Abstract
This work shows that optical switching between the spiro (SP) and merocyanine (MC) states of different photochromes specifically labeled to G-actin can be used to rapidly and reversibly modulate specific dipolar interactions within the conjugate. Members of a common spirobenzopyran photochrome and a related spironaphthoxazine that differ only in the locations of their alkylating groups were selectively labeled to Cys-374 on G-actin. The nature of MC and SP interactions within G-actin was investigated by using optical spectroscopy. The average absorption energy of the highly polarized MC is sensitive to interactions with polar groups on solvents and G-actin; the average absorption energy of the corresponding SP state was found to be relatively constant, consistent with its lower dipole moment compared with MC (5 and 20 D, respectively). Alternate excitation of spirobenzopyran G-actin conjugates with 365 and 546 nm leads to rapid transitions from the SP to MC states and MC to SP states, respectively; optical switching within spirobenzopyran-G-actin occurs with high fidelity and the recovery of specific dipolar interactions between the protein and the MC and SP states. The difference in the free energy for specific dipolar interactions between different MC states within G-actin (6 kcal/mol) is similar to that found for complexes of G-actin and its regulatory proteins. We propose, therefore, that optical switching between SP and MC within an appropriately labeled conjugate could be used to inhibit a functional interaction with a ligand in the MC, but not the SP, state.
Keywords: protein engineering
The increasing need to understand biomolecular and cellular processes in terms of the mechanisms of protein-mediated reactions is driving the development of new optical probes capable of specifically manipulating and mapping protein interactions and activities in complex environments (1-6). We have previously shown that protein interactions and activities can be manipulated in vitro and in vivo by using light-directed activation of caged proteins (3, 6). This perturbation technique can yield important kinetic and functional information on the protein with high temporal and spatial resolution (6). However, the photoisomerization of the 2-nitrophenyl group limits the usefulness of this technique because the reaction is irreversible, slow, and generates toxic photoproducts.
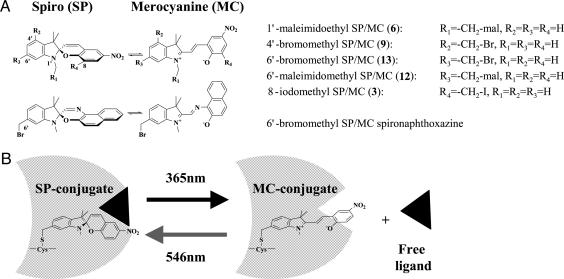
We propose a previously undescribed approach for optical switching of protein interactions (7) that is based on optical control of specific dipolar interactions within proteins specifically labeled with a single photochromic probe. The spirobenzopyran probe is well suited for this task because it undergoes rapid, and reversible, light-directed transitions between a colorless spiro (SP) state and a colorful merocyanine (MC) state without the release of photoproducts (7-13). Optical spectroscopy is used to characterize the interactions of the two switch states within the conjugates and to provide information on their dipolar interactions with G-actin. We argue that if essential dipolar interactions between a ligand and the spirobenzopyran conjugate can be selectively perturbed in the MC, but not SP, state, then optical switching between the two probe states could serve to reversibly modulate the binding of the conjugate with a functional ligand (Fig. 1B). To test this hypothesis, we conducted studies aimed at the following: (i) characterizing the nature of the interactions between SP and MC states specifically labeled within G-actin; (ii) characterizing the properties and fidelity of photochemical transitions between the SP and MC states in G-actin; and (iii) demonstrating the feasibility of engineering spirobenzopyran conjugates whose dipolar interaction with functional ligands is inhibited in only one of the two switch states (Fig. 1B). These studies are facilitated by the introduction of a family of spirobenzopyrans that differ only in the location of the thiol-reactive group and a related spironaphthoxazine (Fig. 1 A).
Fig. 1.
Chemical structures of the thiol-reactive photochromic probes. (A) Chemical structures of the thiol-reactive spirobenzopyrans and the thiol-reactive spironaphthozaxine described in this work showing light-driven transitions between the SP and MC states. (B) Schematic representation of a previously undescribed approach for reversible optical switching of functional interactions and activities of biomolecular conjugates of spirobenzopyran.
