Abstract
We present a large-scale molecular phylogeny that includes 320 of the 761 recognized valid species of the cone snails (Conus), one of the most diverse groups of marine molluscs, based on three mitochondrial genes (COI, 16S rDNA and 12S rDNA). This is the first phylogeny of the taxon to employ concatenated sequences of several genes, and it includes more than twice as many species as the last published molecular phylogeny of the entire group nearly a decade ago. Most of the numerous molecular phylogenies published during the last 15 years are limited to rather small fractions of its species diversity. Bayesian and maximum likelihood analyses are mostly congruent and confirm the presence of three previously reported highly divergent lineages among cone snails, and one identified here using molecular data. About 85 % of the species cluster in the single Large Major Clade; the others are divided between the Small Major Clade (∼ 12%), the Conus californicus lineage (one species), and a newly defined clade (∼ 3%). We also define several subclades within the Large and Small major clades, but most of their relationships remain poorly supported. To illustrate the usefulness of molecular phylogenies in addressing specific evolutionary questions, we analyse the evolution of the diet, the biogeography and the toxins of cone snails. All cone snails whose feeding biology is known inject venom into large prey animals and swallow them whole. Predation on polychaete worms is inferred as the ancestral state, and diet shifts to molluscs and fishes occurred rarely.The ancestor of cone snails probably originated from the Indo-Pacific; rather few colonisations of other biogeographic provinces have probably occurred. A new classification of the Conidae, based on the molecular phylogeny, is published in an accompanying paper.
Keywords: Ancestral state reconstruction, Conidae, Conus, COI, 16SrRNA, 12SrRNA
Graphical abstract
1. Introduction
A molecular phylogeny of a taxon is a hypothesis of its evolutionary patterns and processes, and a framework for clarifying its classification. A strongly supported molecular-based phylogenetic tree can help determine diversification rates, divergence times, ancestral distributions, and community compositions, and it can provide evidence relevant to taxonomic hypotheses. However, many taxa of considerable evolutionary and practical importance have very incomplete species-level molecular phylogenies, based on few species with appropriate genes sequenced, not representative of the diversity of the group, or largely unresolved. The gastropod family Conidae, commonly known as cone snails, includes the widely distributed, mainly tropical genus Conus, a relatively young genus (appearance in Early Eocene) generally considered to be the most diverse of marine animals (Kohn, 1990), with 761 valid Recent species currently (21th January 2014) recognized in the World Register of Marine Species (WoRMS, 2013) and new species usually being described each year. It is also the most rapidly diversifying marine molluscan genus (Kohn, 1990; Stanley, 1975) and is ecologically important especially in coral reef environments where up to 36 species, specialized predators on worms, other molluscs, and fishes, co-occur on a single reef (Kohn, 2001). These latter attributes all likely relate to their extremely diverse peptide venoms that are used to capture prey and that also make the Conidae a most promising source for neurobiologic and therapeutic applications (Biass et al., 2009; Lluisma et al., 2012; Olivera, 2006). Molecular geneticists, evolutionary biologists, pharmacologists, and toxicologists thus all require a robust phylogeny and taxonomy for this group. New drug discovery is particularly likely to benefit from a clear phylogenetic context that permits targeting divergent lineages and thus potential novel toxins (Biggs et al., 2010; Olivera, 2006).
Since the first published molecular phylogenies for Conus (Duda and Palumbi, 1999a; Monje et al., 1999), many others have appeared, either for the cone snails and their relatives (Puillandre et al., 2011a, 2008), or subgroups (Bandyopadhyay et al., 2008; Biggs et al., 2010; Cunha et al., 2008, 2005; Duda and Kohn, 2005; Duda and Palumbi, 2004, 1999b; Duda and Rolan, 2005; Duda et al., 2008, 2001; Espino et al., 2008; Espiritu et al., 2001; Kauferstein et al., 2011, 2004; Kraus et al., 2012, 2011; Nam et al., 2009; Pereira et al., 2010; Puillandre et al., 2010; Williams and Duda, 2008). The most comprehensive includes 138 species, ca. 20% of the known diversity of cone snails (Duda and Kohn, 2005). Ancestral states of morphological, ecological, and developmental traits have been inferred from some of these (Cunha et al., 2005; Duda and Palumbi, 2004, 1999a; Duda et al., 2001; Kohn, 2012) and lineages of toxins with unknown functions identified (Puillandre et al., 2010). However, these authors generally agree that available phylogenies are not complete enough to robustly test hypotheses about how natural history attributes relate to factors that could explain the evolutionary history of the cone snails.
