Abstract
Aims
Perfluoroalkyl Substances (PFAS) are synthetic hydrocarbons shown to preserve pancreatic islet cell viability and reduce islet cell hypoxia and apoptosis. We investigated the relationship of serum PFAS with diabetes, and whether this varied by diabetes type.
Methods
6,460 individuals with and 60,439 without diabetes from the C8 Health Project, were categorized into three groups: Type 1 (n=820), Type 2 (n=4,291), or Uncategorized diabetes (n=1,349, missing data on diabetes type or diabetes based on blood sugar at study entry). Four PFAS were investigated: perfluorohexane sulfonate (PFHxS), perfluoroctanoic acid (PFOA), perfluoroctane sulfonate (PFOS), and perfluorononaoic acid (PFNA).
Results
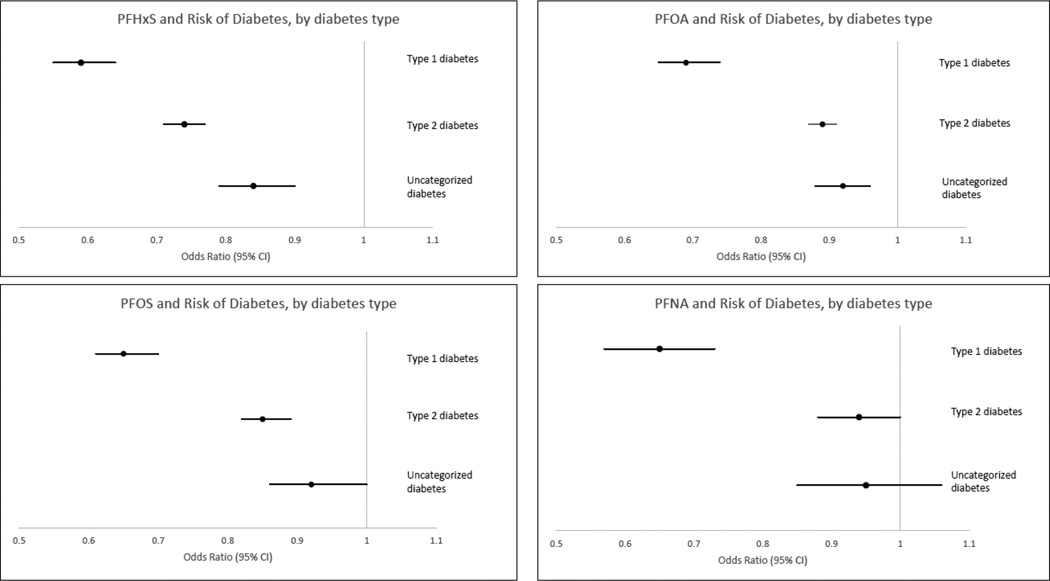
PFAS levels were significantly lower in those with diabetes, and lowest in those with Type 1 diabetes. In age and sex adjusted analyses, ORs (CI) for Type 1, Type 2, and Uncategorized diabetes compared to no diabetes were 0.59 (0.54–0.64), 0.74 (0.71–0.77), 0.84 (0.78–0.90) respectively for PFHxS; 0.69 (0.65–0.74), 0.87 (0.89–0.91), 0.92 (0.88–0.97) respectively for PFOA; 0.65 (0.61–0.70), 0.86 (0.82–0.90), 0.93 (0.86–1.03) respectively for PFOS; and 0.65 (0.57–0.74), 0.94 (0.88–1.00), 0.95 (0.85–1.06), respectively for PFNA. Further adjustment for eGFR and other covariates did not eliminate these inverse associations.
Conclusions
PFAS levels were negatively associated with diabetes. This inverse relationship was strongest for Type 1 diabetes, suggesting the relationship with serum PFAS may vary with the severity of islet cell deficiency.
Keywords: Perfluorocarbons, perfluoroalkyl substances, type 1 diabetes, environmental contaminants, beta cell, islet cell
1. Introduction
Perfluoroalkyl substances (PFAS), also called perfluorocarbons, are synthetic “hydrocarbon” compounds in which the hydrogen atoms have been partially or completely replaced by fluorine. Possessing both hydrophobic and oleophobic characteristics, they are industrial compounds used in the manufacture of Teflon™ cooking products and water-proof surfactants.[1] Because their strong carbon-fluorine bonds resist environmental degradation, PFAS are persistent environmental contaminants that have been found in the serum of the majority of the human populations studied.[1, 2] These environmental pollutants have been linked to adverse health outcomes,[1, 3] including birth defects and certain cancers.[4, 5]
The fluorine substitution of hydrogen makes PFAS highly efficient oxygen carriers. They have a higher oxygen carrying capacity than hemoglobin, with an oxygen solubility reported to be 25 times greater than either blood or water.[6, 7] As such, they have been experimented with as blood substitutes,[8, 9] in the development of synthetic blood, and in the preservation of organs harvested for transplants.[6] The high oxygen transport capacity of PFAS has been shown to reduce the hypoxia-induced damage associated with organ preservation, such as for the kidney and pancreas.[6, 10–12] In particular, PFAS have been shown to preserve pancreatic islet viability, functionality, and extracellular matrix in murine models.[13] They have also been shown to increase insulin mRNA expression[13] and to reduce pancreatic islet cell hypoxia and apoptosis.[14]
Although these environmental pollutants have been associated with certain adverse health effects, their relationship with other chronic diseases and conditions has been mixed. A weak relationship has been reported with kidney disease, and weak to no [15] and protective[16] relationships reported for Type 2 diabetes.[15] In the National Health and Nutrition Examination Survey, PFAS were associated with both increased beta cell function and insulin resistance.[17] The relationship of PFAS with Type 1 diabetes has not been investigated. Type 1 diabetes is a disease of islet cell destruction, characterized pathologically as the nearly complete deficiency of islet cells. As PFAS have been shown to reduce islet cell apoptosis in murine models, using the C8 Health Project population we investigated the association of PFAS with diabetes in a population with exposure to PFAS contaminated drinking water, and whether this relationship varied by diabetes type.
