Abstract
BACKGROUND
In 2011 and 2013, the National Blood Collection and Utilization Survey (NBCUS) revealed declines in blood collection and transfusion in the United States. The objective of this study was to describe blood services in 2015.
STUDY DESIGN AND METHODS
The 2015 NBCUS was distributed to all US blood collection centers, all hospitals performing at least 1000 surgeries annually, and a 40% random sample of hospitals performing 100 to 999 surgeries annually. Weighting and imputation were used to generate national estimates for units of blood and components collected, deferred, distributed, transfused, and outdated.
RESULTS
Response rates for the 2015 NBCUS were 78.4% for blood collection centers and 73.9% for transfusing hospitals. In 2015, 12,591,000 units of red blood cells (RBCs) (95% confidence interval [CI], 11,985,000–13,197,000 units of RBCs) were collected, and 11,349,000 (95% CI, 10,592,000–11,747,000) were transfused, representing declines since 2013 of 11.6% and 13.9%, respectively. Total platelet units distributed (2,436,000; 95% CI, 2,230,000–2,642,000) and transfused (1,983,000; 95% CI, 1,816,000=2,151,000) declined by 0.5% and 13.1%, respectively, since 2013. Plasma distributions (3,714,000; 95% CI, 3,306,000–4,121,000) and transfusions (2,727,000; 95% CI, 2,594,000–2,859,000) in 2015 declined since 2013. The median price paid per unit in 2015—$211 for leukocyte-reduced RBCs, $524 for apheresis platelets, and $54 for fresh frozen plasma—was less for all components than in 2013.
CONCLUSIONS
The 2015 NBCUS findings suggest that continued declines in demand for blood products resulted in fewer units collected and distributed Maintaining a blood inventory sufficient to meet routine and emergent demands will require further monitoring and understanding of these trends.
In the United States, blood donation, distribution, and transfusion services operate within a network of community-based blood collection centers, hospital-based collection centers, and transfusing facilities. The national blood supply is composed of units of red blood cells (RBCs), platelets (PLTs), and plasma, which are derived from whole blood after donation or collected as separate components by apheresis methods. Ensuring that the national supply is adequate to meet routine and emergent demand requires accurate national estimates of annual collections and transfusions. Since 1971, national surveys have been administered intermittently to blood collection centers and transfusing hospitals to assess supply and demand.1–5 In 2003, the Office of the Assistant Secretary for Health launched the biennial National Blood Collection and Utilization Survey (NBCUS), which was administered by the AABB through 2011 and subsequently by the Centers for Disease Control and Prevention.6–9
The 2011 and 2013 surveys revealed declines in both blood collection and utilization.8,9 Since 2010, the AABB has published revised, evidence-based guidance for RBC, PLT, and plasma transfusion with emphasis on conservative thresholds for transfusion when appropriate.10–12 To support implementation of recommended practices, transfusion oversight programs that offer clinical decision support for physicians have proliferated in US hospitals. 13,14 Further, hospital patient blood management programs, which are designed to minimize a patient’s need for transfusion (e.g., through aggressive anemia management before, during, and after surgery), have been broadly implemented.15 Taken together, these shifting clinical norms may explain recent declines in the number of transfusions performed annually since 2008. Yet transfusion of blood products continues to be a lifesaving procedure for a broad range of clinical indications.
Although safely meeting demand for blood products is an ongoing challenge internationally, policy makers in developed countries have expressed concerns over recent declines blood collection and shrinking donor populations. 16–18 To better understand the changing dynamics of blood collection and utilization in the United States, we analyzed data from the 2015 NBCUS survey. Specifically, our objectives were to: quantify blood and blood component collection, distribution, and transfusion in the United States in 2015; describe component processing, costs, and donor characteristics; and compare 2015 national estimates, rates of donation, rates of transfusion, and price paid per unit with previous years.
STUDY DESIGN
The 2015 survey questionnaire consisted of 42 questions, including 14 questions applicable to blood collection centers and 28 applicable to transfusing hospitals. All questions were applicable to hospitals performing both blood collection and transfusion. Survey questions for blood collection centers were similar to those from prior years, with questions designed to elicit the quantity of blood and blood components collected, processed, tested, and distributed as well as the number of donors, donations, and donor adverse events. For transfusing hospitals, questions were consistent with versions from previous years and were designed to collect information on the quantity of blood components transfused, cost paid per unit, recipient adverse events, and hospital-specific practices related to transfusion. The 2015 NBCUS questionnaire did not include sections on patient blood management, tissue services, or cellular therapy.
