Abstract
Computed tomography (CT) lung density is an accepted biomarker for emphysema in alpha-1 antitrypsin deficiency (AATD), although concerns for radiation exposure limit its longitudinal use. Serum proteins associated with emphysema, particularly in early disease, may provide additional pathogenic insights. We investigated whether distinct proteomic signatures characterize the presence and progression of emphysema in individuals with severe AATD and normal forced expiratory volume in 1 second (FEV1). QUANTitative lung CT UnMasking emphysema progression in AATD (QUANTUM-1) is a multicenter, prospective 3-year study of 49 adults with severe AATD and FEV1 post-bronchodilator values (Post-BD) ≥ 80% predicted. All participants received chest CT, serial spirometry, and contributed to the serum biobank. Volumetric imaging display and analysis (VIDA) software defined the baseline 15th percentile density (PD15) which was indexed to CT-derived total lung capacity (TLC). We measured 317 proteins using a multiplexed immunoassay (Myriad Discovery MAP® panel) in 31 individuals with a complete dataset. We analyzed associations between initial PD15/TLC, PD15/TLC annual decline, body mass index (BMI), and protein levels using Pearson’s product moment correlation. C-reactive protein (CRP), adipocyte fatty acid-binding protein (AFBP), leptin, and tissue plasminogen activator (tPA) were found to be associated with baseline emphysema and all but leptin were associated with emphysema progression after adjustments were made for age and sex. All 4 proteins were associated with BMI after further adjustment for multiple comparisons was made. The relationship between these proteins and BMI, and further validation of these findings in replicative cohorts require additional studies.
Keywords: copd, emphysema, chronic obstructive pulmonary disease, biomarkers, alpha-1 antitrypsin deficiency, proteins, lung densitometry, multiplexed assay
Introduction
Alpha-1 antitrypsin deficiency (AATD) predisposes individuals to early chronic obstructive pulmonary disease (COPD). COPD is one of the leading causes of medical hospitalizations and the third leading cause of death worldwide.1 AATD individuals experience even more hospital visits and cost more to the health care system compared to the older and more comorbid cohort of patients with usual COPD.2 The search for biomarkers that inform future clinical events is important. Unfortunately, only a few existing therapies (e.g., smoking cessation and oxygen therapy for hypoxemic individuals) reduce mortality or modify the natural history of disease. In addition, few novel drugs with systemic action are currently in clinical trials. Among the many problems with COPD drug development is the slow progression of disease in the majority of affected individuals. In addition, the many clinical phenotypes of disease complicate a comprehensive disease understanding.
AATD is the most common known genetic cause of COPD3 that affects at least 100,000 Americans and over 3 million individuals worldwide. The blood level of alpha-1 antitrypsin is determined by each of the inherited alleles on SERPINA1, the gene that codes for alpha-1 antitrypsin. Various genotypes exist with the largest burden of disease ascribed to protease inhibitor ZZ (PiZZ) and protease inhibitor SZ (PiSZ).
Individuals with AATD present at an earlier age and have more rapid disease progression than individuals with usual COPD.4 AATD individuals are at increased risk for developing COPD compared to the general population, especially when tobacco or environmental exposures exist, causing disease at younger age. Additionally, individuals with AATD have an emphysema-predominant phenotype that is more homogeneous than usual COPD. Intravenous alpha-1 antitrypsin, known as augmentation therapy, is currently approved by regulatory authorities for AATD individuals with forced expiratory volume in 1 second (FEV1) in the range of 30%-65% predicted.5 Observational studies have noted excess mortality in AATD and have shown a survival benefit in selected subgroups of individuals receiving augmentation therapy.6,7 We hypothesized that biomarkers may characterize AATD individuals with rapid progression of emphysema, thereby creating the possibility of new, targeted therapy.
Most traditional biomarkers of COPD severity and progression have been related to lung function decline, measured as FEV1.8,9 Chest radiography is not sensitive enough to detect early emphysema. Computed tomography (CT) of the chest can reveal early emphysematous changes that are sometimes present in individuals with normal lung function who are minimally symptomatic. Emphysema is usually detected earlier in life and in a higher proportion of AATD affected individuals compared to individuals with usual COPD.