2. Materials and Methods
The C8 Health Project is a community-based study designed to investigate the effects of exposure to perfluorooctanoic acid (PFOA) contaminated drinking water.[18] The C8 Health Project was created as part of a settlement agreement from the case of Jack W. Leach, et al. v. E.I. du Pont de Nemours & Company after it was found that perfluorooctanoic acid had contaminated the drinking water of six water districts in the mid-Ohio Valley between 1950 and 2004. A post-hoc agreement between the settling parties of the class action lawsuit created a population-wide health survey of the individuals affected by the PFOA contamination. From August 2005 to August 2006, baseline data were gathered on 69,030 individuals working or living in six PFOA-contaminated water districts in West Virginia and Ohio, including those exposed to contaminated private-well drinking water. Estimated participation rate in the C8 Health Project among adult residents of the affected water districts was 81%.[19] Data from the C8 Health Project (n=69,030) was obtained for use in the current study.
The enrollment and data collection methods for the C8 Health Project have been described in detail previously.[18] The health survey collected a wide range of serum and anthropometric measures in order to assess the potential link between PFOA and human disease. Brookmar Inc., Parkersburg, West Virginia administered the consent process and data collection. Parents or guardians of those under 18 who were dependents were required to complete the survey for minors. We obtained institutional review board approval at West Virginia University for access to the C8 Health Project de-identified data for the following study.
The presence of diabetes was determined by 1) self- or parent/guardian report of a physician diagnosis of diabetes or 2) having a non-fasting blood-sugar of ≥200 mg/dL or fasting blood glucose of ≥127 mg/dL at study entry. Of the 69,030 study participants, 5,387 reported a physician diagnosis of diabetes and an additional 1,105 were classified as having diabetes based on blood glucose level at study entry. Of the 6,492 with diabetes and 62,538 without diabetes, 32 and 2,099, respectively, had missing data on the four major perfluoroalkyl substances of interest, providing for a final study population of 6,460 with and 60,439 individuals without diabetes. Study participants with diabetes were categorized into three groups: self-reported Type 1 diabetes (n=820), self-reported Type 2 diabetes (n=4,291), or Uncategorized diabetes (n=1,349). The Uncategorized diabetes group was made up of individuals reporting diabetes type as not known and those with missing data on diabetes type (19.1%), or those who were classified as having diabetes based on blood sugar at study entry (81.9%).
Perfluoroalkyl substances, including PFOA, were analyzed at a single commercial laboratory after serum was separated from participant blood samples and shipped on dry ice to the laboratory. The protein precipitation extraction method with reverse phase high-performance liquid chromatography/tandem mass spectrometry was utilized for PFAS assays. A triple quadropole mass spectrometer in pre-selected reaction monitoring mode, monitoring for the M/Z transitions of PFAS species with an internal 13C PFAS standard corresponding to the target compound was utilized for detection of each PFAS. The results from the assay were transferred to the Windows-based information system of the C8 Health Project. Of the 12 PFASs tested, 4 were detectable in the serum of over 90% of project participants. As such, these four compounds, PFHxSs (perfluorohexane sulfonate), PFOA (perfluoroctanoic acid), PFOS (perfluorooctane sulfonate), and PFNA (perfluorononaoic acid) were the focus of the current study.
There were 13,018 children and adolescents under the age of 20 years. Estimated glomerular filtration rate was calculated based on CKD-EPI formula [20] in adults and children age 13 years and older. In children aged less than 13 years, the Schwartz formula[21] was used. In analysis conducted for the population as a whole and in those persons aged 20 years and older (n=54,102), BMI was calculated as weight in kilograms divided by the height in meters squared (kg/m2). When analyses was restricted to 13,018 children and adolescents under the age of 20, a program obtained from the CDC website for growth chart training was used to calculate age- and sex- standardized BMI percentiles, based on the CDC year 2000 standard pediatric population.[22]
General linear models were used to test for differences in continuous variables in the four diabetes groups and the chi square test was used to test for differences in categorical data. Multinomial logistic regression was used to test the independent relationships of the four PFAS with each of the three diabetes groups compared with no diabetes as the reference group. Multinomial logistic regression is a way to simultaneously assess the relationship of an independent variable with an outcome that has several mutually exclusive categories of response. The base multivariable models included terms for diabetes group, age, sex, and race. The fully adjusted multivariable models additionally included BMI, and eGFR. Because of the hypothesized effect of the high oxygen carrying capacity of PFAS on beta cell generation and preservation, serum hemoglobin and serum iron were also controlled for. The criterion for statistical significance was a two-tailed P-value of < 0.05. Statistical analysis was conducted using SAS version 9.3 (Cary, North Carolina). The deviance scores good-ness-of-fit statistics were less than one for all models, indicating that our models fit the data adequately.
3. Results
Characteristics of the study participants by diabetes group are presented in Table 1. For the population as a whole, those with self-reported Type 1 or diabetes or Uncategorized diabetes were more likely to be male. Mean BMI was significantly higher in each of the diabetes groups compared to those without diabetes, with those with Type 2 diabetes having the highest BMI. eGFR was significantly lower in each of the diabetes groups compared to those without diabetes.
