Introduction
The autoimmune destruction of beta cells, eventually emerging in clinical type 1 diabetes, may start early in life and last for several months or years [1–3]. During this period of time, we have an opportunity to prevent or delay further beta-cell death, and thereby the clinical onset of type 1 diabetes. The ultimate goal would be to stop the autoimmune process at an early stage, to allow the individual to be left with a sufficient insulin secretion to handle daily life intake of carbohydrates, and to never require exogenous insulin or risk for short- and long term diabetes complications.
Individuals with progressive autoimmune beta-cell destruction may be identified by the measure of autoantibodies to beta-cell autoantigens – autoantibodies to glutamate decarboxylase (GAD65A), insulinoma-associated protein 2 (IA-2A), insulin (IAA) and the three variants of zinc transporter 8 (ZnT8R/Q/WA) [4, 5]. If two or more of these autoantibodies are found positive, reflecting progressive beta-cell destruction, the risk for type 1 diabetes is high, 70–100 % [4–6]. Individuals with two or ore islet autoantibodies may be eligible for secondary prevention trials of type 1 diabetes.
Background
Skåne is the most southern part of Sweden, with 1.2 million inhabitants. Childhood diabetes is treated at the pediatric clinics in five different hospitals. In 1995, the Skåne study, aiming to include all newly diagnosed children with diabetes in Skåne, was started as collaboration between the principal endocrinologists at the pediatric clinics in Helsingborg, Kristianstad, Lund, Malmö, Ystad and Ängelholm (Figure 1). Samples were taken, before insulin was given, and analyzed not only for islet autoantibodies but also for endomycieautoantibodies (EMA), and autoantibodies against thyroid peroxidase (TPOA) and thyroglobulin (TGA). During follow-up, annual samples for autoimmunity were taken on the participants included in the study. In 2005 the Skåne study emerged into the Better Diabetes Diagnosis study (BDD). BDD is a national study including all pediatric clinics in Sweden, also including HLA typing and islet autoantibody analyses of all newly diagnosed patients (Figure 1). Both those studies have been important for the understanding of autoimmunity and genes for the diagnosis of pediatric diabetes [7, 8], relations between specific autoantibodies and genes [9, 10], discovery of new autoantigens [11–13], prevalence and genetic predisposition for other autoimmune diseases [14, 15]. However, in order to understand the process from development of islet autoimmunity to type 1 diabetes and find factors triggering islet autoimmunity, we needed to follow children before the diagnosis of diabetes. Therefore, a prospective study of children with high risk HLA-genotypes was initiated, the Diabetes Prediction in Skåne (DiPiS) study (Figure 1).
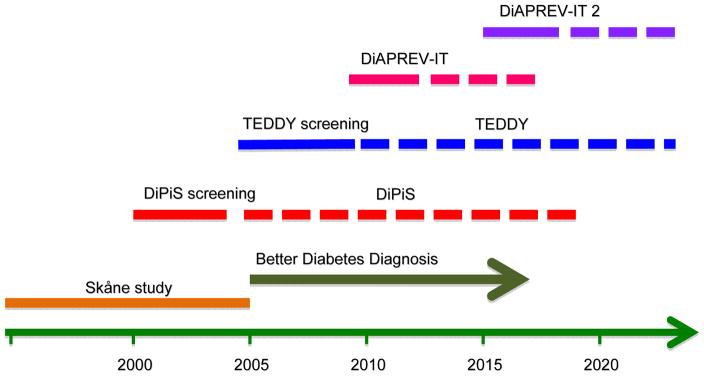
Figure 1.
Timeline of previous and on-going studies in Skåne. The Skåne study in newly diagnosed children with diabetes (start 1995) was succeded by the national Better Diagnosis Study. In the Diabetes Prediction in Skåne (DiPiS) study, we were screening newborn children for risk genes during four years (2000–2004) and follow a risk cohort up to 15 years of age. The Environmental Determinant of Diabetes in the Young (TEDDY) screened newborn children between September 2004 and Februrary 2010 and follow risk children to 15 years of age. The Diabetes Pevention-Immune Tolerance (DiAPREV-IT) and DiAPREV-IT 2 are two secondary prevention studies on autoantibody-positive children. Both are still blinded.
Diabetes Prediction in Skåne - DiPiS
In September 2000, we started to collect cord blood samples of newborns at the five hospitals in Skåne. The samples were analyzed for HLA genotypes and for cord blood islet autoimmunity. During four years we collected 35,000 cord blood samples, i.e. samples from 73 % of the 48,000 children born during the period of time. At two months of age, parents answered a questionnaire on heredity, gestational events, birth data and events during the first two months of the child’s life. A total of 25,000 parents answered those questionnaires. Children with HLA-risk for type 1 diabetes are followed from two years of age annually with questionnaires and blood samples analyzed for autoantibodies (GADA, IA-2A, IAA and ZnT8R/Q/WA). If the child develops more than one islet autoantibody, they are followed more intensive every third month, including sampling for islet autoantibodies, HbA1c and plasma-glucose. Annual oral glucose tolerance test is performed. Currently more than 2000 children are followed in DiPiS [16–18].