Materials and Methods
Instrumentation. Absorption spectra were recorded on a Hewlett-Packard 82152 diode array spectrophotometer or a Shimadzu 1601PC instrument. Fluorescence spectroscopy was performed on an SLM-AB2 instrument (Thermoelectron, Madison, WI) or an ISS PC1 (Champaign, IL). Light-directed switching of the probes described in this work was achieved by irradiating the sample (120-1,000 μl) with the 365- or 546-nm lines of a 100-W Hg-arc lamp (Zeiss).
Synthesis. The synthesis of the five thiol-reactive spirobenzopyrans (compounds 3, 6, 9, 12, and 13) and the thiol-reactive spironaphthoxazine (17) is on modifications of established work (14, 16, 17) and is described in ref. 18.
Labeling of Proteins with Thiol-Reactive Spirobenzopyrans. Rabbit muscle G-actin was purified as described by Marriott (19) in G-buffer (2 mM Tris/0.2 mM CaCl2/0.2 mM ATP, pH 8.0). The concentration of G-actin was determined by absorption spectroscopy using an extinction coefficient of 3,400 M-1·cm-1 at 290 nm (19). One milliliter of a 20 μM solution of G-actin was treated with 20 μl of a 10 mM dimethylformamide stock solution of each thiol-reactive, spirobenzopyran probe (3, 6, 9, 12, and 13) for 2 h at room temperature. The protein was centrifuged for 10 min at 2, 000 × g at 4° and applied to a Bio-Rad PD-10 column equilibrated in G-buffer containing 1 mM DTT. The concentration of spirobenzopyran in each G-actin conjugate was determined from the value of the absorption maximum of the SP form of G-actin conjugates by using an extinction coefficient of 35,000 M-1·cm-1 at 350 nm (20) for the SP state and 52,000 M-1·cm-1 for the MC state (20). The absorption spectra of the MC and SP probes in G-actin were not affected by the labeling ratio (spirobenzopyran/G-actin), which varied from 0.6 to 0.8 in the different conjugates. The G-actin conjugate of the spironaphthoxazine was prepared as described in ref. 18.
Results and Discussion
An important feature in the design of the spirobenzopyran and spironaphthoxazine probes described in this work is the introduction of reactive groups (bromomethyl-, iodomethyl-, and maleimido) to different sites in the probe molecule (Fig. 1 A; see also Fig. 6). This feature provides some control of the orientation of the probe within the bioconjugate. Because the MC state of spirobenzopyran is exquisitely sensitive to changes in specific solvent interactions, these probes and associated techniques provide a previously undescribed approach to survey dipolar interactions and the dipolar landscape of the protein in the vicinity of the thiol attachment site (e.g., Cys-374 on G-actin).
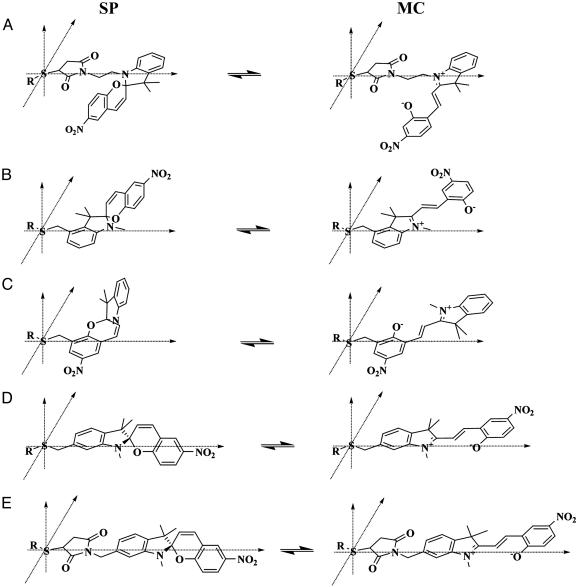
Fig. 6.
Schematic representation of the linkage geometry between the thiol group on a biomolecule and the five thiol-reactive spirobenzopyran probes described in this work as follows: compounds 6 (A), 9 (B), 3 (C), 13 (D), and 12 (E). Two points of reference are used for each probe: the sulfur atom on the biomolecule is fixed at the origin, and the atom harboring the thiol-reactive group on the spirobenzopyran is forced to lie on the x axis. The SP and MC states of the five chromophores have the same stereochemical arrangement.