CT lung density has been utilized as a promising biomarker in AATD.10 In a mixed cohort of both usual COPD and AATD-associated COPD, assessment of emphysema progression was more sensitive by lung densitometry than FEV1 and measurements of gas transfer.11 For this reason, CT density has been allowed as a primary endpoint in trials of augmentation therapy efficacy by U.S. and European regulatory authorities.5 Desmosine is the most promising biomarker in AATD-related COPD,12 although several others have been evaluated, including fibrinogen,13 matrix-metalloprotease-9,14,15 and angiopoietin-like protein 4.16
In 2008, the National Institutes of Health Office of Rare Diseases and the National Heart, Lung, and Blood Institute funded the Rare Lung Disease Consortium to study radiographic emphysema decline in a cohort of PiZZ AATD individuals with normal lung function as defined by post-bronchodilator (post-BD) FEV1% predicted ≥ 80% per the National Health and Nutrition Examination Survey III (NHANES III).17 The primary hypothesis of the study, called QUANTitative lung CT UnMasking emphysema progression in AATD (QUANTUM-1), was to determine whether baseline CT density predicted a more rapid decline in FEV1. Because the large biorepository of this carefully phenotyped cohort is available for additional projects, we analyzed this cohort to identify serum proteins that correlate with CT density decline in individuals with early stage AATD-associated COPD.
Methods
Study Design and Population
The 49 participants in QUANTUM-1 were enrolled between September 9, 2007 and December 11, 2008 following informed consent at 1 of 7 participating U.S. study centers and followed for 3 years. All participants had normal lung function at baseline as defined by post-bronchodilator FEV1% predicted ≥ 80% per the NHANES III.17 Baseline demographics are included in Table 1. No participant smoked during the time of biospecimen collection. At each visit, absence of significant smoke exposure was confirmed in all individuals by urinary cotinine. Participants had bimonthly telephone calls to assess exacerbations and serial site visits (at baseline, 6, 12, 18, 24, and 36 months) with chest CT, pulmonary function tests (spirometry, lung volumes, and diffusing capacity of the lung for carbon monoxide (DLCO) tests, and serum collection to explore the natural history of disease progression over the course of 3 years. Although the protocol specified 50 individuals, funding ended once 49 participants were enrolled.
The annual changes in FEV1 were calculated utilizing baseline, 6, 12, 18, 24, and 36-month visits. The PD15/TLC slope was calculated by endpoint analysis (36 months-baseline) based on acceptability of the CT scans for analysis. The exacerbation frequency for the year preceding the study was assessed at the baseline site visit using the AlphaNet exacerbation questionnaire.18 This AATD-specific questionnaire was developed to address exacerbation type and frequency by a variety of exacerbation definitions and has been used in large cohorts of AATD individuals who receive augmentation therapy through AlphaNet.
Contiguous non-overlapping 0.6-1.0mm slice thickness multi-detector row CT scans were acquired at 80 mAs, 120 kVp. Images were reconstructed using a medium spatial frequency reconstruction algorithm (GE: Standard, Philips: B, Siemens: B30f, Toshiba: FC86). A Gammex phantom was used to assure the accuracy of each CT scanner in the study at 6-month intervals.
We included all participants with acceptable baseline and 36-month CT scans that could be analyzed using Apollo image analysis software (VIDA Diagnostics, Inc.). Briefly, the lungs were segmented in 3-dimensions from the chest wall and mediastinal components using Apollo. The CT-derived total lung capacity (TLC), mean lung density and the lowest percentile density at 15% (PD15) corrected for lung volume was derived from all air density voxels. Of the original cohort of 49 participants, 7 had missing data, and 11 had CT scans that did not follow the specific acquisition protocol. Thirty-one individuals remained eligible for analyses.
Blood Samples
A total of 50 ml of blood was drawn at the initial study visit for serum and plasma analysis. Serum samples were allowed to clot for 15 minutes per protocol, spun at 3500 rpm, and placed into a -80° C freezer until transported on dry ice to the central repository. Serum samples were stored at the University of Florida Alpha-1 Genetics Laboratory or at the Medical University of South Carolina Alpha-1 Registry at -80° C until analyzed.
Primary Outcome Measures
We measured 317 proteins from serum obtained at the baseline visit using a multiplexed immunoassay (Myriad Discovery MAP® panel) in the 31 study individuals with a complete dataset. Values were expressed as mean ± standard deviation. We analyzed the associations between baseline PD15/TLC, PD15/TLC annual decline, and baseline protein levels using Pearson’s product moment correlation. A p-value < 0.05 was considered statistically significant. Correlations were determined to be weak, moderate, or high when the r coefficient was in the range 0.2-0.39, 0.4-0.59, or 0.6-0.79, respectively. The two-tailed correlations were then adjusted for age, and sex. To reduce false positives due to multiple comparisons, we have further adjusted the raw p-values for a global false discovery rate (FDR) of less than 10%.19 This method adjusts up the significant values for individual proteins to ensure that the expected number of false positives among positive results is no more than 10%, which is a common practice in high-throughput data analysis.