Cone snails experienced several episodes of enhanced diversification since their origination (Duda and Kohn, 2005; Kohn, 1990; Williams and Duda, 2008) and exhibit the highest rate of diversification of any marine gastropod or bivalve group (Stanley, 1979), a remarkable radiation that was likely driven by ecological speciation (Stanley, 2008). Currently they occur mostly throughout tropical regions of our world's oceans, although the overwhelming majority of species, both fossil and recent ones, are restricted to single marine biogeographic provinces (e.g., Indo-Pacific, East Pacific, West Atlantic, East Atlantic and South Africa) (Duda and Kohn, 2005). Results from previous molecular phylogenetic analysis suggest that three major lineages arose shortly after the origination of the group: one with extant species mostly occurring in the present-day Indo-Pacific, another with most extant species found in the present-day East Pacific and West Atlantic, and a third that today consists of a single species that is restricted to the East Pacific (Duda and Kohn, 2005). Based on the geographic distributions of species in these clades, there has apparently been very little interchange of lineages among the major marine biogeographic provinces (Duda and Kohn, 2005; Duda and Lessios, 2009). Nonetheless, this work included analyses of sequence data from only one-fifth of the recognized cone snail species and the authors caution that their results are preliminary and the patterns that they observed may change with more complete taxonomic coverage (Duda and Kohn, 2005). Here we examine the biogeography of this group with a much more exhaustive taxonomic and geographic coverage than available previously.
While most cone snail species are vermivorous (i.e., feed on a variety of worms, including mostly polychaetes but also hemichordates), others are either piscivorous or molluscivorous, with few species exhibiting more than one feeding mode. In addition, diets tend to be species-specific, especially in areas where multiple species co-occur (Kohn and Nybakken, 1975; Kohn, 1968, 1959). A previous investigation of the evolution of diets of cone snails reports that major shifts in diet were relatively rare (Duda et al., 2001), although piscivory originated at least twice (Duda and Palumbi, 2004). However, as with all past molecular phylogenetic studies of this group, these studies relied on limited taxonomic coverage. Analyses of a much larger dataset may provide additional insights of the evolution of diet that were not available previously.
We propose here a molecular phylogeny of the Conidae sensu Bouchet et al. (2011), based on three mitochondrial genes (COI, 12S, 16S) sequenced for 329 species (>40% of the known species diversity), and including representatives from the main lineages defined in previous DNA studies: C. californicus, the Small Major Clade and the Large Major Clade (Duda and Kohn, 2005). Tucker and Tenorio (2009) classified the Small Major Clade as the Family Conilithidae – it included C. californicus – and the Large Major Clade as the family Conidae (see Table 1 for a comparison of the recent classifications of cone snails and related species). We then analyse the evolution of three character sets: diet category, biogeographic province and toxin diversity. Previous molecular phylogenetic studies analysed the main evolutionary diet shifts (from worms to fishes or molluscs) (Duda and Kohn, 2005; Duda and Palumbi, 2004; Duda et al., 2001), but never on such a large dataset. Disentangling the evolution of these traits throughout this hyperdiverse taxon should help to generate and critically examine hypotheses of the factors that promoted its exceptional ecological and evolutionary diversification.
Table 1.