Table 1.
Characteristics by Diabetes Status
| Type 1 diabetes n=820 |
Type 2 diabetes n=4,291 |
Uncategorized* n=1,349 |
No diabetes n=60,439 |
|
|---|---|---|---|---|
| Age, years | 52.4 ± 17.4 | 58.4 ±13.4 | 51.5 ±15.3 | 38.2 ± 19.0 |
| Sex, female | 49.0 (402) | 50.1 (2,151) | 41.0 (553) | 52.2 (31,560) |
| Diabetes duration, years | 14.6 ±11.3 | 7.5 ±8.7 | 2.3 ±10.2 | ------------------ |
| PFHxS, ng/mL | 3.4 ±3.8 | 3.8 ±4.6 | 4.2 ±4.9 | 5.2 ±10.4 |
| PFOA, ng/mL | 68.4 ±176.3 | 92.8 ±400.7 | 86.5 ±177.2 | 82.3 ±227.2 |
| PFOS, ng/mL | 21.8 ±17.1 | 25.2 ±17.0 | 25.1 ±16.7 | 23.1 ±15.4 |
| PFNA, ng/mL | 1.4 (0.94) | 1.5 (0.82) | 1.5 (0.77) | 1.6 (0.88) |
| BMI, kg/m2 | 31.9 ±8.8 | 33.2 ±7.4 | 32.0 ±7.1 | 26.9 ±6.4 |
| eGFR, mL/min/1.73m2** | 78.3 ±28.9 | 77.6 ±21.8 | 85.6 ±20.8 | 95.5 ±24.5 |
| <60 mL/min/1.73m2 | 28.5 (225) | 21.7 (903) | 10.1 (134) | 5.7 (3,286) |
| 60–89 mL/min/1.73m2 | 34.9 (275) | 47.0 (1,959) | 47.7 (631) | 37.9 (22,059) |
| 90–119 mL/min/1.73m2 | 29.8 (235) | 29.9 (1,245) | 38.1 (503) | 40.2 (23,374) |
| ≥120 mL/min/1.73m2 | 6.8 (54) | 1.4 (58) | 4.1 (54) | 16.3 (9,470) |
| Hemoglobin, g/dL | 14.0 ±1.6 | 14.1 ±1.5 | 14.9 ±1.5 | 14.4 ±1.4 |
| Iron, µ/dL | 77.3 ±33.8 | 78.8 ±29.4 | 85.0 ±32.2 | 87.2 ±35.0 |
Data are presented as mean ± SD or % (n).
Diabetes type not reported, reported as not known, or based on blood glucose at study entry (n=1,105)
CKD-EPI formula in those age ≥13 years; Schwartz formula in those aged less than 13 years
Characteristics of adults aged twenty years and older (Appendix Table 1a) were similar to those of the population as whole, with the exception that individuals with Uncategorized diabetes were more likely to be male than those without diabetes and had a more similar distribution of non-White participants. Mean eGFR levels were lower in those with Type 1 or Type 2 diabetes and slightly lower in those in the Uncategorized diabetes group compared to those without diabetes.
In children and adolescents under the age of 20 years (Appendix Table 1b), those with diabetes tended to be older with mean age approximately 1 year older for those with Type 1 and Uncategorized diabetes, and approximately 2.5 years older for those with Type 2 diabetes compared to those without diabetes. Those with Type 1 or Uncategorized diabetes were more likely to be male, while those with Type 2 diabetes were more like to be female than those without diabetes. Children and adolescents with Type 1 diabetes had a mean BMI nearly identical to their non-diabetic peers, while those with Type 2 diabetes had a mean BMI approximately 10 kg/m2 higher than their non-diabetic peers. Mean BMI in those in the Uncategorized diabetes group was slightly higher compared to those without diabetes, but still within the normal weight range. Both fasting and non-fasting glucose were highest in children and adolescents with Type 1 diabetes, with those in the Uncategorized diabetes group having mean glucose levels intermediate that of those with Type 1 and Type 2 diabetes. Appendix Figures 1 and 2 show the age and sex adjusted mean PFAS levels by diabetes group in adults and children, respectively.
Figure 1 shows the age and sex-adjusted relationship of the PFASs with each of the three diabetes groups compared to those without diabetes. For each of the four PFAS investigated, higher serum levels were associated with a lower likelihood of diabetes, with the strongest association observed for Type 1 diabetes. While in general the association with Type 2 diabetes was similar to, although with a slightly more suggestive protective association than that of Uncategorized diabetes, there was a clear distinction in apparent risk associated with Type 1 diabetes. The exception was for PFHxS, where the association with Type 2 diabetes was intermediate that of Type 1 and Uncategorized diabetes, with no overlapping confidence intervals.
Figure 1.
Age and sex-adjusted association of perfluoroalkyl substances with diabetes, stratified by diabetes group. Panel a. ORs (95% CIs) for PFHxS with Type 1 diabetes, Type 2 diabetes, and Uncategorized diabetes, respectively: 0.59 (0.54–0.64), 0.74 (0.71–0.77), 0.84 (0.78–0.90). Panel b. ORs (95% CIs) for PFOA with Type 1 diabetes, Type 2 diabetes, and Uncategorized diabetes, respectively: 0.69 (0.65–0.74), 0.87 (0.89–0.91), 0.92 (0.88–0.97). Panel c. ORs (95% CIs) for PFOS with Type 1 diabetes, Type 2 diabetes, and Uncategorized diabetes, respectively: 0.65 (0.61–0.70), 0.86 (0.82–0.90), 0.93 (0.86–1.03). Panel d. ORs (95% CIs) for PFNA with Type 1 diabetes, Type 2 diabetes, and Uncategorized diabetes, respectively: 0.65 (0.57–0.74), 0.94 (0.88–1.00), 0.95 (0.85–1.06).