Optical Spectroscopy of Spirobenzopyran in Solvents. A characterization of the effects of solvent interactions on the spectroscopic properties of MC and SP will be essential for interpreting the nature of MC and SP interactions within a protein conjugate. The average energy of the lowest energy absorption band (S0 - S1) for MC and SP contains information on the dielectric constant of the solvent and the presence of specific solvent interactions, such as H-bonds and dipole-dipole. The ground and excited-state dipole moments for polar aromatic probes such as MC often are defined by the location of polar groups within the conjugated ring system (21). In the case of MC, the two monopoles of the dipole are most likely defined by the positive nitrogen atom and the negative nitro group (Fig. 1 A). The permanent charge on the nitrogen and the highly polarized nitro group probably account for the fact that MC has a very high ground-state dipole moment (20D) (22).
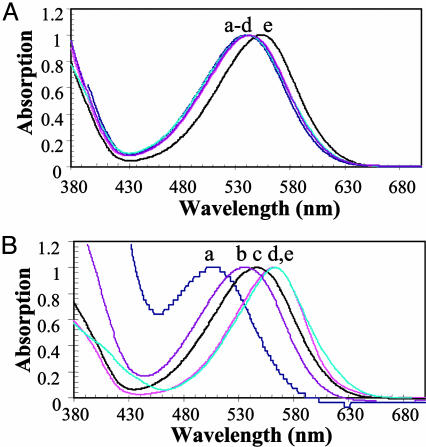
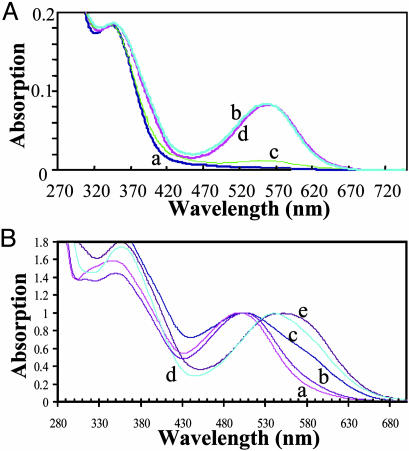
Absorption and fluorescence spectroscopic analysis of optical probes can provide important information on the nature and strength of molecular interactions between the SP and MC states and solvent molecules (20, 21, 23-25). For example, the similarity in the MC absorption spectra for the five spirobenzopyrans (3, 6, 9, 12, and 13) (Fig. 2A) dissolved in ethanol suggests that an alkyl substitution on the MC chromophore does not change its aromaticity or the nature of its interactions with solvent molecules; however, we note that N-alkylation does cause a slight red shift in the absorption spectrum (curve e in Fig. 2 A). We argue, therefore, that any solvent-induced shift in the MC (or SP) absorption spectrum results from differences in specific MC-solvent interactions and/or the difference between their dielectric constants. This argument is supported in this work on the dependence of the MC absorption spectrum (compound 12) on the solvent (water/2-propanol/1,2-propanediol/acetonitrile/dichloromethane; Fig. 2B). In general, the MC absorption spectrum is blue-shifted in polar and red-shifted in apolar solvents. A comparative analysis of these data as described below reveals that the blue shifts are caused by specific dipolar interactions; thus, the MC absorption spectrum in 1,2-propanediol is blue-shifted compared with that in 2-propanol, even though their dielectric constants are reasonably similar (32 and 20.1). We argue that the blue shift reflects a greater H-bonding capacity for 1,2-propanediol vs. 2-propanol. The MC spectrum is further blue-shifted in water and is caused by a combination of the higher dielectric constant of water and stronger H-bonds. Conversely, apolar, low dielectric solvents, such as dichloromethane, lead to a considerable red shift in the MC absorption spectrum. These studies show that the average energy of the MC absorption is highly sensitive to specific dipolar interactions. We argue that nitro group is a key determinant of the large MC dipole moment in spirobenzopyran (and the corresponding solvent-induced shifts in absorption spectrum) because the related spironaphthoxazine (Fig. 1 A), which lacks the nitro group, is insensitive to solvent polarity (18). Finally, Gorner (20) has shown that the quantum yield for optical transitions within the spirobenzopyran photochrome also depends on solvent-photochrome interactions because it changes from 0.75 in apolar solvents (toluene) to 0.1 in the more polar acetonitrile.
Fig. 2.
Absorption spectra of the MC state of the thiol-reactive probes. (A) Absorption spectra of the MC state of the five thiol-reactive probes described in this work in ethanol at a concentration of 20 μM. The maximum absorption value of the lowest energy transition for each probe is normalized to a value of 1.0. The letters a-e refer to compounds 3, 9, 12, 13, and 6, respectively. (B) Absorption spectra of the MC state of compound 12 in the following solvents: curve a, water; curve b, 1,2-propanediol; curve c, 2-propanol; curve d, acetonitrile; and curve e, dichloromethane.