Comparison of recent classifications of cone snails and related species. In this article, the cone snails are restricted to the Conidae sensus Bouchet et al. (2011).
| Taylor et al., 1993 | Bouchet and Rocroi, 2005 | Duda and Kohn, 2005 | Tucker and Tenorio, 2009 | (Bouchet et al., 2011) | New classification | ||
|---|---|---|---|---|---|---|---|
| Coninae | Coninae | Large Major Clade | Taranteconidae | Conidae | Conidae | Conus | |
| Conidae | Coninae | ||||||
| Puncticulinae | |||||||
| Conilithidae | Californiconinae | Californiconus | |||||
| Small Major Clade | Conilithinae | Conasprella | |||||
| Profundiconus | |||||||
| Hemiconidae† | |||||||
| Conorbinae | Conorbinae | Conorbidae | Conorbis† | Conorbidae | |||
| Artemidiconus | |||||||
| Benthofascis | |||||||
| Cryptoconidae | Cryptoconus† | ||||||
| Clathurellinae | Genota | Borsoniidae | |||||
| Genotina | Mangeliidae | ||||||
fossil taxa.
2. Material and Methods
2.1. Sampling
The analysed dataset is the result of a joint effort from several museums and laboratories. The Museum National d'Histoire Naturelle (MNHN), Paris provided 493 specimens collected during several recent expeditions in the Indo-Pacific (details are provided in the appendix A); 88 specimens were collected during the CONCO project in New Caledonia and South Africa, and processed in the University of Frankfurt; 319 specimens were collected and processed by CPM, TFD and BMO or their lab groups. Additionally, sequences from 1207 vouchers were downloaded from GenBank and added to the datasets. Specimens were morphologically identified by the authors and by Eric Monnier, Loïc Limpalaër and Manuel Tenorio; for the GenBank sequences, we followed the identifications provided by the respective authors.
Nine vouchers from GenBank were only identified at the genus level (as “Conus sp.”). For various reasons, the voucher specimens were not available for all the non-GenBank specimens, but in some cases digital images of shells were available (unpublished data) for confirmation of identifications. In most cases, the morphological identification was double- or triple-checked by several taxonomic specialists of the group. We followed the cone snail taxonomy provided in the World Register of Marine Species (WoRMS, version of 14th May 2013) in applying species names to the vouchers: only species names considered as valid in WoRMS were applied. All other species-level names that could have been attributed to the specimens were considered as subspecies, form or variety names, or as synonyms. In total, the 2107 specimens were attributed to 320 species names, representing >40% of the total number of cone snail species considered as valid in WoRMS (Table 2). Additionally, we recognize nine morphospecies as potentially corresponding to undescribed species (numbered from a to i). In total, 1740 COI, 928 16S and 599 12S sequences were analyzed, of which 1523 are newly published (Appendix A).
Table 2.
List of 345 species analysed, with 19 species not included in the concatenated dataset because only one gene over three was available (grey lines) and 16 outgroups (at the end of the list). Voucher numbers in the first column refer to the appendix A. Type of prey (M = Mollusc, F = Fish, W = Worm, S = Shrimps), geographic province (EA = East Atlantic; EP = East Pacific; IP = Indo-Pacific; SA = South Africa; WA = West Atlantic) and GenBank accession numbers for the three genes are indicated.
