Abstract
The St. George’s Respiratory Questionnaire (SGRQ) is a standardized questionnaire for measuring impaired health and perceived well-being in chronic airway disease, but it is not available in the Nepali language. We translated the original SGRQ into Nepali and validated its use in 150 individuals aged 40 to 80 years with and without COPD.We also examined if the SGRQ could be used as a screening tool to identify individuals at risk for COPD. We translated the SGRQ following a standard protocol. The validation study was then conducted in both community and hospital-based settings in Nepal. We enrolled 100 participants from a community setting who were not actively seeking medical care, 50 of which met criteria for chronic obstructive pulmonary disease (COPD) (post-bronchodilator forced expiratory volume in 1 second [FEV1]/ forced vital capacity [FVC]<70%) and 50 who did not. We also enrolled 50 participants with an established diagnosis of COPD who attended outpatient pulmonary clinics. All participants completed the questionnaire. We used linear regressions to compare average SGRQ scores by disease status categories and by lung function values, adjusted for age, sex, height and body mass index (BMI).All 150 participants (mean age 59.8 years, 48% male, mean BMI 20.5 kg/m2) completed the SGRQ. In multivariable regression, the average SGRQ total score was 23.9 points higher in established cases of COPD and 18.1 points higher in community cases of COPD when compared to participants without COPD living in the community (all p<0.001). The SGRQ total score also increased by an average of 2.1 points for each 100 mL decrease in post-FEV1 (p<0.001). The area-under-the-curve for the SGRQ total score as a predictor of COPD was 0.77 (95% confidence interval [CI] 0.68 to 0.85) and the optimal cutoff to identify COPD was 33 points.We developed a Nepali-validated version of SGRQ, which correlated well with both disease status and severity.
Keywords: copd, chronic obstructive pulmonary disease, risk factors, population studies, biomass fuels, quality of life assessment, questionnaire validation
Introduction
Chronic obstructive pulmonary disease (COPD) is an important health problem worldwide and is responsible for significant morbidity in affected individuals.1 It has a profound effect on health status and quality of life, and has proven to have a significant economic burden. Globally, 10% to 20% of the world population, or an estimated 65-80 million, are afflicted with COPD, resulting in more than 3 million deaths each year.2 The number of people affected with COPD is increasing due to tobacco smoking and emerging or previously unrecognized risk factors such as chronic exposure to biomass fuel smoke. In 2002, COPD was the fifth leading cause of death worldwide and it is projected to become the third by 2030.2
The original version of the St. George’s Respiratory Questionnaire (SGRQ) is a standardized, self-completed questionnaire for measuring impaired health and perceived well-being in individuals with chronic airway disease.3 Among many disease specific questionnaires, the SGRQ is easy to use, it gives a comparative measurement of health between populations, and can be used to quantify changes in health following treatment. It also provides a standard metric across settings and cultures once adequately translated and validated. Moreover, it is not limited to individuals with COPD as are other disease-specific questionnaires including the COPD Assessment Test4 and the SGRQ-C. The SGRQ was first developed in British English and has been translated into many languages. Several of these validations have been published and have shown good internal consistency and reliability.5-8
COPD is an often neglected major public health problem in Nepal where 33% of men and 15% of women are smokers, and 85% of all households and 98% of rural households still rely on daily use of biomass fuels for cooking.9,10 It is estimated that COPD accounts for 43% of the non-communicable disease burden and 3% of hospitalizations in Nepal.2 Low levels of COPD awareness occurs not only among individuals but also among health care providers. This has resulted in under-recognition and under-treatment of this condition. Prompt interventions like smoking cessation, reduction to biomass fuel smoke exposure and improvement in awareness of the disease are crucial to reducing the burden of COPD. Having validated tools in Nepali like the SGRQ can thus help with screening and follow-up of COPD patients, and also in research studies. We sought to translate the SGRQ to Nepali and validate its use in a sample of participants with and without documented COPD. In a secondary analysis, we sought to determine if the SGRQ could be used as a screening tool to identify individuals at risk for COPD who may need further evaluation.
Materials and Methods
Study Setting
We conducted our study in 2 separate settings: a community-based setting in the Sarlahi district of southern Nepal in the densely populated low-lying southern plains of the Terai; and, in a hospital-based setting at Norvic International Hospital in Kathmandu, Nepal. The Sarlahi District is predominantly rural and socioeconomically homogenous, and households use predominantly, if not exclusively, biomass fuels for cooking.11,12 In contrast, Kathmandu is the most populous city of Nepal. Households in Kathmandu predominantly use liquid-propane gas or electricity for cooking. The study protocol was approved by the ethics committees of the Bloomberg School of Public Health, Johns Hopkins University in Baltimore, Maryland and the Institute of Medicine, Tribhuvan University and Norvic International Hospital in Kathmandu, Nepal.
