Abstract
Background: Although pain is a common symptom in chronic obstructive pulmonary disease (COPD), pain characteristics such as frequency, duration and type are unclear. The primary study aim was to identify these pain characteristics in individuals with COPD versus healthy control participants. The secondary aim was to explore the clinical and psychological associations with pain in those with COPD.
Methods: Participants with COPD and age and gender-matched, healthy controls completed questionnaires to elicit pain characteristics. Those with COPD also had assessments of dyspnea, health-related quality of life, psychological associations (anxiety and depression) and physical activity.
Results: Sixty-four participants with COPD (mean [standard deviation (SD)] age 71[10] , forced expiratory volume in 1 second [FEV1] 38% predicted) and 64 control participants (mean [SD] age 67 [13] , FEV1 91% predicted) were included. Chronic pain was more prevalent in individuals with COPD compared to control participants (41% versus 29%, p=0.03). The pain was more prevalent in the chest and upper back (p=0.04). COPD participants with chest or upper back pain had a higher total lung capacity (mean difference 2.0L, 95% confidence interval [CI] 0.6 to 3.0L) compared to COPD participants without pain. Greater dyspnea (p<0.001), more depression (p=0.02) and lower physical activity levels (p=0.03) were also present in people with COPD experiencing pain.
Conclusions: Chronic pain is common in COPD. It is associated with higher dyspnea and depression and lower physical activity.
Keywords: copd, chronic obstructive pulmonary disease, quality of life, pain, physical activity, depression
Introduction
This article contains supplemental material.
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality.1 It is characterized by exertional dyspnea, decreased exercise tolerance, fatigue and anxiety, all of which contribute to a reduced health-related quality of life (HRQOL).1 COPD is frequently complicated by the presence of pain, with a reported prevalence ranging from 44% to 88%,2-8 depending on the sample size and study design. In those with moderate to severe COPD, increased pain severity has been linked to poorer HRQOL2,4,9,10 and reduced health status.5 Although the presence of pain has been reported, its other characteristics such as duration, frequency and quality are unclear.11
Pain and dyspnea are both unpleasant sensory and emotional experiences with physiological and psychological consequences.12,13 Although anxiety and depression are common in COPD, their relationship with pain is unclear. In non-respiratory conditions, such as low back pain and osteoarthritis,14-17 increased pain intensity has been associated with pain catastrophizing, an exaggerated negative cognitive and emotional response to actual as well as anticipated pain.14,15 It has been described in cystic fibrosis18 but has not been reported in COPD. Physical activity levels in people with COPD vary directly with pain severity.10 This observation is important, given the links between physical activity, HRQOL and survival benefit in COPD.19,20 A better understanding of the characteristics of pain and its influence on symptoms and physical activity will help inform approaches for managing pain in COPD.
The primary study aim was to determine the characteristics of pain (duration, frequency, location, pain type) in people with clinically stable COPD compared to healthy, age and gender-matched control participants. The secondary aims were to explore the association between pain, dyspnea, psychological symptoms, physical activity and quality of life.
Methods
Study Design
A prospective, observational study was undertaken among patients with moderate to very severe COPD and healthy, age and gender-matched control participants. Consecutive, clinically stable patients who attended respiratory medicine clinics for follow-up medical management or for consideration of pulmonary rehabilitation at West Park Healthcare Centre were approached for recruitment. Eligibility criteria included a diagnosis of COPD (spirometry with FEV1/forced vital capacity[FVC] ratio < 70)1 and a smoking history > 10 pack years. Exclusion criteria were other concurrent respiratory diseases such as bronchiectasis (based on high resolution computed tomography), asthma (clinical diagnosis and reversibility > 12%),21 interstitial lung disease, concurrent malignancy (including lung cancer), acute exacerbation of COPD or soft tissue or musculoskeletal injury within the last 4 weeks. Healthy, age-matched control participants with no respiratory or musculoskeletal history were recruited from within and outside the study center by advertisement. All participants gave written informed consent, with the study approved by the Centre’s Institutional Research Ethics Board.