Table 2 shows the multivariable association of the two most commonly investigated PFASs, PFOA and PFOS, respectively, with the three diabetes types compared to no diabetes. Multivariable adjustment for race, BMI, eGFR, hemoglobin, and iron, in addition to age and sex, attenuated, but did not eliminate this association between PFOA and PFOS with Type 1 diabetes and Type 2 diabetes. The inverse association of PFOS with Uncategorized diabetes was reduced to marginal significance, with an upper confidence interval reaching 1. Similar results were observed for PFHxS and PFNA (data not depicted).
Table 2.
Multivariable Adjusted Association of PFOA with Diabetes, by Diabetes Type
| Type 1 diabetes | Type 2 diabetes | Uncategorized* | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| PFOA | |||
| PFOA, ng/mL** | 0.76 (0.71–0.80) | 0.94 (0.92–0.97) | 0.94 (0.90–0.99) |
| Age, years | 1.03 (1.03–1.04) | 1.07 (1.07–1.08) | 1.05 (1.04–1.05) |
| Sex, female | 0.48 (0.41–0.57) | 0.60 (0.56–0.65) | 0.80 (0.70–0.92) |
| BMI, kg/m2 | 1.10 (1.08–1.11) | 1.13 (1.13–1.14) | 1.10 (1.09–1.11) |
| eGFR, mL/min/1.73m2** | |||
| 90–119 | Ref | Ref | Ref |
| ≥120 | 1.21 (0.77–1.89) | 0.76 (0.55–1.07) | 1.39 (0.97–1.99) |
| 60–89 | 0.85 (0.70–1.03) | 0.69 (0.63–0.75) | 0.72 (0.63–0.82) |
| <60 | 2.40 (1.86–3.10) | 0.75 (0.66–0.85) | 0.63 (0.50–0.79) |
| Hemoglobin, g/dL | 0.77 (0.73–0.82) | 0.84 (0.81–0.87) | 1.27 (1.20–1.34) |
| Iron, µ/dL** | 0.82 (0.68–1.00) | 0.93 (0.84–1.02) | 0.82 (0.70–0.97) |
| PFOS | |||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| PFOS, ng/mL** | 0.73 (0.67–0.79) | 0.92 (0.88–0.96) | 0.95 (0.88–1.03) |
| Age, years | 1.03 (1.03–1.04) | 1.07 (1.07–1.08) | 1.05 (1.04–1.05) |
| Sex, female | 0.49 (0.41–0.57) | 0.60 (0.56–0.65) | 0.81 (0.70–0.93) |
| BMI, kg/m2 | 1.10 (1.09–1.11) | 1.13 (1.13–1.14) | 1.10 (1.09–1.11) |
| eGFR, mL/min/1.73m2** | |||
| 90–119 | Ref | Ref | Ref |
| ≥120 | 1.30 (0.94–1.81) | 0.69 (0.52–0.92) | 1.08 (0.79–147) |
| 60–89 | 0.84 (0.70–1.03) | 0.69 (0.63–0.75) | 0.72 (0.63–0.82) |
| <60 | 2.47 (1.92–3.17) | 0.75 (0.66–0.85) | 0.63 (0.50–0.79) |
| Hemoglobin g/dL | 0.79 (0.74–0.84) | 0.84 (0.82–0.87) | 1.26 (1.19–1.33) |
| Iron, µ/dL** | 0.85 (0.70–1.03) | 0.92 (0.84–1.02) | 0.80 (0.68–0.94) |
Diabetes type not reported, reported as not known, or based on blood glucose at study entry
Natural logarithmically transformed before analysis
When stratified by age 20, higher PFOA levels remained significantly associated with a lower risk of Type 1 diabetes in both children and adults. However for Type 2 and Uncategorized diabetes, the relationship with PFOA differed between children and adults. In those under the age of 20 years, PFOA demonstrated a non-significantly positive association with Type 2 and Uncategorized diabetes. In adults aged 20 years and older, this relationship was inverse. The association of PFOA with Type 1, Type 2, and Uncategorized diabetes stratified by age 20 is presented in Table 3.
Table 3.
