Abstract
Epigenetic reprogramming in Arabidopsis thaliana occurs in developing pollen. The male gametophyte is derived from haploid microspores via two post-meiotic cell divisions to give rise to the gametes (sperm cells, SC) and the vegetative cell (VC). The purification of individual cell types during pollen development coupled with genome-wide DNA methylation analysis and small RNA sequencing has revealed a dynamic regulation of the epigenome during gametogenesis. Interestingly, imprinted loci and previously identified variable epialleles are hypermethylated in the germline; however, their stability after fertilization appears to require targeted demethylation in the neighboring vegetative cell nucleus, possibly by releasing mobile small RNAs that reinforce transcriptional gene silencing and DNA methylation in the gametes. These results have led to a new model for the establishment and transgenerational maintenance of epigenetic marks in flowering plants.
Gametogenesis is the process in sexually reproducing organisms wherein diploid precursor cells undergo meiosis to differentiate into mature haploid gametes. During this process, allelic diversity in the diploid genome is shuffled to create a unique haploid genome in each gamete, which potentiates genetic and epigenetic variation in the germline and in following generations. While meiotic segregation occurs in both plants and animals, an additional source of variation in plants comes with additional rounds of cell division in post-meiotic gametophytes (Boavida et al. 2005) (Fig. 1). In A. thaliana pollen, these mitotic events initiate two distinct but neighboring cell lineages, one vegetative and another generative, that activate different genetic and epigenetic processes (Berger and Twell 2011) as regulatory chromatin modifications are reset or maintained. The companion vegetative cell does not contribute genetic material to the progeny directly in the form of genomic DNA; however, several recent studies indicate the VC as a source of epigenetic information that can be ultimately transmitted through the germline before fertilization (Slotkin et al. 2009; Calarco et al. 2012; Ibarra et al. 2012). The vegetative cell cycle arrests after the first pollen mitosis, and pericentromeric heterochromatin is lost leading to up-regulation of transposons and the accumulation of 21nt small interfering RNAs (siRNAs) (Slotkin et al. 2009). Surprisingly, these epigenetically activated small RNAs accumulate at higher levels in the neighboring sperm cells, where transposons are transcriptionally silenced (Slotkin et al. 2009).
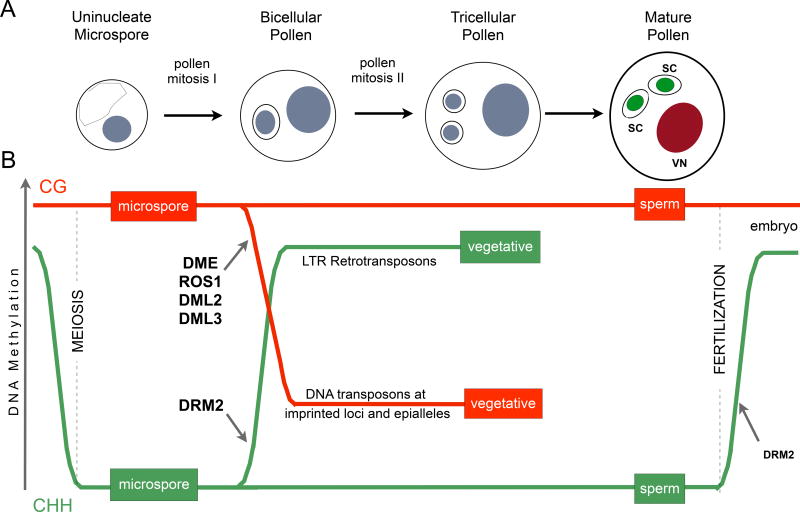
Figure 1. DNA methylation dynamics during male gametophyte development in Arabidopsis thaliana.
