Abstract
The Global initiative for chronic Obstructive Lung Disease (GOLD) Strategy is a valuable tool for clinicians in the diagnosis and management of patients with established chronic obstructive pulmonary disease (COPD). However, there are no recommendations for the evaluation of individuals, exposed to risk factors, who are most likely to develop COPD. Consequently, it is necessary to consider all of the factors that may play a role in the pathogenesis of COPD: genetic factors, gender, socioeconomic status, disadvantageous factors in childhood, lung diseases and exposure to risk factors such as smoking, biomass fuel smoke, occupational hazards and air pollution. Along with the clinical assessment, periodic spirometry should be performed to evaluate lung function and make possible early detection of individuals who will develop the disease through the rate of forced expiratory volume in 1 second (FEV1) decline. The first spirometry, periodicity, and clinically significant decline in FEV1 will encompass the cornerstones of clinical follow up. This approach allows the implementation of important interventions in order to help individuals to cease contact with risk factors and prevent progressive respiratory impairment with the consequent deterioration of quality of life and increased morbidity and mortality.
Keywords: copd, chronic obstructive pulmonary disease, Global initiative for chronic Obstructive Lung Dis, GOLD, risk factors, FEV1 decline
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive inflammatory disease with a high morbidity and mortality, as well as a large economic and social burden throughout the world.1,2 Smoking is the main risk factor, although the use of biomass fuel, air pollution and population ageing also play an important role in the development of the disease.3-5
The Global initiative for chronic Obstructive Lung Disease (GOLD) Strategy was introduced in 2001 in order to increase awareness and reduce morbidity and mortality, improving preventive strategies and the management of patients with COPD.6 In the first publication, the classification of COPD severity encompassed: Stage 0: patients at risk (normal spirometry with chronic symptoms); Stage I: mild COPD (forced expiratory volume in 1 second [FEV1 ]/forced vital capacity [FVC] < 0.70, FEV1 > 80% predictive with or without chronic symptoms); Stage II: moderate COPD (IIA: FEV1/FVC < 0.70, FEV1 50-80% predictive and IIB: FEV1/FVC < 0.70, FEV1 30-50% predictive, in both cases with or without chronic symptoms); Stage III: severe COPD (FEV1/FVC < 0.70, FEV1 < 30% predictive or the presence of respiratory failure or signs of right heart failure). In the 2006 update, Stage 0, the presence of symptoms and right heart failure were suppressed, shifting Stage II to Stage I, Stage IIA to Stage II, Stage IIB to Stage III and Stage III to Stage IV.7 While in the following updates, including the last update in April 2015,8 there is a brief recommendation for smoking cessation under the chapter “Therapeutic Options,” there are no recommendations for patients exposed to risk factors, symptoms like cough and sputum production, and no recommendation for those who have a decline in lung function without fulfilling GOLD criteria for airway obstruction and COPD. Also, there are no considerations about what population is more likely to develop COPD, how often these individuals should be tested with spirometry, and what criteria we should use to define a patient with abnormal lung function decline. The GOLD strategy does not emphasize screening of patients exposed to risk factors who also have enhancing factors, are more likely to develop COPD, and in whom we should make an effort to implement specific interventions to avoid irreversible lung damage.
Hence, in the evaluation of individuals at risk for COPD we should assess: 1) risk factors (mainly tobacco), 2) enhancing factors that place individuals in a group where COPD is more likely to occur, and 3) lung growth, ageing and lung function decline. Thus, apart from risk factors, other elements should be taken into account in the clinical setting which, if present, configure important factors that make individuals more susceptible to COPD. Finally, lung growth, ageing and lung function decline must be addressed in order to determine normal and abnormal FEV1 decline that will identify individuals with higher chances of developing COPD.
Risk Factors
Tobacco and Other Inhalants
The GOLD 2015 guidelines state that “COPD, a common preventable and treatable disease, is characterized by airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases.”8
Smoking is the most important risk factor for the development of COPD.9-12 Nicotine is a potent, addictive alkaloid inhaled when smoking tobacco and reaches the nervous system within a few seconds stimulating nicotinic receptors of acetylcholine generating addiction through complex mechanisms.13 Approximately 15% of smokers develop COPD14 so it is clear that there are many other factors that contribute to the presence of the disease. However, multiple studies demonstrate that more than 15% of smokers will develop chronic airway obstruction with COPD criteria, with a range of 25%-50%.15-17 Second hand smoke, i.e., ambient cigarette smoke inhaled by non-smokers, represents another important risk factor.18,19
Macrophages may be activated by cigarette smoke and other irritants to release neutrophil-chemotactic factors, such as leukotriene B4 (LTB4) and interleukin (IL)-8. Neutrophils and macrophages release multiple proteinases that break down connective tissue in the lung parenchyma resulting in emphysema, and stimulate mucus secretion.20
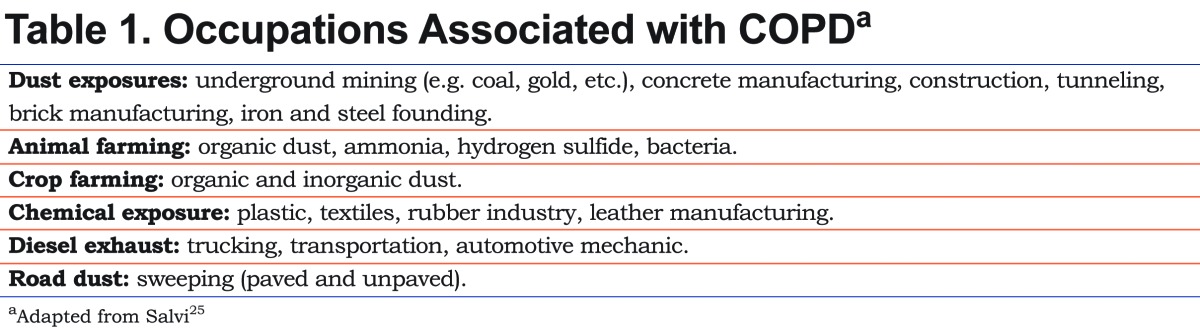
While the use of tobacco is responsible for most cases of COPD, there are other inhalational agents responsible for the development of this disease like biomass fuel smoke, still used in developing countries and estimated to affect 3 billion people worldwide.3,4,21 Also, occupational exposure to dust, fumes and smoke are related to the increased prevalence of chronic symptoms, accelerated decline in FEV1 and establishment of COPD (Table 1).22-25
Enhancing Factors
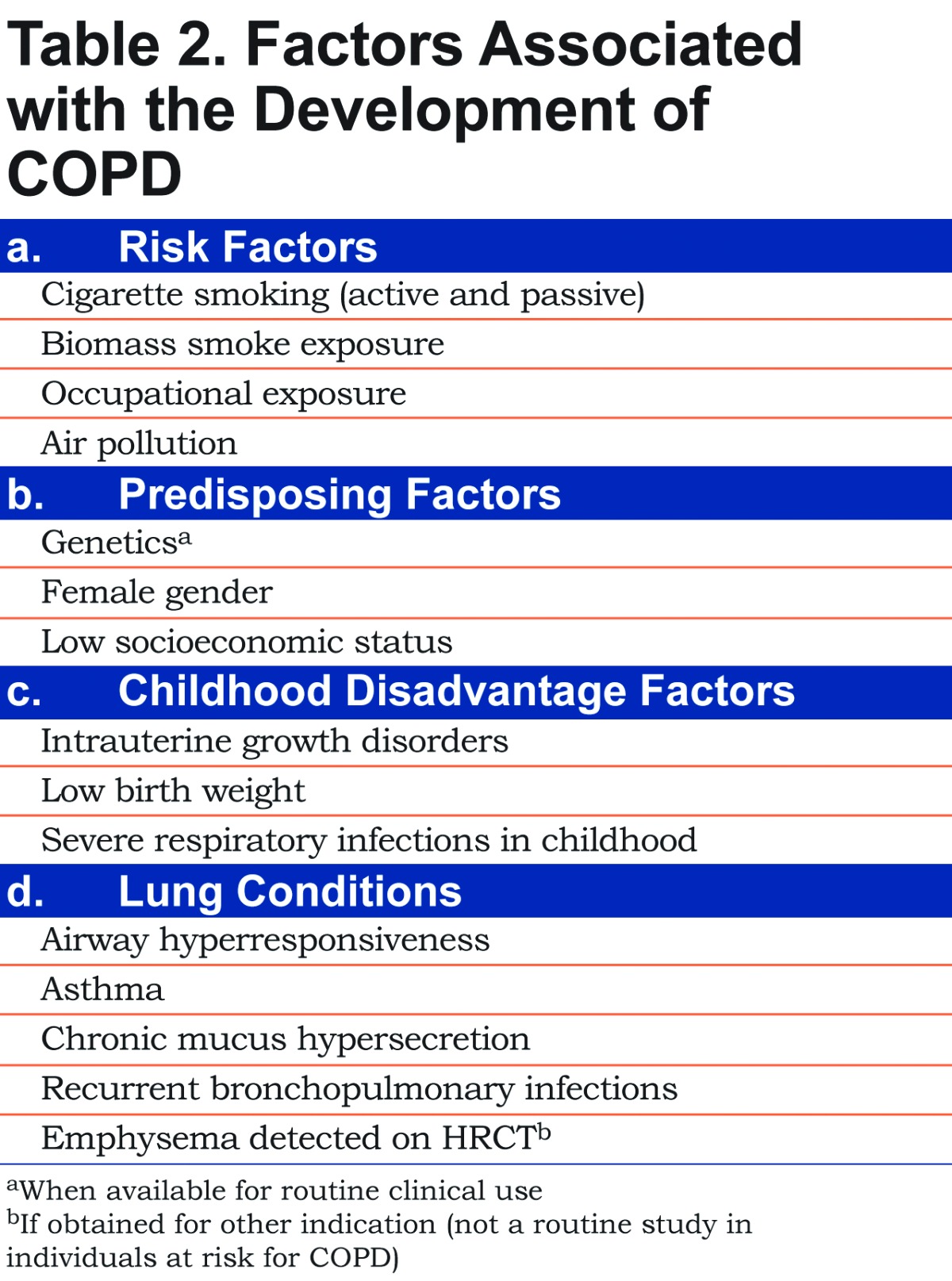
Even though inhaled substances are the most identifiable risk factors for the development of COPD, there are other factors that, when present in an individual exposed to risk factors, make COPD more likely to occur. These enhancing factors could be listed as predisposing factors, childhood disadvantage factors and lung conditions (Table 2). In the evaluation of individuals exposed to risk factors, enhancing factors should always be assessed in order to have an integrative view of the patient and detect potential cases at higher risk for COPD.