Multivariable Adjusted Association of PFOA with Diabetes, by Age and Diabetes Type
| Type 1 diabetes | Type 2 diabetes | Uncategorized* | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Adults age ≥ 20 years | |||
| PFOA, ng/mL** | 0.74 (0.70–0.79) | 0.91 (0.89–0.94) | 0.92 (0.88–0.96) |
| Age, years | 1.03 (1.02–1.03) | 1.06 (1.06–1.06) | 1.05 (1.04–1.05) |
| Sex, female | 0.50 (0.43–0.60) | 0.66 (0.61–0.71) | 0.90 (0.78–1.03) |
| eGFR, mL/min/1.73m2** | |||
| 90–119 | Ref | Ref | Ref |
| ≥120 | 1.15 (0.73–1.79) | 0.70 (0.51–0.97) | 1.36 (0.95–1.94) |
| 60–89 | 0.87 (0.72–1.06) | 0.70 (0.64–0.76) | 0.71 (0.62–0.81) |
| <60 | 2.54 (1.97–3.29) | 0.79 (0.70–0.90) | 0.61 (0.48–0.77) |
| Hemoglobin, g/dL | 0.80 (0.76–0.85) | 0.87 (0.84–0.89) | 1.31 (1.25–1.38) |
| Iron, µ/dL** | 0.64 (0.53–0.77) | 0.69 (0.63–0.75) | 0.63 (0.54–0.74) |
| Children and adolescents age <20 years | |||
| PFOA, ng/mL** | 0.72 (0.54–0.97) | 1.13 (0.82–1.56) | 1.18 (0.90–1.55) |
| Age, years | 1.00 (0.91–1.10) | 1.27 (1.11–1.46) | 1.05 (0.95–1.16) |
| Sex, female | 1.01 (0.50–2.04) | 4.43 (1.53–12.81) | 0.33 (0.14–0.74) |
| eGFR, mL/min/1.73m2** | 4.65 (0.60–35.7) | 5.15 (0.35–77.8) | 0.28 (0.05–1.62) |
| Hemoglobin, g/dL | 1.54 (1.13–2.10) | 1.15 (0.79–1.69) | 0.89 (0.65–1.23) |
Diabetes type not reported, reported as not known, or based on blood glucose at study entry
Natural logarithmically transformed before analysis
Similar relationships were observed for PFOS, although the inverse association of PFOS with Type 1 diabetes appeared to be substantially stronger in children and adolescents than in adults. In children and adolescents, a one unit increase in the natural log of serum PFOS levels was associated with nearly half the likelihood of Type 1 diabetes (OR=0.52, 95% CI=0.37–0.73), while the same unit increase was associated with an approximately 25% lower likelihood in adults (OR=0.77, 95% CI=0.71–0.84, p-value for multiplicative interaction =0.09), data not depicted.
4. Discussion
Perfluoroalkyl substances are hydrogen carbon chains in which the hydrogen atoms have been replaced by fluorine. This results in a complex with a high ability to bind and transfer molecular oxygen, exceeding even that of hemoglobin. It has been hypothesized that oxygen induces the differentiation of endocrine cells,[23–25] and within endocrine cells beta cells and alpha cells,[24] in both adult and embryonic pancreata.[23, 25] Hyperoxia has been shown to upregulate this process,[25] while hypoxia has been shown to induce islet cell apoptosis. Our data resulting from a natural experiment in which humans were chronically exposed to PFOA contaminated drinking water suggests the hypothesis that enhanced oxygenation from PFAS may be protective against Type 1 diabetes, a condition manifest by beta cell deficiency.
PFAS, made up of the strongest single bonds found in organic chemistry,[2] are persistent environmental contaminants that are orally absorbed, but not metabolized, and stored in the kidney, liver, and blood serum, with half-lives of approximately four to eight years in humans depending on the PFAS.[1] We have shown that PFAS, specifically the long chain length perfluoroalkyl substances PFHxS, PFOA, PFOS, and PFNA are associated with a reduced odds of having diabetes, with the strongest association observed in both children and adults with Type 1 diabetes. These associations were independent of age, sex, and importantly, eGFR (a marker of clearance through the kidney) and hemoglobin and iron levels (markers of hypoxia). Adults with Type 1 diabetes had the lowest kidney function, hemoglobin and iron levels of all four diabetes groups while children and adolescents with Type 1 diabetes had the highest. Our results in adults, where the greatest inverse association was observed for Type 1 diabetes, followed by Type 2 diabetes, and the least association observed for those with Uncategorized diabetes suggests a gradient of beta cell presence, function, and/or viability with the degree of PFAS exposure. Our results showed a clear separation in the apparent risk associated with Type 1 diabetes compared to that associated with the other two diabetes risk groups. While there was overlap in the association of the PFAS with Type 2 and Uncategorized diabetes, the strong inverse association observed for Type 1 diabetes showed no overlap in apparent risk with the other diabetes groups. The null and non-significantly positive relationship of PFAS with Type 2 and Uncategorized diabetes, respectively, may be due to the greater heterogeneity in insulin resistance and beta cell deficiency in these populations. Early stages of Type 2 diabetes are characterized by insulin resistance, which is positively associated with PFAS,[17] and higher than normal insulin levels.
Few population studies have investigated the relationship of PFAS with diabetes, and the few that have generally have found a null to inverse relationship. Melzer et al found no significant relationship between PFOA or PFOS with self-reported diabetes in the NHANES population, though generally higher quartiles had a non-significantly lower risk compared to the first quartile of PFAS levels.[26] In a population of elderly adults, PFAS demonstrated no cross-sectional relationship with diabetes, with the exception of PFNA, which exhibited a curvilinear positive relationship.[27] In the C8 Health cohort, a previous cross-sectional study in adults has shown an apparent inverse relationship between the higher deciles compared to the first decile of PFOA and Type 2 diabetes,[28] consistent with the relationship observed in the current analyses of the same cohort. A prospective follow-up of this population found no association between PFOA and the incidence of Type 2 diabetes in adults;[15] however, the population for that follow-up study comprised only 56% of the original adult cohort. Our multivariable adjusted results are consistent with the weak to null relationship with Type 2 diabetes generally observed by others. However, to our knowledge, we are the first to investigate the relationship of PFAS with Type 1 diabetes in adults, or with diabetes of any type in children.