The uninucleate microspore divides asymmetrically giving rise to bicellular pollen, which consists of a larger vegetative cell embedding a smaller generative cell. A second mitotic division of the generative cell originates two sperm cells, that will be delivered to the embryo sac to perform double fertilization. SC - Sperm Cell, VN - Vegetative Nucleus. The postmeiotic microspore and sperm cells retain CG methylation. However, CHH methylation is lost from retrotransposons in microspores and sperm cells and restored both in the vegetative nucleus and in the embryo after fertilization, when the activity of the de novo DNA methyltransferase DRM2 is restored. In the vegetative nucleus, CG methylation is lost from targets of the DNA glycosylases DME, ROS1, DML2 and DML3, which include imprinted loci and recurrent epialleles that accumulate corresponding small RNA in sperm. Genome reprogramming in pollen might thus contribute to epigenetic inheritance, transposon silencing, and genomic imprinting. Reproduced (with permission) from Calarco et al., 2012.
Transposable elements (TEs) are epigenetically silenced by repressive chromatin marks such as DNA methylation and histone tail modifications (Slotkin and Martienssen 2007), which in animals are extensively reprogrammed in primordial germ cells and during early embryo development (Feng et al. 2010). DNA methylation in plants is more widespread, and occurs in three different sequence contexts (CG, CHG and CHH, where H=A, C or T), all of which can be established de novo by a mechanism known as RNA-directed DNA methylation (RdDM), guided by small RNAs. CG methylation can be maintained during replication by the DNA METHYLTRANSFERASE 1 (MET1, orthologous to mammalian Dnmt1), while CHG maintenance requires the activity of CHROMOMETHYLASE 3 (CMT3) which recognizes H3K9me2 in a self-reinforcing loop mechanism (Du et al. 2012). In contrast, CHH methylation is asymmetric and must be re-established de novo after each cell division, directed via small interfering RNAs (siRNAs) and dependent on the activity of DNA methyltransferase DOMAINS REARRANGED METHYLTRANSFERASE 2 (DRM2, ortholog to mammalian Dnmt3) (Law and Jacobsen 2010). Although CHH methylation is highly correlated with small RNA accumulation (Lister et al. 2008), a recent study suggested that the histone methyltransferases KRYPTONITE (KYP/SUVH4), SUVH5/6 are also able to regulate CHH methylation independent of, or in parallel with siRNAs (Stroud et al. 2013).
The advantage of employing covalent chromatin modifications to regulate gene expression is their reversibility, and in Arabidopsis many of the pathways that mediate this process are beginning to be well understood. Cytosine methylation, for example, can be actively removed by the DNA glycosylases DEMETER (DME), REPRESSOR OF SILENCING 1 (ROS1) AND DEMETER-LIKE 2 (DML2) AND 3 (DML3) by a base excision repair mechanism (Law and Jacobsen 2010). Loss-of-function mutations in the Arabidopsis DNA glycosylases revealed ectopic gains of DNA methylation at hundreds of sites throughout the genome (Stroud et al. 2013; Hsieh et al. 2009; Gehring et al. 2009; Lister et al. 2008), however, it is still not clear what targets DNA demethylation, or how it interacts with pathways that establish or maintain DNA methylation.
Dynamics of DNA methylation throughout pollen development
Much of our current understanding of epigenetic reprogramming of TEs in the plant germline comes from studies in pollen, where it has recently become possible to isolate the two differentiated cell types, as well as the progenitor microspore, by flow cytometry (Borges et al. 2008; 2012; Schoft et al. 2009). Strikingly, most CHH methylation is lost in the haploid microspore following meiosis and re-established only in the vegetative cell, while the resulting sperm cells remain largely hypomethylated at CHH (Fig. 1) (Calarco et al. 2012; Ibarra et al. 2012). In contrast, CG methylation is maintained throughout plant development and in the differentiating germline (Fig. 1). The absence of CHH methylation in the germline is particularly striking in pericentromeric regions where LTR retrotransposons are more abundant, and suggests that re-establishment of most CHH methylation in the paternal genome might occur only after fertilization, guided by maternal small interfering RNAs (siRNAs) (Calarco et al. 2012). However, CHH methylation remains in the microspore and sperm cells at particular TEs neighboring imprinted genes, particularly those that are maternally expressed in the endosperm (Calarco et al. 2012). A parallel study showed that at least some of this CHH methylation in sperm cells depends on the activity of DME at imprinted loci, as DME is expressed exclusively in the vegetative cell of mature pollen, and dme/+ sperm cells have reduced levels of CHH in comparison with the wild-type (Ibarra et al. 2012). These observations indicate that DNA demethylation in the vegetative nucleus is somehow important to reinforce CHH methylation in sperm, but the mechanism responsible remains unknown.