Procedures
All participants attended for 1 visit, at which time the following measures were undertaken:
Brief Pain Inventory (BPI) (long form): evaluates pain experiences over 1 week.22 This instrument collates information on pain intensity using numerical rating scales, with a higher score denoting greater intensity. It is a valid and reliable measure of pain,22 previously applied in COPD.2-4,8
Locations of pain from the body chart of the BPI were determined by standardized body regions based on 45 anatomical areas.23
Pain duration and frequency was established in response to 3 questions. For overall duration, participants were asked the overall length of time for which daily pain symptoms had been experienced, with the options ranging from less than 3 months to greater than 5 years.24 Chronic pain was defined as daily pain for more than 3 months.9 Duration and frequency of pain episodes were referenced to the preceding week.25
Extended Aberdeen Back Pain Scale (EABPS) provides a reliable and valid measure of neck, upper and lower back pain from 35 questions with an overall total score.26
Self-reported Leeds Assessment of Neuropathic Symptoms and Signs pain scale (S-LANSS) identifies neuropathic pain. It consists of 9 items identifying the presence or absence of clinical signs and symptoms with a score of ≥ 12 indicative of neuropathic pain.27
Pain Catastrophizing Scale (PCS) assesses the presence and extent of catastrophic thoughts or feelings accompanying pain experiences from 13 questions. Dimensions of rumination, magnification and helplessness are measured, with a high score representing greater catastrophizing.28 The PCS is reliable and valid in those with chronic low back pain, osteoarthritic pain or fibromyalgia28,29 and has been applied in patients with cystic fibrosis.18
Spirometry was measured in all participants, according to standardized guidelines,30 and lung volumes were measured in those with COPD. Assessment of total lung capacity and residual volume were applied as measures of hyperinflation. Demographic factors of age and body mass index (BMI) were collated from self-reports and clarified with medical records.
Participants with COPD also completed the following measures.
Chronic Respiratory Questionnaire (CRQ) is a disease-specific HRQOL measurement tool assessing dyspnea (sensory-perceptual experience and symptom impact), fatigue, emotional function and mastery over 2 weeks. It is reliable and valid in COPD.31
Hospital Anxiety and Depression Scale (HADS) measures the severity of anxiety and depression from 14 statements, with a higher score indicating greater anxiety or depression.32
Multidimensional Dyspnea Profile (MDP) assesses the sensory-perceptual experience of dyspnea (intensity and sensory quality) and emotional response (affective distress) to dyspnea over one week.33,34 It is comprised of 11 items and is valid as well as reliable in COPD.34,35
Physical activity was measured using the StepWatch Activity Monitor (SAM) (Orthocare Innovations, Seattle, Washington). The SAM is an instrument used to measure ambulatory activity during daily life. It is a small, waterproof, self-contained device worn on the ankle, which records the number of strides taken every minute. The monitor is programmed with a standard computer via a docking station and provides no feedback to the participant. Step detection accuracy exceeds 98% both for unimpaired gait and movement styles.36,37 Measurements included average steps, percentage of time inactive, percentage of time at low activity, moderate and high activity as well as number of steps at low, moderate or high activity (Online supplement (13KB, pdf) ). It has been previously applied to measure physical activity in COPD.38 The sensitivity of the SAM was tuned to each participant through their height and gait characteristics. Participants wore the SAM for 7 days, after which data was downloaded for analysis. Weekends and weekdays with less than 8 hours of wearing time were excluded and participants had to have a minimum of 4 valid weekdays of measurement.39
Data Analysis
Analysis was undertaken using SPSS for windows (version 22.0; SPSS Inc, Chicago, Illinois). Based on 2 recent studies, in which the prevalence of pain was 72% in individuals with COPD2 and 34% in healthy control participants,3 with a power of 0.8 and an alpha of 0.05, a sample size of 64 participants per group was required to detect a difference in pain prevalence between control and COPD participants. Participants with or without COPD were matched for age (± 10 years) and gender, as pain is more prevalent in females.40 Due to the potential influence of seasonal variation on physical activity levels,41 participants were also matched for season of recruitment. Data was checked for normal distribution. Comparisons between groups (COPD and control; COPD with or without pain) for continuous measurements were analyzed using paired t-tests, independent t-test or Mann-Whitney U test, with categorical data analyzed using Chi Square test. Relationships between variables were explored using Spearman’s rank correlation coefficient. The significance of these relationships was interpreted according to Cohen’s effect size for correlation coefficients.42 Alpha was set at <0.05.
Results
Demographics
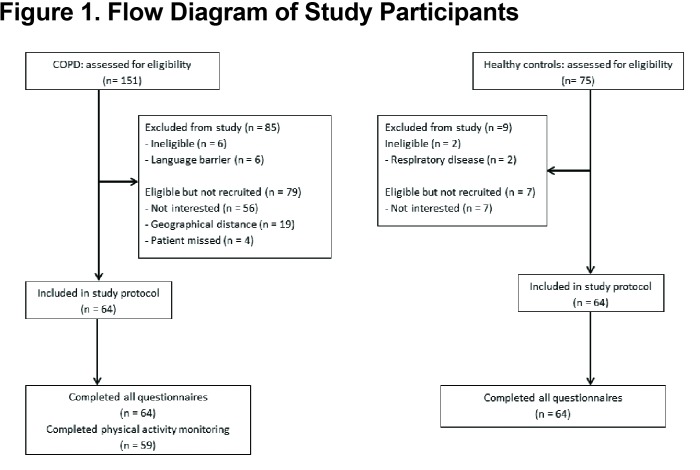
From July 2014 to April 2015, a total of 151 potential participants with COPD and 75 controls were approached (Figure 1). A total of 64 participants with COPD and 64 healthy, control participants were recruited, with no difference in age, gender or BMI between groups (Table 1). Age ranged from 48 to 91 years in individuals with COPD and 47 to 94 years in healthy control participants. All participants with COPD and 57 control participants completed lung function measurements. Five control participants had an unacceptable technique and 2 did not undertake the measurement due to equipment unavailability. There was a significant difference in spirometry between groups (Table 1); those with COPD were classified as having severe to very severe airflow limitation. Of the control participants, 12 were past smokers (less than 5 pack years). Fifty-nine of the participants with COPD wore the activity monitor for the appropriate number of days and hours.