Pathway analysis of proteins correlated with PD15/TLC and/or its slope was performed using the UniProt number and Homo sapiens Reactome pathway analysis software.20
Results
The 31 eligible PiZZ participants had a mean age of 52 ± 10 years, a body mass index (BMI) of 30 ± 7 kg/m2 (39% had BMI in the obese range), FEV1/ forced vital capacity (FVC) post-BD of 0.76 ± 0.1, post-BD FEV1 of 101 ± 12% predicted, baseline DLCO adjusted for hemoglobin 23 ± 6, and baseline PD15 of -937 ± 27 Hounsfield units (HU). A slight majority of participants were women (58%). No differences in baseline characteristics between the 31 included and the 18 excluded participants were found (Table 1). There were changes in radiographic and spirometric measurements over the 3-year study course, verifying progression of emphysema. Annual changes included a decline of FEV1 of 58 ± 85 ml/year, decline of PD15 of 4 ± 13HU/year, increase of CT volume (CT TLC) of 80 ± 371 ml/year, decrease of PD15/ CT TLC of 0.75 ± 4.4 HU/ml/year, decline in DLCO of 0.5 ± 0.8, and rise in post-bronchodilator TLC measured by lung volumes of 212 ± 600 ml.
Exacerbation questionnaires at the baseline visit revealed that 47% of individuals had at least one exacerbation in the preceding year. However, over the 3 years of observation, only 51% had one or more events. A bivariate analysis of FEV1 slope and PD15 slope adjusted for TLC in 31 individuals confirmed a significant association between spirometry and CT measures. However, adjustment for age and sex, extinguished the significant correlation between FEV1 decline and emphysema progression (p=0.21 after adjustment). Baseline emphysema showed wide variation among participants at baseline. All but 5 individuals had emphysema present, as defined by PD15 ≤ -910 HU, with a range of PD15 of -966 HU to -892 HU. Three of 5 participants with no emphysema by this definition were extremely obese (BMI>39), potentially affecting PD15 measurements.
From the 317 proteins evaluated, 31 were associated with baseline emphysema (Table 2) and 14 were associated with emphysema progression (Table 3) when unadjusted for age, sex, and multiple comparisons. Most of these proteins showed a moderate degree of correlation as measured by Pearson product moment correlation. In unadjusted analyses, 3 proteins (C-reactive protein [CRP], adipocyte fatty acid binding protein [AFBP], and sex hormone-binding globulin) were associated with both baseline emphysema and emphysema progression as measured by the PD15/TLC and PD15/TLC slope, respectively (Table 4). Angiopoietin-related protein 4 showed a moderate correlation (r=-0.477, p=0.007) with emphysema progression. CRP and AFBP correlated strongly with BMI.
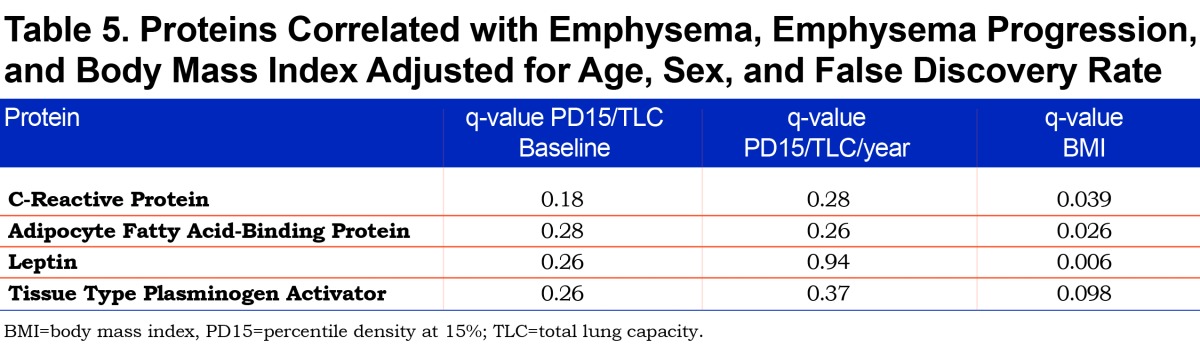
CRP, AFBP, leptin, and tissue plasminogen activator (tPA) were found to be significantly associated with BMI after controlling for multiple comparisons at global FDR < 10% and adjusting for age and sex (Table 5). If these proteins were not adjusted for multiple comparisons, all 4 were statistically significantly associated with baseline emphysema and all but leptin were associated with emphysema progression after adjustments were made for age and sex (Table 6 and Figure 1). In summary, the adjustment for multiple comparisons caused the strength of the associations of CT-measured emphysema with biomarkers to lessen such that none correlated with CT measures of emphysema (q value range 0.18-0.94).