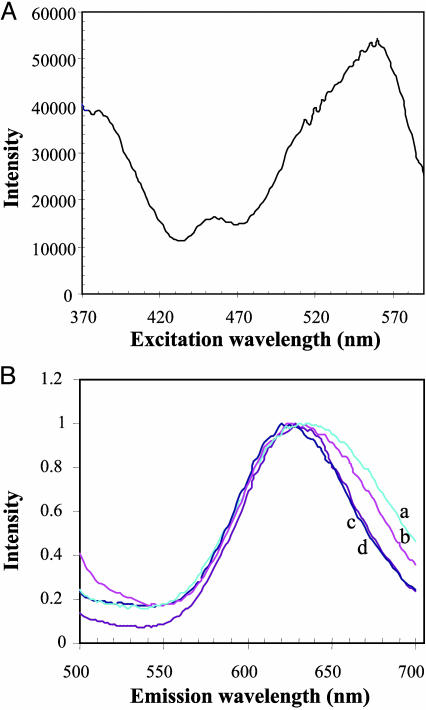
Further information on the nature of MC-solvent interactions can be derived from studies of its fluorescence emission in different molecular environments (21). Given the high quantum yield for the photochemical conversion of MC to SP (20), it is not surprising to find that fluorescence emission of MC is weak (Fig. 3A). We found that the fluorescence emission from the 1MC* state is best studied by exciting the spirobenzopyran solution with 365-nm light (S0 - S2 transition; see the Jablonski diagram in Fig. 7, which is published as supporting information on the PNAS web site); this condition generates a uniform population of excited-state molecules for fluorescence spectroscopic analysis. Contrary to the findings from the MC absorption studies, the average energy of the MC fluorescence was found to be insensitive to the polarity of the solvent (water/2-propanol/1,2-propanediol/acetonitrile; data not shown) and to the orientation of the MC probe within G-actin conjugates (Fig. 3B).
Fig. 3.
Fluorescence spectra of the thiol-reactive probes. (A) Technical fluorescence excitation spectrum of the G-actin conjugate of compound 12, showing the S0 - S2 (centered at 370 nm) and S0 - S1 (centered at 558 nm) excitation bands of MC. The emission was monitored at an emission wavelength of 650 nm. (B) Normalized, technical emission spectra of G-actin conjugates of the following compounds: curve a, compound 3 (624 nm); curve b, compound 12 (622 nm); curve c, compound 6 (628 nm); and curve d, compound 13 (630 nm). The emission from the MC state in each conjugate was generated by using an excitation wavelength of 370 nm.
The anomalous polar solvent-induced blue shifts in the MC absorption spectrum and the insensitivity of MC fluorescence to polar solvents can be explained by considering the unusual property of the MC dipole moment, which is lower in the excited state (14 D) compared with the ground state (20 D) (22). The inversion of the strength of the dipole moment in the excited state will lead to interactions between MC and solvent or protein dipoles that are stronger in the ground state (Figs. 2B and 4B) compared with the excited-state, as seen in the constancy of the emission spectra for different MC-G-actin conjugates (Fig. 3B). An energy level diagram consistent with these results is schematized in the supporting information. Our results indicate that the nitro group is an important contributor to the observed solvent-dependent absorption spectral shifts for spirobenzopyran, because the spectrum of a related and red-shifted spironaphthoxazine (18) (Fig. 1 A) is far less sensitive to solvent polarity (data not shown).
Fig. 4.
Absorption spectra of spirobenzopyran G-actin conjugates. (A) Optical switching between the SP and MC states on G-actin. Absorption spectra of compound 13 attached to Cys-374 on G-actin in response to sequential irradiation with UV and visible light are shown. Curve a shows preirradiated SP state; curve b shows 30-s illumination of the conjugate with 365-nm light; curve c shows 30-s illumination of the MC conjugate (spectrum b) with 546-nm light; and curve d shows 30-s illumination of the SP conjugate (spectrum c) with 365-nm light. (B) Normalized absorption spectra for the lowest energy transition of the MC state of the five thiol-reactive probes attached to Cys-374 on actin. Curves a-e refer to the compounds 9, 13, 3, 6, and 12, respectively.