The strong inverse relationship we observed between PFAS and Type 1 diabetes is consistent with the inhibition of immune activation, reduction of insulitis, and reduced risk of Type 1 diabetes after hyperbaric oxygen therapy in the NOD mouse model of diabetes.[29] It is also consistent with the hypothesized effect of oxygen on pancreatic endocrine tissue differentiation.[23–25] In vitro studies have also shown that hyperoxia induces preferential differentiation of endocrine cells as opposed to exocrine cells, and increased differentiation of beta cells compared to alpha cell.[24] In vitro studies have also shown that pancreatic progenitor cells bathed in oxygen diffusion membranes incorporating perfluorocarbons enhance pancreas endocrine cell differentiation compared with such membranes not incorporating perfluorocarbons.[24] Our results showing a cross-sectionally inverse association of PFAS with Type 1 diabetes are also consistent with these in vitro studies of perfluorocarbons on enhanced endocrine cell, and specifically, beta cell differentiation.
Controlled experimental studies have shown beneficial effects of perfluorocarbons on extracted pancreatic tissue, perhaps due to enhanced oxygenation. In rat psuedoislets cultured with perfluoroctyl bromide, insulin levels and insulin stimulation index were preserved three days after stimulation with buffer containing glucose.[14] While insulin levels and insulin stimulation index were similar in controls and in cells cultured with perfluoroctyl bromide on day 1, levels on day 3 in control cells were reduced compared to day 1, but remained similar to day 1 in treated cells. Markers of hypoxia, hypoxia inducible factor-alpha and VEGF mRNA levels significantly increased after three days in the control cells but remained similar throughout the experiment in cells cultured with perfluroctyl bromide.[14] Ramachandran et al showed that in human donor pancreata, islet cells from pancreas preserved in perfluorocarbon solution demonstrated decreased expression of the proapoptotic genes bad, bax, caspases, TRAF5, and TNF-β/LTB compared to control cells after prolonged cold storage.[30] In contrast, there was an increased expression of the anti-apoptotic genes IAP2 and survivin in these islets isolated from pancreata stored in perfluorocarbon solution.[30] Improved islet yield has been observed from human pancreata preserved in perfluorocarbon solution.[31–33] Data from these studies indicate that preservation of the pancreas in perfluorocarbons reduces hypoxia and inhibits apoptosis of pancreatic islet cells, consistent with the lower likelihood of Type 1 diabetes in individuals with increased serum levels of PFAS observed in our study.
Experimental studies in murine models have shown the strongest antidiabetic relationship for the median chain length perfluorocarbons.[34, 35] In ob/ob mice, the perfluorocarbons C7 and C8 perfluoroacyl chains C8 (PFOA) and C9PFNA), respectively, demonstrated the greatest glucose lowering and insulin lowering after the 75 gram oral glucose tolerance test. With the exception of C6 (PFHxS), which was not investigated, longer and shorter length chain perfluorocarbons did not eliminate hyperglycemia in this mouse model.[34] Kees et at found the strongest glucose lowering effect for PFOA in the ob/ob mouse, but there was also the possible suggestion of liver toxicity associated with this perfluorocarbon.[35] In the db/db mouse model of Type 2 diabetes, perfluorocarbon treatment attenuated polydipsia; treatment with PFOA and PFNA completely eliminated it in this mouse model, providing further evidence of the greater anti-diabetic effect of perfluorocarbons of these chain lengths.[34]
As reported by others,[17] we found higher concentrations of PFAS in children and adolescents than in our adult population. This is thought to be due to the greater water consumption per kg body weight in children compared to adults, resulting in greater mean serum levels of PFAS. In addition to the higher levels of PFAS in children than in adults in our population, mean levels of PFOA in children without diabetes in the C8 Health Project population were approximately 10 times higher than those of children in the US general population.[36] Levels of PFOA in our population with Type 1 diabetes were still six to seven times higher than general population norms.[36] Levels in our children with non-Type 1 diabetes were approximately 15 times higher than general population norms[36] and it is possible that there may have been some hepatoxicity leading to impaired glucose homeostasis, particularly in those in the Uncategorized diabetes group made up largely of those classified as having diabetes based on blood glucose at study entry.
The strengths of the study include the population-based design, the large sample size, and the high study participation rates (>80% of affected population for this study) in an Appalachian region.[16] Additional strengths include our ability to evaluate persistent biomarkers of PFAS exposure obtained concurrently with survey information regarding diagnosis of diabetes and other conditions, and the measurement of a wide array of biomarkers, including serum hemoglobin, iron, and creatinine. Finally, our study included a large number of children and adolescents under the age of 20 years, which allowed for stratified analyses of the adult and pediatric populations.
Our study has a number of limitations as well. Most important, the cross-sectional nature of the data preclude determination of temporal and causal relationships. Thus, reverse causality remains a possibility. PFAS are excreted via the kidney and thus an increased filtration rate will result in lower serum concentrations of PFAS. Contrary to expectations of an increased prevalence of hyperfiltration in Type 1 diabetes, but consistent with our recent data on the association of PFAS with eGFR in this population,[37] the prevalence of hyperfiltration was highest in those without diabetes, i.e. twice that of those with Type 1 diabetes, the group with the next highest prevalence of hyperfiltration. However, adjustment for stage of eGFR did not alter the inverse association of PFAS with diabetes, suggesting that diabetes-associated abnormalities in renal clearance did not explain the observed relationships. The relationship of the PFAS with the diabetes groups in children, where reverse causality due to decreased renal clearance resulting from kidney damage is even less likely to be operant, further suggests a gradient in the relationship between PFAS exposure and beta cell presence, function, and/or viability, as evidenced by the clear linear trend of serum PFAS concentration across the diabetes groups, particularly for PFHxS and PFOS (Appendix Figure 2). In addition, experimental studies in animal models and in human prancreata have shown PFAS to improve beta cell function, possibly through enhanced oxygenation, suggesting an anti-diabetic effect of PFAS is plausible. For the primary analyses, ascertainment of diabetes was based on participant-reported physician diagnosis, which may have introduced misclassification bias. However, agreement between self-report and medical record-verified data for diabetes in this study population was good (over 80%)[16]. This is further supported by the divergent relationship of hemoglobin concentration with diabetes type, where very poor blood glucose control in those with undiagnosed diabetes likely leads to hemoconcentration and thus the positive association between hemoglobin concentration and odds of having undiagnosed diabetes. Data on HbA1c was not available in this population and thus we could not assess whether differences in glycemic control among the subgroups with diabetes partially accounted for the differential relationship of PFAS with diabetes between the groups. Finally, participants were drawn from an Appalachian population exposed to PFOA drinking water contamination; generalizability to other populations may thus be limited. However, our findings are broadly consistent with those of other large, population-based studies. Furthermore, while mean levels of PFOA were approximately five-fold higher than that observed in the general population, mean serum levels of PFOS were similar to that observed in the general population yet the same strong inverse association with Type 1 diabetes was observed.