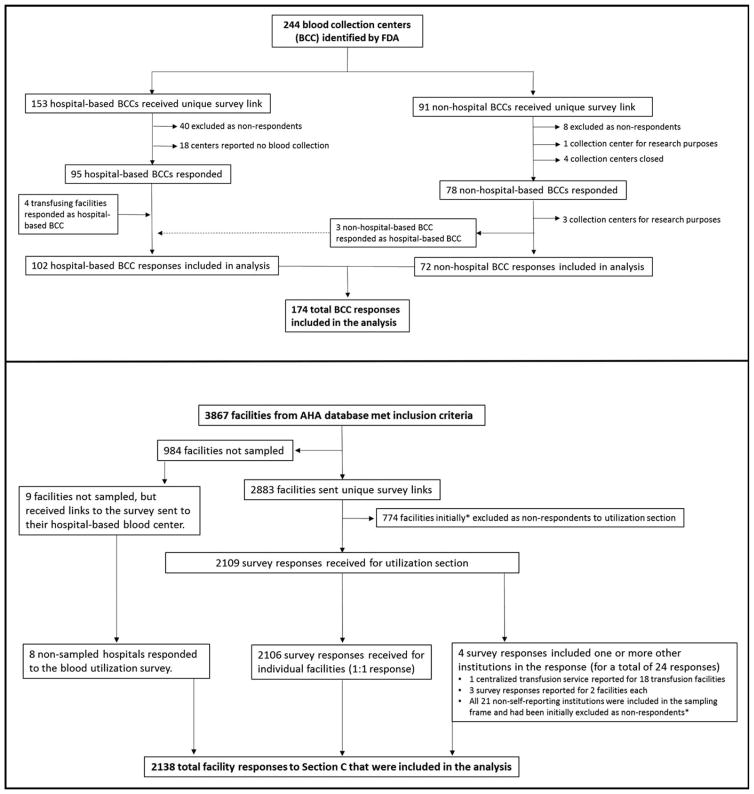
Blood collection centers were identified from the Food and Drug Administration Blood Establishment Registration (FDA-BER) database and from the America’s Blood Centers membership list. Military facilities and facilities collecting only cord blood were excluded; the remaining 91 nonhospital-based (i.e., community) and 153 hospital-based blood collection centers were sent surveys (Fig. 1). Transfusing hospitals were identified from the 2013 American Hospital Association annual survey database. Hospitals performing fewer than 100 surgical procedures; military, Department of Justice, psychiatric, rehabilitation, long-term acute care, and specialty treatment institutions; and facilities located in US territories were excluded from the sampling frame. Of the 3867 facilities included in the sampling frame, 40% of hospitals performing 100 to 999 surgeries were selected at random for participation and were sent surveys, and 100% of hospitals performing 1000 or more surgeries annually were sent surveys. This sampling strategy is consistent with previous NBCUS methodological approaches.8
Fig. 1.
Flow diagram depicting identification, stratification, sampling, exclusion, and recategorization of 2015 National Blood Collection and Use Survey respondents. AHA=American Hospital Association.
The survey was administered in a web-based electronic format. In March 2016, administrative contacts from nonhospital-based blood collection centers, hospital-based blood collection centers, and selected transfusing hospitals were sent e-mails with a unique, facility-specific link to the NBCUS survey portal, where responses could be entered. Letters were also sent via US mail to chief executive officers at each facility describing the survey, requesting participation, and providing directions for survey portal access. Nonrespondents were contacted by e-mail, US mail, and telephone from March through June 2016, to maximize response. Data collection concluded in June 2016. Follow-up for clarification on select responses continued through August 2016. Unlike previous NBCUS surveys, hospital respondents, with few exceptions, could not include aggregate data from multiple facilities on a single survey response.
National estimates for the number of units of blood and blood components collected, distributed, processed, transfused, and outdated were calculated in units and rounded to the nearest 1000. For weighting and imputation purposes, blood collection centers were stratified based on anticipated levels of activity in 2015. Community-based, nonhospital blood collection centers were stratified into four categories based on expected annual volume of whole blood and RBC donations: less than 50,000, from 50,000 to 199,999, from 200,000 to 399,000, and 400,000 or more units. Hospital-based blood centers were stratified into three categories based on annual inpatient surgical volume: less than 1000, from 1000 to 7999, and 8000 or more surgeries. Transfusing hospitals were stratified into six categories based on annual surgical volume: from 100 to 999, from 1000 to 1399, from 1400 to 2399, from 2400 to 4999, from 5000 to 7999, and 8000 or more surgical procedures annually.
Responses were weighted to adjust for nonresponse within strata. Sample weights were calculated for blood collection centers by dividing the total number of eligible participants by the number of actual respondents for each stratum, according to the stratification scheme described above. Blood collection centers with an expected collection volume of more than 400,000 units were assigned a weight of 1.0; all other collection centers and transfusing hospitals were weighted according to strata-specific, inverse response rates. For transfusing hospitals, weighting was conducted in a similar manner, except that stratification was used for both surgical volume and public health service region. Confidence intervals (CIs) for national collection and transfusion estimates were calculated using the Taylor Series method.19
Multiple imputation was performed for the following variables related to collection: whole blood and apheresis RBCs collected, distributed, rejected upon testing, rejected for other reasons (e.g., insufficient volume), and outdated; and apheresis PLTs, plasma, and cryoprecipitate units collected. Imputed variables related to utilization included: allogeneic (nondirected), autologous, and directed whole blood and apheresis RBCs, PLTs, plasma, and cryoprecipitate units transfused and outdated. All imputed variables were continuous and non-normally distributed. A two-stage imputation procedure was performed for variables with distributions skewed toward zero.20 According to established multiple imputation logic, imputation factors were considered for each variable to assure that the variables used for imputation had distributions that were similar to those of the variables requiring imputation.21
Both mean and median cost per unit for blood and blood components paid by transfusing hospitals were calculated using nonweighted data. Medians were preferred over means for comparing differences in unit costs for both 2013 and 2015 due to the presence of outliers, particularly from more remote locations where blood components are more expensive than in the rest of the United States.