Study Design
We aimed to enroll 150 adults with and without COPD: 50 patients without COPD (post-bronchodilator forced expiratory volume in 1 second [FEV1 ]/ forced vital capacity [FVC] ≥ 70%) and 50 with spirometry-confirmed COPD (post-bronchodilator FEV1/FVC < 70%) in the community setting located in Sarlahi, and 50 outpatients with an established diagnosis of COPD who attended the pulmonary clinic at Norvic International Hospital. Community participants were not actively seeking medical care and were randomly selected from a longitudinal cohort of adult participants aged ≥40 years nested into a larger study.13 We defined COPD as a post-bronchodilator FEV1/FVC<70%.14 Inclusion criteria for all participants included age 40 to 80 years and ability to provide informed consent. Exclusion criteria included currently having active pulmonary tuberculosis or a self-reported history of pulmonary infection in the last 60 days. Because of high illiteracy rates, all participants provided verbal informed consent.
After informed consent, all participants were then interviewed using the Nepali version of the SGRQ developed by our team. Participants from the community setting in Sarlahi underwent spirometry using a portable spirometer (SpiroPro, Jaeger, Hoechberg, Germany) following standard guidelines.15 Patients were enrolled in a consecutive manner until 50 patients with spirometry-confirmed COPD and 50 patients without COPD were reached. Participants with an established diagnosis of COPD at Norvic International Hospital had documented pre- and post-bronchodilator spirometry, and the most recent values in the last year were used.