Predisposing Factors
Genetic Risks
Genetics is an important factor in the development of COPD.26 There is a close relationship between alpha-1 antitrypsin deficiency and COPD as it is a known abnormality that gives support to the theory of loss of the proteinase/antiproteinase balance, especially in the variant homozygous for the Z allele (PiZZ).27-29 Familial cases suggest that hereditary factors play an important role in the development of COPD.30,31 Of the many genetic changes described as associated with the development of the disease, only 4 genetic variants are significantly associated with its pathogenesis: glutathione-S-transferase (GST) M1 null variant, rs 1800470 in transforming growth factor beta 1 (TGFβ1), rs 1800629 in tumor necrosis factor alpha (TNFα), and rs 1799896 in superoxide dismutase-3 (SOD3).32,33 For this reason, it is not surprising that race and ethnicity play an equally important role. This was demonstrated by the COPD Genetic Epidemiology (COPDGene) study where 42% of African-Americans developed early (<55 years) and severe COPD (FEV1 <50% predictive) compared with 14% of non-Hispanic whites.34 In a cohort of smokers and former smokers of New Mexico it was found that Hispanic ethnicity and those with Native American ancestors had a lower risk of developing COPD.35
Recently, the genetic study done by the United Kingdom Biobank Lung Exome Variant Evaluation confirmed the strong association with the nicotinic acetylcholine receptors CHRNA3 and CHRNA5 at the 15q25 locus, and discovered 5 novel regions of association in or near neural cell adhesion molecule 1 (NCAM1), testis expressed 41/poly (A) binding protein, cytoplasmic 1 pseudogene 2 (TEX41/PABPC1P2), dynein axonemal heavy chain 8 (DNAH8), nucleolar protein 4-like (NOL4L), and lipid phosphate phosphatase-related protein type 5 (LPPR5) implicated in nicotine addiction.36 The investigators also discovered 5 new genetic loci of lung function: nephronectin (NPNT), tet methylcytosine dioxygenase 2 (TET2), major histocompatibility complex, class II, DQ beta 1/ major histocompatibility complex, class II, DQ alpha 2 (HLA-DQB1/HLA-DQA2), KAT8 regulatory NSL complex subunit 1 (KANSL1), and tRNA splicing endonuclease subunit 54 (TSEN54). The lead single nucleotide polymorphisms (SNPs) at these loci were more strongly associated with low FEV1 in never smokers than in heavy smokers, and were also associated with COPD, which would explain the different trajectories in lung function decline in the pathogenesis of COPD.36
In a recent work by Cho et al, a genome-wide association study was performed on quantitative emphysema and airway imaging phenotypes in the COPDGene, ECLIPSE, NETT and GenKOLS studies and on percentage gas trapping in COPDGene. Significant genome-wide associations with loci previously linked to COPD or airflow limitation were identified, including 15q25, hedgehog interacting protein (HHIP), and advanced glycoslation end product-specific receptor (AGER) loci. The study also describes a genome-wide association with emphysema and variants near SERPINA10. One of the most important associations with emphysema was a novel locus located in the gene DLC1 (deleted in liver cancer 1), highly expressed in the lungs.37
Although the advances in genetics and serum markers for COPD38-40 are important steps forward in better understanding the disease, these measurements may still not be available in clinical practice for routine study of patients at risk for COPD. As genetic factors are better understood and test panels are widely available, it may become invaluable information in the future evaluation of individuals at risk for COPD.
Gender
While it is very difficult to compare the decline in lung function between men and women because of bias, there is increasing evidence that women are more likely to develop COPD.41 In a meta-analysis of 55,079 individuals assessed at least twice with spirometry, it was observed that women who smoked had a more rapid decline in lung function between 45 to 50 years of age compared with men smokers.42 Experimental studies indicate that estrogen may account for the increased susceptibility of women through an effect on TGFβ release in small airways leading to fibrosis.43,44
Leptin, a pro-inflammatory adipokine that affects innate and adaptive immunity, is a protein synthesized and secreted by white adipose tissue. Systemic leptin is increased in COPD patients, particularly in women. Leptin, expressed in bronchial epithelial cells, type II pneumocytes and macrophages, differentially increases T helper (Th) 1 cytokine production, suppresses Th2 cytokine production, and increases the release of vascular endothelial growth factor (VEGF). Systemic and airway leptin concentrations may be associated with greater odds of COPD prevalence, particularly among women, and reflect greater airway inflammation and disease severity.45 In a study investigating sex-related differences in adipokines in relation to systemic inflammatory biomarkers in stable COPD patients and body mass index-matched controls, COPD patients were characterized by systemic inflammation, and leptin secretion increased with increasing fat mass, especially in women COPD patients in whom leptin and C-reactive protein were significantly correlated .46 On the other hand, estrogens and progesterone in young women have an alveolar-maintaining effect, and perhaps alveolar-regenerating ability of ovarian hormones. Menopause, which is age related, is an important cause of accelerated alveolar and lung function loss.47
Socioeconomic Status
Socioeconomic status (SES), and therefore the level of education, are particularly important in the development of and increased mortality from COPD and is the second most important risk factor after smoking.48,49 In a study of 410 non-smoking men it was reported that the difference in FEV1 between the lowest and highest social class was 400 ml in favor of the latter .50 In a Belgian study of 59,562 individuals, a low level of education (as surrogate for SES) was a clear risk factor for the presence of COPD.51 This factor is intrinsically related to other risk factors listed in the following sections as abnormal intrauterine growth and low birth weight, nutritional status and development, access to health care, vaccines and respiratory tract infections, as SES affects the social environment on COPD development, diagnosis and outcome.52
Childhood Disadvantage Factors
Normal lung growth is closely related to processes occurring during gestation, childhood and adolescence.53-55 Barker et al showed that deaths from COPD in adulthood are associated with low weight at birth and at the first year because of alterations of lung development.56 Infections such as bronchitis, pneumonia and whooping cough in childhood further reduce lung function in adulthood.57
One of the most important studies in this regard is from Svanes et al which coined the term childhood disadvantage factors (CDF) and include parental asthma, maternal asthma, childhood asthma, severe respiratory infections before the age of 5 and maternal smoking. Comparing the FEV1 attained in adulthood of individuals who showed no CDF against those who had one or more CDFs, it was noted that the latter reached an average FEV1 -95 ml (men) and -60 ml (women). When comparing those who had 3 CDFs, attained FEV1 was -274 ml (men) and -208 ml (women). Furthermore, it was observed that the presence of CDF correlated with greater decline in FEV1 and higher incidence of COPD.58
Lung Conditions
Airway Hyperresponsiveness