To calculate the national rates of whole blood and RBC collection per 1000 population, the total estimated number of units collected before the removal of rejected units was divided by the 2015 US population ages 16 to 64 years; this denominator was used for consistency in comparison with previous years (Fig. 2c). The 2015 national transfusion rate per 1000 population was calculated using the entire US population. All population estimates were derived using US Census Bureau state-specific and age-specific estimates for 2015.22
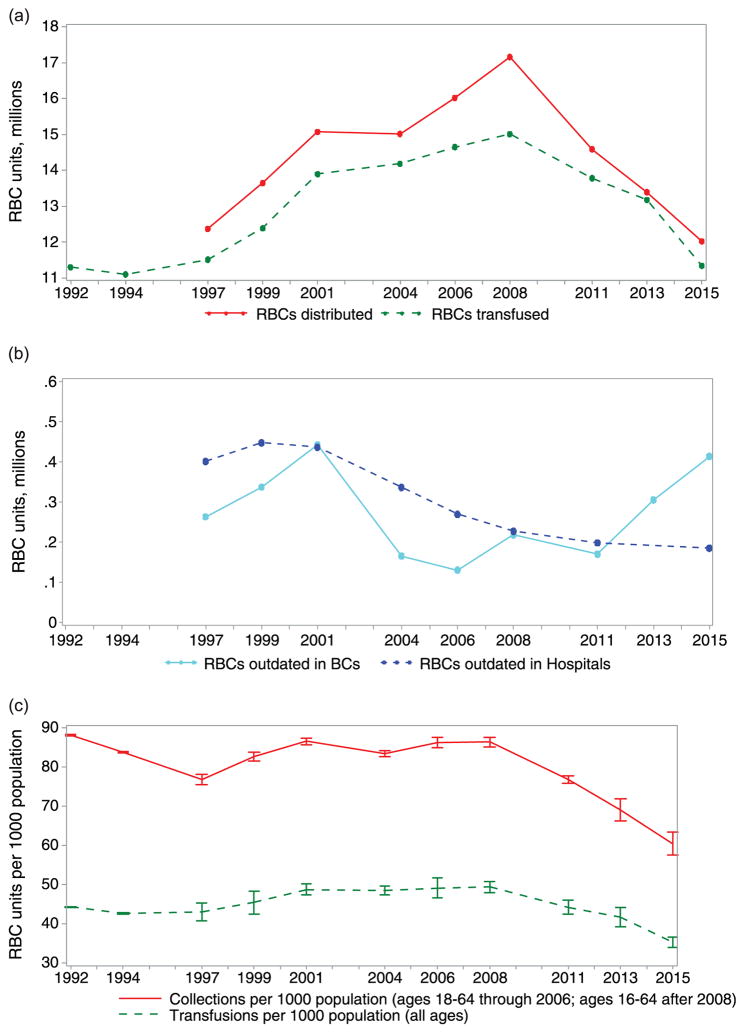
Fig. 2.
Trends in (a) RBC distributions and transfusions, (b) RBC units outdated in blood centers and hospitals, and (c) RBC collections and transfusion per 1000 population.
To determine whether differences in the collection and utilization estimates between the 2013 and 2015 surveys were caused by sampling and response rates, a subset of transfusing hospitals was created including only respondents who had completed both the 2013 and 2015 surveys. This matched subset of NBCUS respondents allowed a sensitivity analyses to assess whether differences observed between the two survey years were consistent or disparate when holding the respondents constant. All analyses were conducted using SAS statistical software (version 9.4; SAS Institute).
RESULTS
Participation
Of the 91 nonhospital-based blood collection centers that were sent surveys, 11 were excluded because of closures, misclassification, or status as a research only collection center (Fig. 1); of the 80 correctly classified and active, nonhospital-based collection centers in the sampling frame, 72 (90.0%) responded. Of the 153 hospital-based collection centers that were sent a survey link, 18 reported that they no longer collected blood and were excluded, and seven hospitals were added to the sampling frame after original misclassification as nonhospital-based collection centers or as transfusing hospitals only (i.e., without a collection center). Of the 142 correctly classified and active, hospital-based blood centers, 102 (71.8%) responded to the survey (Fig. 1). In total, 3867 hospitals met the inclusion criteria for participation in the utilization section of the survey. All hospitals performing 1000 or more surgeries annually were sent surveys. A 40% sample of hospitals performing from 100 to 999 surgeries annually were selected at random and were also sent surveys; of the 984 (60%) hospitals in this category that were not sampled, 9 were sent surveys because they had been designated as hospital-based blood collection centers and thus were sent both collection and utilization portions of the survey. Of the 2892 hospitals that were sent surveys, 2138 (73.9%) responded to the utilization section. Compared with NBCUS response rates in 2013, the response rates in 2015 were 38.9% higher for nonhospital blood collection centers, 74.8% higher for hospital-based collection centers, and 121.9% higher for hospitals that provided utilization data.8,9
Whole blood and RBC collections and transfusions
In 2015, 12,591,000 whole blood and apheresis RBC units (95% CI, 11,985,000–13,197,000 units) were collected in the United States. (Table 1). This estimate reflects an 11.6% decline in RBC collections since 2013, when 14,237,000 whole blood and apheresis RBC units (95% CI, 13,639,000–14,835,000 units) were collected. Approximately 95.5% of all RBC units collected in 2015 were collected at nonhospital-based collection centers. Of all whole blood units collected, 10,748,000 (95% CI, 10,176,000–11,321,000 units), or 99.6%, were the result of allogeneic, nondirected donations. There were 1,797,000 apheresis RBC units collected in 2015 (95% CI, 1,551,000–2,043,000 units), which is 12.1% fewer than were collected in 2013 (2,043,000 units; 95% CI, 1,659,000–2,427,000 units). In 2015, apheresis RBC collections accounted for 14.3% of all RBC units collected. The total whole blood and RBC supply available in 2015 after excluding units that were rejected was 12,028,000 (95% CI, 11,454,000–12,603,000 units), which is 10.2% fewer units than were available in 2013 (13,395,000 units; 95% CI, 12,823,000–13,966,000 units). Of the RBC units rejected after collection in 2015, 9.4% (53,000 units; 95% CI, 42,000–63,000 units) were rejected upon testing for transfusion-transmissible infections, whereas 90.6% (510,000 units; 95% CI, 457,000–562,000 units) were rejected for “other reasons,” such as insufficient volume.
TABLE 1.
