pract. 1989;30:298–301. [Google Scholar]
- 27.Liu L, Skinner M, McDonough S, Mabire L, Baxter GD. Acupuncture for low back pain: An overview of systematic reviews. Evid Based Complement Alternat Med. 2015 doi: 10.1155/2015/328196. Available from: doi: 10.1155/2015/328196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin XY, Li XX, Berghea F, Suteanu S. Comparative study on Chinese medicine and western medicine for treatment of osteoarthritis of the knee in Caucasian patients. Zhongguo Zhen Jiu. 2008;28:459–462. [PubMed] [Google Scholar]
- 29.Teixeira LR, Luna SPL, Matsubara LM, et al. Owner assessment of chronic pain intensity and results of gait analysis of dogs with hip dysplasia treated with acupuncture. J Am Vet Med Assoc. 2016;249:1031–1039. doi: 10.2460/javma.249.9.1031. [DOI] [PubMed] [Google Scholar]
- 30.Hadianfard M, Bazrafshan E, Momeninejad H, Jahani N. Efficacies of acupuncture and anti-inflammatory treatment for carpal tunnel syndrome. J Acupunct Meridian Stud. 2014;8:229–235. doi: 10.1016/j.jams.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol. 2013;9:654–664. doi: 10.1038/nrrheum.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]