Airway hyperresponsiveness (AHR) is an independent risk factor for accelerated decline in FEV1. In an analysis of 25 years of follow-up of 1139 individuals, Rijcken et al showed that men and women smokers, former smokers and non-smokers, with a positive AHR test (PC10 ≤ 16 mg/ml histamine), had a greater annual decline in FEV1 than non-responders.59 In another sub-analysis of the cohort of patients from the Lung Health Study, it was found that between 5733 smokers with mild and moderate obstruction, reactivity to methacholine was an important predictor of progression of airflow obstruction in early COPD, regardless of the value of baseline lung function.60 By contrast, post bronchodilator FEV1 reversibility as surrogate of AHR has not proved to be a predictor of lung function worsening in patients with COPD.61
Asthma
Asthma is an entity that has pathophysiologic mechanisms clearly different from COPD, but FEV1 decline in patients with asthma is higher than in non-asthmatic individuals.62 Furthermore, it has been shown that airway inflammation in severe asthma has predominantly more neutrophils and alterations of the extracellular matrix, a characteristic shared with COPD and has been termed airway remodeling.63,64 These alterations in severe asthma lead to a chronic irreversible obstruction generating an indistinguishable clinical picture from COPD. Finally, although the asthma-COPD overlap syndrome (ACOS) was described several years ago,65 only in recent years has it gained importance and was described in more detail as patients with ACOS have more symptoms and exacerbations than patients with asthma and COPD.66-68
Chronic Mucus Hypersecretion
Chronic mucus hypersecretion (CMH) is characterized by an increased number of goblet cells in the bronchial mucosa with consequent excess mucus.69 Harmful agents for bronchial mucosa (tobacco, particles, infections, etc.) trigger a complex inflammatory mechanism where mediators like TNF-α, IL-1β, IL-6 and IL-13 cause up-regulation of mucin 5AC (MUC5AC) and mucin 5B (MUC5B) gene expression that induces goblet cell hyperplasia and mucus hypersecretion.70,71
Several studies show that CMH is associated with an accelerated decline in lung function.72-74 Using data from the Copenhagen City Heart Study on 5354 women and 4081 men comparing spirometry at 5-year intervals, an excess decline in FEV1 of 22.8 ml/year in men with CMH compared with the decline of FEV1 in men without CMH after adjusting for age, height, weight change, and smoking was demonstrated. In women, the excess decline was not significant (12.6 ml/year).73 In the context of the European Community Respiratory Health Survey II, in 5002 individuals without asthma with normal lung function and followed for a period of 12 years, it was observed that CMH was a statistically significant independent predictor of COPD.75 New concepts in pathophysiology of COPD demonstrate this strong association with chronic bronchitis.76
Recurrent Bronchopulmonary Infections
COPD exacerbations are related to worsening of lung function77,78 but the decline in FEV1 is slower as the disease worsens, as evidenced by data from 2163 patients from the ECLIPSE study evaluated during 3 years where GOLD stage II patients had an average decline in FEV1 of 35 ± 1 ml/year, GOLD stage III patients of 33 ± 1 ml/year and GOLD stage IV patients 25 ± 2 ml/year.79 Considering that the decline in FEV1 is substantially higher in young smokers80 and that infections negatively affect lung function, such infections may also have a negative impact on smokers with normal lung function constituting another factor to consider.
Emphysema
Emphysema is the result of exposure of the lungs to noxious particles or gases. The mechanism by which this process takes place is very complex and involves inflammatory processes in the peripheral airways (bronchioles) and pulmonary parenchyma. Bronchioles become obstructed by fibrosis and macrophage infiltration, cytotoxic T lymphocytes (CD8+) and neutrophils.20,81 Several inflammatory mediators, such as LTB4, TNFα and CXCL8 (chemokine [C-X-C motif] ligand 8), together with proteinase/antiproteinase imbalance, and increased oxidative stress are also involved.82-87
Although high resolution computed tomography (HRCT) is not a routine investigation in smokers or patients with COPD, in 2085 smokers and former heavy smokers enrolled in a screening test for lung cancer and evaluated with spirometry and chest HRCT at baseline and 3 years later, it was noted that the severity of emphysema detected in images correlates with lower FEV1 and greater decline in lung function, even in those without obstruction at baseline examination, indicating that emphysema detected in HRCT may predict which smokers without spirometric alteration will develop airway obstruction.88Additionally, several studies demonstrate the association between the presence of emphysema and decline in lung function.89,90 Furthermore, the predominance of emphysema in the upper lobes is associated with a faster decline of lung function.91,92 In summary, in the evaluation of patients at risk for COPD, an HRCT, obtained for other indications, demonstrating the presence of emphysema places the patient in a group more likely to develop COPD.
Lung Growth, Ageing, and Lung Function Decline
The lungs grow progressively up to the age of 25 with a plateau until about the age of 35-40, then starting an ageing process with a gradual decline of FEV1.93-95 The estimated average decline in FEV1 is 30 ml/year in men and 23 ml/year in women although there is considerable inter-individual variability.96,97
FEV1 Decline and Smoking
Peat et al showed that FEV1 decline is faster in smokers than in nonsmokers and that the difference between the 2 groups is significant from the age of 30.80 The Lung Health Study 3 assessed 4194 individuals with spirometry who participated in the Lung Health Study 11 years earlier and who were divided into 3 groups according to their smoking history: 1) sustained quitters (16.7%), 2) intermittent quitters (57.4) and 3) continuing smokers (25.9%). Eleven years after the first evaluation, the decline in FEV1 was 27 ml/year, 48 ml/year and 60 ml/year respectively, being somewhat lower in women.98 In the Lovelace Smokers Cohort study of 1170 patients evaluated for 36 months, it was observed that 32% were rapid decliners (annual decline of FEV1> 40 ml), 34% had normal decline of FEV1 (annual decline of FEV1 between 20-39.9 ml) and the remaining 34% were non decliners (annual decline of FEV1 0-19.9 ml), concluding that the decline of FEV1 among smokers is not homogeneous, but that individuals with rapid decline starting from a normal FEV1, had an increased risk of developing COPD, setting the 36-month period as the appropriate time to control the decline of FEV1.99
Kohansal et al, analyzing the Framingham Offspring Cohort (n=4391, men=2121), showed that the annualized rate of decline for healthy never-smoking men was 19.6 ml/year and 17.6 ml/year in women. Additionally, 33% of continuous-smoker men and 24.2% of continuous-smoker women developed airflow obstruction during follow-up, higher than the proportions observed in the group of never-smokers, 7.4% of men and 5.6% of women. Finally, continuous smoking increased the rate of FEV1 decline (versus never smokers), in men (38.2 ml/year), and women (23.9 ml/year).100