Estimated numbers of whole blood and RBC units collected, transfused, and outdated in 2015 (expressed in thousands)
| Blood centers | Hospitals | Combined totals | 95% CI | 2013 Totals* | % Change 2015–2013 | |
|---|---|---|---|---|---|---|
| Collections | ||||||
| Whole blood units | ||||||
| Allogeneic, nondirected | 10,220 | 529 | 10,748 | 10,176–11,321 | 12,109 | −11.2 |
| Autologous | 18 | 7 | 25 | 19–31 | 61 | −59.0 |
| Directed | 11 | 10 | 21 | 14–28 | 24 | −12.3 |
| Apheresis RBC units† | 1,773 | 24 | 1,797 | 1,551–2,043 | 2,043 | −12.1 |
| Total supply | 12,022 | 569 | 12,591 | 11,985–13,197 | 14,237 | −11.6 |
| Rejected on testing | 46 | 7 | 53 | 42–63 | 98§ | |
| Rejected for other reasons‡ | 480 | 30 | 510 | 457–562 | 744§ | |
| Total available supply | 11,496 | 532 | 12,028 | 11,454–12,603 | 13,395 | −10.2 |
| Transfusions | ||||||
| Allogeneic, nondirected | 11,264 | 10,868–11,659 | 13,093 | −14.0 | ||
| Autologous | 20 | 8–32 | 44 | −54.2 | ||
| Directed | 66 | 35–96 | 43 | 52.3 | ||
| Total transfusions | 11,349 | 10,952–11,747 | 13,180 | −13.9 | ||
| Outdated whole blood or RBCs | 414 | 186 | 600 | 542–658 | ||
Totals for 2013 were obtained from Chung et al.9
Apheresis RBC units include allogeneic, autologous, directed, and concurrent collections.
Units rejected for other reasons do not include outdated units.
Rejected units for 2015 are restricted to whole blood and apheresis red blood cell collections, whereas the 2013 survey did not specify rejected units by component type or collection method.
In 2015, an estimated 11,349,000 whole blood-derived and apheresis RBC units were transfused at US acute care hospitals (95% CI, 10,952,000–11,747,000 units), constituting a 13.9% decline since 2013. Approximately 11,264,000 units (95% CI, 10,868,000–11,659,000 units), or 99.3%, of whole blood-derived and apheresis RBCs transfused originated from allogeneic, nondirected donations in 2015, which is nearly equivalent to the proportion of allogenic RBC units transfused in 2013 (99.2%). Approximately 414,000 RBC units (95% CI, 380,000–450,000 units) outdated on the shelves (i.e., “expired”) of nonhospital blood centers in 2015, which is a 35.6% increase from the 306,000 RBC units outdated in nonhospital blood centers (95% CI, 269,000–343,000 units) in 2013. (Note that the number of outdated units was available for both nonhospital-based and hospital-based blood centers in 2015, but only from nonhospital-based blood centers in 2013; therefore, the comparison of blood center outdates is based on nonhospital blood collection centers only.)
The previously reported declines in RBC collections and transfusions since 2008 continued in 2015 (Fig 2a), whereas outdated units at nonhospital blood centers increased (Fig. 2b). Population trends in donation and transfusion of RBCs continue to decline (Fig. 2c).
PLT, plasma, and cryoprecipitate distribution and transfusion
In 2015, 2,436,000 PLT units were distributed from blood collection centers in the United States (95% CI, 2,230,000–2,642,000 units), which is 0.5% fewer units than were distributed in 2013 (2,448,000 units; 95% CI, 2,237,000–2,659,000 units). Of all PLT units distributed, 93.9% (2,234,000 units; 95% CI, 2,040,000–2,429,000 units) were collected by apheresis; the remaining 202,000 units distributed (95% CI, 146,000–257,000 units) were whole blood-derived.
In total, 3,714,000 plasma units (95% CI, 3,306,000–4,121,000 units) were distributed in 2015, which included fresh-frozen plasma, plasma frozen within 24 hours of collection, cryoprecipitate-reduced plasma, and liquid plasma. Total plasma distributed in 2015 declined 14.4% since 2013, when 4,338,000 units (95% CI, 3,432,000–5,244,000 units) were distributed. Also in 2015, a total of 1,857,000 units of cryoprecipitate were distributed (95% CI, 1,605,000–2,109,000 units), an 89.9% increase since 2013 (978,000; 95% CI, 798–1,157,000 units of cryoprecipitate).
In addition, a total of 1,983,000 whole blood-derived and apheresis PLT units (95% CI, 1,816,000–2,151,000 units) were transfused nationally, which is 13.1% fewer than were transfused in 2013 (2,281,000 units; 95% CI, 1,915,000–2,646,000 units). Apheresis PLT units transfused (1,807,000 units; 95% CI, 1,670,000–1,943,000 units) declined by 15.4% since 2013 (2,137,000 units; 95% CI, 1,773,000–2,500,000 units). Whole blood-derived PLT units transfused (172,000 units; 95% CI, 84,000–258,000 units) increased by 33.7% since 2013 (128,000 units; 95% CI, 83,000–171,000 units), although, of all PLT units transfused, only 8.6% were whole blood-derived. Approximately 2,727,000 units of plasma were transfused (95% CI, 2,594,000–2,859,000 units), which is a 24.8% decrease since 2013. Cryoprecipitate transfusions in 2015 (1,167,000 transfusions; 95% CI, 1,021,000–1,314,000 transfusions) increased by 6.6% since 2013.
Of all PLT, plasma, and cryoprecipitate components produced for distribution in 2015, 242,000 (95% CI, 211,000–273,000) outdated at blood collection centers, which is a 1.2% increase since 2013. The number and percentage of units outdated at blood centers in 2015 included: 176,000 apheresis PLT units (7.9% of apheresis PLT units), 37,000 whole blood-derived apheresis equivalents (18.3%), 21,000 plasma units (0.57%), and 7000 (0.4%) cryoprecipitate units. Component outdates at transfusing hospitals in 2015 (426,000 outdates; 95% CI, 392,000–461,000 outdates) increased 7.9% since 2013. Components outdated at hospitals before transfusion in 2015 included 171,0000 apheresis PLT units (9.5% of the units on hospital shelves), 14,000 whole blood-derived apheresis equivalents (8.2%), 165,000 plasma units (6.1%), and 77,000 cryoprecipitate units (6.6%).