Comparisons Between COPD and Control Groups
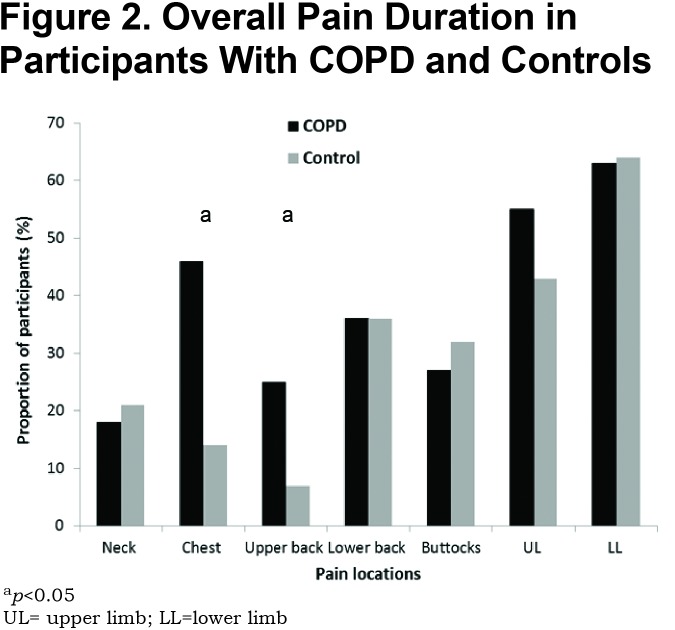
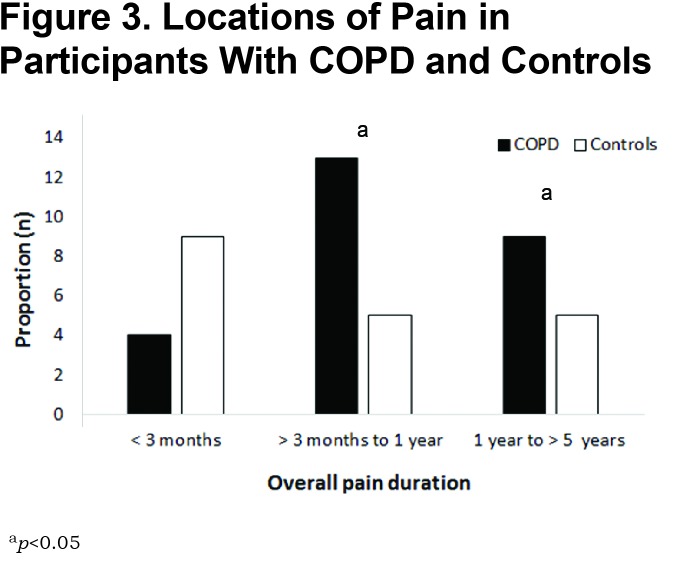
The prevalence of pain was 41% in the group with COPD versus 29% in control participants (p=0.03). Both chest pain and upper back pain were more common in COPD (Figure 2) and the pain was of greater intensity (p=0.04) (Table 2). Chronic pain (daily pain for greater than 3 months) experienced for greater than 3 and up to 12 months (p=0.03) or experienced for more than 12 months (p=0.04) was more common in those with COPD (Figure 3) who also reported more episodes of daily pain (p=0.03), of longer duration (all day) (p=0.04) than control participants. Back pain severity based on EABPS was higher in COPD compared to control participants (p=0.003). There were no differences in neuropathic pain. Seven participants with COPD and 2 control participants reported a clinically significant PCS of ≥ 30 (p=0.04).28

COPD Only: Pain Versus No Pain
In the COPD group, the most common descriptors of pain were aching (30%), tiring (17%) and penetrating (17%). No difference in age, pack years, BMI or spirometry was evident in those with pain compared to those without pain. Those with chest or upper back pain had a higher total lung capacity (mean difference 2.0L, 95% CI 0.6 to 3.0L) compared to those without pain.