Translation and Linguistic Validation of the St. George’s Respiratory Questionnaire
We followed the widely used approach advocated by MAPI Group (http://www.mapigroup.com/) for the validation of SGRQ in Nepali. Our validation process was comprised of 6 steps: conceptual definition, forward translation, pilot testing, backward translation, internal harmonization and proof-reading. Two pairs of study team members (SS/CS and RP/AK) who are native speakers of the Nepali language, proficient in English and living in Nepal, independently did a forward translation of the English SGRQ into Nepali. The 2 questionnaires were then compared, reconciled and proof-read into 1 agreed version (by CS). The reconciled version was pilot-tested on 5 COPD patients, followed by a focus group discussion with team members fluent in Nepali from our community setting in Sarlahi, and a few words of the questionnaire items were modified. The final version was then translated back into English by a native English speaker who was also proficient in Nepali (SL), and we confirmed that the context and meaning were maintained between the original English version and the translated version.
Biostatistical Methods
We compared the SGRQ total score by disease category status using linear regression adjusted for age, sex, height and body mass index (BMI). We then calculated the area under the receiver operating characteristic curve when using the SGRQ total score as a means to identify COPD, and calculated the optimal cutoff point based on the equality of sensitivity and specificity.16 We compared baseline characteristics and spirometry results using chi-square tests or analysis of variance (ANOVA) for categorical and continuous variables, as appropriate. We used percent-predicted (% predicted) post-bronchodilator FEV1 values to assess severity by Global initiative for chronic Obstructive Lung Disease (GOLD) staging. Since there are no established reference equations for the Nepalese, we utilized reference equations for the Global Lung Function Initiative (GLI) mixed ethnic population.17 Statistical analyses were conducted in R (www.r-project.org).
Results
Participant Characteristics
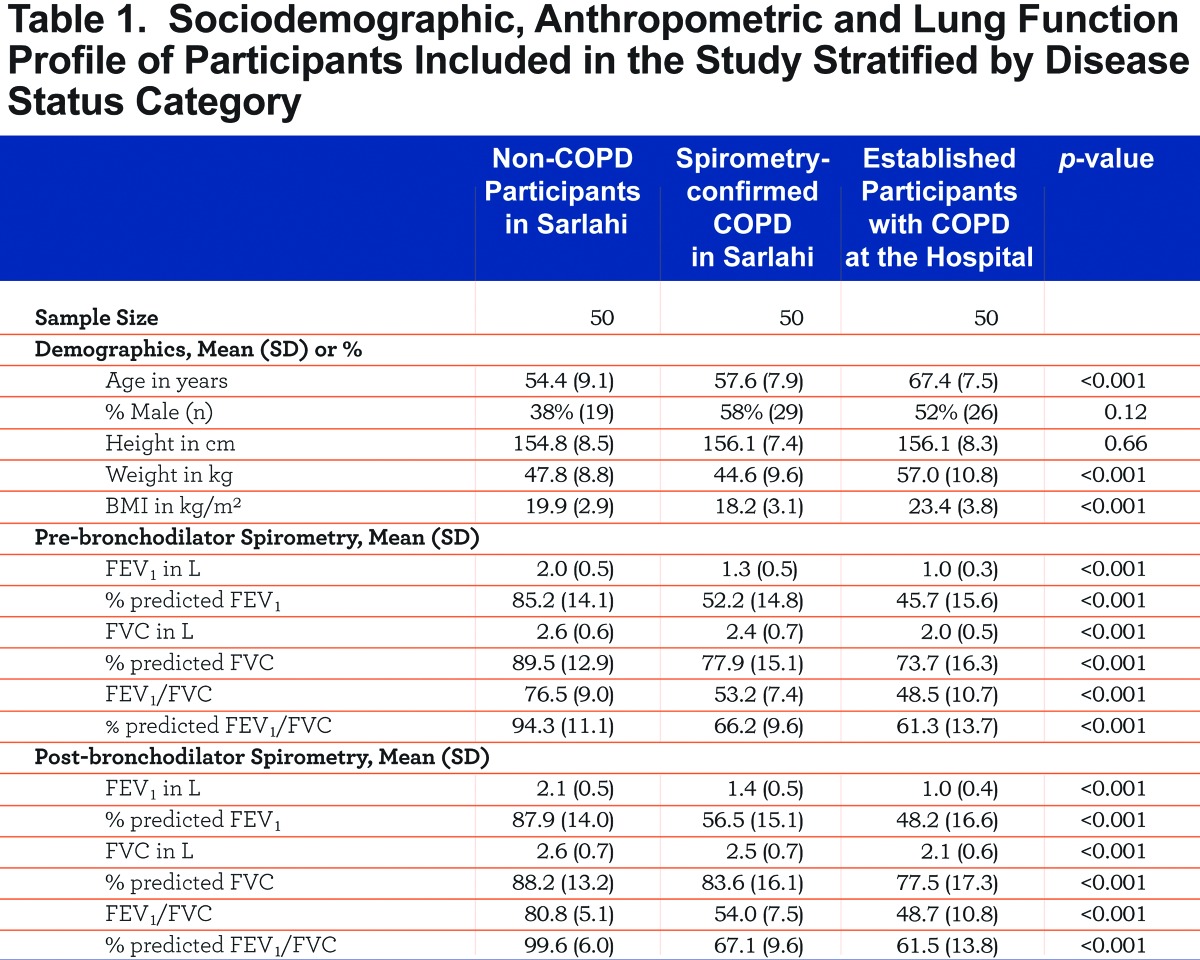
A total of 150 participants were enrolled in the study, of which 72 (48%) were male. One hundred participants were enrolled in a community setting of which 50 had spirometry-confirmed COPD and 50 did not. The remaining 50 participants had established COPD and were enrolled from the pulmonary clinic at Norvic International Hospital. All participants completed the questionnaire. Average age was 59.8 years (SD=9.9) and average BMI was 20.5 kg/m2 (SD=3.9). Hospital-based participants