Effect of Linkage Geometry on the Absorption Spectrum of Spirobenzopyran Conjugates. Having shown that the MC absorption spectrum is sensitive to changes in specific dipolar interactions, we performed parallel studies on spirobenzopyran protein conjugates to characterize the nature of the interactions between MC and SP and the protein matrix.
G-Actin. The region around Cys-374 in G-actin is involved in regulatory interactions with actin and actin-binding proteins (15, 24, 26). Absorption spectroscopic analysis of the five spirobenzopyrans differentially linked to G-actin was used to reveal information on dipolar interactions between MC and SP and G-actin and the nature of the molecular environment around Cys-374.
The introduction of thiol-reactive groups at different sites on a common spirobenzopyran scaffold (Fig. 1 A) allows control of the orientation of the MC probe and its dipole moment within the G-actin conjugate. The different labeling geometries generated within the spirobenzopyran family of G-actin conjugates forces MC and SP probes to occupy slightly different sites in the volume bounded by Cys-374. The results from an absorption spectroscopic analysis of these conjugates suggest that differentially linked MC probes engage in a diverse array of polar interactions with polar groups on the protein within a small-volume element around Cys-374. Although we cannot attribute changes in the MC absorption spectra to specific types of interactions, we expect that the polar, aromatic probe will engage in Van der Waals contacts and stronger dipolar interactions with peptide bonds, polar side groups, surface water molecules, and charged amino acid residues (25).
Although the average energy of the MC (or SP) absorption spectrum in G-actin does not represent the true binding energy of the probe-protein complex, differences in the average energy of the absorption spectrum of a common MC probe differentially linked to the same thiol group in a protein conjugate will reflect the contributions from different polar and apolar interactions between MC and the protein matrix, e.g., H-bonding, charge-dipole, or dipole-dipole. If the protein interior could be considered as a homogeneous dielectric, then the average energy of the MC absorption band would be independent of the MC linkage geometry as is seen for the different MC probes in ethanol (Fig. 2 A). The following results, however, show that the nature and strength of dipolar interactions in the small volume around Cys-374 that is surveyed by the five MC probes vary enormously. For example, the average energy of the MC absorption in the five G-actin conjugates (Fig. 4B) varies from 20,000 cm-1 (500 nm) for compound 9, to 18,132 cm-1 (551.5 nm) for compound 12, corresponding to an energy difference of 1,868 ± 27 cm-1 (1 nm at 525.75 nm), or nearly 10 kT. Interestingly, the difference in the average energy of the MC absorption between compounds 12 and 13 on G-actin is also large at 1,400 cm-1, despite the fact that the thiol-reactive groups are linked to an identical atom on the chromophore. This result suggests that the further displacement of the MC dipole from Cys-374 caused by the maleimido-group of compound 12 positions the MC dipole at a site on G-actin where it engages in much stronger dipolar interactions compared with compound 13. On the basis of this analysis of these MC-absorption data, we argue that the dipole environment around Cys-374 in G-actin is highly complex and changes dramatically within a small-volume element around Cys-374.
Absorption spectroscopic analysis of spirobenzopyran-G-actin conjugates was used to investigate the nature of the interaction of SP and G-actin. Contrary to findings derived from the absorption spectra of MC-G-actin, the wavelength at the maximum SP absorption value is almost constant within the five SP-G-actin conjugates (e.g., Fig. 4A, curves a and c). For example, the maximum shifts from 348 nm (28,736 cm-1) in the SP-state in G-actin conjugates of compounds 6 and 13 to 342 nm (29,240 cm-1) for compound 3 and 29,070 cm-1 (344 nm) for compound 12 (data and spectra not shown). A maximum could not be determined with confidence from the flat spectrum measured for the conjugate with compound 9. The largest difference in energy for the five differentially linked SP states on G-actin is 504 ± 85 cm-1 (±1 nm at 345 nm) between compounds 3 and 6 and 12. This value represents only 27% of the equivalent difference found for the most divergent MC states within G-actin, and, interestingly, this value scales with the strength of the dipole moments of SP and MC of 5 and 20 D, respectively (22). We note that the difference in energy between the most divergent MC conjugates (1,868 cm-1) and the most divergent SP conjugates (504 cm-1) is 1,363 cm-1, which is comparable to the binding energy of G-actin with regulatory proteins and ligands. This finding means that an optical transition between the MC and SP states therefore would release >6 kT of interaction energy from polar groups within G-actin within ≈20 μs (20) that would be available for interactions with a functional interaction with a ligand or protein, which, we argue, demonstrates the feasibility of extending our approach to optical switch dipolar interactions between a spirobenzopyran conjugate and a functional binding partner.