Clinical Associations in COPD
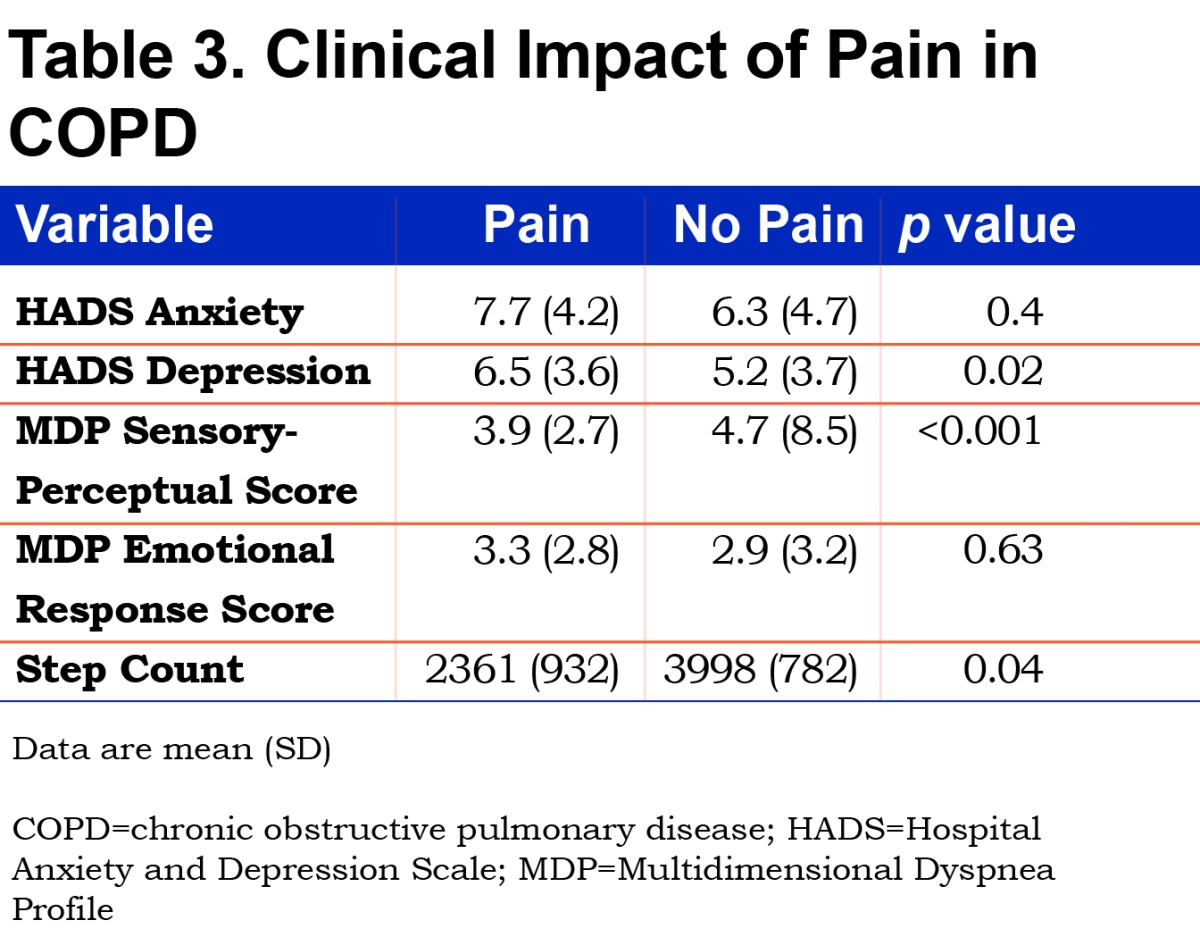
Those with pain had higher dyspnea scores in the CRQ (Figure 4) and higher depression scores on the HADS (Table 3). Based on the MDP, those with pain had a higher sensory-perceptual response. A higher average pain intensity was moderately related to sensory-perceptual experience of dyspnea (rs=0.47) and weakly related to emotional response to dyspnea (rs=0.27). Those with pain had a lower step count (mean difference -1637 steps [95% CI -2673.3 to - 600.8 steps]) and spent a greater proportion of time doing no or low activity and less time doing medium or high intensity activity (Figure 5).
Discussion
This study found a greater prevalence of chronic pain among participants with COPD compared to persons of similar age and gender without lung disease. Those with COPD who reported pain had greater hyperinflation (greater total lung capacity), higher dyspnea scores, more depression and reduced physical activity compared to those without pain. Pain catastrophizing was evident in a small number of participants with COPD.