| Voucher | Species | Prey | Geography | COI | 16S | 12S |
|---|---|---|---|---|---|---|
| 00002 | abbreviatus | W | IP | AY588148.1 | KJ550551 | KJ550957 |
| 00003 | achatinus | F | KJ549854 | KJ550552 | KJ550958 | |
| 00006 | acutangulus | W* | IP | KJ549855 | KJ550553 | KJ550959 |
|
| ||||||
| alconnelli | ||||||
|
| ||||||
| 01605 | alisi | IP | KJ550120 | KJ550790 | KJ551198 | |
| 01607 | ammiralis | M | IP | KJ550122 | KJ550791 | KJ551132 |
| 00010 | amphiurgus | W | WA | KJ549856 | KJ550554 | KJ551049 |
| 00011 | anabathrum | W | WA | KJ549857 | KJ550555 | KJ550999 |
| 02348 | andamanensis | IP | KJ550549 | KJ550950 | KJ551230 | |
| 01619 | andremenezi | IP | KJ550125 | KJ550794 | KJ551205 | |
| 00013 | anemone | W | IP | AY588149.1 | AF174141.1 | KJ551346 |
| 00014 | antoniomonteiroi | EA | AY588150.1 | KJ550557 | ||
| 00015 | aphrodite | W* | IP | JF496229.1 | JF496218.1 | JF496207.1 |
|
| ||||||
| araneosus | ||||||
|
| ||||||
| 00017 | arangoi | WA | KJ549859 | KJ550558 | KJ550955 | |
| 00018 | archon | W* | EP | KJ549860 | KJ550559 | KJ550965 |
| 00019 | arcuatus | W* | EP | KJ549861 | KJ550560 | KJ551327 |
| 00043 | ardisiaceus | IP | KJ549873 | KJ551304 | ||
| 00021 | arenatus | W | IP | KJ549863 | KJ550562 | KJ551317 |
| 01635 | aristophanes | W | IP | KJ550129 | KJ550796 | KJ551115 |
| 00023 | articulata | IP | JF496231.1 | JF496220.1 | JF496209.1 | |
| 00024 | ateralbus | W* | EA | AY588154.1 | AY381998.1 | |
| 01636 | augur | W* | IP | KJ550797 | KJ551214 | |
| 00026 | aulicus | M* | IP | KJ549864 | KJ550564 | KJ551283 |
| 00027 | aureus | M | IP | AY588155.1 | AF174145.1 | |
| 00028 | auricomus | M* | IP | KJ549865 | KJ550565 | KJ551297 |
| 00029 | aurisiacus | IP | GU134371.1 & FJ868111.1 | EU078943.1 | EU682276.1 | |
| 01637 | australis | W* | IP | KJ550130 | KJ550798 | KJ551089 |
| 01641 | baileyi | W* | IP | KJ550133 | KJ550801 | KJ551155 |
| 01646 | balteatus | W | IP | KJ550134 | KJ550802 | KJ551082 |
| 01648 | bandanus | M | IP | KJ550136 | KJ550803 | KJ551073 |
| 00034 | barthelemyi | F* | IP | AY588158.1 | AY382000.1 | KJ551352 |
| 00035 | bartschi | W* | EP | AY588159.1 | AY382001.1 | KJ551362 |
| 00036 | bengalensis | M* | IP | KJ550568 | KJ550967 | |
| 00037 | betulinus | W | IP | KJ549869 | KJ550569 | KJ550968 |
| 00038 | biliosus | W | IP | KJ549870 | KJ550570 | KJ551273 |
| 00040 | blanfordianus | IP | KJ549871 | KJ550571 | KJ550969 | |
| 00490 | boavistensis | EA | AY726442.1 | AY726442.1 | ||
| 01652 | boeticus | W | IP | KJ550139 | KJ550804 | KJ551104 |
| 01656 | boholensis | IP | KJ550142 | KJ550805 | KJ551196 | |
| 00493 | borgesi | W* | EA | NC013243.1 | NC013243.1 | NC013243.1 |
| 01667 | boucheti | IP | KJ550150 | KJ550806 | KJ551199 | |
| 00046 | brunneus | W | EP | KJ549875 | AF174149.1 | KJ550971 |
| 01673 | bruuni | IP | KJ550155 | KJ550807 | KJ551192 | |