with established COPD were older and had a higher BMI than participants from the community, but no differences in height were noted (Table 1). FEV1 and FEV1/FVC ratio values were lower in hospital-based participants with established COPD versus those with spirometry-confirmed COPD in the community and those without COPD (Table 2). Outpatients with an established diagnosis of COPD had slightly more severe disease than spirometry-confirmed COPD in the community: 60% versus 40% had GOLD Stage III or greater (p=0.07) and 14% versus 2% had GOLD Stage IV or greater (p=0.06), respectively.
Validation Stage
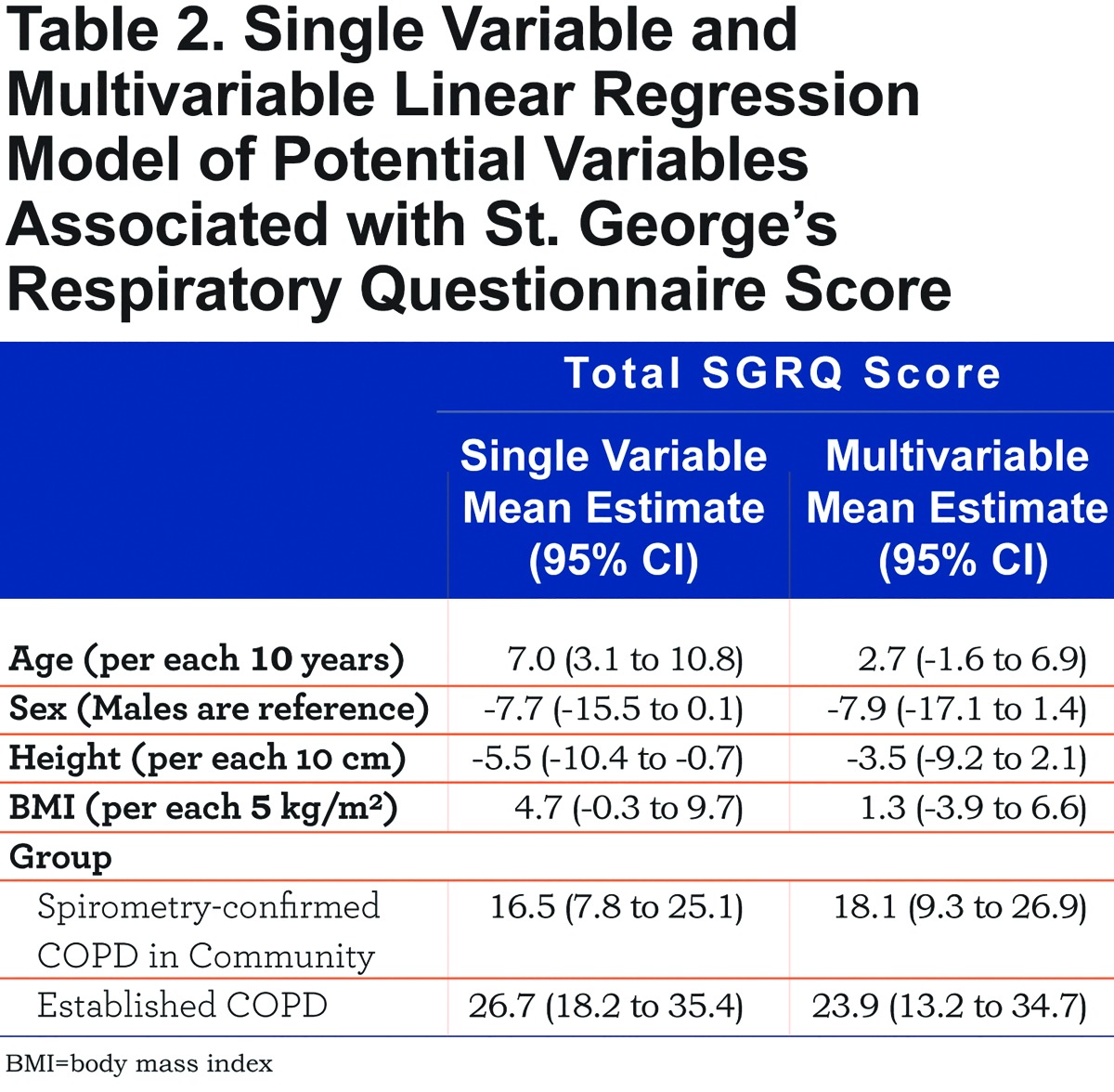
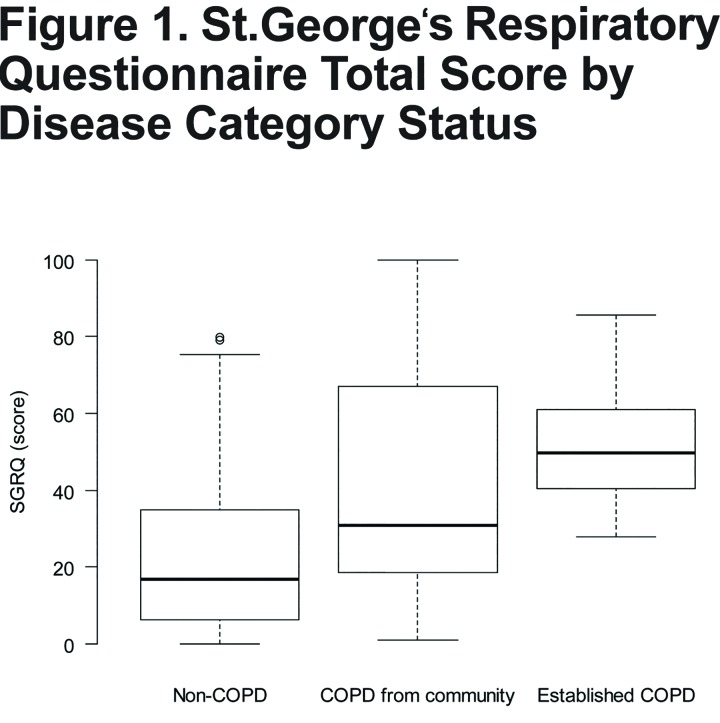
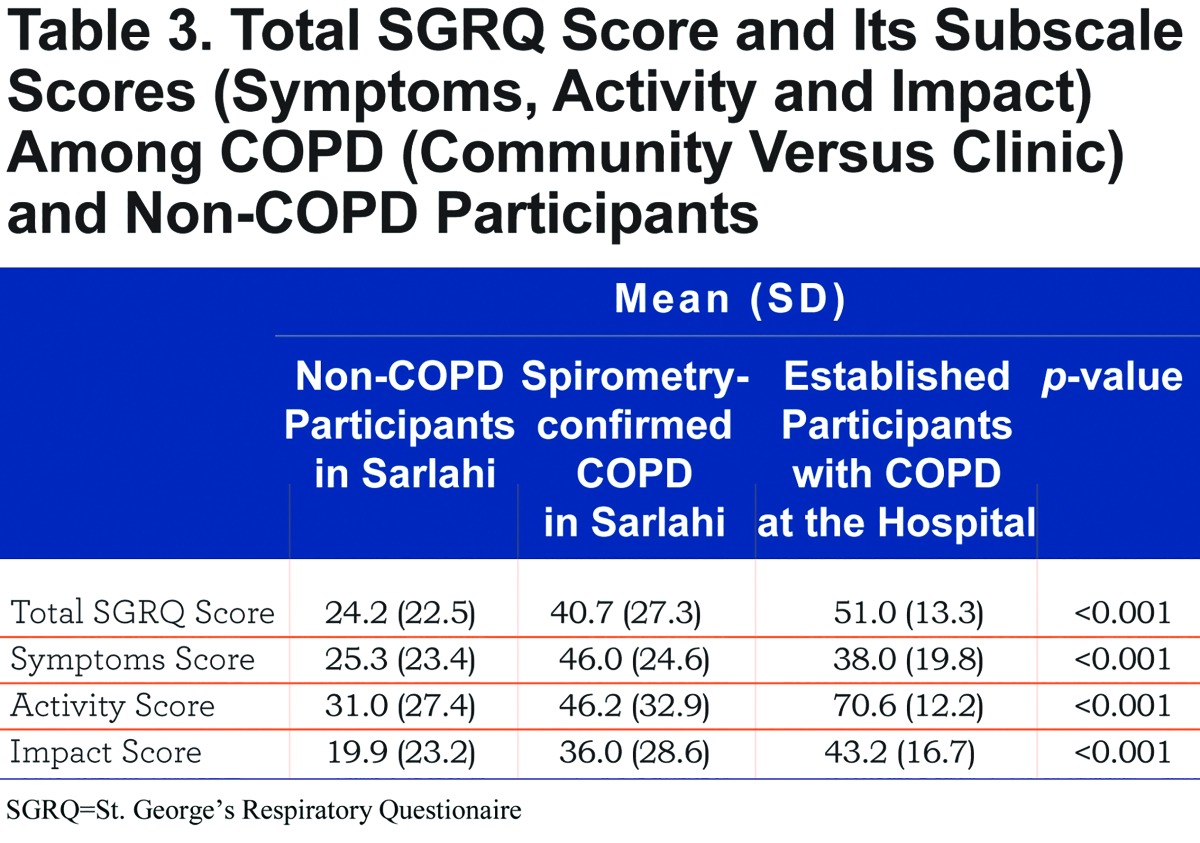
We show the distribution of SGRQ scores by group in Figure 1. On average, SGRQ total score was 23.9 points higher in participants with established COPD (p<0.001) and 18.1 points higher in spirometry-confirmed COPD from the community than in participants without COPD living in the community (p<0.001), adjusted for age, sex, height and BMI (Table 2). Each of the 3 components of the SGRQ score was higher in participants with established COPD than in participants from the community (Table 3). While participants with spirometry-confirmed COPD in the community had higher activity and impact scores than participants without COPD living in the community, they had similar overall symptoms scores.