In conclusion, we found that higher serum concentrations of PFAS were associated with a lower frequency of diabetes, particularly Type 1 diabetes. This lower frequency of Type 1 diabetes was observed in both children and adults. Although our cross-sectional data limit the evaluation of causal relationships, the consistency of our findings with experimental studies is consistent with a biologically plausible causal relationship of our findings. Any true protective relationship of PFAS with diabetes, particularly Type 1 diabetes, may reflect enhanced oxygenation of pancreatic tissue due to higher serum PFAS concentrations circulating through pancreatic blood vessels. Further study is indicated due to the adverse associations of PFAS with some health outcomes and the lack of longitudinal data on the relationship of PFAS with Type 1 diabetes.
Supplementary Material
Appendix Figure 1. Mean Age and Sex Adjusted Levels of Perfluoroalkyl Substances by Diabetes Group in Adults.
Appendix Figure 2. Mean Age and Sex Adjusted Levels of Perfluoroalkyl Substances by Diabetes Group in Children.
Acknowledgments
Baqiyyah Conway conceived the study, analyzed the data, and wrote the manuscript. Karen Innes conceived the study and contributed to the discussion. Dustin Long contributed to the data analyses and critically reviewed the manuscript for scientific content. Baqiyyah Conway is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported in part by the National Institutes of Health grant U54GM1049 to the West Virginia University CTSI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References
- 1.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicological sciences : an official journal of the Society of Toxicology. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 2.Krafft MP, Riess JG. Perfluorocarbons: Life Sciences and Biomedical Uses. J Polymer Science: Part A: Polymer Chemistry. 2007;45:1185–1198. [Google Scholar]
- 3.Kennedy GL, Butenhoff JL, Olsen GW, et al. The toxicology of perfluorooctonaoate. Crit Rev Toxicol. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- 4.Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environmental health perspectives. 2013;121:1313–1318. doi: 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein CR, Savitz DA, Elston B, Thorpe PG, Gilboa SM. Perfluorooctanoate exposure and major birth defects. Reproductive toxicology (Elmsford, NY) 2014;47:15–20. doi: 10.1016/j.reprotox.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosgood SA, Nicholson ML. The role of perfluorocarbon in organ preservation. Transplantation. 2010;89:1169–1175. doi: 10.1097/TP.0b013e3181da6064. [DOI] [PubMed] [Google Scholar]
- 7.Hancock JB, Davidson S, Guinn C, Zachary R. Using liquid ventilation to improve lung function in patients with respiratory distress syndrome: a comprehensive review of the literature. AANA journal. 2004;72:218–224. [PubMed] [Google Scholar]
- 8.Spahn DR. Blood Substitutes: Artificial oxygen carriers: Perfluorocarbon emulsions. Critical Care. 1999;3:R93–R97. doi: 10.1186/cc364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riess JG. Perfluorocarbon-based oxygen delivery. Artificial cells, blood substitutes, and immobilization biotechnology. 2006;34:567–580. doi: 10.1080/10731190600973824. [DOI] [PubMed] [Google Scholar]
- 10.Reznik ON, Bagnenko SF, Loginov IV, Iljina VA, Ananyev AN, Moysyuk YG. The use of oxygenated perfluorocarbonic emulsion for initial in situ kidney perfusion. Transplantation proceedings. 2008;40:1027–1028. doi: 10.1016/j.transproceed.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 11.Atias S, Mizrahi SS, Shaco-Levy R, Yussim A. Preservation of pancreatic tissue morphology, viability and energy metabolism during extended cold storage in two-layer oxygenated University of Wisconsin/perfluorocarbon solution. The Israel Medical Association journal : IMAJ. 2008;10:273–276. [PubMed] [Google Scholar]
- 12.Squifflet JP, LeDinh H, de Roover A, Meurisse M. Pancreas Preservation for Pancreas and Islet Transplantation: A Minireview. 2011:3398–3401. doi: 10.1016/j.transproceed.2011.09.052. [DOI] [PubMed] [Google Scholar]
- 13.Maillard E, Sanchez-Dominguez M, Kleiss C, et al. Perfluorocarbons: new tool for islets preservation in vitro. Transplantation proceedings. 2008;40:372–374. doi: 10.1016/j.transproceed.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Maillard E, Juszczak MT, Langlois A, et al. Perfluorocarbon emulsions prevent hypoxia of pancreatic beta-cells. Cell transplantation. 2012;21:657–669. doi: 10.3727/096368911X593136. [DOI] [PubMed] [Google Scholar]
- 15.Karnes C, Winquist A, Steenland K. Incidence of type II diabetes in a cohort with substantial exposure to perfluorooctanoic acid. Environmental research. 2014;128:78–83. doi: 10.1016/j.envres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 16.MacNeil J, Steenland NK, Shankar A, Ducatman A. A cross-sectional analysis of type II diabetes in a community with exposure to perfluorooctanoic acid (PFOA) Environmental research. 2009;109:997–1003. doi: 10.1016/j.envres.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Lin CY, Chen PC, Lin YC, Lin LY. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care. 2009;32:702–707. doi: 10.2337/dc08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisbee SJ, Brooks AP, Jr, Maher A, et al. The C8 health project: design, methods, and participants. Environmental health perspectives. 2009;117:1873–1882. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steenland K, Tinker S, Shankar A, Ducatman A. Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with Uric Acid among Adults with Elevated Community Exposure to PFOA. Environ Health Perspect. 2010;118:229–233. doi: 10.1289/ehp.0900940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 22.CDC. A SAS Program for the CDC Growth Charts. 2011 Available from http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm2014.