| 00047 | bullatus | F* | IP | KJ549876 | KJ550574 | KJ551261 |
|
| ||||||
| buxeus | ||||||
|
| ||||||
| 01678 | byssinus | W* | EA | KJ550808 | KJ551206 | |
| 00048 | cacao | W* | EA | KJ549877 | KJ550575 | KJ551358 |
| 00504 | calhetae | EA | AY726474.1 | AY726474.1 | ||
| 00049 | californicus | W+M+F+S | EP | KJ549878 | KJ550576 | KJ550992 |
| 00052 | cancellatus | W | WA | KJ549879 | KJ550579 | KJ550993 |
| 00053 | canonicus | M | IP | KJ549880 | KJ550580 | KJ551298 |
| 01686 | capitanellus | W* | IP | KJ550163 | KJ550811 | KJ551194 |
| 00055 | capitaneus | W | IP | KJ549882 | KJ550582 | KJ551275 |
| 00056 | caracteristicus | W* | IP | KJ549883 | KJ550583 | KJ551306 |
| 01691 | catus | F | IP | KJ550165 | KJ550812 | KJ551108 |
| 00059 | cedonulli | W | WA | KJ549885 | KJ550586 | KJ551017 |
| 00062 | cervus | IP | KJ549886 | KJ550587 | KJ550996 | |
| 01698 | chaldaeus | W | IP | KJ550170 | KJ550813 | KJ551179 |
| 01700 | chiangi | W* | IP | KJ550172 | KJ550814 | KJ551130 |
|
| ||||||
| cinereus | ||||||
| circumactus | ||||||
|
| ||||||
| 01701 | circumcisus | F | IP | EU015749 | KJ550815 | KJ551060 |
| 01709 | coelinae | W* | IP | KJ550176 | KJ550816 | KJ551066 |
| 01711 | coffeae | W | IP | KJ550178 | KJ550817 | KJ551095 |
| 01713 | comatosa | W* | IP | GU131299 | GU131286 | GU131274 |
|
| ||||||
| compressus | ||||||
|
| ||||||
| 01714 | consors | F | IP | EU015751 | HQ401672 | HQ401605 |
| 00072 | corallinus | W* | IP | KJ549891 | KJ550593 | KJ551003 |
| 01718 | coriolisi | IP | GU131298 | KJ550818 | KJ551072 | |
| 01740 | coronatus | W | IP | KJ550193 | KJ550819 | KJ551172 |
| 00074 | crocatus | M* | IP | EU733512.1 | EU682300.1 | EU682280.1 |
| 00595 | crotchii | SA | AY726445.1 | AY726445.1 | ||
| 00075 | cuneolus | W* | EA | AY588166.1 | AY382003.1 | |
|
| ||||||
| 00076 | curassaviensis | W | WA | KJ549893 | KJ550595 | KJ550963 |
| 01753 | cuvieri | IP | KJ550203 | KJ550820 | KJ551213 | |
|
| ||||||
| cylindraceus | ||||||
|
| ||||||
| 00078 | dalli | M | EP | EU733513.1 | EU078935.1 | EU682281.1 |
| 00080 | damottai | EA | AY588168.1 | KJ550596 | ||
|
| ||||||
| 00083 | daucus | W | WA | AY588169.1 | AY382005.1 | KJ551364 |
| 01754 | dayriti | IP | KJ550821 | KJ551156 | ||
| 00604 | decoratus | EA | AY726449.1 | AY726449.1 | ||
| 00085 | delanoyae | W* | EA | AY588170.1 | KJ550600 | KJ551335 |
| 00086 | delessertii | W* | WA | KJ549896 | KJ550601 | KJ551334 |
| 00087 | derrubado | EA | AY588171.1 | KJ550602 | ||
|
| ||||||
| 00088 | diadema | W | EP | AY588172.1 | AY382006.1 | KJ551353 |
| 00089 | diminutus | EA | AY588173.1 | KJ550603 | ||
|
| ||||||
| 01757 | distans | W | IP | KJ550205 | KJ550822 | KJ551120 |
| 02347 | dorotheae | EA | KJ550548 | KJ550949 | KJ551229 | |
| 00091 | dorreensis | W | IP | KJ549898 | AF174163.1 | KJ551354 |
| 00092 | dusaveli | IP | KJ549899 | KJ550605 | KJ551018 | |