The analyses of the absorption spectra for differentially placed MC probes in G-actin show that the distribution of dipoles within the protein matrix is complex and can vary tremendously in polarity over the very small volumes surveyed by the MC probes in these conjugates. These data also show that optical switching of the SP and MC states in these conjugates occurs with high fidelity and positions the SP and MC probes to the same location in the conjugate where they engage in identical dipolar interactions with polar groups in the protein.
Optical Switching of Dipolar Interactions in Spirobenzopyran Protein Conjugates. Irradiation of the five spirobenzopyran-labeled G-actin conjugates with 365-nm light for 30 s or less generates the highly colored MC conjugate (curve b in Fig. 4A). Conversely, excitation of the MC conjugate with 546-nm light for 30 s or less leads to a photochemical transition of MC back to the colorless SP state (curve c in Fig. 4A). Optical control of the MC and SP states on G-actin is efficient and reversible and can be repeated over many 365/546-nm excitation cycles (curve d in Fig. 4A). Importantly, the MC (and SP) absorption spectrum within G-actin is very similar at the end of each switch cycle. Because the MC-absorption spectrum is highly sensitive to changes in specific dipolar interactions, the full reversibility of the these spectra after each irradiation cycle suggests that optical switching between the SP and MC states occurs with high fidelity and faithfully positions MC and SP to the same locations in conjugate where they engage in identical interactions with polar groups in the protein; we refer to this effect as optical switching of specific dipolar interactions within proteins.
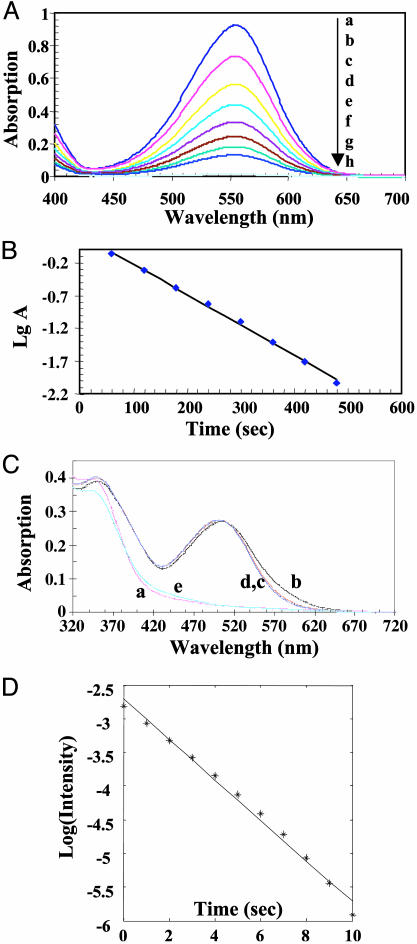
Thermally Driven Transitions Between MC and SP. The rate of the thermal equilibration of the MC to the SP state was measured in ethanol and in the dark by using the decrease in the maximum MC absorption value as a function of time (Fig. 5 A and B). These data are fit by using a single exponential decay function and yield a time constant of 270 s, which is sufficiently fast as to preclude some applications of optical switches in cell biology. To determine the effect of specific MC-protein interactions on the rate of thermal equilibration, we performed a similar experiment by using MC probes differentially linked to G-actin. In almost all cases, the MC absorption spectrum either was unchanged or decreased slightly during the 60-min measurement period (the example shown in Fig. 5C is the G-actin conjugate of compound 9). These results suggest that the thermally induced MC-to-SP transition is greatly impaired within the G-actin conjugates. Given the findings of our absorption studies on these conjugates; we suggest that the thermally driven MC-to-SP transition in G-actin does not occur because of strong and specific dipolar interactions and/or steric effects between the MC state and the conjugate. Support for this argument comes from a study of the thermally driven MC-to-SP transition in the less polar spironaphthoxazine labeled to Cys-374 in G-actin, which has a far more rapid time constant of 6 s (Fig. 5D). We argue that the remarkable stability of the spirobenzopyran MC-protein complex is due to specific ground-state dipolar interactions, which can be overcome, however, after excitation of the MC probe by means of a triplet-state MC-to-SP transition (20). This point is important because it allows exclusive optical control of the two states of the switch. Although ground-state transitions between the two switch states are extremely slow at physiological temperature, Görner (20) has shown that the triplet-state MC and SP transition occurs with a time constant on the order of 10 μs. If the same photochemical mechanism existed within spirobenzopyran conjugates, then our approach for reversible, optical switching of specific dipolar interactions would represent the fastest method for perturbing specific interactions on proteins.