A higher prevalence of pain in COPD compared to the general population has been previously reported, with variations among studies related to sample size, study design and pain definitions.2-5,7,8 Our study population has included those with severe and very severe COPD. The chronic nature of the pain is reflected by the proportion of those who experienced daily pain for greater than 3 months to greater than 5 years, as well as their experience of more frequent and longer lasting episodes of pain.
The increased back pain severity (according to the EABPS) in COPD participants compared to control participants may relate to the higher prevalence of upper back pain in this group, a finding which is consistent with a recent study that noted thoracic pain in 54% of people with COPD.5 In the current study, the age-matching of control participants and COPD participants reduces the likelihood of age alone accounting for pain. This study is limited by the absence of reports of the exact anatomical location of the back pain (central versus lateral spine) and accompanying radiological studies to identify degenerative changes, compression fractures or alterations in kyphosis. Therefore, we cannot comment on underlying causes of upper back pain.
We noted that in individuals with COPD, chest and upper back pain was associated with greater resting hyperinflation, likely because of the attendant impact on rib orientation, ligamentous strain, excessive joint force, an increased work of breathing and postural dysfunction.43-45 This is similar to the trend towards a higher total lung capacity in those with thoracic pain compared to those without pain.5 Coughing, a common symptom of COPD can also trigger or aggravate back pain.6 However, in the absence of measurements of cough in the current study, the precise relationship between respiratory mechanics, coughing and pain requires further study.
The reduced time engaged in high or medium intensity physical activity among those with pain in COPD is consistent with reports of back and lower limb pain being barriers to attending pulmonary rehabilitation.46 Lower levels of function have also been described in individuals with chronic pain without lung disease.47,48 The observation that participants with pain also experienced more dyspnea suggests that these symptoms co-modulate and amplify perception of each other.12,13,49 The association between pain and inactivity is important, given the impact of a sedentary lifestyle on 7-year survival in COPD.20
Pain catastrophizing was reported in COPD for the first time. However, as the total PCS score was clinically significant in only a few patients, the inference of this finding is difficult to interpret. The observation that people with COPD and pain had higher levels of depression is in keeping with findings of the general population suffering from chronic pain.49 It is also consistent with clinically relevant higher depression scores in people with COPD experiencing thoracic pain.5 Pain management is best undertaken using psychological, physical, behavioral and pharmacological strategies.50 It may therefore be a goal of the multidisciplinary rehabilitation team.
This study evaluated the presence of pain in a cohort of stable participants with severe or very severe COPD. Although it was intended to include those with moderate COPD, the disease severity of participants is the reflection of individuals reviewed by respiratory medicine or referred to pulmonary rehabilitation at the time of recruitment. Therefore, our observations are applicable to those with severe or very severe disease, although the prevalence noted is similar to reports which included participants with moderate COPD.3,4,8 A limitation was the absence of detail as to the exact cause of the pain. Previous studies have provided an association between the presence of comorbidities (including osteoarthritis, cardiovascular conditions, cancer, diabetes)7,8 and the presence of pain in patients with COPD. However, it is difficult to determine whether these comorbidities are actual causes of pain, based on health care diagnostic and clinical evaluation or patient perception. The strength of associations reported was influenced by the proportion of individuals with COPD (41%) with pain. To accurately understand the independent contributions of dyspnea, depression and low physical activity to the perception of pain, a larger sample size is required. In females without COPD, pain is of a higher prevalence and intensity than in males40,51,52 whereas males report a poorer HRQOL.51,52 Lower pain intensity has also been noted among elderly individuals,53 but in the current study the lack of gender difference between groups and the mean age of greater than 65 years makes these factors unlikely to influence these study findings. As clinically stable individuals were studied, the impact of an acute exacerbation could not be assessed. Nor was sleep quality, although poor quality sleep can negatively influence perception of pain.54 Another potential limitation might have been patient recall, as the BPI is dependent on identifying pain experiences within the last week.22
In conclusion, chronic pain is common in those with COPD and is associated with higher levels of hyperinflation, dyspnea, depression and reduced physical activity. Knowledge of the pain experience in patients with COPD is an important step to informing strategies to minimize its impact.
Abbreviations
chronic obstructive pulmonary disease, COPD; standard deviation, SD; forced expiratory volume in 1 second, FEV1; confidence interval, CI; health-related quality of life, HRQL; forced vital capacity, FVC; brief pain inventory, BPI; Extended Aberdeen Back Pain Scale, EABPS; Self-reported Leeds Assessment of Neuropathic Symptoms and Signs, S-LANSS; Pain Catastrophizing Scale, PCS; body mass index, BMI; Chronic Respiratory Questionnaire, CRQ; Hospital Anxiety and Depression Scales, HADS; Multidimensional Dyspnea Profile, MDP; StepWatch Activity Monitor, SAM
Funding Statement
DB is supported by a Canadaian Research Chair.