The Environmental Determinants of Diabetes in the Young - TEDDY
In September 2004, the screening of newborns in Skåne for type 1 diabetes risk continued within The Environmental Determinants of Diabetes in the Yong (TEDDY) study. This multicenter-study, financed by NIH and NIDDK and composed of six centers, three in Europe (Finland, Germany and Sweden) and three in US (Colorado, Florida/Georgia and Washington), follows more than 6000 children with high risk for type 1 diabetes up to 15 years of age [19, 20]. The aim is to define the triggers of autoimmunity and progression to type 1 diabetes. In total, 421,000 newborn children were screened at birth for risk genotypes for type 1 diabetes and a selected cohort with increased genetic risk is followed [20]. TEDDY children are followed from 3 months of age with questionnaires, blood and plasma samples, stool samples, urin samples and saliva for analysis of factors that may trigger islet autoimmunity and progression to type 1 diabetes. TEDDY children who develop islet autoimmunity are additionally followed with HbA1c, plasma-glucose and OGTT, and are diagnosed at an early stage of disease, often without symptoms, through the TEDDY clinics [21, 22].
The Diabetes Prevention – Immune Tolerance (DiAPREV-IT) studies
While following a large number of children with multiple islet autoantibodies and a high risk for type 1 diabetes in the predictive studies, we could not offer any prevention for the disease. In 2008 we got the opportunity to start an investigator initiated clinical trial with Alum-formulated GAD65 in children at risk. The drug had proven safe in both preclinical and clinical studies, and animal studies indicated a possible preventive effect [23]. In both a phase II study in LADA patients and in a study of newly diagnosed children with type 1 diabetes, a promising effect on saving remaining beta cell capacity had been observed [24] [25], although we now know that phase III studies in newly diagnosed did not reach the efficacy goals [26]. In February 2009 we had all the needed approvals from regulatory authorities to start the first investigator initiated Diabetes Prevention – Immune Tolerance (DiAPREV-IT) study, the first prevention study where Alum-formulated GAD65 was given to children who had not yet developed diabetes (EudraCT 2008-007484-16, NCT01122446) (Figure 1). During 2009–2011 50 children from 4 years of age, with GADA and at least one more islet autoantibody were included in this clinical trial. Two doses of 20 microgram Alum-formulated GAD65 was given to half of the children (n=25) while half of the children (n=25) received placebo. The children were at baseline staged with both intravenous and oral glucose tolerance tests (IvGTT and OGTT) and are after treatment followed according to a strict protocol during five years with alternating OGTT’s ad IvGTT’s. Additionally cellular analyses are performed on fresh cells and PBMC’s frozen for future analyses.
The study will be un-blinded and results analyzed in December 2016 when all children have been followed for five full years after treatment.
The study was designed as phase II study, with only 50 participants, and with safety as the primary endpoint and development of diabetes as the secondary endpoint. Indeed, safety has up to date not been an issue. However, the small number of participants may be a problem when analyzing efficacy. As eligibility for inclusion was based only upon autoantibodies, we ended up with two groups of children – one with impaired glucose metabolism and one with normal glucose metabolism at baseline screening. The children with impaired glucose metabolism, especially with low first phase insulin release measured in IvGTT and elevated 30, 60 and 90 min glucose values at OGTT, had a higher rate of progression to type 1 diabetes than the ones with normal glucose metabolism [27]. We also found that children with high IA-2A was at higher risk of progression to diabetes [27].
After discussions during 2014, we concluded that we wanted to expand the trial. However, since the group of children now was older, and since we wanted to adapt the protocol, the solution was to include children in a new study, as similar to the current DiAPREV-IT so that results could be merged to a meta-analysis. In May 2015 the first children were included in DiAPREV-IT 2 (EudraCT 2014-003755-64, NCT02387164) (Figure 1). The inclusion criteria remained the same, but randomization is stratified by glucose metabolism at the baseline IvGTT and OGTT. We also introduced a secondary endpoint - impaired glucose metabolism for the ones that had normal glucose metabolism at baseline screening. In addition, all children will be treated with Vitamin D, 2000 IE daily, through the full study period as a promotor of the immune tolerance.