| 01765 | ebraeus | W | IP | KJ550208 | KJ550823 | KJ551174 |
| 01776 | eburneus | W | IP | KJ550217 | KJ550825 | KJ551096 |
| 01805 | elokismenos | IP | GU131318 | GU131294 | GU131282 | |
| 00096 | emaciatus | W | IP | KJ549903 | AF174166.1 | KJ551032 |
| 01810 | episcopatus | M | IP | KJ550234 | KJ550829 | KJ551069 |
| 00098 | ermineus | F | EA,WA | KJ549905 | KJ550610 | KJ551033 |
| 01820 | eucoronata (aff.) | IP | KJ550241 | KJ550834 | KJ551147 | |
| 02306 | eugrammata | W* | IP | EU015734 | EU685782 | EU685489 |
| 00099 | evorai | W* | EA | AY588177.1 | KJ550611 | |
|
| ||||||
| 01825 | excelsus | IP | KJ550243 | KJ550836 | KJ551189 | |
| 00101 | eximius | W* | IP | KJ549907 | KJ550613 | KJ551035 |
| 00102 | fantasmalis | EA | AY588178.1 | KJ550614 | ||
|
| ||||||
| 00780 | felitae | W* | EA | AY726456.1 | AY726456.1 | |
| 00103 | fergusoni | W* | EP | AY588179.1 | AY382007.1 | KJ551339 |
| 01841 | ferrugineus | W* | IP | KJ550258 | KJ550839 | KJ551128 |
| 00105 | figulinus | W | IP | KJ549908 | AF160702.1 | KJ550977 |
| 00106 | flavescens | W | WA | AY588236.1 | AY382034.1 | KJ551349 |
| 00108 | flavidus | W | IP | KJ549909 | KJ550617 | KJ551294 |
| 00109 | flavus | IP | KJ549910 | EU794326.1 | EU794315.1 | |
| 01843 | floccatus | IP | KJ550259 | KJ550840 | KJ551085 | |
| 01844 | floridulus | W* | IP | KJ550260 | KJ550841 | KJ551081 |
| 00111 | fontonae | W* | EA | AY588181.1 | KJ550619 | |
|
| ||||||
| franciscoi | ||||||
|
| ||||||
| 01849 | frigidus | W | IP | KJ550264 | KJ550842 | KJ551103 |
| 01853 | fumigatus | IP | KJ550268 | KJ550843 | KJ551212 | |
| 01854 | furvus | M | IP | KJ550269 | KJ550844 | KJ551125 |
| 00116 | fuscoflavus | EA | AY588182.1 | KJ550623 | ||
| 00117 | gauguini | IP | FJ868117.1 | EU078944.1 | FJ868047.1 | |
| 01864 | generalis | W | IP | KJ550273 | KJ550847 | KJ551094 |
| 00796 | genuanus | W* | EA | AY726459.1 | AY726459.1 | |
| 00120 | geographus | F | IP | FJ868152.1 | FJ868141.1 | EU794316.1 = FJ868126.1 |
| 00123 | gladiator | W | EP | AY588185.1 | KJ550625 | KJ551356 |
| 00124 | glans | W | IP | KJ549918 | KJ550626 | KJ551333 |
| 01871 | gloriamaris | M* | IP | KJ550275 | KJ550848 | KJ551068 |
| 01875 | gondwanensis | W* | IP | KJ550278 | KJ550849 | KJ551162 |
| 00127 | gradatus | EP | KJ549921 | KJ550629 | KJ550964 | |
| 00814 | grahami | EA | AY726460.1 | AY726460.1 | ||
| 00128 | grangeri | IP | KJ550630 | KJ550978 | ||
| 00129 | granum | IP | KJ549922 | KJ550631 | KJ551041 | |
| 00816 | guanche | W* | EA | AY726461.1 | AY726461.1 | |
| 01879 | gubernator | F* | IP | KJ550281 | KJ550850 | KJ551178 |
| 02346 | guidopoppei | IP | KJ550547 | KJ550948 | KJ551228 | |
|
| ||||||
| guinaicus (aff.) | ||||||
|
| ||||||
| 01882 | hamamotoi | IP | KJ550283 | KJ550851 | KJ551157 | |
| 00130 | hieroglyphus | W* | WA | KJ549923 | KJ550632 | KJ551042 |