Recently, Lange et al conducted a sub-analysis of 2864 patients (2207 with a normal FEV1 at baseline) enrolled in the Framingham Offspring Cohort and Copenhagen City Heart Study studies who performed spirometry at least twice, the first before the age of 40 for inclusion in the studies and another after an average of 22 years of observation. In all cohorts analyzed, regardless of baseline FEV1, the annual decline of FEV1 was higher in those who developed COPD. At the end of the study, 332 patients (12%) had COPD GOLD stage II or higher. Of this group, 158 (48%) had a normal baseline FEV1 (7% of the group with normal baseline FEV1) with an annual decline of FEV1 of 53 ml/year, and 174 (52%) had a baseline FEV1 <80% predictive (26% of the group with FEV1 <80% of predictive) with an annual decline of FEV1 of 27 ml/year. In the 812 participants who never smoked, an average decline of FEV1 was 18 ml/year. Of this group, only 27 developed COPD, including 7 starting from a normal FEV1 (annual decline of FEV1 = 37 ml/year) and 20 individuals starting from a FEV1 <80% of predictive (annual decline of FEV1 = 23 ml/year).101 These results are consistent with a previous study by the same authors which shows similar data on FEV1 decline in young individuals evaluated for 25 years, where those who developed airflow obstruction had a fall of FEV1 of 51 ml/year (basal FEV1> 80% predictive) versus 29 ml/year (basal FEV1 <80% predictive).102
Filling in the Blanks
Emphysema and chronic obstructive bronchitis are implicated as the major pathological entities responsible for COPD. However, many patients have chronic bronchitis whose diagnosis is clinical and others have emphysema detected in HRCT, but in both cases without airway obstruction on spirometry, an essential requirement for the diagnosis of COPD.103,104 These patients have risk factors (primarily smoking), and are most often asymptomatic, but with typical pathological findings of COPD in the airways, parenchyma and vascular structures.105,106 Only when emphysema or chronic bronchitis progress to alter respiratory physiology with the appearance of airflow obstruction that meets GOLD criteria, do these conditions change their name to be called COPD, keeping its distinctive features recognized in the different phenotypes: emphysema phenotype or chronic bronchitis phenotype. Consequently, the question arises whether a patient with one or more risk factors, who has significantly decreased FEV1 compared to previous spirometry but has not fallen below 80% of predictive and FEV1/FVC remains above 0.70 has a different disease from COPD. Clearly the answer is that they are the same disease in different developmental stages, but COPD is used to describe a group of patients in whom spirometric values have fallen below the values stipulated by GOLD. Hence, there is an ongoing debate about whether to continue using the FEV1/FVC <0.70 as the cut-off value since COPD is underdiagnosed in younger people and overestimated in the elderly, and if we should continue to use the predictive FEV1 value or should use the lower limit of normal (LLN).107-111 This question becomes more vigorous considering that the decline in FEV1 is faster in the early stages of the disease.112
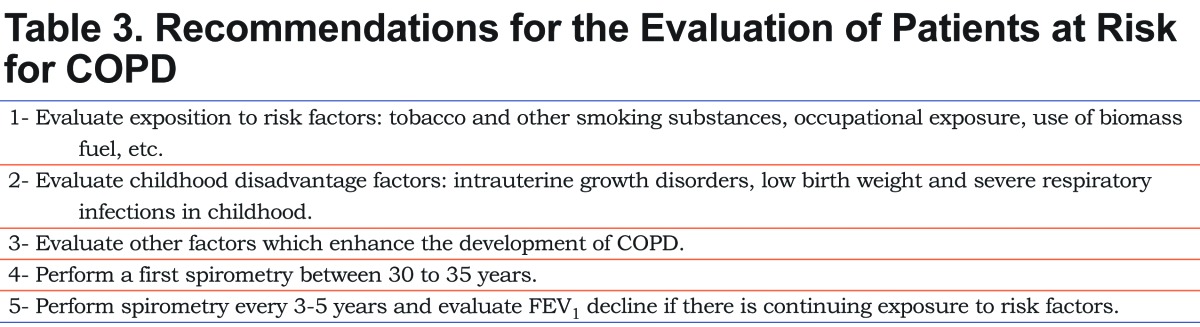
The lack of recommendations for individuals with risk factors for the development of COPD without spirometric criteria for obstruction lead each health care professional to have different approaches to these cases given there is no consensus. In view of these facts, every patient exposed to risk factors without spirometric abnormalities is at risk for COPD, but the chances increase if other enhancing factors are present and if lung function declines excessively between 2 spirometric assessments. The studies reviewed in this paper show that individuals exposed to risk factors who did not reach adequate lung growth (low attained FEV1) and those who achieved normal lung growth (FEV1 around 100% predictive) have an accelerated loss of lung function when FEV1 decline is at a rate of more than 30 ml/year and 50-60 ml/year respectively98,99,101,102 which coincides with other studies such as EUROSCOPE (-69 ml/year) and the ISOLDE study (-59 ml/year) study,113,114 keeping in mind that the speed of decline of FEV1 is greater in the early stages of the disease and decelerate as FEV1 is reduced further. For this reason, some authors recommend using the calculation of FEV1 decline in percentages rather than absolute values,115 and others suggest that there is an excessive decrease in FEV1 when the percentage change in FEV1 (%-ΔFEV1) between 2 measurements is below the 5th percentile.97 In this controversial point, a reasonable estimation of an accelerated decline of FEV1 would be >50 ml/year in patients with FEV1 >80% of predictive and >30 ml/year in those with an FEV1 <80% of the predictive at baseline screening. Since the decline in lung function in smokers is more pronounced than in nonsmokers, and this difference is significant from the age of 30,80 it would be advisable to perform a first spirometric evaluation on individuals exposed to risk factors (primarily smoking) between 30 and 35 years, to assess if there was an adequate lung growth and record a baseline FEV1 for comparison with subsequent tests. At this age, most individuals are likely to have been smoking for more than 10 years. Following Wang, Petersen and Vestbo the suitable interval for the assessment of FEV1 decline would be 3 to 5 years.97,99,116 A 3-year interval between spirometry measurements is reasonable for patients with a low attained FEV1 at baseline and for those with an accelerated FEV1 decline during follow up, whereas a 5-year interval is appropriate for patients with normal FEV1 at baseline and for those with 30-49 ml/year FEV1 decline during follow up.
Consequently, in the evaluation of young and middle-aged patients with enhancing factors for the development of COPD it would be appropriate to consider the recommendations detailed in Table 3.
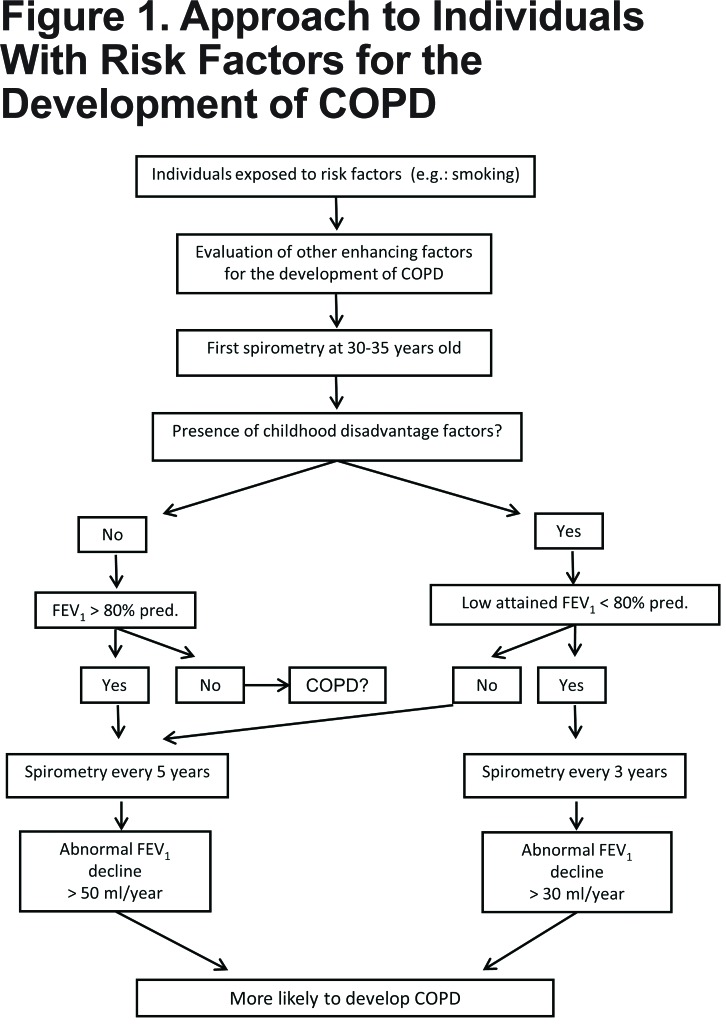
This approach to individuals exposed to risk factors allows for the detection of patients at early stages of the disease and gives the chance to adopt measures to eliminate eradicable factors (Figure 1). It is known that smoking cessation has a beneficial effect on lung function and symptoms.117-121 Similarly, reducing exposure to biomass fuel slows FEV1 decline and improves symptoms,122,123 thus early intervention would result in less loss of lung function.
Conclusions
COPD is a progressive disease with great detrimental impact among the global population. GOLD guidelines establish recommendations for patients with impaired lung function but do not include recommendations on how to evaluate individuals with risk factors or individuals who, without meeting COPD spirometric criteria, have a decline in lung function that inevitably leads to the establishment of the disease with the onset of symptoms and limitations in their daily activities. Similarly, a U.S. Preventive Services Task Force panel concluded that adults would not be screened for COPD using spirometry, as no evidence is available for the later prevention of COPD exacerbations or for improvements in respiratory-related health status.124 On the other hand, recent data demonstrate that, although there is heterogeneity in COPD prevalence, underdiagnosis of COPD is universally high.125
However, considering all factors implicated in the development of COPD makes the population at risk more identifiable and follow up more personalized. In accordance with Soriano and Price, a program of early detection of COPD meets the 3 criteria necessary to receive funding for its implementation: 1) undetected disease would go on to cause substantial morbidity and mortality; 2) treatment of risk factors has a major impact upon the subsequent development of disease; and 3) an objective test that is relatively simple, affordable and safe is available to confirm the disease.126 Moreover, periodic controls and its findings may persuade patients at risk to abandon harmful habits, mainly smoking.