Leukocyte reduction and irradiation of components
In 2015, 7,939,000 (95% CI, 7,511,000–8,366,000), or 71.3%, of whole blood-derived RBC units were leukocyte reduced before arrival at the recipient’s bedside, which is similar to the 2013 proportion (72.0%). For whole blood-derived PLTs, 56.2% (95,000 units; 95% CI, 54,000–135,000 units) were leukocyte reduced before arrival at bedside in 2015, reflecting an 18.5% increase since 2013, when the proportion was 35.0%. For whole blood-derived RBCs and whole blood-derived PLTs, the proportions that were leukocyte filtered at bedside in 2015 were 0.8% and 1.1%, respectively; these proportions equate to 85,000 RBC units (95% CI, 43,000–127,000 units) and 2000 PLT units (95% CI, 0–5000 units) that were leukocyte filtered at bedside in the United States. An estimated 15.8% (1,761,000; 95% CI, 1,507,000–2,015,000) of whole blood-derived RBC units were irradiated, which is a slight decrease from 2013, when 16.8% of units were irradiated. For apheresis PLTs and whole blood-derived PLTs, 58.0% (1,233,000 units; 95% CI, 970,000–1,495,000 units) and 36.2% (61,000 units; 95% CI, 29,000–93,000units) were irradiated, respectively.
Component prices paid by hospitals
The median price paid per unit by transfusing hospitals for a single leukocyte-reduced RBC unit in 2015 was $211 (interquartile range [IQR], $197–$228), which is $10 less than the median price paid per unit in 2013 ($221; IQR, $205–$240). For a single nonleukocyte-reduced unit, hospitals paid a median price of $204 (IQR, $185–$205). In 2015, hospitals paid a median price of $54 per unit of plasma frozen within 8 hours of phlebotomy (IQR, $45–$64), which is $5 less than the price paid per unit in 2013 ($59; IQR, $50–$68). For plasma frozen between 8 and 24 hours after phlebotomy, hospitals paid a median price of $52 (IQR, $45–$60) per unit, which is $6 less than the price paid per unit in 2013 ($59; IQR, $48–$65). For a single apheresis PLT unit, the median price paid per unit in 2015 was $524 (IQR, $495–$560), a $16 decrease compared with the median price paid in 2013 ($540; IQR, $510–$590).
Donor deferrals
In 2015, an estimated 13,225,000 persons presented to donate blood in the United States (Table 3). Approximately 14.3% of these donors (1,886,000; 95% CI, 1,774,000–1,997,000 donors) were deferred, meaning that they did not donate or had their donation discarded after collection. In 2013, 15,237,000 persons presented for donation, of which 15.5% were deferred. Over one-half (51.7%) of all donor deferrals in 2015 were due to low hemoglobin (975,000 donors; 95% CI, 911,000–1,040,000 donors). Deferrals for other medical reasons (e.g., blood pressure outside of an acceptable range, certain types of cancer, uncontrolled or untreated medical conditions, etc.) contributed to 26.7% of all deferrals (504,000; 95% CI, 463,000–544,000 deferrals). Prescription medication use contributed to 2.9% of deferrals. In 2015, 8000 donors (95% CI, 7000–10,000 donors) were deferred because of their status as men who have sex with men, which constituted 0.4% of all deferrals. Travel-related deferrals (113,000; 95% CI, 105,000–122,000 deferrals) represented 6.0% of all deferrals.
TABLE 3.
Estimated number of donors and deferrals in the United States, 2015 (expressed in thousands)†
| % of Total | % Change 2015–2013 | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Variable | All facilities (95% CI), 2015 | All facilities, 2013 | 2015 | 2013 | |
| Donor deferrals | |||||
| Low hemoglobin | 975 (911–1,040) | 1,188 | 51.7 | 50.2 | 1.5 |
| Prescription drug use | 54 (46–62) | 70 | 2.9 | 3.0 | −0.1 |
| Other medical reasons | 504 (463–544) | 472 | 26.7 | 19.9 | 6.8 |
| High-risk behavior, MSM only | 8 (7–10) | 8 | 0.4 | 0.3 | 0.1 |
| High-risk behavior, all other behaviors | 16 (11–21) | 14 | 0.9 | 0.6 | 0.3 |
| Travel | 113 (105–122) | 173 | 6.0 | 7.3 | −1.3 |
| Tattoo/piercing | 49 (44–54) | 76 | 2.6 | 3.2 | −0.6 |
| Other | 166 (125–206) | 367 | 8.8 | 15.5 | −6.7 |
| Total deferrals | 1,886 (1,774–1,997) | 2,368* | |||
| Total presenting to donate | 13,225 | 15,237 | |||
The 2013 percentage of total deferrals was obtained from Chung et al.9
Excludes directed and autologous donors.
MSM=men who have sex with men.
Rates of blood collection and utilization
Whole blood and RBC collections per 1000 population in the United States declined in 2015 (60.4 per 1000 population ages 16–64 years) by 12.5% compared with 2013 (69.0 per 1000 population ages 16–64 years) and by 21.3% compared with (76.7 per 1000 population ages 16–64 years) (Fig. 2c). Similarly, whole blood and RBC transfusions per 1000 population in the United States declined in 2015 (35.3 per 1000 population, all ages) by 15.3% compared with 2013 (41.7 per 1000 population, all ages) and by 19.8% compared with 2011 (44.0 per 1000 population, all ages).
Matched set analysis for RBC transfusion
In a subanalysis of 594 hospitals that provided allogenic RBC utilization data for both 2013 and 2015, the median difference between 2013 and 2015 was a 12.2% decline. When comparing all (i.e., nonmatched) RBC utilization data from the two survey years, the decline was 15.8%. In this matched subset, declines since 2013 in the number of units transfused were noted across all surgical volume-based strata (Table 4). Hospitals performing the fewest surgeries annually (100–999 surgeries) showed the greatest decline in RBC transfusions between 2013 and 2015 (median difference, −22.4%). Conversely, the smallest decline in RBC transfusions occurred among hospitals that performed 8000 or more surgeries annually (median difference, −3.9%).
TABLE 4.