Transgenerational Inheritance of DNA Methylation
In mammals, chromatin modifications established during development are almost entirely reset in the germline (Feng et al. 2010), allowing the zygote to become pluripotent before it initiates embryonic development. Hence, DNA methylation marks are reset once during sperm maturation, and again during early embryo development (Popp et al. 2010; Feng et al. 2010). In Arabidopsis embryos, as in sperm cells, the levels of CG and CHG methylation seem to be stable during early development (Jullien et al. 2012), suggesting that symmetric methylation is broadly maintained throughout the Arabidopsis life cycle. These results are in agreement with the stable expression of the DNA methyltransferases MET1 and CMT3 in all the tissues and cell types analyzed (Jullien et al. 2012). However, the striking decrease of CHH methylation in sperm is reversed in the developing embryo (Calarco et al. 2012). The de novo DNA methyltransferases DRM1 and 2 are expressed in the egg cell and through embryo development (Jullien et al. 2012), correlating with a progressive remethylation of loci that were CHH hypomethylated in sperm. Seemingly then, CHH methylation is the only type of DNA modification that shows a striking fluctuation between germ cells and embryo. As a result, the production and transmission of siRNAs, whose importance in directing RdDM is well established, becomes an attractive potential mechanism for reprogramming.
Analysis of Pol IV-dependent transcription profiles, the RNA polymerase with a specific function in the RdDM pathway, would be helpful in understanding the spatial and temporal control of siRNA production in the gametophytes and embryo/endosperm. Furthermore, a combined analysis of siRNAs present in the sperm, egg cells and developing seed would be important in understanding the mechanism of re-methylation and parental contributions. A previous study reported a very low abundance of Pol IV-derived 24nt siRNAs in the embryo, in contrast to the endosperm and seed coat, where much higher levels of siRNAs were detected (Mosher et al. 2009). In the same study, reciprocal crosses with different ecotypes and single nucleotide polymorphism (SNP) analyses showed that 24nt siRNAs in the endosperm are mostly maternal in origin. Thus hypomethylated CHH of pericentromeric retrotranpsosons in sperm would need to be restored by maternal siRNA after fertilization - this would become interesting in outcrosses with related species.
The low levels of siRNA in the embryo indicate that maternal siRNAs might move from the neighboring endosperm/seed coat into the embryo, a possibility that has been tested experimentally (Ibarra et al. 2012). Nonetheless, we found that in mature sperm cells 24nt siRNAs match to a few hundred CHH hypomethylated regions (Calarco et al. 2012), suggesting that paternal siRNAs might play a role in methylation after fertilization. It is possible that these siRNAs were so low in abundance by comparison with the maternal tissues of the seed coat, that they were not detected in whole seed extracts (Mosher et al. 2009). Further studies of isolated embryos and endosperm are required to clarify the parental contributions of small RNAs, but it seems that DNA methylation dynamics in the gametophytes and a differential distribution of parental siRNAs might dictate the extent of epigenetic reprogramming that occurs during seed development.
Targeted Demethylation and Transposon Expression in the Vegetative Nucleus and Endosperm: A Role in Imprinting?
Genomic imprinting occurs in both plants and animals, which entails restricting the expression of a particular locus to one parental allele (Feil and Berger 2007). In mammals, it relies heavily on Dnmt3a and Dnmt3b which perform de novo deposition of DNA methylation in a sex-specific manner (Bartolomei 2009; Ferguson-Smith 2011). Once established, the allele specific patterns of DNA methylation of each parental allele are preserved in the developing embryo by the maintenance DNA methyltransferase Dnmt1. In addition, monoallelic expression can also be regulated via targeting with Polycomb Group (PcG) proteins that establish and recognize the chromatin mark histone H3K27me3 (Feil and Berger 2007).