Discussion
Until we have identified the mechanisms that trigger the first appearance of the first islet autoantibody and the subsequent progressive autoimmunity, eventually leading to type 1 diabetes, it will be difficult to design primary prevention studies. During the time between the initiation of the autoimmune process and development of type 1 diabetes, secondary prevention of the disease can be tested. However, several issues may complicate the trials. First, when we include the children they may be on different stages of the disease process – some may be very close to type 1 diabetes onset, while some may have years left. Second, we know from studies that follow both first degree relatives or children with high risk HLA that the autoimmune destruction of the beta-cells may occur at different rates. Third, the beta-cell capacity may be fluctuating during the process and also after development of type 1 diabetes – i.e. it is not a steady slope of beta-cell loss. Forth, the best markers of beta-cell capacity we have are glucose metabolism, and also this may be fluctuating between the times of measure. For example a child may have a diabetic OGTT on week and one with only impaired glucose metabolism the next week. Fifth, the autoimmune process may start as early as at 6 months of age. Therefore prevention has to start early and at a low age. Sixth, since the autoimmune process may take years, prevention studies tend to be long-term projects.
The majority of children developing type 1 diabetes are not close relatives to type 1 diabetes patients. Therefore, clinical trials aiming to prevent type 1 diabetes, only including type 1 diabetes relatives, will miss a large number of potential participants. In our prediction studies we screened children already at birth for HLA-mediated risk for type 1 diabetes, and followed the ones with increased risk for development of islet autoimmunity. However, outside studies, this is both ethically complicated and expensive. One possibility could be to screen children at low age for islet autoimmunity and include those positive in prevention trials. However, since autoantibodies develop at different ages, the screening has to be done several times to pick up all children at risk for type 1 diabetes.
Although no drug tested for secondary prevention of type 1 diabetes up to date has shown effective, there may be possibilities to combine different drugs to enhance the effect. Small studies, short studies with mechanistic endpoints, studies with different combinations of therapies will be important. Moreover, data from the current, on-going trials, may lead us to a better knowledge of the natural history of the autoimmune process and make it possible to find new therapies to test.
In conclusion, secondary prevention may be possible in children with islet autoimmunity but not yet diabetes, but the studies are complicated by the variability of glucose metabolism and beta-cell loss. Although the current ongoing studies may turn out to be ineffective in preventing beta-cell loss, additional knowledge about the pattern of progression to diabetes will be gained that may help us to design more effective secondary prevention studies.
References
- 1.Kimpimaki T, Kupila A, Hamalainen AM, et al. The first signs of beta-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. The Journal of clinical endocrinology and metabolism. 2001;86:4782–4788. doi: 10.1210/jcem.86.10.7907. [DOI] [PubMed] [Google Scholar]
- 2.Stene LC, Witso E, Torjesen PA, et al. Islet autoantibody development during follow-up of high-risk children from the general Norwegian population from three months of age: design and early results from the MIDIA study. Journal of autoimmunity. 2007;29:44–51. doi: 10.1016/j.jaut.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Mrena S, Savola K, Kulmala P, Akerblom HK, Knip M Childhood Diabetes in Finland Study G. Natural course of preclinical type 1 diabetes in siblings of affected children. Acta Paediatr. 2003;92:1403–1410. doi: 10.1111/j.1651-2227.2003.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 4.Siljander HT, Veijola R, Reunanen A, Virtanen SM, Akerblom HK, Knip M. Prediction of type 1 diabetes among siblings of affected children and in the general population. Diabetologia. 2007;50:2272–2275. doi: 10.1007/s00125-007-0799-5. [DOI] [PubMed] [Google Scholar]
- 5.LaGasse JM, Brantley MS, Leech NJ, et al. Successful prospective prediction of type 1 diabetes in schoolchildren through multiple defined autoantibodies: an 8-year follow-up of the Washington State Diabetes Prediction Study. Diabetes care. 2002;25:505–511. doi: 10.2337/diacare.25.3.505. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA : the journal of the American Medical Association. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson C, Kolmodin M, Ivarsson SA, et al. Islet cell antibodies (ICA) identify autoimmunity in children with new onset diabetes mellitus negative for other islet cell antibodies. Pediatric diabetes. 2014;15:336–344. doi: 10.1111/pedi.12093. [DOI] [PubMed] [Google Scholar]