Median percentage difference in allogeneic RBC units transfused in 2013 and 2015 from matched hospitals, stratified by surgical volume
| Surgical volume: Surgeries per year | No.* | Median (mean) 2015 allogeneic RBC units* | Median % difference† | IQR of % difference† |
|---|---|---|---|---|
| 100–999 | 106 | 397 (561) | −22.4 | 38.5 |
| 1,000–1,399 | 79 | 1,271 (1,426) | −15.8 | 22.4 |
| 1,400–2,399 | 108 | 1,993 (2,248) | −13.0 | 26.5 |
| 2,400–4,999 | 155 | 3,791 (4,056) | −11.8 | 18.5 |
| 5,000–7,999 | 72 | 6,094 (7,069) | −10.3 | 14.4 |
| ≥8,000 | 74 | 13,096 (15,442) | −3.9 | 20.8 |
| Total | 594 | 2,796 (4,538) | −12.2 | 23.8 |
Based on matched facilities reporting allogeneic red blood cells in both 2013 and 2015 National Blood Collection and Utilization Surveys.
The percentage difference is calculated as 100*(2015–2013)/2013.
IQR=interquartile range (75th to 25th percentile).
DISCUSSION
Findings from the 2015 NBCUS suggest a continued decline in both blood collection and blood utilization in the United States. Since 2013, the number of units transfused decreased substantially across the three primary blood component types, including declines of 13.9%, 13.1%, and 24.8% in RBC, PLT, and plasma transfusions, respectively. Although PLT collections remained relatively stable (0.5% decline since 2013), allogenic RBC collections declined by 11.1%, and plasma collections declined by 14.4%. Since 2013, the median cost paid per unit by hospitals to blood collection centers for leukocyte-reduced RBCs and apheresis PLTs decreased by $10 and $16, respectively. Declining prices could result from decreasing demand from hospitals, which is supported by the trend toward more outdated units at blood centers (Fig. 2b).
The decline in utilization of RBCs and plasma likely reflects technological innovation (e.g., laparoscopic surgery) and implementation of patient blood management programs to reduce the need for transfusion, 15,23 as well as efforts toward more systematic ordering and dosing of transfusions.11,12,24 The shift toward transfusion of fewer blood products nationally, in part, can be attributed to a growing evidence base that supports the judicious use of blood and blood products. For example, inconsistent use of blood products during various surgical procedures, including coronary artery bypass grafting, has heightened scrutiny over the appropriateness of transfusion across medical and surgical procedures.25–28 Recent studies also have described an association between liberal transfusion policies and increased incidence of health care-associated infections,29 including surgical site infections and sepsis.30,31 Other studies have implicated high PLT dosing as a risk factor for transfusion-associated adverse events,32,33 and transfusions in patients with certain gastrointestinal and other metastatic diseases may be associated with reduced chances of remission and survival. 34–37 It is possible that clinical recommendations supported by these studies have motivated the decreased demand for blood products.
Currently, the risk of transfusion-transmitted infections in the United States blood supply is very low.38 However, blood centers continue to face challenges in maintaining the safety of the blood supply, including bacterial contamination of PLT products and transfusion-transmission risks associated with emerging pathogens, such as Zika virus and babesiosis.39,40 Technologies to mitigate these risks are available either as FDA-approved, commercially available products or as investigational protocols. These include pathogen-reduction technology (PRT) for apheresis PLTs and plasma, rapid tests for bacterial detection in PLTs, and investigational screening assays for Zika virus and Babesia spp. Implementation of these technologies in the United States to further enhance the safety of the blood supply has been the focus of recent discussions.39–41 Currently, PRT has been adopted in several other industrialized countries.41,42 The considerable operational costs of PRT implementation by blood centers in the United States is prohibitive for many blood centers when combined with current testing requirements. Furthermore, PRT can reduce the potency of PLT units, which could result in the need for more units, thereby increasing costs.43 The challenges faced by blood centers in incorporating and absorbing the cost of new technologies are reinforced by evidence that even currently available technologies to improve safety, such as leukocyte reduction, have not been universally adopted.9 In the United States, the relatively low proportion of blood products subjected to leukocyte reduction is likely due to cost along with the lack of regulatory mandate despite evidence supporting reduction in transfusion-related adverse reactions.
In addition to meeting routine medical needs, maintaining resiliency in the blood supply is critical to addressing surge demands for blood during public health emergencies. Recently, because of FDA guidance to mitigate the threat of transfusion-transmitted Zika virus infection, blood collections ceased in Puerto Rico from February 2016 until nucleic acid testing could be implemented under investigational new drug protocols in April 2016. This resulted in the importation of blood products to meet clinical demand in Puerto Rico from non-Zika–affected blood centers in the mainland United States.44 Ensuring continued resiliency through maintenance of a surplus capacity in the blood collection system is integral to meeting future challenges that may arise due to public health emergencies.
Findings from the 2015 NBCUS suggest that the cost of maintaining this surplus is borne primarily by community-based blood collection centers rather than hospitals. For example, the number of RBC units outdated at community-based blood collection centers increased since 2011 but declined in hospitals (Fig. 2b), suggesting that nontransfused units are increasingly more likely to expire on the shelf of a blood collection center than a transfusing hospital. These trends in outdates suggest that transfusing facilities are more tightly managing their inventory of blood products, with blood centers bearing the costs of unutilized units. Whether a sufficient reserve in blood products will be maintained in the context of declining demand is unknown. In 2015, 56.9% of all units were collected at the five largest blood collection centers in the United States. The impact of consolidating blood collection to fewer high-volume blood collection centers on stability of the blood supply is unknown.
These findings are subject to the following limitations. First, imputation and weighting had to be used to generate final estimates, so comparisons with the 2013 survey results could be biased by differences in sampling and response rate. To address this potential bias, a matched set of respondents was created that contained only respondents who had participated in both surveys, and inconsistencies were reported where relevant. Despite these limitations, recruitment, follow-up, and survey responses were more robust for 2015 than for previous years. The survey was not distributed to outpatient facilities or to military and specialty hospitals, which may transfuse substantial numbers of blood and blood components. The survey was also not distributed to military collection facilities. This may result in underestimations of collection and utilization.