Since FEV1 decline is determinant in the development of COPD, its measurement and periodic evaluation is advisable in patients with risk factors for COPD. FEV1 decline is not homogeneous in all cases so there are different rates of decline and every single patient should be monitored individually. In this aspect, spirometry is essential in the diagnosis of COPD and good-quality tests should be implemented in order to make spirometry a simple, reliable, noninvasive, safe and inexpensive test in the evaluation of airway obstruction.127,128 Perhaps in the future there will be new diagnostic strategies for COPD (e.g., imaging), but at present GOLD guidelines define COPD by its symptoms along with airway obstruction and these are the tools available for the diagnosis in the clinical setting.
These recommendations target those patients who, at the age of 35, have been exposed to risk factors for 10 years or more and expand to the time that COPD is established. This means that the present recommendations encompass patients between 35 to 50-55 years old so there is a 15-20-year period of screening with a spirometry every 3 to 5 years, a simple and affordable test that can help doctors to identify patients at serious risk of COPD and implement specific strategies to encourage patients to quit smoking, replace biomass fuel or avoid jobs where pollution inevitably causes lung damage. In the future, disease-modifying treatments that suppress the underlying inflammation and remodeling may be developed and would be most effective if given early in the disease process.
Perhaps new prospective studies on populations at risk may improve identification of individuals who will develop COPD with a better understanding of the predisposing factors and lung conditions that enhance the settlement of COPD. More consensus about the decline in lung function in these groups, whether expressed in absolute or relative values, will be of great help in the detection of incipient cases.
In summary, patients exposed to risk factors for COPD should be evaluated considering all of the enhancing factors for the development of COPD. Spirometry, a simple and inexpensive test, should be done for the first time between the age of 30-35 years and repeated every 3 to 5 years according to baseline results and rate of FEV1 decline. Patients should be encouraged to avoid risk factors at all time, but specifically when faster FEV1 decline is detected.
Abbreviations
Global initiative for chronic Obstructive Lung Disease, GOLD; chronic obstructive pulmonary disease, COPD; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; leukotriene B4, LTB4; interleukin, IL; glutathione-s-transferase, GST; transforming growth factor beta, TGFβ; tumor necrosis factor alpha, TNFα; superoxide dismutase-3, SOD3; COPD Genetic Epidemiologist, COPDGene; nicotinic acetylcholine receptors, CHRNA; neural cell adhesion molecule 1, NCAM1; testis expressed 41/poly (A) binding protein, cytoplasmic 1 pseudogene 2, TEX41/PABPC1P2; dynein axonemal heavy chain 8, DNAH8; nucleolar protein 4-like, NOL4L; lipid phosphate phosphatase-related protein type 5, LPPR5; nephronectin, NPNT; tet methylcytosine dioxygenase 2, TET2; Major histocompatibility complex, class II, DQ beta 1/ major histocompatibility complex, class II, DQ alpha 2, HLA-DQB1/HLA-DQA2; KAT8 regulatory NSL complex subunit 1, KANSL1; tRNA splicing endonuclease subunit 54, TSEN54; single nucleotide polymorphisms, SNPs; hedgehog interacting protein, HHIP; advanced glycoslation end product-specific receptor, AGER; T helper cell, Th; Vascular endothelial growth factor, VEGF; socioeconomic status, SES; childhood disadvantage factors, CDF; airway hyperresponsiveness, AHR; asthma-COPD overlap syndrome, ACOS; chronic mucus hypersecretion, CMH; Mucin 5AC, MUC5AC; Mucin 5B, MUC5B; cytotoxic T lymphocytes, CD8+; chemokine [C-X-C motif] ligand 8, CXCL8; high resolution computed tomography, HRCT; lower limit of normal, LLN; percentage change in FEV1, %-ΔFEV1
References
- 1. Mathers CD,Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442-e442. doi: http://dx.doi.org/10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lopez AD,Shibuya K,Rao C,et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27(2):397-412. doi: http://dx.doi.org/10.1183/09031936.06.00025805 [DOI] [PubMed] [Google Scholar]
- 3. Regalado J,Pérez-Padilla R,Sansores R,et al. The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am J Respir Crit Care Med. 2006;174(8):901-5. doi: http://dx.doi.org/10.1164/rccm.200503-479OC [DOI] [PubMed] [Google Scholar]
- 4. Jaganath D,Miranda JJ,Gilman RH,et al. Prevalence of chronic obstructive pulmonary disease and variation in risk factors across four geographically diverse resource-limited settings in Peru. Respir Res. 2015;16:40-40. doi: http://dx.doi.org/10.1186/s12931-015-0198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacNee W,Rabinovich RA,Choudhury G. Ageing and the border between health and disease. Eur Respir J. 2014;44(5):1332-52. doi: http://dx.doi.org/10.1183/09031936.00134014 [DOI] [PubMed] [Google Scholar]
- 6. Pauwels RA,Buist AS,Calverley PMA,Jenkins CR,Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(5):1256-76. doi: http://dx.doi.org/10.1164/ajrccm.163.5.2101039 [DOI] [PubMed] [Google Scholar]
- 7. Global initiative for chronic Obstructive Pulmonary Disease (GOLD) Global initiative for chronic Obstructive Disease: Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, updated November, 2006. GOLD website. http://wwwgoldcopdorg/uploads/users/files/GOLDReport2006_0122pdf Published 2006 Accessed August 27, 2015. [Google Scholar]
- 8. Global initiative for chronic Obstructive Disease (GOLD) Global initiative for chronic Obstructive Disease: Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, updated 2015. GOLD website. http://wwwgoldcopdorg/uploads/users/files/GOLD_Report_2015pdf Published 2015 Accessed Aug 27, 2015. [Google Scholar]
- 9. Antó JM,Vermeire P,Vestbo J,Sunyer J. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-94. doi: http://dx.doi.org/10.1183/09031936.01.17509820 [DOI] [PubMed] [Google Scholar]
- 10. Laniado-Laborín R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21(st) century. Int J Environ Res Public Health. 2009;6(1):209-24. doi: http://dx.doi.org/10.3390/ijerph6010209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forey BA,Thornton AJ,Lee PN. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med. 2011;11:36-36. doi: http://dx.doi.org/10.1186/1471-2466-11-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zamarro Garcia C,Bernabe Barrios MJ,Santamaria Rodriguez B,Rodriguez Hermosa JL. Tabaquismo en la enfermedad pulmonar obstructiva crónica. Arch Bronconeumol. 2011;47(Suppl8):3-9. doi: http://dx.doi.org/10.1016/S0300-2896(11)70059-X [DOI] [PubMed] [Google Scholar]
- 13. Ortells MO,Arias HR. Neuronal networks of nicotine addiction. Int J Biochem Cell Biol. 2010;42(12):1931-1935. doi: http://dx.doi.org/10.1016/j.biocel.2010.08.019 [DOI] [PubMed] [Google Scholar]
- 14. Fletcher C,Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645-1648. doi: http://dx.doi.org/10.1136/bmj.1.6077.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rennard SI,Vestbo J. COPD: the dangerous underestimate of 15%. Lancet. 2006;367(9518):1216-1219. doi: http://dx.doi.org/10.1016/S0140-6736(06)68516-4 [DOI] [PubMed] [Google Scholar]
- 16. Lundbäck B,Lindberg A,Lindström M,et al. Not 15 but 50% of smokers develop COPD?—Report from the Obstructive Lung Disease in Northern Sweden studies. Respir Med. 2008;97(2):115-122. doi: http://dx.doi.org/10.1053/rmed.2003.1446 [DOI] [PubMed] [Google Scholar]
- 17. Løkke A,Lange P,Scharling H,Fabricius P,Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61(11):935-939. doi: http://dx.doi.org/10.1136/thx.2006.062802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sandler DP,Comstock GW,Helsing KJ,Shore DL. Deaths from all causes in non-smokers who lived with smokers. Am J Public Health. 1989;79(2):163-167. doi: http://dx.doi.org/10.2105/AJPH.79.2.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalandidi A,Trichopoulos D,Hatzakis A,Tzannes S,Saracci R. Passive smoking and chronic obstructive lung disease. Lancet. 1987;330(8571):1325-1326. doi: http://dx.doi.org/10.1016/S0140-6736(87)91210-4 [DOI] [PubMed] [Google Scholar]