References
- 1. Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2001.Update 2015 GOLD website. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html Published 2015 Accessed June 2015. [Google Scholar]
- 2. Borge CR,Wahl AK,Moum T.Pain and quality of life with chronic obstructive pulmonary disease. Heart Lung. 2011;40(3):90-101. doi: https://doi.org/10.1016/j.hrtlng.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 3. Bentsen SB,Rustoen T,Miaskowski C.Prevalence and characteristics of pain in patients with chronic obstructive pulmonary disease compared to the Norwegian general population. J Pain. 2011;12(5):539-545. doi: https://doi.org/10.1016/j.jpain.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 4. HajGhanbari B,Holsti L,Road JD,Reid WD.Pain in people with chronic obstructive pulmonary disease (COPD). Respir Med. 2012;106(7):998-1005. doi: https://doi.org/10.1016/j.rmed.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 5. Janssen DJA,Wouters EFM,Lozano Parra Y,Stakenborg K,Franssen FME.Prevalence of thoracic pain in patients with chronic obstructive pulmonary disease and relationship with patient characteristics: a cross-sectional observational study. BMC Pulm Med. 2016;16:47. doi: https://doi.org/10.1186/s12890-016-0210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bentsen SB,Rustoen T,Miaskowski C.Differences in subjective and objective respiratory parameters in patients with chronic obstructive pulmonary disease with and without pain. Int J COPD. 2012;7:137-143. doi: https://doi.org/10.2147/COPD.S28994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roberts MH,Mapel D,Harty A,Von Worley A,Thomson H.Chronic pain and pain medication use in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thor Soc. 2013;10(4):290-298. doi: https://doi.org/10.1513/AnnalsATS.201303-040OC [DOI] [PubMed] [Google Scholar]
- 8. HajGhanbari B,Yamabayashi C,Garland SJ,Road JD,Reid WD.The relationship between pain and comorbid health conditions in people with chronic obstructive pulmonary disease. Cardiopulm Phys Ther J. 2014;25(1):29-35. [Google Scholar]
- 9. Lohne V,Heer HC,Andersen M,Miaskowski C,Kongerud J,Rustoen T.Qualitative study of pain of patients with chronic obstructive pulmonary disease. Heart Lung. 2010;39(3):226-234. doi: https://doi.org/10.1016/j.hrtlng.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 10. HajGhanbari H,Garland SJ,Road JD,Reid WD.Pain and physical performance in people with COPD. Respir Med. 2013;107(11):1692-1699. doi: https://doi.org/10.1016/j.rmed.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 11. Merskey H,Bogduk N.Classification of chronic pain descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. Task force on Taxonomy of the International Association for the Study of Pain. Seattle: IASP Press; 1994. [Google Scholar]
- 12. Niishino T,Yashiro E,Yogo H,Isono S,Shinozuka N,Ishikawa T.Experience of pain can intensity the sensation of dyspnoea. Comparisons of pain and dyspnoea perceptual responses in healthy subjects. Pain. 2010;148(3):426-430. doi: https://doi.org/10.1016/j.pain.2009.11.024 [DOI] [PubMed] [Google Scholar]
- 13. von Leupoldt A,Sommer T,Kegat S,et al. Dyspnoea and pain share emotion-related brain network. Neuroimage. 2009;48(1):200-206. doi: https://doi.org/10.1016/j.neuroimage.2009.06.015 [DOI] [PubMed] [Google Scholar]
- 14. Jensen M,Turner J,Romano J,Karoly P.Coping with chronic pain: a critical review of the literature. Pain. 1991;47:249-283. doi: https://doi.org/10.1016/0304-3959(91)90216-K [DOI] [PubMed] [Google Scholar]
- 15. Quartana PJ,Campbell CM,Edwards RR.Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9:745-758. doi: https://doi.org/10.1586/ern.09.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sullivan M,Thorn B,Haythornthwaite JA,et al. Theorectical perspectives on the relation between catastrophising and pain. Clin J Pain. 2001;17(1):52-64. doi: https://doi.org/10.1097/00002508-200103000-00008 [DOI] [PubMed] [Google Scholar]
- 17. Meyer K,Tschopp A,Sprott H,Mannion AF.Association between catastrophising and self-rated pain and disability in patients with chronic low back pain. J Rehab Med. 2009;41(8):620-625. doi: https://doi.org/10.2340/16501977-0395 [DOI] [PubMed] [Google Scholar]