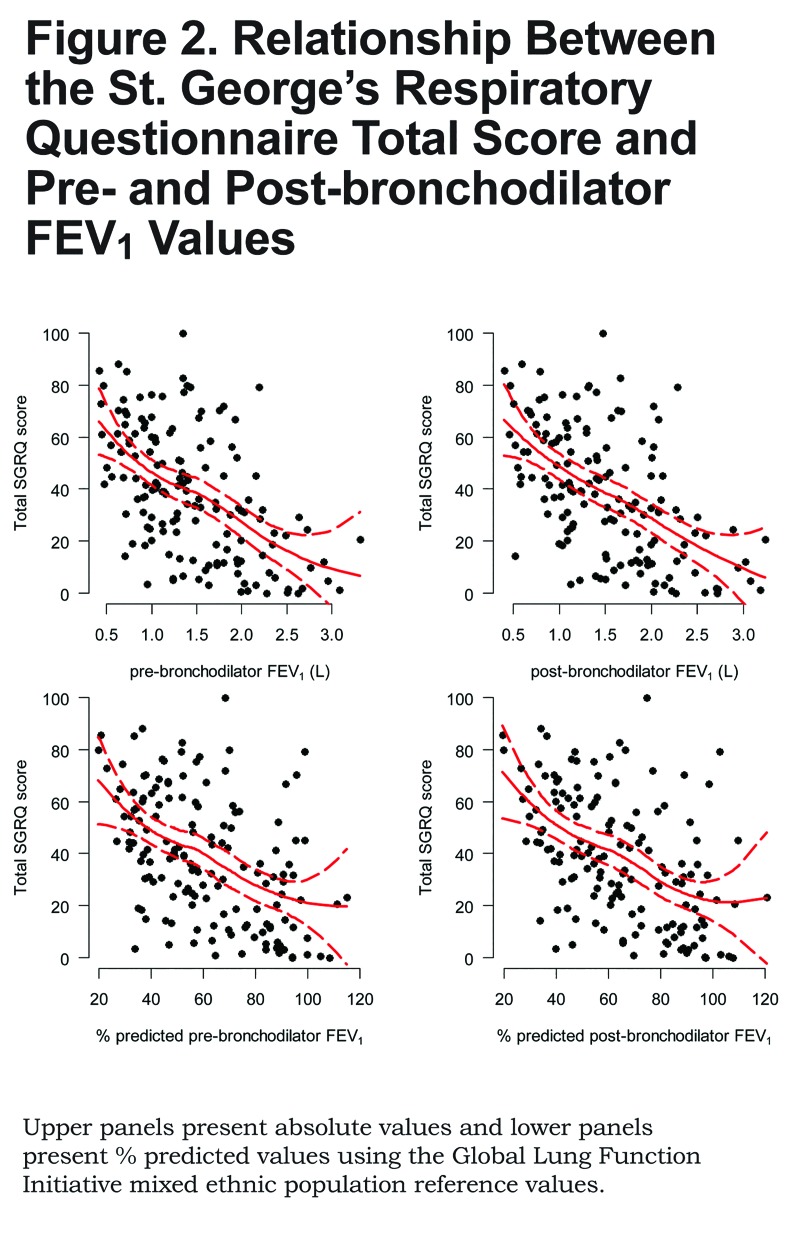
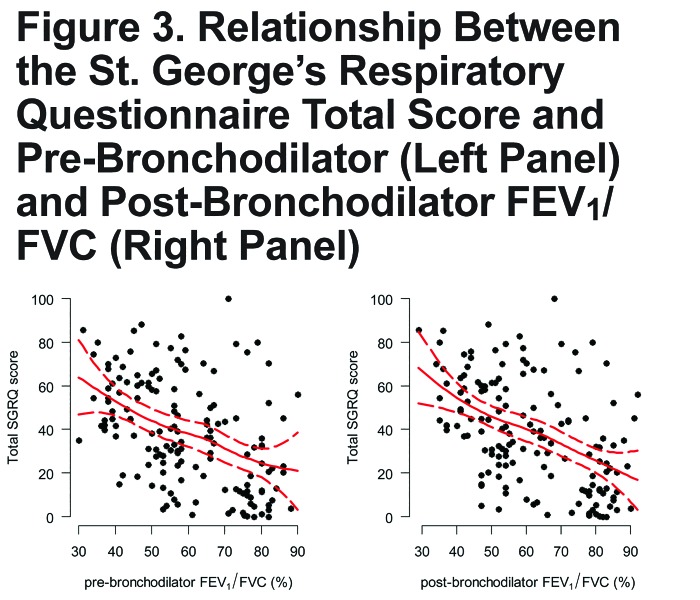
There was a linear relationship between SGRQ total score and pre- and post-bronchodilator values of FEV1, either absolute or % predicted (Figure 2). Specifically, we found that for every 100 mL decrease in post-bronchodilator FEV1 there was a 2.1 point increase (95% CI 1.4 to 2.7) in total SGRQ score; and, for every 5% decrease in % predicted post-bronchodilator FEV1 there was a 2.3 point increase (95% CI 1.6 to 3.1). We saw a similar linear relationship between pre- or post-bronchodilator FEV1/FVC and SGRQ total score (Figure 3). Specifically, for every 5% decrease in post-bronchodilator FEV1/FVC, there was 3.4 point increase in SGRQ total score (95% CI 2.2 to 4.5).
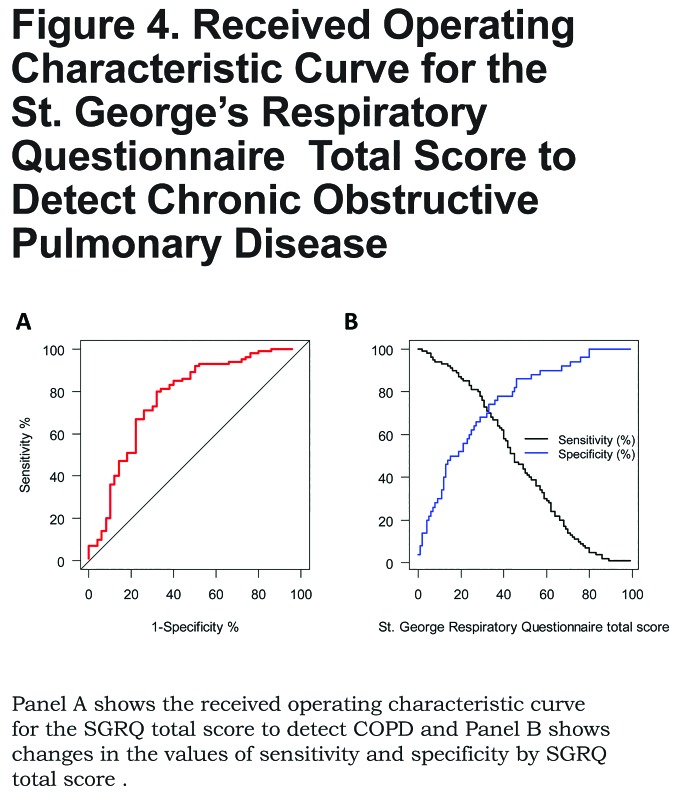
The area under the receiver operating characteristic curve for the SGRQ total score as a predictor of COPD was 0.77 (95% CI 0.68 to 0.85), and the optimal cutoff to identify COPD was 33 points (Figure 4).
Discussion
This is the first study to validate the SGRQ in the Nepali language, and we found that the total score obtained from this questionnaire was a good proxy for both disease status and lung function. Specifically, the SGRQ discriminated well between individuals with and without the condition as reflected by total higher scores among COPD patients. Moreover, the total score for the SGRQ was linearly and inversely associated with both FEV1 and FEV1/FVC.