While the role of de novo DNA methylation with regards to genomic imprinting is well established in mammals, its function in plants has been less clear. Endogenous repression of MET1 (dnmt1) in the central cell and endosperm, together with active DNA demethylation by DEMETER (DME) (Gehring et al. 2006), partially accounts for the activation of maternal alleles, suggesting that the paternal allele is repressed by DNA methylation (Jullien and Berger 2010). DNA methylation profiles in purified sperm cells correlate well with this idea, as the paternal copy of maternally expressed imprinted loci were shown to be hypermethylated in the CG context (Calarco et al. 2012; Ibarra et al. 2012). However, alternative pathways might exist, as predicted for the regulation of the maternally expressed gene MEDEA (MEA), whose maternal activation also depends on DME/MET1, but whose paternal silencing is apparently governed by polycomb (Wöhrmann et al. 2012).
DME is also expressed in the pollen vegetative cell resulting in DNA demethylation at a few hundred loci (Calarco et al. 2012; Schoft et al. 2011) and potentially the movement of small RNAs into the sperm cells (see above). Most of the recently discovered imprinted loci (Gehring et al. 2011; Wolff et al. 2011; Hsieh et al. 2011; McKeown et al. 2011) are surrounded by short repetitive sequences and/or DNA transposons that are transcriptionally silenced by siRNAs, suggesting that RdDM might also regulate genomic imprinting (T.Vu and F.Berger, unpublished). Thus RdDM is associated with silencing of imprinted genes with flanking transposons, while imprinted loci without TEs might be primarily controlled by other mechanisms such as Polycomb. However, it is also possible that both mechanisms are intrinsically related: in met1 mutants, genome-wide loss of CG methylation leads to the increase of H3K27me3 (Deleris et al. 2012). In contrast, PcG targeted regions in wild-type tend to lose H3K27me3 in met1 mutants, which is often replaced by H3K9me2 and DNA hypermethylation (Deleris et al. 2012).
Is not clear though how CG loss results in PcG recruitment, but one possibility is that H3K27me3 might represent a transient repressive histone mark that is somehow able to exclude the RdDM machinery from some PcG targets. This interplay between DNA methylation and the PcG complex has been also reported in the Arabidopsis endosperm, as H3K27me3 was detected at TEs that are actively targeted for DNA demethylation by DME (Weinhofer et al. 2010). The implications of these mechanisms in the regulation of genomic imprinting in the endosperm and in pollen are still poorly understood, but one idea is that monoallelic expression in the endosperm might be the outcome of gene expression programs in the developing gametophytes.
Targeted Demethylation and Transposon Expression in the Vegetative Nucleus and Endosperm: A Role in Epiallele Formation?
Even broader implications can be drawn from the important finding of DNA methylation re-establishment in the embryo with regards to the formation of ‘epialleles’. Two recent studies provided additional genome-wide DNA methylation profiling data over multiple generations from single seed descend in Arabidopsis, and uncovered a number of variable epialleles (Becker et al. 2011; Schmitz et al. 2011). The origins of this variation are still unclear, but it was proposed that the gains and losses in DNA methylation might be pre-programmed in the germline (Fig. 2) (Becker et al. 2011; Schmitz et al. 2011; Calarco et al. 2012). Thus, the reduction and re-establishment of CHH methylation during gamete development and early embryogenesis, respectively, could represent a pathway for the erasure or establishment of repressive marks on specific epialleles.
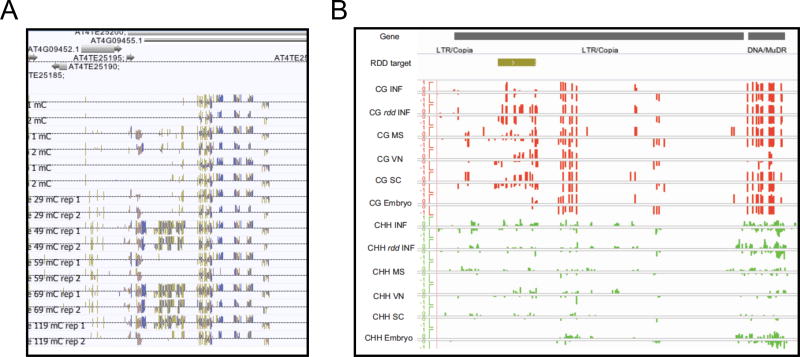
Figure 2. Epilalelle formation in the germline.