- 20. Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343(4):269-280. doi: http://dx.doi.org/10.1056/NEJM200007273430407 [DOI] [PubMed] [Google Scholar]
- 21. Assad NA,Balmes J,Mehta S,Cheema U,Sood A. Chronic obstructive pulmonary disease secondary to household air pollution. Semin Respir Crit Care Med. 2015;36(03):408-421. doi: http://dx.doi.org/10.1055/s-0035-1554846 [DOI] [PubMed] [Google Scholar]
- 22. Korn RJ,Dockery DW,Speizer FE,Ware JH,Ferris BG. Occupational exposures and chronic respiratory symptoms: a population-based study. Am Rev Respir Dis. 1987;136(2):298-304. doi: http://dx.doi.org/10.1164/ajrccm/136.2.298 [DOI] [PubMed] [Google Scholar]
- 23. Bergdahl IA,Torén K,Eriksson K,et al. Increased mortality in COPD among construction workers exposed to inorganic dust. Eur Respir J. 2004;23(3):402-406. doi: http://dx.doi.org/10.1183/09031936.04.00034304 [DOI] [PubMed] [Google Scholar]
- 24. Humerfelt S,Gulsvik A,Skjaerven R,et al. Decline in FEV1 and airflow limitation related to occupational exposures in men of an urban community. Eur Respir J. 1993;6(8):1095-1103. [PubMed] [Google Scholar]
- 25. Salvi S. Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):17-27. doi: http://dx.doi.org/10.1016/j.ccm.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 26. Ingebrigtsen T,Thomsen SF,Vestbo J,et al. Genetic influences on chronic obstructive pulmonary disease - a twin study. Respir Med. 2010;104(12):1890-1895. doi: http://dx.doi.org/10.1016/j.rmed.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 27. Stoller JK,Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet. 2005;365(9478):2225-2236. doi: http://dx.doi.org/10.1016/S0140-6736(05)66781-5 [DOI] [PubMed] [Google Scholar]
- 28. DeMeo D,Silverman E. α(1)-Antitrypsin deficiency 2: Genetic aspects of α(1)-antitrypsin deficiency: phenotypes and genetic modifiers of emphysema risk. Thorax. 2004;59(3):259-264. doi: http://dx.doi.org/10.1136/thx.2003.006502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stockley RA. Alpha-1antitrypsin review. Clin Chest Med. 2014;35(1):39-50. doi: http://dx.doi.org/10.1016/j.ccm.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 30. Silverman EK,Chapman HA,Drazen JM,et al. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(6):1770-1778. doi: http://dx.doi.org/10.1164/ajrccm.157.6.9706014 [DOI] [PubMed] [Google Scholar]
- 31. McCloskey SC,Patel BD,Hinchliffe SJ,Reid ED,Wareham NJ,Lomas DA. Siblings of patients with severe chronic obstructive pulmonary disease have a significant risk of airflow obstruction. Am J Respir Crit Care Med. 2001;164(8):1419-1424. doi: http://dx.doi.org/10.1164/ajrccm.164.8.2105002 [DOI] [PubMed] [Google Scholar]
- 32. Castaldi PJ,Cho MH,Cohn M,et al. The COPD genetic association compendium: a comprehensive online database of COPD genetic associations. Hum Mol Genet. 2010;19(3):526-534. doi: http://dx.doi.org/10.1093/hmg/ddp519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marciniak SJ,Lomas DA. Genetic susceptibility. Clin Chest Med. 2014;35(1):29-38. doi: http://dx.doi.org/10.1016/j.ccm.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 34. Foreman MG,Zhang L,Murphy J,et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene study. Am J Respir Crit Care Med. 2011;184(4):414-420. doi: http://dx.doi.org/10.1164/rccm.201011-1928OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bruse S,Sood A,Petersen H,et al. New Mexican hispanic smokers have lower odds of chronic obstructive pulmonary disease and less decline in lung function than non-Hispanic whites. Am J Respir Crit Care Med. 2011;184(11):1254-1260. doi: http://dx.doi.org/10.1164/rccm.201103-0568OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wain LV,Shrine N,Miller S,et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir Med. 2015;3(10):769-781. doi: http://dx.doi.org/10.1016/S2213-2600(15)00283-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cho MH,Castaldi PJ,Hersh CP,et al. A genome-wide association study of emphysema and airway quantitative imaging phenotypes. Am J Respir Crit Care Med. 2015;192(5):559-569. doi: http://dx.doi.org/10.1164/rccm.201501-0148OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim DK,Cho MH,Hersh CP,et al. Genome-wide association analysis of blood biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(12):1238-1247. doi: http://dx.doi.org/10.1164/rccm.201206-1013OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laucho-Contreras ME,Polverino F,Gupta K,et al. Protective role for club cell secretory protein-16 (CC16) in the development of COPD. Eur Respir J. 2015;45(6):1544-1556. doi: http://dx.doi.org/10.1183/09031936.00134214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu L,Di PY,Wu R,Pinkerton KE,Chen Y. Repression of CC16 by cigarette smoke (CS) exposure. PLoS One. 2015;10(1):e0116159-e0116159. doi: http://dx.doi.org/10.1371/journal.pone.0116159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han MK,Postma D,Mannino DM,et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med. 2007;176(12):1179-1184. doi: http://dx.doi.org/10.1164/rccm.200704-553CC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gan WQ,Man SFP,Postma DS,Camp P,Sin DD. Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res. 2006;7(1):52-52. doi: http://dx.doi.org/10.1186/1465-9921-7-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tam A,Churg A,Wright JL,et al. Sex differences in airway remodeling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193(8):825-834. doi: http://www.atsjournals.org/doi/abs/10.1164/rccm.201503-0487OC [DOI] [PubMed] [Google Scholar]
- 44. Barnes PJ. Sex differences in chronic obstructive pulmonary disease mechanisms. Am J Respir Crit Care. 2016;193(8):813-814. doi: http://dx.doi.org/10.1164/rccm.201512-2379ED [DOI] [PubMed] [Google Scholar]
- 45. Assad NA,Sood A. Leptin, adiponectin and pulmonary diseases. Biochimie. 2012;94(10):2180-2189. doi: http://dx.doi.org/10.1016/j.biochi.2012.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Breyer MK,Rutten EP,Vernooy JH,et al. Gender differences in the adipose secretome system in chronic obstructive pulmonary disease (COPD): a pivotal role of leptin. Respir Med. 2011;105(7):1046-1053. doi: http://dx.doi.org/10.1016/j.rmed.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 47. Massaro D,Massaro GD. toward therapeutic pulmonary alveolar regeneration in humans. Proc Am Thorac Soc. 2006;3(8):709-712. doi: http://dx.doi.org/10.1513/pats.200605-127SF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gershon AS,Dolmage TE,Stephenson A,Jackson B. chronic obstructive pulmonary disease and socioeconomic status: a systematic review. COPD. 2012;9(3):216-226. doi: http://dx.doi.org/10.3109/15412555.2011.648030 [DOI] [PubMed] [Google Scholar]
- 49. Prescott E,Vestbo J. Socioeconomic status and chronic obstructive pulmonary disease. Thorax. 1999;54(8):737-741. doi: http://dx.doi.org/10.1136/thx.54.8.737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stebbings J. Chronic respiratory disease among nonsmokers in Hagerstown, Maryland. Social class and chronic respiratory disease. Environ Res. 1971;4(3):213-232. doi: http://dx.doi.org/10.1016/0013-9351(71)90024-7 [DOI] [PubMed] [Google Scholar]
- 51. Van den,Bosch K,Geerts J,Willemé P. Long-term care use and socio-economic status in Belgium: a survival analysis using health care insurance data. Arch Public Health. 2013;71(1):1-1. doi: http://dx.doi.org/10.1186/0778-7367-71-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eisner MD,Blanc PD,Omachi TA,et al. Socioeconomic status, race and COPD health outcomes. J Epidemiol Community Health. 2011;65(1):26-34. doi: http://dx.doi.org/10.1136/jech.2009.089722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martinez FD. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc. 2009;6(3):272-277. doi: http://dx.doi.org/10.1513/pats.200808-092RM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Postma DS,Bush A,van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2008;385(9971):899-909. doi: http://dx.doi.org/10.1016/S0140-6736(14)60446-3 [DOI] [PubMed] [Google Scholar]