| 01883 | hirasei | IP | KJ550284 | KJ550852 | KJ551201 | |
| 01884 | hopwoodi | W* | IP | KJ550285 | KJ550853 | KJ551110 |
|
| ||||||
| hybridus | ||||||
|
| ||||||
| 01909 | ichinoseana | IP | KJ550307 | KJ550855 | KJ551087 | |
| 00132 | immelmani | M*? | SA | EU781489.1 | EU781488.1 | |
| 01914 | imperialis | W | IP | KJ550308 | KJ550857 | KJ551067 |
| 00136 | infinitus | EA | AY588187.1 | KJ550635 | ||
| 00137 | infrenatus | W* | SA | KJ549925 | KJ550636 | KJ551043 |
| 00138 | inscriptus | W* | IP | KJ549926 | AY382010.1 | KJ551045 |
| 01923 | ione | IP | KJ550312 | KJ550859 | KJ551190 | |
| 00140 | irregularis | EA | AY588188.1 | KJ550637 | ||
| 00141 | jacarusoi | WA | KJ549927 | KJ550638 | KJ550956 | |
| 01942 | janus | IP | KJ550325 | KJ551164 | ||
| 00144 | jaspideus | W | WA | KJ549930 | KJ550641 | KJ551046 |
| 01926 | joliveti | IP | GU131313 | GU131290 | GU131278 | |
| 00147 | josephinae | EA | AY588190.1 | KJ550643 | ||
| 00148 | jucundus | WA | KJ549932 | KJ550644 | KJ550953 | |
| 01769 | judaeus | W | IP | KJ550211 | KJ550824 | KJ551184 |
| 00362 | kanakinus | IP | KJ550052 | KJ550771 | ||
| 01934 | kimioi | W* | IP | KJ550320 | KJ550860 | KJ551161 |
| 00150 | kinoshitai | W* | IP | FJ937341.1 | FJ937345.1 | FJ937337.1 |
| 00151 | kintoki | W* | IP | KJ549934 | EU794328.1 | EU794317.1 |
| 00152 | klemae | W | IP | KJ549935 | KJ550645 | KJ551323 |
| 00020 | koukae | IP | KJ549862 | KJ550561 | KJ551279 | |
| 01938 | laterculatus | W* | IP | KJ550323 | KJ550861 | KJ551137 |
| 00154 | legatus | M | IP | KJ549936 | KJ550646 | KJ551289 |
| 01941 | lenavati | W* | IP | KJ550324 | KJ550862 | KJ551124 |
| 00156 | leopardus | W | IP | KJ549937 | KJ550648 | KJ551328 |
| 01957 | lischkeanus | W | IP | KJ550332 | KJ550865 | KJ551163 |
| 01960 | litoglyphus | W | IP | KJ550334 | KJ550866 | KJ551061 |
| 01965 | litteratus | W | IP | KJ550338 | KJ550867 | KJ551101 |
| 00161 | lividus | W | IP | HQ852591 | AF174178.1 | KJ551365 |
| 01986 | locumtenens | M* | IP | KJ550868 | KJ551210 | |
| 00867 | lohri | GQ424495.1 | GQ424508.1 | |||
| 00163 | longilineus | EA | AY588193.1 | KJ550654 | ||
| 01987 | longurionis | W* | IP | KJ550351 | KJ550869 | KJ551165 |
| 01995 | lozeti | IP | KJ550358 | KJ550871 | ||
| 02002 | luciae | IP | KJ550364 | KJ550872 | KJ551193 | |
|
| ||||||
| lucidus | ||||||
|
| ||||||
| 00874 | lugubris | EA | AY726467.1 | AY726467.1 | ||
| 00167 | luquei | EA | AY588195.1 | KJ550657 | ||
| 00168 | luteus | IP | KJ549942 | KJ550658 | KJ551305 | |
| 00169 | lynceus | IP | KJ549943 | KJ550659 | KJ550980 | |
| 01829 | madecassina | IP | KJ550246 | KJ550837 | KJ551171 | |
| 00172 | magnificus | M* | IP | AY588197.1 | AY382013.1 | KJ550995 |
| 00173 | magus | F | IP | KJ549945 | KJ550662 | KJ551258 |
| 00174 | mahogani | EP | AY588198.1 | KJ550663 | KJ551366 | |