Discussion
In individuals with rare diseases such as AATD, large, prospective, controlled, clinical trials are difficult to perform. Therefore, characterizing the correlation between biomarkers and important clinical features is of paramount importance, as the availability of reliable biomarkers might limit the size of required clinical trials and inform emphysema research about biologically relevant pathways.
Baseline PD15 values in our cohort (N=47) were -937 ± 25 HU, which is abnormal even in comparison to smoking participants with normal gas trapping.21
In individuals with AATD and preserved lung function, our unadjusted analysis showed that of 317 proteins assessed, 31 proteins were associated with baseline emphysema extent and 14 proteins were correlated with its progression. Only 3 of these 42 proteins correlated both with baseline emphysema extent and its progression: CRP, AFBP, and sex hormone-binding globulin. These 3 proteins are not related in pathway analysis; however, each has a previously described connection to emphysema.22-26
One of the important questions in the field of COPD and emphysema is whether the longitudinal change in FEV1 correlates with the emphysema progression measured by PD15 utilizing CT. In our cohort of 31 individuals followed for 3 years we found no association between decline in spirometry and CT emphysema measures in analyses adjusted for age and sex. More importantly, once corrected for age, sex, and multiple comparisons, striking associations between CT features and CRP, AFBP, leptin, and tPA with BMI were found. This finding is significant as we previously found that obesity (BMI>30) was associated with more exacerbations in this cohort of individuals.23 Exacerbations are important patient-reported outcomes and are known to negatively affect COPD individuals and portend poor prognosis.24 Our study lacked sufficient power for formal statistical pathway analysis. However, proteins correlated with PD15/TLC or its slope were often interrelated with broad connections to known pathways of emphysema pathogenesis. These involved complement activation, retinoic acid transport, wingless-related integration site (WNT) signaling, pathways of hemostasis, and chemokine production. As discussed below, some of the pathways involved in metabolism of lipids and lipoproteins were also overexpressed.
CRP has been proposed as a biomarker for COPD because of clinical associations with FEV1, exacerbations, comorbidities, hospitalization risk, and mortality.22-24 Biochemically, CRP promotes complement fixation and may scavenge nuclear material from damaged circulating cells. In addition, CRP has several functions associated with host defense. The complement cascade is critical in connecting the innate and adaptive immune response and has been studied in COPD.25 Because CRP has been extensively studied in cardiac disease as a risk factor biomarker, the lack of specificity for emphysema will be an issue in further advancing this observation toward the clinic.
AFBP is a lipid transport protein in adipocytes which binds both long-chain fatty acids and retinoic acid. Given the long history of interest in retinoic acid therapy and alveolar growth, the association with rate of emphysema progression is interesting.
TPA plays an important role in tissue remodeling and degradation, by converting plasminogen to plasmin. Prior research confirms that the tPA levels are predictive of cardiovascular events26 and recent publications reveal the potential of fibrinolytic agents as treatments for chronic respiratory diseases.27
Also, earlier analyses revealed that exacerbation frequency is increased in obese individuals with AATD.23 In this exploratory analysis, we found that some proteins had very strong correlations with BMI (CRP and AFBP). This association is not surprising as some view obesity as a chronic inflammatory state that further perpetuates many chronic diseases. In a clinical context, the prevalence of obesity is disproportionately high in individuals with COPD28 and the cause and consequences of this association remains speculative. To date, studies investigating the impact of obesity on mortality in COPD individuals suggest that obesity is associated with a 30%-34% increase in relative risk of all-cause mortality in individuals with mild or moderate COPD compared to individuals with normal weight and comparable disease severity.29 How much of the associated mortality from high BMI is from COPD comorbidities and how much may be from an impact on emphysema progression remains unknown. Recent analysis of the Behavioral Risk Factor Surveillance System data collected by the Centers for Disease Control and Prevention confirmed that comorbidities are commonly present in COPD individuals.30
Body composition abnormalities with higher measurements of adiposity are related to greater functional limitations, particularly in women.31 One of the reasons that obesity may be associated with worse outcomes in COPD may relate to systemic inflammation.32 In the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort, patients exhibiting high circulating levels of 6 inflammatory biomarkers had worse health outcomes and were more obese compared to non-inflamed individuals.33 In our study, metabolism of lipids and lipoproteins were implicated by finding different expression of adipocyte fatty acid-binding protein, aldose reductase, and angiopoietin-related protein 4. Angiopoietin-related protein 4 plays a role in several plausible AATD-associated COPD pathways34 involving inflammation, lipid metabolism, and metabolic dysregulation. Therefore, linkage between lipid transport and systemic inflammation in COPD 35 is supported by these observations in the well-phenotyped QUANTUM-1 cohort of AATD individuals.