(A) A representative Differentially Methylated Region (DMR) in which both biological replicates of descendant lines gain DNA methylation. Adapted from Schmitz et al. (2011) (B) The AtCopia51 retrotransposon is a target of ROS1 and its homologs DML2 and DML3 (RDD). Tracks represent the RDD target region, and methylation levels in CG and CHH contexts in microspores (MS), vegetative nucleus (VN), and sperm cells (SC), along with inflorescence (INF) and embryo. CG methylation at the RDD target site is found in rdd triple mutant inflorescence (rdd INF) and in pollen but not in inflorescence or embryo. Adapted from Calarco et al. (2012).
Interestingly, a majority of these epialleles are targeted by DME and ROS1 in the vegetative nucleus, suggesting that DNA demethylation could represent the source of epigenetic variation (Schmitz and Ecker 2012; Calarco et al. 2012). Expression of at least some of these genes is essential for pollen tube growth, as well as response to external factors such as biotic or abiotic stress (Dowen et al. 2012). These same RdDM targeted loci were recently found among the most stably methylated loci in several Arabidopsis accessions (Schmitz et al. 2013). Thus targeted demethylation in pollen (Calarco et al. 2012; Ibarra et al. 2012) and restoration of methylation in the developing embryo (Jullien et al. 2012) might indeed reinforce RdDM in the germ cells and after fertilization.
Concluding Remarks
The faithful transmission of epigenetic information through cell division is essential to the cell’s ability to regulate gene expression, to control transposable elements and to regulate small RNA biogenesis and function. Though mutations that eliminate these mechanisms entirely have dire consequences, it will be interesting to see if subtle modulations in these pathways have biological effects in the next generation, suggesting a reversible mechanism for epigenetic inheritance. Thus the misregulation of epigenetic modifications in the germline might affect multiple subsequent generations. Active DNA demethylation and TE expression in the developing endosperm have been intensively studied for their importance in regulating parent-of-origin expression in plants. However, the fact that the same mechanisms are found in the pollen vegetative cell, suggests broader implications for epiallele formation as well as imprinting (Martínez and Slotkin 2012).
Acknowledgments
JPC was supported by a graduate student fellowship from NSERC and by a grant from the Fred C. Gloeckner Foundation. RM is a Howard Hughes Medical Institute and Gordon and Betty Moore Foundation Investigator in plant biology. This work was supported by NIH grant R01 GM067014 to RM.
References
- Bartolomei MS. Genomic imprinting: employing and avoiding epigenetic processes. Genes & Development. 2009;23:2124–2133. doi: 10.1101/gad.1841409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Hagmann J, Müller J, Koenig D, Stegle O, Borgwardt K, Weigel D. Spontaneous epigenetic variation in the Arabidopsis thaliana methylome. Nature. 2011:1–7. doi: 10.1038/nature10555. [DOI] [PubMed] [Google Scholar]
- Berger F, Twell D. Germline Specification and Function in Plants. Annu Rev Plant Biol. 2011;62:461–484. doi: 10.1146/annurev-arplant-042110-103824. [DOI] [PubMed] [Google Scholar]
- Boavida LC, Becker JD, Feijó JA. The making of gametes in higher plants. Int J Dev Biol. 2005;49:595–614. doi: 10.1387/ijdb.052019lb. [DOI] [PubMed] [Google Scholar]