In conclusion, the continued decline in demand for blood and blood products in 2015 could have resulted in fewer units collected and distributed. A simultaneous decline in revenue could preclude the adoption of existing or new safety interventions by blood centers, such as universal leukocyte reduction, donor screening, and product modification strategies, which can mitigate the risk of transfusion-transmitted infection. Continued vigilance is necessary to maintain resiliency in the blood supply and to meet disaster preparedness and other public health challenges.
TABLE 2.
Estimated number of platelets, plasma, and cryoprecipitate units distributed, transfused, and outdated in 2015 (expressed in thousands)
| Variable | Blood centers | Hospitals | Combined totals | 95% CI | 2013 Totals* | % Change 2015–2013 |
|---|---|---|---|---|---|---|
| Distributed | ||||||
| Apheresis platelets | 2034 | 200 | 2234 | 2040–2429 | 2318 | −3.6 |
| Whole-blood–derived PLTs† | 189 | 13 | 202 | 146–257 | 130 | 55.0 |
| Total platelets | 2223 | 213 | 2436 | 2230–2642 | 2448 | −0.5 |
| Total plasma | 3450 | 264 | 3714 | 3306–4121 | 4338 | −14.4 |
| Cryoprecipitate‡ | 1694 | 163 | 1857 | 1605–2109 | 978 | 89.9 |
| Blood center outdates§ | 217 | 25 | 242 | 211–273 | 239 | 1.2 |
| Transfused | ||||||
| Apheresis platelets | 1807 | 1670–1943 | 2137 | −15.4 | ||
| Whole-blood–derived PLTs† | 171 | 84–258 | 128 | 33.7 | ||
| Total platelets (includes directed units) | 1983 | 1816–2151 | 2281 | −13.1 | ||
| Total plasma | 2727 | 2594–2859 | 3624 | −24.8 | ||
| Cryoprecipitate‡ | 1167 | 1021–1314 | 1095 | 6.6 | ||
| Hospital outdates|| | 426 | 392–461 | 395 | 7.9 | ||
Totals for 2013 were obtained from Chung et al.9
Whole-blood–derived PLTs are expressed as apheresis equivalents.
Cryoprecipitates are expressed as individual unit equivalents.
Blood center outdates are units that were outdated at nonhospital-based and hospital-based blood centers.
Hospital outdates are units that were outdated at transfusing hospitals.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflicts of interest.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention. The use of trade names is for identification purposes only and does not constitute endorsement by the US Centers for Disease Control and Prevention or the Department of Health and Human Services.
References
- 1.Surgenor DM, Wallace EL, Hao SH, et al. Collection and transfusion of blood in the United States, 1982–1988. N Engl J Med. 1990;322:1646–51. doi: 10.1056/NEJM199006073222306. [DOI] [PubMed] [Google Scholar]
- 2.Wallace EL, Surgenor DM, Hao HS, et al. Collection and transfusion of blood and blood components in the United States, 1989. Transfusion. 1993;33:139–44. doi: 10.1046/j.1537-2995.1993.33293158046.x. [DOI] [PubMed] [Google Scholar]
- 3.Wallace EL, Churchill WH, Surgenor DM, et al. Collection and transfusion of blood and blood components in the United States, 1992. Transfusion. 1995;35:802–12. doi: 10.1046/j.1537-2995.1995.351096026360.x. [DOI] [PubMed] [Google Scholar]
- 4.Wallace EL, Churchill WH, Surgenor DM, et al. Collection and transfusion of blood and blood components in the United States, 1994. Transfusion. 1998;38:625–36. doi: 10.1046/j.1537-2995.1998.38798346630.x. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan MT, McCullough J, Schreiber GB, et al. Blood collection and transfusion in the United States in 1997. Transfusion. 2002;42:1253–60. doi: 10.1046/j.1537-2995.2002.00203.x. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan MT, Wallace EL. Blood collection and transfusion in the United States in 1999. Transfusion. 2005;45:141–8. doi: 10.1111/j.1537-2995.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan MT, Cotten R, Read EJ, et al. Blood collection and transfusion in the United States in 2001. Transfusion. 2007;47:385–94. doi: 10.1111/j.1537-2995.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 8.US Department of Health and Human Services. The 2011 National Blood Collection and Utilization Survey Report. Washington (DC): US Department of Health and Human Services; 2013. [Google Scholar]
- 9.Chung KW, Basavaraju SV, Mu Y, et al. Declining blood collection and utilization in the United States. Transfusion. 2016;56:2184–92. doi: 10.1111/trf.13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316:2025–35. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205–13. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 12.Roback JD, Caldwell S, Carson J, et al. Evidence-based practice guidelines for plasma transfusion. Transfusion. 2010;50:1227–39. doi: 10.1111/j.1537-2995.2010.02632.x. [DOI] [PubMed] [Google Scholar]
- 13.Xydas S, Magovern CJ, Slater JP, et al. Implementation of a comprehensive blood conservation program can reduce blood use in a community cardiac surgery program. J Thorac Cardiovasc Surg. 2012;143:926–35. doi: 10.1016/j.jtcvs.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Tavares MM, Diquattro PJ, Sweeney JD. Reduction in red blood cell transfusion associated with engagement of the ordering physician. Transfusion. 2014;54:2625–30. doi: 10.1111/trf.12552. [DOI] [PubMed] [Google Scholar]
- 15.Goodnough LT. Blood management: transfusion medicine comes of age. Lancet. 2013;381:1791–2. doi: 10.1016/S0140-6736(13)60673-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borkent-Raven BA, Janssen MP, Van Der Poel CL. Demographic changes and predicting blood supply and demand in the Netherlands. Transfusion. 2010;50:2455–60. doi: 10.1111/j.1537-2995.2010.02716.x. [DOI] [PubMed] [Google Scholar]