- 23.Fraker CA, Ricordi C, Inverardi L, Dominguez-Bendala J. Oxygen: a master regulator of pancreatic development? Biology of the cell/under the auspices of the European Cell Biology Organization. 2009;101:431–440. doi: 10.1042/BC20080178. [DOI] [PubMed] [Google Scholar]
- 24.Fraker CA, Alvarez S, Papadopoulos P, et al. Enhanced oxygenation promotes beta-cell differentiation in vitro. Stem cells (Dayton, Ohio) 2007;25:3155–3164. doi: 10.1634/stemcells.2007-0445. [DOI] [PubMed] [Google Scholar]
- 25.Cechin S, Alvarez-Cubela S, Giraldo JA, et al. Influence of in vitro and in vivo oxygen modulation on beta cell differentiation from human embryonic stem cells. Stem cells translational medicine. 2014;3:277–289. doi: 10.5966/sctm.2013-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environmental health perspectives. 2010;118:686–692. doi: 10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lind L, Zethelius B, Salihovic S, van Bavel B, Lind PM. Circulating levels of perfluoroalkyl substances and prevalent diabetes in the elderly. Diabetologia. 2014;57:473–479. doi: 10.1007/s00125-013-3126-3. [DOI] [PubMed] [Google Scholar]
- 28.MacNeil J, Steenland NK, Shankar A, Ducatman A. A cross-sectional analysis of type II diabetes in a community with exposure to perfluorooctanoic acid (PFOA) Environmental research. 2009;109:997–1003. doi: 10.1016/j.envres.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Faleo G, Fotino C, Bocca N, et al. Prevention of autoimmune diabetes and induction of beta-cell proliferation in NOD mice by hyperbaric oxygen therapy. Diabetes. 2012;61:1769–1778. doi: 10.2337/db11-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramachandran S, Desai NM, Goers TA, et al. Improved islet yields from pancreas preserved in perflurocarbon is via inhibition of apoptosis mediated by mitochondrial pathway. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:1696–1703. doi: 10.1111/j.1600-6143.2006.01368.x. [DOI] [PubMed] [Google Scholar]
- 31.Ricordi C, Fraker C, Szust J, et al. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation. 2003;75:1524–1527. doi: 10.1097/01.TP.0000058813.95063.7A. [DOI] [PubMed] [Google Scholar]
- 32.Hering BJ, Matsumoto I, Sawada T, et al. Impact of two-layer pancreas preservation on islet isolation and transplantation. Transplantation. 2002;74:1813–1816. doi: 10.1097/00007890-200212270-00033. [DOI] [PubMed] [Google Scholar]
- 33.Tsujimura T, Kuroda Y, Kin T, et al. Human islet transplantation from pancreases with prolonged cold ischemia using additional preservation by the two-layer (UW solution/perfluorochemical) cold-storage method. Transplantation. 2002;74:1687–1691. doi: 10.1097/00007890-200212270-00007. [DOI] [PubMed] [Google Scholar]
- 34.Kees KL, Cheeseman RS, Prozialeck DH, Steiner KE. Perfluoro-N-[4-(1H -tetrazol-5-ylmethyl)phenyl]-alkanamides. A New Class of Oral Antidiabetic Agents. J Med Chem. 1989;32:11–13. doi: 10.1021/jm00121a003. [DOI] [PubMed] [Google Scholar]
- 35.Kees KL, Smith TM, McCaleb ML, et al. Perfluorocarbon-based antidiabetic agents. J Med Chem. 1992;35:944–953. doi: 10.1021/jm00083a021. [DOI] [PubMed] [Google Scholar]
- 36.Kato K, Calafat AM, Wong LY, Wanigatunga AA, Caudill SP, Needham LL. Polyfluoroalkyl compounds in pooled sera from children participating in the National Health and Nutrition Examination Survey 2001–2002. Environmental science & technology. 2009;43:2641–2647. doi: 10.1021/es803156p. [DOI] [PubMed] [Google Scholar]
- 37.Conway B, Costacou T, Innes K, Arthur J. Environmental Contaminant Perfluorooctane Sulfonate and Kidney Function by Diabetes Status. Diabetes. 2015;64:LB6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 1. Mean Age and Sex Adjusted Levels of Perfluoroalkyl Substances by Diabetes Group in Adults.
Appendix Figure 2. Mean Age and Sex Adjusted Levels of Perfluoroalkyl Substances by Diabetes Group in Children.