- 18. Kelemen L,Lee AL,Button BM,Presnell S,Wilson JW,Holland AE.Pain impacts on quality of life and interferes with treatment in adults with cystic fibrosis. Physiother Res Inter. 2012;17(3):132-141. doi: https://doi.org/10.1002/pri.524 [DOI] [PubMed] [Google Scholar]
- 19. Esteban C,Quintana JM,Aburto M,et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010;36(2):292-300. doi: https://doi.org/10.1183/09031936.00021409 [DOI] [PubMed] [Google Scholar]
- 20. Garcia-Aymerich J,Lange P,Benet M,Schnohr P,Anto JM.Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175(5):458-463. doi: https://doi.org/10.1164/rccm.200607-896OC [DOI] [PubMed] [Google Scholar]
- 21. Global Initiative for Asthma (GINA) Pocket Guide for health professionals. Update 2015. GINA website. http://www.ginasthma.org/local/uploads/files/GINA_Pocket_2015.pdf Published 2015 Accessed June 2015. [Google Scholar]
- 22. Keller S,Bann C,Dodd S,Schein J,Mendoza TR,Cleeland CS.Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309-318. doi: https://doi.org/10.1097/00002508-200409000-00005 [DOI] [PubMed] [Google Scholar]
- 23. Margolis RB,Tait RC,Krause SJ.A rating system for use with patient pain drawings. Pain. 1986;24:57-65. doi: https://doi.org/10.1016/0304-3959(86)90026-6 [DOI] [PubMed] [Google Scholar]
- 24. Toblin RL,Mack KA,Perveen G,Paulozzi LJ.A population-based survey of chronic pain and its treatment with prescription drugs. Pain. 2011;152(6):1249-1255. doi: https://doi.org/10.1016/j.pain.2010.12.036 [DOI] [PubMed] [Google Scholar]
- 25. Koh J,Harrison D,Palermo T,Turner H,McGraw T.Assessment of acute and chronic pain symptoms in children with cystic fibrosis. Ped Pulmonol. 2005;40(4):330-335. doi: https://doi.org/10.1002/ppul.20292 [DOI] [PubMed] [Google Scholar]
- 26. Williams NH,Wilkinson C,Russell IT.Extending the Aberdeen Back Pain Scale to include the whole spine: a set of outcome measures for the neck, upper and lower back. Pain. 2001;94(3):261-274. doi: https://doi.org/10.1016/S0304-3959(01)00360-8 [DOI] [PubMed] [Google Scholar]
- 27. Bennett M,Smith BH,Torrance N,Potter J.The S-LANSS Score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6(3):149-153. doi: https://doi.org/10.1016/j.jpain.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 28. Sullivan M,Bishop S,Pivik J.The pain catastrophising scale: development and validation. Psych Assess. 1995;7(4):524-532. doi: https://doi.org/10.1037/1040-3590.7.4.524 [Google Scholar]
- 29. Keefe FJ,Lefebvre JC,Egert JR,Affleck G,Sullivan MJ,Caldwell DS.The relationship of gender to pain, pain behavior and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87(3):325-334. doi: https://doi.org/10.1016/S0304-3959(00)00296-7 [DOI] [PubMed] [Google Scholar]
- 30. Wanger J,Clausen JL,Coates A,et al. Standardization of the measurement of lung volumes. Eur Respir J. 2005;26(3):511-522. doi: https://doi.org/10.1183/09031936.05.00035005 [DOI] [PubMed] [Google Scholar]
- 31. Williams JE,Singh S,Sewell L,Guyatt GH,Morgan MD.Development of a self-reported chronic respiratory questionnaire (CRQ-SR). Thorax. 2001;56(12):954-959. doi: https://doi.org/10.1136/thorax.56.12.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Snaith RP.The Hospital Anxiety and Depression Scale. Health Qual Life Out. 2003;1:29. doi: https://doi.org/10.1186/1477-7525-1-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Banzett RB,O'Donnell CR,Guilfoyle TE,et al. Multidimensional Dyspnea Profile: an instrument for clinical and laboratory research. Eur Respir J. 2015;45(6):1526-1528. doi: https://doi.org/10.1183/09031936.00038914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parshall MB,Schwartzstein RM,Adams L,et al. An official Thoracic Society Statement: Update on the mechanism, assessment and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452. doi: https://doi.org/10.1164/rccm.201111-2042ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meek PM,Banzett RB,Parshall MB,Gracely RH,Schwartzstein RM,Lansing R.Reliability and validity of the multidimensional dyspnea profile (MDP). Chest. 2012;141(6):1546-1553. doi: https://doi.org/10.1183/09031936.00038914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Langer D,Gosselink R,Sena R,Burtin C,Decramer M,Troosters T.Validation of two activity monitors in patients with COPD. Thorax. 2009;64(7):641-642. doi: https://doi.org/10.1136/thx.2008.112102 [DOI] [PubMed] [Google Scholar]