Sex hormone-binding globulin was also associated with emphysema and its progression, although the degree of correlation was smaller. This molecule has been associated with androgen binding and could participate in emphysema pathogenesis by altering the clearance rate of steroid hormones. We are unaware of other pathway associations for COPD.
The other 39 proteins that were associated with either baseline emphysema extent or emphysema progression play roles in many pathways that have been previously implicated in emphysema pathogenesis. Associations with programmed cell death showed activity for apoptotic signaling that has been well studied in emphysema.36 We also found strong signals for retinoic acid transport. Although clinical trials of all-trans retinoic acid have proved negative37 the retinoid cycle has been implicated in COPD pathogenesis. In this context, 2 proteins in our analysis regarded the retinoid cycle: retinol-binding protein 4 and transthyretin, both with significant correlations to baseline emphysema (of moderate and strong degrees).
Very little is known about the types of inflammatory events and their initiation, especially in early onset emphysema. In this regard, our finding that some chemokine receptors and chemokines were associated with emphysema extent raises the possibility that they contribute to the pathogenesis of emphysema. The associated proteins in this study include 6Ckine, stromal cell-derived factor-1, macrophage inflammatory protein 3 beta, growth-regulated alpha protein, and macrophage-derived chemokine.
Linkages to autoimmunity have been studied extensively in clinical and experimental COPD.38 Proteins involved in WNT signaling (sclerostin and dickkopf-related protein 1) were moderately expressed, as was metalloproteinase 3, a metalloproteinase previously identified to play a role in AATD-related emphysema.39
The hemostatic protein fibrinogen is the first serum biomarker validated in the COPD Biomarkers Qualification Consortium40 and has known involvement in hemostatic pathways.41 Fibrinogen reflects both COPD severity and progression by association with adverse clinical outcomes (e.g., exacerbation, hospitalization, and mortality) and is now a drug development tool in COPD. We saw significant upregulation (negative correlation with PD15/TLC) for tissue type plasminogen activator and downregulation of its inhibitor plasminogen activator inhibitor 1, signaling a pro-thrombotic state associated with emphysema.
This study has several limitations. First, the sample size was small and included only 31 individuals of the 49 participants originally enrolled in the study. The fact that included participants did not differ from excluded participants regarding demographic features lessens concerns about selection bias. Second, the associations reported were based on single measurements but not serial measures. Furthermore, the extent of inter-individual and intra-individual variation in the biomarker protein measurements in COPD patients is currently unclear. Few exacerbations were seen over the 3 years of the study, limiting correlations with this clinical outcome. Obesity with BMI >40 was present in 4 of 31 individuals and can impact CT density independent of chest restriction by radiation scatter. As such, though our findings are provocative and novel, their validity and generalizability will require replication in other cohorts.
Variation in CT measurement of lung volumes posed a challenge in this study, as it has characteristically in other studies of individuals with AATD.5 Our study used state-of-the-art techniques to control CT acquisition, which have not materially advanced since 2008 when the images in this study were obtained. Although new methodologies that allow registration of the same segments during inspiration and expiration to differentiate between small airways disease and emphysema are available, these were not used in this study as they have not been fully validated.42
Projects designed for discovery of novel biologic pathways that impact clinical disease are high risk with the hope of high reward. The challenge with rare disease cohorts such as AATD is that validation cohorts are limited and data are costly to acquire. Recognizing that validation of these findings is needed before routine use of these biomarkers in clinical trials can be endorsed, our findings are novel and, we believe, promising.