- 17.Drackley A, Newbold KB, Paez A, et al. Forecasting Ontario’s blood supply and demand. Transfusion. 2012;52:366–74. doi: 10.1111/j.1537-2995.2011.03280.x. [DOI] [PubMed] [Google Scholar]
- 18.Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion. 2015;56:139–45. doi: 10.1111/trf.13342. [DOI] [PubMed] [Google Scholar]
- 19.Woodruff R. A simple method for approximating the variance of a complicated estimate. J Am Stat Assoc. 1971;66:411–4. [Google Scholar]
- 20.He YRT. Tukey’s gh distribution for multiple imputation. Am Stat Assoc. 2006;60:251–6. [Google Scholar]
- 21.Rubin D. Multiple imputation for nonresponse in surveys. Hoboken (NJ): Wiley; 2004. [Google Scholar]
- 22.US Census Bureau. Population Estimates by State and Age. Suitland (MD): US Census Bureau; 2015. [Google Scholar]
- 23.Goodnough LT, Shander A. Patient blood management. Anesthesiology. 2012;116:1367–76. doi: 10.1097/ALN.0b013e318254d1a3. [DOI] [PubMed] [Google Scholar]
- 24.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 25.Bennett-Guerrero E, Zhao Y, O’Brien SM, et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304:1568–75. doi: 10.1001/jama.2010.1406. [DOI] [PubMed] [Google Scholar]
- 26.Likosky DS, Al-Attar PM, Malenka DJ, et al. Geographic variability in potentially discretionary red blood cell transfusions after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2014;148:3084–9. doi: 10.1016/j.jtcvs.2014.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherwood MW, Wang Y, Curtis JP, et al. Patterns and outcomes of red blood cell transfusion in patients undergoing percutaneous coronary intervention. JAMA. 2014;311:836–43. doi: 10.1001/jama.2014.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Triulzi D, Gottschall J, Murphy E, et al. A multicenter study of plasma use in the United States. Transfusion. 2015;55:1313–9. doi: 10.1111/trf.12970. quiz 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317–26. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen SJ, Braun Y, Wood KB, et al. Allogeneic blood transfusions and postoperative infections after lumbar spine surgery. Spine J. 2015;15:901–9. doi: 10.1016/j.spinee.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Kim JL, Park JH, Han SB, et al. Allogeneic blood transfusion is a significant risk factor for surgical-site infection following total hip and knee arthroplasty: a meta-analysis. J Arthroplasty. 2016;32:320–5. doi: 10.1016/j.arth.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman RM, Assmann SF, Triulzi DJ, et al. Transfusion-related adverse events in the Platelet Dose study. Transfusion. 2015;55:144–53. doi: 10.1111/trf.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A, Mhaskar R, Grossman BJ, et al. Platelet transfusion: a systematic review of the clinical evidence. Transfusion. 2015;55:1116–27. doi: 10.1111/trf.12943. quiz 1115. [DOI] [PubMed] [Google Scholar]
- 34.Squires MH, 3rd, Kooby DA, Poultsides GA, et al. Effect of perioperative transfusion on recurrence and survival after gastric cancer resection: a 7-institution analysis of 765 patients from the US Gastric Cancer Collaborative. J Am Coll Surg. 2015;221:767–77. doi: 10.1016/j.jamcollsurg.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Schiergens TS, Rentsch M, Kasparek MS, et al. Impact of perioperative allogeneic red blood cell transfusion on recurrence and overall survival after resection of colorectal liver metastases. Dis Colon Rectum. 2015;58:74–82. doi: 10.1097/DCR.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 36.Janssen SJ, Braun Y, Ready JE, et al. Are allogeneic blood transfusions associated with decreased survival after surgery for long-bone metastatic fractures? Clin Orthop Relat Res. 2015;473:2343–51. doi: 10.1007/s11999-015-4167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aquina CT, Blumberg N, Becerra AZ, et al. Association among blood transfusion, sepsis, and decreased long-term survival after colon cancer resection [published online ahead of print 2016 Sep 14] Ann Surg. doi: 10.1097/SLA.0000000000001990. [DOI] [PubMed] [Google Scholar]
- 38.Harvey AR, Basavaraju SV, Chung KW, et al. Transfusion-related adverse reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2010 to 2012. Transfusion. 2015;55:709–18. doi: 10.1111/trf.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US Food and Drug Administration (FDA), Blood Products Advisory Committee. Strategies for implementation of serological and nucleic acid testing for Babesia microti in blood donors. Silver Spring (MD): FDA; 2015. [Google Scholar]
- 40.US Food and Drug Administration (FDA) Revised recommendations for reducing the risk of Zika virus transmission by blood and blood components. Silver Spring (MD): FDA; 2016. [Google Scholar]
- 41.Snyder EL, Stramer SL, Benjamin RJ. The safety of the blood supply--time to raise the bar [letter] N Engl J Med. 2015;373:882. doi: 10.1056/NEJMc1507761. [DOI] [PubMed] [Google Scholar]
- 42.Girona-Llobera E, Jimenez-Marco T, Galmes-Trueba A, et al. Reducing the financial impact of pathogen inactivation technology for platelet components: our experience. Transfusion. 2014;54:158–68. doi: 10.1111/trf.12232. [DOI] [PubMed] [Google Scholar]
- 43.Hess JR, Pagano MB, Barbeau JD, et al. Will pathogen reduction of blood components harm more people than it helps in developed countries? Transfusion. 2016;56:1236–41. doi: 10.1111/trf.13512. [DOI] [PubMed] [Google Scholar]
- 44.Vasquez AM, Sapiano MR, Basavaraju SV, et al. Survey of blood collection centers and implementation of guidance for prevention of transfusion-transmitted Zika virus infection–Puerto Rico, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:375–8. doi: 10.15585/mmwr.mm6514e1. [DOI] [PubMed] [Google Scholar]