Fig. 5.
Thermally driven transitions between MC and SP states. (A) Time-dependent absorption spectra of the lowest energy transition of MC for compound 12 dissolved in ethanol at a concentration of 20 μM at 20°C in the dark. The spectra, from top to bottom, were recorded at the following times: a, 0; b, 60 s, c, 120 s; d, 180 s; e, 240 s; f, 360 s; g, 420 s; and h, 480 s. The lowermost curve is the spectrum of the nonirradiated SP state. (B) The rate for the thermally driven MC-to-SP transition was determined by analyzing the log of the maximum absorption value for each spectrum shown in A as a function of time. The reaction rate of 270 s-1 was calculated by using a least-squares fitting procedure. (C) Absorption spectra of the MC-G-actin conjugate (compound 9) as a function of time in the dark. Spectra are as follows: a, before irradiation; b, after 30-s irradiation with 365-nm light; c, after 5 min in the dark at 20°C; d, after 22 min in the dark at 20°C; and e, after irradiation with 546-nm light for 30 s. (D) Plot of the logarithm change in MC absorption vs. time for the thermally driven MC-to-SP transition of spironaphthoxazine-labeled G-actin in G-buffer/50% propylene glycol at 20°C.
Control of Spirobenzopyran Geometry Within Protein Conjugates. We believe that a consideration of the relative orientations assumed by differentially linked SP and MC probes within a protein conjugate is useful in helping to understand the origin of the different dipolar interactions that exist between the MC and SP states and the protein (25). A qualitative comparison of the relative orientations of the MC and SP probes within a hypothetical protein is shown in Fig. 6. The orientations of the SP and MC probes were generated through the following considerations: (i) the sulfur atom on a single Cys residue in the protein is fixed at the origin of the probe-protein reference coordinate (x,y,z); (ii) the position of the spirobenzopyran molecule is constrained in this reference coordinate by forcing the atom in the aromatic ring harboring the thiol-reactive group to lie on the x axis; and (iii) the structures of the SP and MC probes are identical within each conjugate and are the same as those derived from crystallographic studies (10). Under these conditions, the direction of the MC dipole would be reversed in the protein conjugates of compounds 6 and 9, whereas the MC probes in the conjugates harboring compounds 3, 12, and 13 would survey a considerable volume of the protein matrix around the Cys residue. As we argued earlier, if the protein interior conforms to a homogeneous dielectric, then the average energy of the MC absorption spectrum should be independent of the MC-protein linkage geometry. Our data, however, show that the dipolar environment within a protein is remarkably diverse, in particular, the projection of the same MC dipole to slightly different locations around Cys-374 in G-actin. For example, in Fig. 4B we show that the MC probe is exposed to molecular environments that are as polar as water or apolar as dichloromethane (Fig. 2B); the dipolar interactions between the MC probe and the protein within these extreme environments differs by almost 10 kT or ≈6 kcal/mol. However, the corresponding difference for the SP probe, which has a smaller dipole moment (5 D), is at least four times lower than that of MC. The significant differences in the nature and energy of dipolar interactions between the SP and MC states within a protein conjugate satisfy an important requirement in our approach for optical switching of functional interactions within spirobenzopyran conjugates (schematized in Fig. 1B).
In summary, the difference in the energy of the dipolar interactions of G-actin in the MC and SP states are significant in terms of the free energy of complex formation between G-actin and actin-binding proteins. The studies described here demonstrate the feasibility of engineering specifically labeled spirobenzopyran conjugates whose interactions with a regulatory protein or ligand are selectively inhibited by dipolar interactions with the MC state, but not the SP state: optical switching between the SP and MC states would provide a means to rapidly and reversibly manipulate functional interactions in proteins within complex molecular environments.
This work was supported in part by National Institutes of Health Grant R01 HL069970-01 (to G.M.).
Supplementary Material
Author contributions: T.S. and G.M. designed research; T.S. and G.M. performed research; T.S. and G.M. contributed new reagents/analytic tools; T.S., Y.Y., and G.M. analyzed data; and G.M. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MC, merocyanine; SP, spiro.