- 8.Andersson C, Vaziri-Sani F, Delli A, et al. Triple specificity of ZnT8 autoantibodies in relation to HLA and other islet autoantibodies in childhood and adolescent type 1 diabetes. Pediatric diabetes. 2013;14:97–105. doi: 10.1111/j.1399-5448.2012.00916.x. [DOI] [PubMed] [Google Scholar]
- 9.Andersson C, Larsson K, Vaziri-Sani F, et al. The three ZNT8 autoantibody variants together improve the diagnostic sensitivity of childhood and adolescent type 1 diabetes. Autoimmunity. 2011;44:394–405. doi: 10.3109/08916934.2010.540604. [DOI] [PubMed] [Google Scholar]
- 10.Delli AJ, Vaziri-Sani F, Lindblad B, et al. Zinc transporter 8 autoantibodies and their association with SLC30A8 and HLA-DQ genes differ between immigrant and Swedish patients with newly diagnosed type 1 diabetes in the Better Diabetes Diagnosis study. Diabetes. 2012;61:2556–2564. doi: 10.2337/db11-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skarstrand H, Vaziri-Sani F, Delli AJ, et al. Neuropeptide Y is a minor autoantigen in newly diagnosed type 1 diabetes patients. Pediatric diabetes. 2014 doi: 10.1111/pedi.12222. [DOI] [PubMed] [Google Scholar]
- 12.Vaziri-Sani F, Delli AJ, Elding-Larsson H, et al. A novel triple mix radiobinding assay for the three ZnT8 (ZnT8-RWQ) autoantibody variants in children with newly diagnosed diabetes. Journal of immunological methods. 2011;371:25–37. doi: 10.1016/j.jim.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanatsuna N, Taneera J, Vaziri-Sani F, et al. Autoimmunity against INS-IGF2 protein expressed in human pancreatic islets. The Journal of biological chemistry. 2013;288:29013–29023. doi: 10.1074/jbc.M113.478222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonsdottir B, Andersson C, Carlsson A, et al. Thyroid autoimmunity in relation to islet autoantibodies and HLA-DQ genotype in newly diagnosed type 1 diabetes in children and adolescents. Diabetologia. 2013;56:1735–1742. doi: 10.1007/s00125-013-2934-9. [DOI] [PubMed] [Google Scholar]
- 15.Larsson K, Carlsson A, Cederwall E, et al. Annual screening detects celiac disease in children with type 1 diabetes. Pediatric diabetes. 2008;9:354–359. doi: 10.1111/j.1399-5448.2008.00367.x. [DOI] [PubMed] [Google Scholar]
- 16.Lernmark B, Elding-Larsson H, Hansson G, Lindberg B, Lynch K, Sjoblad S. Parent responses to participation in genetic screening for diabetes risk. Pediatric diabetes. 2004;5:174–181. doi: 10.1111/j.1399-543X.2004.00070.x. [DOI] [PubMed] [Google Scholar]
- 17.Larsson HE, Lynch K, Lernmark B, et al. Diabetes-associated HLA genotypes affect birthweight in the general population. Diabetologia. 2005;48:1484–1491. doi: 10.1007/s00125-005-1813-4. [DOI] [PubMed] [Google Scholar]
- 18.Larsson HE, Lynch K, Lernmark B, Hansson G, Lernmark A, Ivarsson SA. Relationship between increased relative birthweight and infections during pregnancy in children with a high-risk diabetes HLA genotype. Diabetologia. 2007;50:1161–1169. doi: 10.1007/s00125-007-0648-6. [DOI] [PubMed] [Google Scholar]
- 19.Group TS. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatric diabetes. 2007;8:286–298. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 20.Hagopian WA, Erlich H, Lernmark A, et al. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatric diabetes. 2011;12:733–743. doi: 10.1111/j.1399-5448.2011.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elding Larsson H, Vehik K, Bell R, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes care. 2011;34:2347–2352. doi: 10.2337/dc11-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elding Larsson H, Vehik K, Gesualdo P, et al. Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatric diabetes. 2014;15:118–126. doi: 10.1111/pedi.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tisch R, Liblau RS, Yang XD, Liblau P, McDevitt HO. Induction of GAD65-specific regulatory T-cells inhibits ongoing autoimmune diabetes in nonobese diabetic mice. Diabetes. 1998;47:894–899. doi: 10.2337/diabetes.47.6.894. [DOI] [PubMed] [Google Scholar]
- 24.Agardh CD, Lynch KF, Palmer M, Link K, Lernmark A. GAD65 vaccination: 5 years of follow-up in a randomised dose-escalating study in adult-onset autoimmune diabetes. Diabetologia. 2009;52:1363–1368. doi: 10.1007/s00125-009-1371-2. [DOI] [PubMed] [Google Scholar]
- 25.Ludvigsson J, Faresjo M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. The New England journal of medicine. 2008;359:1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 26.Ludvigsson J, Krisky D, Casas R, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. The New England journal of medicine. 2012;366:433–442. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 27.Elding Larsson H, Larsson C, Lernmark A, Di A-ITsg. Baseline heterogeneity in glucose metabolism marks the risk for type 1 diabetes and complicates secondary prevention. Acta diabetologica. 2015;52:473–481. doi: 10.1007/s00592-014-0680-1. [DOI] [PubMed] [Google Scholar]