Like some previous validations of the SGRQ into other languages, 5,8,18-20 our study showed a negative correlation between the SGRQ total score and lung function; however, we found only 1 other study that quantified the magnitude of this association. When compared with the meta-analysis conducted by Westwood et al,19 our findings of the relationship between total score and lung function were similar. Specifically, Westwood et al reported a change in 2.5 points in total score per 100 mL of FEV1 using data from multiple studies whereas our study reported a change in 2.1 points per 100 mL in FEV1. Another small study conducted in Korea found that there was a 5.8 increase in SGRQ total score per each 5% decrease in % predicted FEV1, which is similar in magnitude to our findings when we used % predicted FEV1.21
The average total SGRQ scores in our study sample were high, even among the group of participants from the community who did not have COPD. Specifically, in the latter, the average SGRQ score was 3 times higher than the mean normative value of a general population of Spanish adults aged 50-59 years.22 A likely explanation for this increase is that participants in the community setting are exposed to biomass fuel smoke on a daily basis. A small intervention trial that used an improved, ventilated cookstove conducted in Bolivia found that adult women (average age 48 years) who were chronically exposed to biomass fuel smoke had average scores ranging from 29.9 post-intervention to 54.3.23 The average values for the total SGRQ score obtained in our study among participants with COPD were similar in range to those in a large, multi-center study of COPD patients across Asia.24
Our validation study has several strengths. First, our study sample size was slightly larger than that of previous validation studies in other languages.5,8,18-20 Second, unlike previous validations, we included a group of healthy individuals without COPD. By including this group, we found that the SGRQ total score was able to discriminate well between healthy and diseased participants, suggesting it could be used as a screening tool in our setting for COPD. Third, previous validation studies also selected patients with an established COPD diagnosis from a hospital or clinic settings.5,8,18 In contrast, in our study we enrolled participants from both hospital and community settings.
Our study also has some shortcomings. First, while our sample size is larger than previous validation studies, we were still limited by our sample size in examining for differences between participants to identify sub-populations of participants with COPD based on severity. Second, it would have been ideal to perform a longitudinal follow-up and examine for the correlation between COPD exacerbation and SGRQ total score within individual patients as done by other investigators.25,26 Third, our sample consisted of the community participants in a rural setting who were predominantly, if not exclusively, exposed daily to biomass fuel smoke whereas those in the hospital were enrolled in Kathmandu and were more likely to use liquid-propane gas or other clean fuels for cooking. However, we did not collect this information. Therefore, it is unclear how environmental exposures may have affected our results when comparing patients with established COPD and COPD from the community. Fourth, we did not conduct serial tests to assess repeatability. However, our questionnaire is sufficiently reliable to statistically distinguish between participants with and without COPD. Moreover, we also documented dose-response relationship between SGRQ and increasing disease severity measured objectively with lung function values and subjectively by groups of participants (i.e., non-COPD participants in Sarlahi versus spirometry-confirmed COPD in Sarlahi versus established participants with COPD at the hospital). Finally, we did not quantify tobacco smoking consumption or biomass fuel smoke exposure in the study sample. However, while we did not collect qualitative or quantitative information on environmental exposures for the participants in this study, we have documented via field observations that participants in our study site in Sarlahi use biomass fuels predominantly if not exclusively for cooking.27,28
In summary, the Nepali-validated SGRQ can be used in epidemiological studies as a measure of quality of life in chronic respiratory disease, and it may also be a good screening survey to identify people with COPD who may need further evaluation with spirometry.
Abbreviations
St. George’s Respiratory Questionnaire, SGRQ; chronic obstructive pulmonary disease, COPD; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; body mass index, BMI; confidence interval, CI; analysis of variance, ANOVA; percent predicted, % predicted; Global initiative for chronic Obstructive Lung Disease, GOLD; Global Lung Function Initiative, GLI
Funding Statement
This project has been funded in whole with Federal funds from the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268200900033C.
References
- 1. Mannino DM,Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765-773. doi:http://dx.doi.org/10.1016/S0140-6736(07)61380-4 [DOI] [PubMed] [Google Scholar]
- 2. Bhandari R,Sharma R. Epidemiology of chronic obstructive pulmonary disease: a descriptive study in the mid-western region of Nepal. Int J Chron Obstruct Pulmon Dis. 2012;7:253-257. doi:http://dx.doi.org/10.2147/COPD.S28602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones PW,Quirk FH,Baveystock CM,Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321-1327. doi:http://dx.doi.org/10.1164/ajrccm/145.6.1321 [DOI] [PubMed] [Google Scholar]
- 4. Jones PW,Harding G,Berry P,Wiklund I,Chen WH,Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648-654. doi:http://dx.doi.org/10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 5. Katsoulas TA,Skordilis EK,Myrianthefs P,Fildisis G,Theodosopoulou E,Baltopoulos G. Validity of St. George's Respiratory Questionnaire for Greek patients with chronic obstructive pulmonary disease. Percept Mot Skills. 2010;110:772-788. doi:http://dx.doi.org/10.2466/pms.110.3.772-788 [DOI] [PubMed] [Google Scholar]