- 55. Bolton CE. COPD as a consequence of premature birth? Controversies in COPD. ERS Monogr. 2015;69:26-34. [Google Scholar]
- 56. Barker DJ,Godfrey KM,Fall C,Osmond C,Winter PD,Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303(6804):671-675. doi: http://dx.doi.org/10.1136/bmj.303.6804.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lopez Bernal JA,Upton MN,Henderson AJ,et al. Lower respiratory tract infection in the first year of life is associated with worse lung function in adult life: prospective results from the Barry Caerphilly Growth study. Ann Epidemiol. 2013;23(7):422-427. doi: http://dx.doi.org/10.1016/j.annepidem.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 58. Svanes C,Sunyer J,Plana E,et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65(1):14-20. doi: http://dx.doi.org/10.1136/thx.2008.112136 [DOI] [PubMed] [Google Scholar]
- 59. Rijcken B,Schouten JP,Xu X,Rosner B,Weiss ST. Airway hyperresponsiveness to histamine associated with accelerated decline in FEV1. Am J Respir Crit Care Med. 1995;151(5):1377-1382. doi: http://dx.doi.org/10.1164/ajrccm.151.5.7735588 [DOI] [PubMed] [Google Scholar]
- 60. Tashkin DP,Altose MD,Connett JE,Kanner RE,Lee WW,Wise RA. Methacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive pulmonary disease. The Lung Health Study Research Group. Am J Respir Crit Care Med. 1996;153(6):1802-1811. doi: http://dx.doi.org/10.1164/ajrccm.153.6.8665038 [DOI] [PubMed] [Google Scholar]
- 61. Hansen EF,Phanareth K,Laursen LC,Kok-Jensen A,Dirksen A. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(4):1267-1271. doi: http://dx.doi.org/10.1164/ajrccm.159.4.9807121 [DOI] [PubMed] [Google Scholar]
- 62. Peat JK,Woolcock AJ,Cullen K. Rate of decline of lung function in subjects with asthma. Eur Respir J. 1987;70(3):171-179. [PubMed] [Google Scholar]
- 63. Postma DS,Timens W. Remodeling in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(5):434-439. doi: http://dx.doi.org/10.1513/pats.200601-006AW [DOI] [PubMed] [Google Scholar]
- 64. Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1(3):176-183. doi: http://dx.doi.org/10.1513/pats.200402-009MS [DOI] [PubMed] [Google Scholar]
- 65. Guerra S. Overlap of asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2005;11(1):7-13. doi: http://dx.doi.org/10.1097/01.mcp.0000146780.33963.bf [DOI] [PubMed] [Google Scholar]
- 66. Papaiwannou A,Zarogoulidis P,Porpodis K,et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis. 2014;6(Suppl1):S146-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barrecheguren M,Esquinas C,Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21(1):74-79. doi: http://dx.doi.org/10.1097/MCP.0000000000000118 [DOI] [PubMed] [Google Scholar]
- 68. Nielsen M,Barnes CB,Ulrik CS. Clinical characteristics of the asthma-COPD overlap syndrome--a systematic review. Int J Chron Obstruct Pulmon Dis. 2015;10:1443-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Riise GC,Larsson S,Andersson BA. A bronchoscopic brush biopsy study of large airway mucosal pathology in smokers with chronic bronchitis and in healthy nonsmokers. Eur Respir J. 1992;5(4):382-386. [PubMed] [Google Scholar]
- 70. Voynow JA,Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135(2):505-512. doi: http://dx.doi.org/10.1378/chest.08-0412 [DOI] [PubMed] [Google Scholar]
- 71. Turner J,Jones CE. Regulation of mucin expression in respiratory diseases. Biochem Soc Trans. 2009;37:877-881. doi: http://dx.doi.org/10.1042/BST0370877 [DOI] [PubMed] [Google Scholar]
- 72. Sherman CB,Xu X,Speizer FE,Ferris BG Jr. ,Weiss ST,Dockery DW. Longitudinal lung function decline in subjects with respiratory symptoms. Am Rev Respir Dis. 1992;146(4):855-859. doi: http://dx.doi.org/10.1164/ajrccm/146.4.855 [DOI] [PubMed] [Google Scholar]
- 73. Vestbo J,Prescott E,Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med. 1996;153(5):1530-1535. doi: http://dx.doi.org/10.1164/ajrccm.153.5.8630597 [DOI] [PubMed] [Google Scholar]
- 74. Ramos FL,Krahnke JS,Kim V. Clinical issues of mucus accumulation in COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:139-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. de Marco R,Accordini S,Cerveri I,et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med. 2007;175(1):32-39. doi: http://dx.doi.org/10.1164/rccm.200603-381OC [DOI] [PubMed] [Google Scholar]
- 76. Kim V,Rogers TJ,Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):478-485. doi: http://dx.doi.org/10.1513/pats.200802-014ET [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kanner RE,Anthonisen NR,Connett JE. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164(3):358-364. doi: http://dx.doi.org/10.1164/ajrccm.164.3.2010017 [DOI] [PubMed] [Google Scholar]
- 78. Donaldson GC,Seemungal TAR,Bhowmik A,Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847-852. doi: http://dx.doi.org/10.1136/thorax.57.10.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vestbo J,Edwards LD,Scanlon PD,et al. Changes in Forced Expiratory Volume in 1 Second over Time in COPD. N Engl J Med. 2011;365(13):1184-1192. doi: http://dx.doi.org/10.1056/NEJMoa1105482 [DOI] [PubMed] [Google Scholar]
- 80. Peat JK,Woolcock AJ,Cullen K. Decline of lung function and development of chronic airflow limitation: a longitudinal study of non-smokers and smokers in Busselton, Western Australia. Thorax. 1990;45(1):32-37. doi: http://dx.doi.org/10.1136/thx.45.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Finkelstein R,Fraser RS,Ghezzo H,Cosio MG. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med. 1995;152(5):1666-1672. doi: http://dx.doi.org/10.1164/ajrccm.152.5.7582312 [DOI] [PubMed] [Google Scholar]
- 82. Hill AT,Bayley D,Stockley RA. The interrelationship of sputum inflammatory markers in patients with chronic bronchitis. Am J Respir Crit Care Med. 1999;160(3):893-898. doi: http://dx.doi.org/10.1164/ajrccm.160.3.9901091 [DOI] [PubMed] [Google Scholar]
- 83. Keatings VM,Collins PD,Scott DM,Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153(2):530-534. doi: http://dx.doi.org/10.1164/ajrccm.153.2.8564092 [DOI] [PubMed] [Google Scholar]
- 84. Stockley RA. Neutrophils and protease/antiprotease imbalance. Am J Respir Crit Care Med. 1999;160(5):S49-S52. doi: http://dx.doi.org/10.1164/ajrccm.160.supplement_1.13 [DOI] [PubMed] [Google Scholar]
- 85. Mocchegiani E,Giacconi R,Costarelli L. Metalloproteases/anti-metalloproteases imbalance in chronic obstructive pulmonary disease: genetic factors and treatment implications. Curr Opin Pulm Med. 2011;17(Suppl1):S11-9. doi: http://dx.doi.org/10.1097/01.mcp.0000410743.98087.12 [DOI] [PubMed] [Google Scholar]
- 86. Repine JE,Bast A,Lankhorst I,et al. Oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156(2):341-357. doi: http://dx.doi.org/10.1164/ajrccm.156.2.9611013 [DOI] [PubMed] [Google Scholar]
- 87. MacNee W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(1):50-60. doi: http://dx.doi.org/10.1513/pats.200411-056SF [DOI] [PubMed] [Google Scholar]
- 88. Mohamed Hoesein FAA,de Hoop B,Zanen P,et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011;66(9):782-787. doi: http://dx.doi.org/10.1136/thx.2010.145995 [DOI] [PubMed] [Google Scholar]
- 89. Cerveri I,Corsico AG,Grosso A,et al. The rapid FEV1 decline in chronic obstructive pulmonary disease is associated with predominant emphysema: a longitudinal study. COPD. 2012;10(1):55-61. [DOI] [PubMed] [Google Scholar]
- 90. Turato G,Zuin R,Miniati M,et al. Airway inflammation in severe chronic obstructive pulmonary disease: relationship with lung function and radiologic emphysema. Am J Respir Crit Care Med. 2002;166(1):105-110. doi: http://dx.doi.org/10.1164/rccm.2111084 [DOI] [PubMed] [Google Scholar]
- 91. Mohamed Hoesein FAA,van Rikxoort E,van Ginneken B,et al. Computed tomography-quantified emphysema distribution is associated with lung function decline. Eur Respir J. 2012;40(4):844-850. doi: http://dx.doi.org/10.1183/09031936.00186311 [DOI] [PubMed] [Google Scholar]