- Borges F, Gardner R, Lopes T, Calarco JP, Boavida LC, Slotkin RK, Martienssen RA, Becker JD. FACS-based purification of Arabidopsis microspores, sperm cells and vegetative nuclei. Plant Methods. 2012;8:44. doi: 10.1186/1746-4811-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges F, Gomes G, Gardner R, Moreno N, McCormick S, Feijo JA, Becker JD. Comparative Transcriptomics of Arabidopsis Sperm Cells. Plant Physiol. 2008;148:1168–1181. doi: 10.1104/pp.108.125229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarco JP, Borges F, Donoghue MTA, Van Ex F, Jullien PE, Lopes T, Gardner R, Berger F, Feijó JA, Becker JD, et al. Reprogramming of DNA Methylation in Pollen Guides Epigenetic Inheritance via Small RNA. Cell. 2012 doi: 10.1016/j.cell.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleris A, Stroud H, Bernatavichute Y, Johnson E, Klein G, Schubert D, Jacobsen SE. Loss of the DNA methyltransferase MET1 Induces H3K9 hypermethylation at PcG target genes and redistribution of H3K27 trimethylation to transposons in Arabidopsis thaliana. PLoS Genet. 2012;8:e1003062. doi: 10.1371/journal.pgen.1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowen RH, Pelizzola M, Schmitz RJ, Lister R, Dowen JM, Nery JR, Dixon JE, Ecker JR. Widespread dynamic DNA methylation in response to biotic stress. Proceedings of the National Academy of Sciences. 2012;109:E2183–91. doi: 10.1073/pnas.1209329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zhong X, Bernatavichute YV, Stroud H, Feng S, Caro E, Vashisht AA, Terragni J, Chin HG, Tu A, et al. Dual Binding of Chromomethylase Domains to H3K9me2-Containing Nucleosomes Directs DNA Methylation in Plants. Cell. 2012;151:167–180. doi: 10.1016/j.cell.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Berger F. Convergent evolution of genomic imprinting in plants and mammals. Trends Genet. 2007;23:192–199. doi: 10.1016/j.tig.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Feng S, Jacobsen SE, Reik W. Epigenetic Reprogramming in Plant and Animal Development. Science. 2010;330:622–627. doi: 10.1126/science.1190614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Smith AC. Genomic imprinting: the emergence of an epigenetic paradigm. Nature Reviews Genetics. 2011;12:565–575. doi: 10.1038/nrg3032. [DOI] [PubMed] [Google Scholar]
- Gehring M, Bubb KL, Henikoff S. Extensive Demethylation of Repetitive Elements During Seed Development Underlies Gene Imprinting. Science. 2009;324:1447–1451. doi: 10.1126/science.1171609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M, Huh JH, Hsieh T-F, Penterman J, Choi Y, Harada JJ, Goldberg RB, Fischer RL. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell. 2006;124:495–506. doi: 10.1016/j.cell.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M, Missirian V, Henikoff S. Genomic analysis of parent-of-origin allelic expression in Arabidopsis thaliana seeds. PLoS ONE. 2011;6:e23687. doi: 10.1371/journal.pone.0023687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T-F, Shin J, Uzawa R, Silva P, Cohen S, Bauer MJ, Hashimoto M, Kirkbride RC, Harada JJ, Zilberman D, et al. Regulation of imprinted gene expression in Arabidopsis endosperm. Proceedings of the National Academy of Sciences. 2011;108:1755–1762. doi: 10.1073/pnas.1019273108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TF, Ibarra CA, Silva P, Zemach A, Eshed Williams L, Fischer RL, Zilberman D. Genome-Wide Demethylation of Arabidopsis Endosperm. Science. 2009;324:1451–1454. doi: 10.1126/science.1172417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra CA, Feng X, Schoft VK, Hsieh T-F, Uzawa R, Rodrigues JA, Zemach A, Chumak N, Machlicova A, Nishimura T, et al. Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science. 2012;337:1360–1364. doi: 10.1126/science.1224839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien PE, Berger F. DNA methylation reprogramming during plant sexual reproduction? Trends in Genetics. 2010;26:394–399. doi: 10.1016/j.tig.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Jullien PE, Susaki D, Yelagandula R, Higashiyama T, Berger F. DNA methylation dynamics during sexual reproduction in Arabidopsis thaliana. Curr Biol. 2012;22:1825–1830. doi: 10.1016/j.cub.2012.07.061. [DOI] [PubMed] [Google Scholar]
- Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nature Reviews Genetics. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, O’Malley RC, Tonti-Filippini J, Gregory BD, Berry CC, Millar AH, Ecker JR. Highly Integrated Single-Base Resolution Maps of the Epigenome in Arabidopsis. Cell. 2008;133:523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez G, Slotkin RK. Developmental relaxation of transposable element silencing in plants: functional or byproduct? Current Opinion in Plant Biology. 2012;15:496–502. doi: 10.1016/j.pbi.2012.09.001. [DOI] [PubMed] [Google Scholar]
- McKeown PC, Laouielle-Duprat S, Prins P, Wolff P, Schmid MW, Donoghue MTA, Fort A, Duszynska D, Comte A, Lao NT, et al. Identification of imprinted genes subject to parent-of-origin specific expression in Arabidopsis thaliana seeds. BMC Plant Biology. 2011;11:113. doi: 10.1186/1471-2229-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher RA, Melnyk CW, Kelly KA, Dunn RM, Studholme DJ, Baulcombe DC. Uniparental expression of PolIV-dependent siRNAs in developing endosperm of Arabidopsis. Nature. 2009:1–5. doi: 10.1038/nature08084. [DOI] [PubMed] [Google Scholar]
- Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M, Jacobsen SE, Reik W. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature. 2010;463:1101–1105. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz RJ, Ecker JR. Epigenetic and epigenomic variation in Arabidopsis thaliana. Trends in Plant Science. 2012;17:149–154. doi: 10.1016/j.tplants.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz RJ, Schultz MD, Lewsey MG, O'Malley RC, Urich MA, Libiger O, Schork NJ, Ecker JR. Transgenerational Epigenetic Instability Is a Source of Novel Methylation Variants. Science. 2011;334:369–373. doi: 10.1126/science.1212959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz RJ, Schultz MD, Urich MA, Nery JR, Pelizzola M, Libiger O, Alix A, McCosh RB, Chen H, Schork NJ, et al. Patterns of population epigenomic diversity. Nature. 2013:1–8. doi: 10.1038/nature11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoft VK, Chumak N, Choi Y, Hannon M, Garcia-Aguilar M, Machlicova A, Slusarz L, Mosiolek M, Park J-S, Park GT, et al. Function of the DEMETER DNA glycosylase in the Arabidopsis thaliana male gametophyte. Proceedings of the National Academy of Sciences. 2011;108:8042–8047. doi: 10.1073/pnas.1105117108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoft VK, Chumak N, Mosiolek M, Slusarz L, Komnenovic V, Brownfield L, Twell D, Kakutani T, Tamaru H. Induction of RNA-directed DNA methylation upon decondensation of constitutive heterochromatin. EMBO Rep. 2009;10:1015–1021. doi: 10.1038/embor.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nature Reviews Genetics. 2007;8:272–285. doi: 10.1038/nrg2072. [DOI] [PubMed] [Google Scholar]
- Slotkin RK, Vaughn M, Borges F, Tanurdzić M, Becker JD, Feijó JA, Martienssen RA. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell. 2009;136:461–472. doi: 10.1016/j.cell.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud H, Greenberg MVC, Feng S, Bernatavichute YV, Jacobsen SE. Comprehensive Analysis of Silencing Mutants Reveals Complex Regulation of the Arabidopsis Methylome. Cell. 2013;152:352–364. doi: 10.1016/j.cell.2012.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhofer I, Hehenberger E, Roszak P, Hennig L, Köhler C. H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation. In: Kakutani T, editor. PLoS Genet. Vol. 6. 2010. p. e1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff P, Weinhofer I, Seguin J, Roszak P, Beisel C, Donoghue MTA, Spillane C, Nordborg M, Rehmsmeier M, Köhler C. High-Resolution Analysis of Parent-of-Origin Allelic Expression in the Arabidopsis Endosperm. In: Kakutani T, editor. PLoS Genet. Vol. 7. 2011. p. e1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhrmann HJP, Gagliardini V, Raissig MT, Wehrle W, Arand J, Schmidt A, Tierling S, Page DR, Schöb H, Walter J, et al. Identification of a DNA methylation-independent imprinting control region at the Arabidopsis MEDEA locus. Genes & Development. 2012;26:1837–1850. doi: 10.1101/gad.195123.112. [DOI] [PMC free article] [PubMed] [Google Scholar]