References
- 1.Zhang, J., Campbell, R. E., Ting, A. Y. & Tsien, R. Y. (2002) Nat. Rev. Mol. Cell Biol. 3, 906-918. [DOI] [PubMed] [Google Scholar]
- 2.Yan, Y. & Marriott, G. (2003) Curr. Opin. Chem. Biol. 7, 635-640. [DOI] [PubMed] [Google Scholar]
- 3.Roy, P., Rajfur, Z., Jones, D., Marriott, G., Loew, L. & Jacobson, K. (2001) J. Cell Biol. 153, 1035-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyawaki, A. & Tsien, R. Y. (2000) Methods Enzymol. 327, 472-500. [DOI] [PubMed] [Google Scholar]
- 5.Marriott, G., Miyata, H. & Kinosita, K., Jr. (1992) Biochem. Int. 26, 943-951. [PubMed] [Google Scholar]
- 6.Marriott, G. & Heidecker, M. (1996) Biochemistry 35, 3170-3174. [DOI] [PubMed] [Google Scholar]
- 7.Willner, I., Rubin, S., Wonner, J., Effenberger, F. & Baeuerle, P. (1992) J. Am. Chem. Soc. 114, 3150-3151. [Google Scholar]
- 8.Medintz, I. L., Trammell, S. A., Mattoussi, H. & Mauro, J. M. (2004) J. Am. Chem. Soc. 126, 30-31. [DOI] [PubMed] [Google Scholar]
- 9.Berkovich, G., Krongauz, V. & Weiss, V. (2000). Chem. Rev. 100, 1741-1753. [DOI] [PubMed] [Google Scholar]
- 10.Inouye, M. (1994) Mol. Cryst. Liq. Cryst. A 246, 169-172. [Google Scholar]
- 11.Giordano, L., Jovin, T. M., Irie, M. & Jares-Erijman, E. A. (2002) J. Am. Chem. Soc. 124, 7481-7489. [DOI] [PubMed] [Google Scholar]
- 12.Angelini, N., Corrias, B., Fissi, A., Pieroni, O. & Lenci, F. (1998) Biophys. J. 74, 2601-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song, L., Jares-Erijman, E. A. & Jovin, T. M. (2002) J. Photochem. Photobiol. A 150, 177-185. [Google Scholar]
- 14.Inouye, M., Ueno, M., Tsuchiya, K., Nakayama, N., Konishi, T. & Kitao, T. (1992) J. Org. Chem. 57, 5377-5383. [Google Scholar]
- 15.Tanaka, J., Yan, Y., Choi, J., Bai, J., Klenchin, V. A., Rayment, I. & Marriott, G. (2003) Proc. Natl. Acad. Sci. USA 100, 13851-13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raymo, F. M., Giordani, S., White, A. J. & Williams, D. J. (2003) J. Org. Chem. 68, 4158-4169. [DOI] [PubMed] [Google Scholar]
- 17.Walker, M. A. (1995) J. Org. Chem. 60, 5352-5355. [Google Scholar]
- 18.Sakata, T., Yan, Y. & Marriott, G. (2005) J. Org. Chem., in press. [DOI] [PMC free article] [PubMed]
- 19.Marriott, G. (1994) Biochemistry 33, 9092-9097. [DOI] [PubMed] [Google Scholar]
- 20.Gorner, H. (2001) Phys. Chem. Chem. Phys. 3, 416-423. [Google Scholar]
- 21.Weber, G. & Farris, F. J. (1979) Biochemistry 18, 3075-3078. [DOI] [PubMed] [Google Scholar]
- 22.Bletz, M., Pfeifer-Fukumura, U., Kolb, U. & Baumann, W. (2002) J. Phys. Chem. A 106, 2232-2236. [Google Scholar]
- 23.Chibisov, A. K. & Goerner, H. (1997) J. Phys. Chem. A 101, 4305-4312. [Google Scholar]
- 24.Marriott, G., Zechel, K. & Jovin, T. M. (1988) Biochemistry 27, 6214-6220. [DOI] [PubMed] [Google Scholar]
- 25.Macgregor, R. B. & Weber, G. (1986) Nature 319, 70-73. [DOI] [PubMed] [Google Scholar]
- 26.Kouyama, T. & Mihashi, K. (1981) Eur. J. Biochem. 114, 33-38. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.