- 92. Hughes JA,Hutchison DC,Bellamy D,Dowd DE,Ryan KC,Hugh-Jones P. Annual decline of lung function in pulmonary emphysema: influence of radiological distribution. Thorax. 1982;37(1):32-37. doi: http://dx.doi.org/10.1136/thx.37.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sharma G,Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1(3):253-260. doi: http://dx.doi.org/10.2147/ciia.2006.1.3.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bosse R,Sparrow D,Rose CL,Weiss ST. Longitudinal effect of age and smoking cessation on pulmonary function. Am Rev Respir Dis. 1981;123(4Pt1):378-381. [DOI] [PubMed] [Google Scholar]
- 95. Griffith KA,Sherrill DL,Siegel EM,Manolio TA,Bonekat HW,Enright PL. Predictors of loss of lung function in the elderly: the Cardiovascular Health Study. Am J Respir Crit Care Med. 2001;163(1):61-68. doi: http://dx.doi.org/10.1164/ajrccm.163.1.9906089 [DOI] [PubMed] [Google Scholar]
- 96. Kerstjens HA,Rijcken B,Schouten JP,Postma DS. Decline of FEV1 by age and smoking status: facts, figures, and fallacies. Thorax. 1997;52(9):820-827. doi: http://dx.doi.org/10.1136/thx.52.9.820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang ML,Avashia BH,Petsonk EL. Interpreting periodic lung function tests in individuals*: The relationship between 1- to 5-year and long-term fev1 changes. Chest. 2006;130(2):493-499. doi: http://dx.doi.org/10.1016/S0012-3692(15)51866-7 [DOI] [PubMed] [Google Scholar]
- 98. Anthonisen NR,Connett JE,Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166(5):675-679. doi: http://dx.doi.org/10.1164/rccm.2112096 [DOI] [PubMed] [Google Scholar]
- 99. Petersen H,Sood A,Meek PM,et al. Rapid lung function decline in smokers is a risk factor for COPD and is attenuated by angiotensin-converting enzyme inhibitor use. Chest. 2014;145(4):695-703. doi: http://dx.doi.org/10.1378/chest.13-0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kohansal R,Martinez-Camblor P,Agustí A,Buist AS,Mannino DM,Soriano JB. The natural history of chronic airflow obstruction revisited. Am J Respir Crit Care Med. 2009;180(1):3-10. doi: http://dx.doi.org/10.1164/rccm.200901-0047OC [DOI] [PubMed] [Google Scholar]
- 101. Lange P,Celli B,Agusti A,et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111-122. doi: http://dx.doi.org/10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 102. Lange P,Marott JL,Vestbo J. Natural history of development of airflow limitation. Eur Respir J. 2014;44(Suppl58) [Google Scholar]
- 103. Clark KD,Wardrobe-Wong N,Elliott JJ,Gill PT,Tait NP,Snashall PD. Patterns of lung disease in a "normal" smoking population*: Are emphysema and airflow obstruction found together?. Chest. 2001;120(3):743-747. doi: http://dx.doi.org/10.1378/chest.120.3.743 [DOI] [PubMed] [Google Scholar]
- 104. Lutchmedial SM,Creed WG,Moore AJ,Walsh RR,Gentchos GE,Kaminsky DA. How common is airflow limitation in patients with emphysema on CT scan of the chest?. Chest. 2015;148(1):176-184. doi: http://dx.doi.org/10.1378/chest.14-1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pipavath SN,Schmidt RA,Takasugi JE,Godwin JD. Chronic obstructive pulmonary disease: radiology-pathology correlation. J Thorac Imaging. 2009;24(3):171-180. doi: http://dx.doi.org/10.1097/RTI.0b013e3181b32676 [DOI] [PubMed] [Google Scholar]
- 106. Stewart JI,Criner GJ. The small airways in chronic obstructive pulmonary disease: pathology and effects on disease progression and survival. Curr Opin Pulm Med. 2013;19(2):109-115. doi: http://dx.doi.org/10.1097/MCP.0b013e32835ceefc [DOI] [PubMed] [Google Scholar]
- 107. Fabbri LM,Boschetto P,Mapp CE. COPD Guidelines. Am J Respir Crit Care Med. 2007;176(6):527-528. doi: http://dx.doi.org/10.1164/rccm.200706-854ED [DOI] [PubMed] [Google Scholar]
- 108. Hardie JA,Buist AS,Vollmer WM,Ellingsen I,Bakke PS,Morkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J. 2002;20(5):1117-1122. doi: http://dx.doi.org/10.1183/09031936.02.00023202 [DOI] [PubMed] [Google Scholar]
- 109. Enright P,Brusasco V. Counterpoint: should we abandon FEV(1)/FVC < 0. 70 to detect airway obstruction? Yes. Chest. 2010;138(5):1040-1042. [DOI] [PubMed] [Google Scholar]
- 110. Celli BR,Halbert RJ. Point: should we abandon FEV(1)/FVC <0. 70 to detect airway obstruction? No. Chest. 2010;138(5):1037-1040. doi: http://dx.doi.org/10.1378/chest.10-2049 [DOI] [PubMed] [Google Scholar]
- 111. Sorino C,Battaglia S,Scichilone N,et al. Diagnosis of airway obstruction in the elderly: contribution of the SARA study. Int J Chron Obstruct Pulmon Dis. 2012;7:389-395. doi: http://dx.doi.org/10.2147/COPD.S31630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Celli BR,Thomas NE,Anderson JA,et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178(4):332-338. doi: http://dx.doi.org/10.1164/rccm.200712-1869OC [DOI] [PubMed] [Google Scholar]
- 113. Pauwels RA,Löfdahl C-G,Laitinen LA,et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med. 1999;340(25):1948-1953. doi: http://dx.doi.org/10.1056/NEJM199906243402503 [DOI] [PubMed] [Google Scholar]
- 114. Burge PS,Calverley PMA,Jones PW,Spencer S,Anderson JA,Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297-1303. doi: http://dx.doi.org/10.1136/bmj.320.7245.1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Thomsen LH,Dirksen A,Shaker SB,Skovgaard LT,Dahlback M,Pedersen JH. Analysis of FEV1 decline in relatively healthy heavy smokers: implications of expressing changes in FEV1 in relative terms. COPD. 2014;11(1):96-104. doi: http://dx.doi.org/10.3109/15412555.2013.830096 [DOI] [PubMed] [Google Scholar]
- 116. Vestbo J,Lange P. Natural history of COPD: Focusing on change in FEV1. Respirology. 2016;21(1):34-43. [DOI] [PubMed] [Google Scholar]
- 117. Peto R,Speizer FE,Cochrane AL,et al. The relevance in adults of air-flow obstruction, but not of mucus hypersecretion, to mortality from chronic lung disease. Results from 20 years of prospective observation. Am Rev Respir Dis. 1983;128(3):491-500. doi: http://dx.doi.org/10.1164/arrd.1983.128.3.491 [DOI] [PubMed] [Google Scholar]
- 118. Scanlon PD,Connett JE,Waller LA,et al. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161(2Pt1):381-390. doi: http://dx.doi.org/10.1164/ajrccm.161.2.9901044 [DOI] [PubMed] [Google Scholar]
- 119. Willemse BW,Postma DS,Timens W,ten Hacken NH. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J. 2004;23(3):464-476. doi: http://dx.doi.org/10.1183/09031936.04.00012704 [DOI] [PubMed] [Google Scholar]
- 120. Brown CA,Crombie IK,Smith WC,Tunstall-Pedoe H. The impact of quitting smoking on symptoms of chronic bronchitis: results of the Scottish Heart Health Study. Thorax. 1991;46(2):112-116. doi: http://dx.doi.org/10.1136/thx.46.2.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lange P,Groth S,Nyboe G,et al. Effects of smoking and changes in smoking habits on the decline of FEV1. Eur Respir J. 1989;2(9):811-816. [PubMed] [Google Scholar]
- 122. Romieu I,Riojas-Rodriguez H,Marron-Mares AT,Schilmann A,Perez-Padilla R,Masera O. Improved biomass stove intervention in rural Mexico: impact on the respiratory health of women. Am J Respir Crit Care Med. 2009;180(7):649-656. doi: http://dx.doi.org/10.1164/rccm.200810-1556OC [DOI] [PubMed] [Google Scholar]
- 123. Zhou Y,Zou Y,Li X,et al. Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: a 9-year prospective cohort study. PLoS Med. 2014;11(3):e1001621-e1001621. doi: http://dx.doi.org/10.1371/journal.pmed.1001621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. US Preventive Services Task Force; Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534. doi: http://dx.doi.org/10.7326/0003-4819-148-7-200804010-00212 [DOI] [PubMed] [Google Scholar]
- 125. Lamprecht B,Soriano JB,Studnicka M,et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148(4):971-985. doi: http://dx.doi.org/10.1378/chest.14-2535 [DOI] [PubMed] [Google Scholar]
- 126. Soriano JB,Price D. Screening and case findings. Controversies in COPD. ERS Monogr. 2015;69:1-25. [Google Scholar]
- 127. Celli BR,MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946. doi: http://dx.doi.org/10.1183/09031936.04.00014304 [DOI] [PubMed] [Google Scholar]
- 128. Sims EJ,Price D. Spirometry: an essential tool for screening, case-finding, and diagnosis of COPD. Prim Care Respir J. 2012;21(2):128-130. doi: http://dx.doi.org/10.4104/pcrj.2012.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]