- 6. Barr JT,Schumacher GE,Freeman S,LeMoine M,Bakst AW,Jones PW. American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin Ther. 2000;22:1121-1145. doi:http://dx.doi.org/10.1016/S0149-2918(00)80089-2 [DOI] [PubMed] [Google Scholar]
- 7. Engstrom CP,Persson LO,Larsson S,Sullivan M. Reliability and validity of a Swedish version of the St George's Respiratory Questionnaire. Eur Respir J. 1998;11:61-66. doi:http://dx.doi.org/10.1183/09031936.98.11010061 [DOI] [PubMed] [Google Scholar]
- 8. Aggarwal AN,Gupta D,Kumar T,Singh N,Jindal SK. Validation of Hindi translation of St. George's respiratory questionnaire in Indian patients with chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci. 2007;49:87-91. [Google Scholar]
- 9. Ministry of Health and Population, Government of Nepal; Brief profile on tobacco control in Nepal. World Health Organization website. http://apps.who.int/fctc/reporting/party_reports/nepal_2012_annex2_tobacco_profile.pdf Published2012 AccessedAugust2014. [Google Scholar]
- 10. Pandey MR,Smith KR,Boleij JSM,Wafula EM. Indoor air pollution in developing countries and acute respiratory infection in children. Lancet. 1989;333: 427-429. doi:http://dx.doi.org/10.1016/S0140-6736(89)90015-9 [DOI] [PubMed] [Google Scholar]
- 11. Checkley W,West KP Jr. ,Wise RA,et al. Maternal vitamin A supplementation and lung function in offspring. N Engl J Med. 2010;362:1784-1794. doi:http://dx.doi.org/10.1056/NEJMoa0907441 [DOI] [PubMed] [Google Scholar]
- 12. Checkley W,West KPJ,Wise RA,et al. Supplementation with vitamin A early in life and subsequent risk of asthma. Eur Respir J. 2011;38:1310-1319. doi:http://dx.doi.org/10.1183/09031936.00006911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tielsch JM,Katz J,Zeger SL,et al. Designs of two randomized, community-based trials to assess the impact of alternative cookstove installation on respiratory illness among young children and reproductive outcomes in rural Nepal. BMC Public Health. 2014;14(1):1271. doi:http://dx.doi.org/10.1186/1471-2458-14-1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vestbo J,Hurd SS,Agustí AG,et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347-365. doi:http://dx.doi.org/10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 15. Miller MR,Hankinson J,Brusasco V,et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338. doi:http://dx.doi.org/10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 16. Hosmer DW Jr. ,Lemeshow S. Applied Logistic Regression. John Wiley & Sons: New York, NY: 2004. [Google Scholar]
- 17. Quanjer PH,Stanojevic S,Cole TJ,et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324-1343. doi:http://dx.doi.org/10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu W-X,Zhang Y-J,Hu B,Ma Y,Zhu Y-J. Application of St George's respiratory questionnaire in evaluating the life quality of Chinese patients with chronic obstructive pulmonary disease [in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi. 2003;26:195-198. [PubMed] [Google Scholar]
- 19. Westwood M,Bourbeau J,Jones PW,Cerulli A,Capkun-Niggli G,Worthy G. Relationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respir Res. 2011;12:40. doi:http://dx.doi.org/10.1186/1465-9921-12-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El Rhazi K,Nejjari C,Benjelloun MC,et al. Validation of the St. George's Respiratory Questionnaire in patients with COPD or asthma in Morocco. Int J Tuberc Lung Dis. 2006;10:1273-1278. [PubMed] [Google Scholar]
- 21. Kim MA,Kim DK,Lee CH,Chung HS. The correlation of brain natriuretic peptide (BNP), pulmonary arterial pressure, and St. George Respiratory Questionnaire (SGRQ) and their changes with a trial of an angiotensin converting enzyme inhibitor. Tuberc Respir Dis. 2010;68:273-279. doi:http://dx.doi.org/10.4046/trd.2010.68.5.273 [Google Scholar]
- 22. Ferrer M,Villasante C,Alonso J,et al. Interpretation of quality of life scores from the St George's Respiratory Questionnaire. Eur Respir J. 2002;19:405-413. doi:http://dx.doi.org/10.1183/09031936.02.00213202 [DOI] [PubMed] [Google Scholar]
- 23. Alexander D,Linnes JC,Bolton S,Larson T. Ventilated cookstoves associated with improvements in respiratory health-related quality of life in rural Bolivia. J Public Health (Oxf). 2014;36(3):460-466. doi:http://dx.doi.org/10.1093/pubmed/fdt086 [DOI] [PubMed] [Google Scholar]
- 24. Oh YM,Bhome AB,Boonsawat W,et al. Characteristics of stable chronic obstructive pulmonary disease patients in the pulmonology clinics of seven Asian cities. Int J Chron Obstruct Pulmon Dis. 2013;8:31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim T,Huh J,Oh Y,Lee S. The factors influencing the acute exacerbation of COPD in 1 year follow-up: The experience of KOLD Cohort. Am J Res Crit Care Med. 2009;179: A1509. doi:http://dx.doi.org/10.1164/ajrccm-conference.2009.179.1_MeetingAbstracts.A1509 [Google Scholar]
- 26. Miravitlles M,Ferrer M,Pont A. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004;59:387-395. doi:http://dx.doi.org/10.1136/thx.2003.008730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rhodes EL,Dreibelbis R,Klasen EM,et al. Behavioral attitudes and preferences in cooking practices with traditional open-fire stoves in Peru, Nepal, and Kenya: implications for improved cookstove interventions. Int J Environ Res Public Health. 2014;11:10310-10326. doi:http://dx.doi.org/10.3390/ijerph111010310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tielsch JM,Katz J,Zeger SL,et al. Designs of two randomized, community-based trials to assess the impact of alternative cookstove installation on respiratory illness among young children and reproductive outcomes in rural Nepal. BMC Public Health. 2014;14:1271. doi:http://dx.doi.org/10.1186/1471-2458-14-1271 [DOI] [PMC free article] [PubMed] [Google Scholar]