- 37. Resnick B,Nahm ES,Orwig D,Zimmerman SS,Magaziner J.Measurement of activity in older adults: Reliability and validity of the step activity monitor. J Nurs Meas. 2001;9:275-290. [PubMed] [Google Scholar]
- 38. Ng LWC,Jenkins S,Hill K.Accuracy and responsiveness of the stepwatch activity monitor and ActivPAL in patients with COPD when walking with and without a rollator. Disab Rehabil. 2012;34(15):1317-1322. doi: https://doi.org/10.3109/09638288.2011.641666 [DOI] [PubMed] [Google Scholar]
- 39. Trost SG,Mclver KL,Pate RR.Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37:S531-S43. doi: https://doi.org/10.1249/01.mss.0000185657.86065.98 [DOI] [PubMed] [Google Scholar]
- 40. Davis J,Robinson R,Le TK,Xie J.Incidence and impact of pain conditions and comorbid illnesses. J Pain Res. 2011;2011(4):331-345. doi: https://doi.org/10.2147/JPR.S24170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sewell L,Singh SJ,Williams JE,Morgan MD.Seasonal variations affect physical activity and pulmonary rehabilitation outcomes. J Cardiopulm Rehabil Prev. 2010;30(5):329-333. doi: https://doi.org/10.1097/HCR.0b013e3181e175f2 [DOI] [PubMed] [Google Scholar]
- 42. Cohen J.Statistical analysis for the behaviour sciences. New Jersey; Lawrence Erlbaum Associates Inc: 1988. [Google Scholar]
- 43. Heneghan N,Adab P,Jackman S,Balanos G.Musculoskeletal dysfunction in chronic obstructive pulmonary disease (COPD). An observational study. Int J Therapy Rehabil. 2015;22(3):119-128. doi: https://doi.org/10.12968/ijtr.2015.22.3.119 [Google Scholar]
- 44. Massery M.Musculoskeletal and neuromuscular interventions: a physical approach to cystic fibrosis. J Roy Soc Med. 2005;98(Suppl45):55-66. [PMC free article] [PubMed] [Google Scholar]
- 45. Janssens L,Brumagne S,Polspoel K,Troosters T,McConnell A.The effect of inspiratory muscles fatigue on postural control in people with and without recurrent low back pain. Spine. 2010;35(10):1088-1094. doi: https://doi.org/10.1097/BRS.0b013e3181bee5c3 [DOI] [PubMed] [Google Scholar]
- 46. Keating A,Lee AL,Holland AE.Lack of perceived benefit and inadequate transport influence uptake and completion of pulmonary rehabilitation in people with chronic obstructive pulmonary disease: a qualitative study. J Physiother. 2011;57(3):183-190. doi: https://doi.org/10.1016/S1836-9553(11)70040-6 [DOI] [PubMed] [Google Scholar]
- 47. Bair MJ,Robinson RL,Katon W,Kroenke K.Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(2):2433-2445. doi: https://doi.org/10.1001/archinte.163.20.2433 [DOI] [PubMed] [Google Scholar]
- 48. Holmes A,Christelis N,Arnold C.Depression and chronic pain. Med J Aust. 2012;1(Suppl4):17-20. [DOI] [PubMed] [Google Scholar]
- 49. Cai B,Oderda GM.The association between pain and depression and some determinants of depression for the general population of the United States. J Pain Palliat Care Pharmacother. 2012;26(3):257-265. doi: https://doi.org/10.3109/15360288.2012.703292 [DOI] [PubMed] [Google Scholar]
- 50. Turk D,Stanos ST,Palermo TM,et al. Interdisciplinary pain management. American Pain Society website. http://americanpainsociety.org/uploads/about/position-statements/interdisciplinary-white-paper.pdf Published 2009 Accessed April 2017. [Google Scholar]
- 51. Rustoen T,Wahl AK,Hanestad BR,Lerdal A,Paul S,Miaskowski C.Gender differences in chronic pain – findings from a population-based study of Norwegian adults. Pain Manag Nurs. 2004;5(3):105-107. doi: https://doi.org/10.1016/j.pmn.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 52. Rustoen T,Wahl AK,Hanestad BR,Lerdal A,Paul S,Miaskowski C.Prevalence and characteristics of chronic pain in the general Norwegian population. Eur J Pain. 2004;8(6):555-565. doi: https://doi.org/10.1016/j.ejpain.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 53. Galiese L,Malzack R.Age differences in the quality of chronic pain: a preliminary study. Pain Res Manag. 1997;2:157-163. doi: https://doi.org/10.1155/1997/709054 [Google Scholar]
- 54. Sayar K,Arikon M,Yantem J.Sleep quality in chronic pain patients. Can J Psychiatry. 2002;47:844-848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplemental material.