Conclusions
Prior publications confirm that PiZZ AATD individuals are at increased risk for developing COPD compared to the normal population and typically develop emphysema at a younger age, especially if exposed to tobacco smoke. We have shown that baseline CT density varies widely in the QUANTUM-1 cohort, which includes individuals with advanced emphysema despite normal lung function tests and some individuals with normal lung density as measured by densitometry. In addition, these preliminary data suggest that, at baseline, there is poor correlation between the rate of FEV1 decline and the radiographic presence of emphysema.
In this cohort of AATD individuals, leptin, CRP, AFBP, and tPA were associated with baseline emphysema and all but leptin were associated with emphysema progression after controlling for age and sex. Strong correlations of these proteins with BMI were also found after adjustments for multiple comparisons were made.
While analysis of CRP and leptin in COPD is ongoing,22,43,44 the observation regarding AFBP may prompt investigation regarding the regulation of systemic glucose and lipid metabolism in the pathogenesis of COPD. Similarly, more research investigating plasminogen activation in COPD is needed. Such investigations could suggest promising targets for COPD therapy.
Abbreviations
alpha-1 antitrypsin deficiency, AATD; chronic obstructive pulmonary disease, COPD; protease inhibitor ZZ, PiZZ; protease inhibitor SZ, PiSZ; forced expiratory volume in 1 second, FEV1; computed tomography, CT; -post-bronchodilator, post-BD; National Health and Nutrition Examination Survey III, NHANES III; QUANTitative lung CT UnMasking emphysema progression in AATD, QUANTUM-1; diffusing capacity of the lung for carbon monoxide, DLCO; total lung capacity, TLC; percentile density at 15%, PD15; false discovery rate, FDR; body mass index, BMI; forced vital capacity, FVC; Hounsfield units, HU;C-reactive protein, CRP;adipocyte fatty acid-binding protein, AFBP; tissue plasminogen activator, tPA; wingless-related integration, WNT; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints, ECLIPSE
Funding Statement
The QUANTUM-1 Study was supported by the National Heart Lung and Blood Institute, the Office of Rare Diseases through the Rare Lung Disease Clinical Research Network (1 U54 RR019498-01, Trapnell PI), and the Alpha-1 Foundation. The protein analysis was performed by a grant from the Chest Foundation (Beiko, PI).
References
- 1. Lozano R,Naghavi M,Foreman K,et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-2128. doi: https://doi.org/10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zacherle E,Noone JM,Runken MC,Blanchette CM.Health care cost and utilization associated with alpha-1 antitrypsin deficiency among a cohort of Medicare beneficiaries with COPD. Value Health. 2015;18(7):A664. doi: https://doi.org/10.1016/j.jval.2015.09.2419 [Google Scholar]
- 3. Stoller JK,Aboussouan LS.A review of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185(3):246-259. doi: https://doi.org/10.1164/rccm.201108-1428CI [DOI] [PubMed] [Google Scholar]
- 4. Stockley RA.Alpha1-antitrypsin review. Clin Chest Med. 2014;35(1):39-50. doi: https://doi.org/10.1016/j.ccm.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 5. Chapman KR,Burdon JG,Piitulainen E,et al. Intravenous augmentation treatment and lung density in severe alpha1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9991):360-368. doi: https://doi.org/10.1016/S0140-6736(15)60860-1 [DOI] [PubMed] [Google Scholar]
- 6. Stoller JK,Tomashefski J Jr.,Crystal RG,et al. Mortality in individuals with severe deficiency of alpha1-antitrypsin: findings from the National Heart, Lung, and Blood Institute Registry. Chest. 2005;127(4):1196-1204. doi: https://doi.org/10.1378/chest.127.4.1196 [DOI] [PubMed] [Google Scholar]
- 7. Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin; The Alpha-1-Antitrypsin Deficiency Registry Study Group. Am J Respir Crit Care Med. 1998;158(1):49-59. doi: https://doi.org/10.1164/ajrccm.158.1.9712017 [DOI] [PubMed] [Google Scholar]
- 8. Stockley RA.Biomarkers in chronic obstructive pulmonary disease: confusing or useful?. Int J Chron Obstruct Pulmon Dis. 2014;9:163-177. doi: https://doi.org/10.2147/COPD.S42362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vestbo J,Agusti A,Wouters EF,et al. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. Am J Respir Crit Care Med. 2014;189(9):1022-1030. doi: https://doi.org/10.1164/rccm.201311-2006PP [DOI] [PubMed] [Google Scholar]
- 10. Dirksen A,Piitulainen E,Parr DG,et al. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J. 2009;33(6):1345-1353. doi: https://doi.org/10.1183/09031936.00159408 [DOI] [PubMed] [Google Scholar]
- 11. Stolk J,Putter H,Bakker EM,Shaker SB,Parr DG,Piitulainen E,et al. Progression parameters for emphysema: a clinical investigation. Respir Med. 2007;101(9):1924-1930. doi: https://doi.org/10.1016/j.rmed.2007.04.016 [DOI] [PubMed] [Google Scholar]
- 12. Ma S,Lin YY,Cantor JO,et al. The effect of alpha-1 proteinase inhibitor on biomarkers of elastin degradation in alpha-1 antitrypsin deficiency: An analysis of the RAPID/RAPID Extension trials. Chronic Obstr Pulm Dis. 2017;4(1):34-44. doi: https://doi.org/10.15326/jcopdf.4.1.2016.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stolk J,Nieuwenhuizen W,Stoller JK,Aboussouan L.High dose intravenous AAT and plasma neutrophil derived fibrinogen fragments. Thorax. 2005;60(1):84. [PMC free article] [PubMed] [Google Scholar]
- 14. Omachi TA,Eisner MD,Rames A,Markovtsova L,Blanc PD.Matrix metalloproteinase-9 predicts pulmonary status declines in alpha1-antitrypsin deficiency. Respir Res. 2011;12:35. doi: https://doi.org/10.1186/1465-9921-12-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koepke J,Dresel M,Schmid S,et al. Therapy with plasma purified alpha1-antitrypsin (Prolastin(R)) induces time-dependent changes in plasma levels of MMP-9 and MPO. PLoS One. 2015;10(1):e0117497. doi: https://doi.org/10.1371/journal.pone.0117497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frenzel E,Wrenger S,Immenschuh S,et al. Acute-phase protein alpha1-antitrypsin--a novel regulator of angiopoietin-like protein 4 transcription and secretion. J Immunol. 2014;192(11):5354-5362. doi: https://doi.org/10.4049/jimmunol.1400378 [DOI] [PubMed] [Google Scholar]
- 17. Hankinson JL,Odencrantz JR,Fedan KB.Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179-187. doi: https://doi.org/10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 18. Hoth KF,Ford DW,Sandhaus RA,Strange C,Wamboldt FS,Holm KE.Alcohol use predicts ER visits in individuals with alpha-1 antitrypsin deficiency (AATD) associated COPD. COPD. 2012;9(4):417-425. doi: https://doi.org/10.3109/15412555.2012.684414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benjamini Y.Controlling the false discovery rate: a practical and powerful approach to multiple testing. R Stat Soc Series B Stat Methodol B. 1995;57:289-300. [Google Scholar]
- 20. Reactome: ; A Curated Pathway Database. Reactome website. Availablefrom: http://www.reactome.org Accessed June 2017. [Google Scholar]
- 21. Pompe E,van Rikxoort EM,Schmidt M,et al. Parametric response mapping adds value to current computed tomography biomarkers in diagnosing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(9):1084-1086. doi: https://doi.org/10.1164/rccm.201411-2105LE [DOI] [PubMed] [Google Scholar]
- 22. Mannino DM,Ford ES,Redd SC.Obstructive and restrictive lung disease and markers of inflammation: data from the Third National Health and Nutrition Examination. Am J Med. 2003;114(9):758-762. doi: https://doi.org/10.1016/S0002-9343(03)00185-2 [DOI] [PubMed] [Google Scholar]
- 23. Tatsiana B,Suchit K,Alan FB,Mark B,James KS,Robert S,et al. Body mass index predicts exacerbation frequency in alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2015;191:A2808-A. [Google Scholar]
- 24. Soler-Cataluna JJ,Martinez-Garcia MA,Roman Sanchez P,Salcedo E,Navarro M,Ochando R.Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925-931. doi: https://doi.org/10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ricklin D,Hajishengallis G,Yang K,Lambris JD.Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785-797. doi: https://doi.org/10.1038/ni.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tofler GH,Massaro J,O'Donnell CJ,et al. Plasminogen activator inhibitor and the risk of cardiovascular disease: The Framingham Heart Study. Thromb Res. 2016;140:30-35. doi: https://doi.org/10.1016/j.thromres.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schuliga M,Westall G,Xia Y,Stewart AG.The plasminogen activation system: new targets in lung inflammation and remodeling. Curr Opin Pharmacol. 2013;13(3):386-393. doi: https://doi.org/10.1016/j.coph.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 28. Liu Y,Pleasants RA,Croft JB,et al. Body mass index, respiratory conditions, asthma, and chronic obstructive pulmonary disease. Respir Med. 2015;109(7):851-859. doi: https://doi.org/10.1016/j.rmed.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Landbo C,Prescott E,Lange P,Vestbo J,Almdal TP.Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856-1861. doi: https://doi.org/10.1164/ajrccm.160.6.9902115 [DOI] [PubMed] [Google Scholar]
- 30. Cunningham TJ,Ford ES,Rolle IV,Wheaton AG,Croft JB.Associations of self-reported cigarette smoking with chronic obstructive pulmonary disease and co-morbid chronic conditions in the united states. COPD. 2015;12(3):276-286. doi: https://doi.org/10.3109/15412555.2014.949001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eisner MD,Blanc PD,Sidney S,Yelin EH,Lathon PV,Katz PP,et al. Body composition and functional limitation in COPD. Respir Res. 2007;8:7. doi: https://doi.org/10.1186/1465-9921-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gan WQ,Man SF,Senthilselvan A,Sin DD.Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574-580. doi: https://doi.org/10.1136/thx.2003.019588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Agusti A,Edwards LD,Rennard SI,et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi: https://doi.org/10.1371/journal.pone.0037483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frenzel E,Wrenger S,Brugger B,et al. Alpha1-antitrypsin combines with plasma fatty acids and induces angiopoietin-like protein 4 expression. J Immunol. 2015;195(8):3605-3616. doi: https://doi.org/10.4049/jimmunol.1500740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suzuki M,Makita H,Ostling J,et al. Lower leptin/adiponectin ratio and risk of rapid lung function decline in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(10):1511-1519. doi: https://doi.org/10.1513/AnnalsATS.201408-351OC [DOI] [PubMed] [Google Scholar]
- 36. Demedts IK,Demoor T,Bracke KR,Joos GF,Brusselle GG.Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006;7:53. doi: https://doi.org/10.1186/1465-9921-7-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mao JT,Goldin JG,Dermand J,et al. A pilot study of all-trans-retinoic acid for the treatment of human emphysema. Am J Respir Crit Care Med. 2002;165(5):718-723. doi: https://doi.org/10.1164/ajrccm.165.5.2106123 [DOI] [PubMed] [Google Scholar]
- 38. Kheradmand F,Shan M,Xu C,Corry DB.Autoimmunity in chronic obstructive pulmonary disease: clinical and experimental evidence. Expert Rev Clin Immunol. 2012;8(3):285-292. doi: https://doi.org/10.1586/eci.12.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McAloon CJ,Wood AM,Gough SC,Stockley RA.Matrix metalloprotease polymorphisms are associated with gas transfer in alpha 1 antitrypsin deficiency. Ther Adv Respir Dis. 2009;3(1):23-30. doi: https://doi.org/10.1177/1753465809102263 [DOI] [PubMed] [Google Scholar]
- 40. Casaburi R,Celli B,Crapo J,et al. The COPD Biomarker Qualification Consortium (CBQC). COPD. 2013;10(3):367-377. doi: https://doi.org/10.3109/15412555.2012.752807 [DOI] [PubMed] [Google Scholar]
- 41. Miller BE,Tal-Singer R,Rennard SI,et al. Plasma fibrinogen qualification as a drug development tool in COPD: Perspective of the COPD Biomarker Qualification Consortium. Am J Respir Crit Care Med. 2016.doi: https://doi.org/10.1164/rccm.201509-1722PP [DOI] [PubMed] [Google Scholar]
- 42. Martinez CH,Diaz AA,Meldrum C,et al. Age and small airway imaging abnormalities in subjects with and without airflow obstruction in SPIROMICS. Am J Respir Crit Care Med. 2016.doi: https://doi.org/10.1164/rccm.201604-0871OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hurst JR,Donaldson GC,Perera WR,et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174(8):867-874. doi: https://doi.org/10.1164/rccm.200604-506OC [DOI] [PubMed] [Google Scholar]
- 44. Emerging Risk Factors Collaboration; C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132-140. doi: https://doi.org/10.1016/S0140-6736(09)61717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]