| 00176 | maioensis | EA | AY588199.1 | KJ550664 | ||
| 00179 | mappa | W | WA | KJ549949 | KJ550666 | KJ551052 |
| 00180 | marmoreus | M | IP | KJ549950 | KJ550667 | KJ551320 |
| 00181 | mazei | W | WA | KJ550668 | KJ551053 | |
| 02008 | medoci | IP | KJ550370 | KJ551177 | ||
| 00182 | melvilli | W* | IP | KJ549951 | KJ550669 | KJ551330 |
| 00183 | memiae | W* | IP | FJ868154.1 = JF496236.1 | FJ868143.1 = JF496225.1 | FJ868128.1 = JF496214.1 |
| 00184 | mercator | W* | IP | AY588200.1 | KJ550670 | KJ551360 |
| 00185 | messiasi | EA | AY588201.1 | KJ550671 | ||
| 02025 | miles | W | IP | KJ550379 | KJ550876 | KJ551055 |
| 02033 | miliaris | W | IP | KJ550380 | KJ550878 | KJ551182 |
| 00191 | mindanus | W* | WA | KJ549956 | KJ550676 | KJ551054 |
| 02037 | miniexcelsus | IP | KJ550384 | KJ550879 | KJ551153 | |
| 00192 | miruchae | EA | AY588204.1 | KJ550677 | ||
| 00193 | mitratus | IP | KJ549957 | KJ550678 | KJ551014 | |
| 00194 | moluccensis | W* | IP | KJ549958 | KJ550679 | KJ550981 |
| 00195 | monile | W* | IP | KJ549959 | KJ550680 | KJ551267 |
| 00196 | mordeirae | W* | EA | AY588205.1 | KJ550681 | |
| 00198 | moreleti | W | IP | KJ549960 | KJ550683 | KJ551291 |
| 00385 | mozambicus | W* | SA | KJ550075 | KJ550774 | KJ551243 |
| 02041 | mucronatus | F* | IP | KJ550385 | KJ550880 | KJ551136 |
| 02042 | muriculatus | W | IP | KJ550386 | KJ550881 | KJ551086 |
| 00203 | mus | W | WA | KJ549962 | KJ550687 | KJ551000 |
| 00204 | musicus | W | IP | EU423417.1 | EU423321.1 | KJ551307 |
| 00205 | mustelinus | W | IP | KJ549963 | KJ550688 | KJ551264 |
|
| ||||||
| n. sp. a | ||||||
|
| ||||||
| 01593 | n. sp. b | IP | KJ550112 | KJ550789 | KJ551146 | |
| 01989 | n. sp. c | W* | IP | KJ550352 | KJ550870 | KJ551151 |
|
| ||||||
| n. sp. d | ||||||
|
| ||||||
| 01859 | n. sp. E | IP | KJ550272 | KJ550845 | KJ551187 | |
| 01949 | n. sp. f | IP | KJ550327 | KJ550863 | KJ551166 | |
|
| ||||||
| n. sp. g | ||||||
|
| ||||||
| 01680 | n. sp. h | IP | KJ550159 | KJ550810 | KJ551129 | |
| 02316 | n. sp. i | IP | KJ550533 | KJ550941 | KJ551202 | |
| 02055 | namocanus | W | IP | KJ550390 | KJ550882 | KJ551215 |
| 00208 | nanus | W | IP | EU423427.1 | EU423346.1 | KJ551282 |
|
| ||||||
| natalis | ||||||
|
| ||||||
| 01084 | navarroi | EA | AY726475.1 | AY726475.1 | ||
| 02345 | neptunus | W* | IP | KJ550546 | KJ550947 | KJ551227 |
|
| ||||||
| nimbosus | ||||||
|
| ||||||
| 00211 | nobilis | W* | IP | KJ550692 | KJ550987 | |
| 00213 | nucleus | IP | KJ549966 | KJ550694 | KJ551220 | |
| 02060 | nussatella | W* | IP | KJ550392 | KJ550883 | KJ551092 |
| 00215 | nux | W | EP | EU423428.1 | EU423351.1 | KJ551326 |
| 00216 | obscurus | F | IP | KJ549967 | KJ550695 | KJ551260 |
| 02063 | ochroleucus | W* | IP | KJ550395 | KJ550884 | KJ551080 |
| 00218 | omaria | M | IP | KJ549969 | KJ550696 | KJ551265 |
| 02082 | orbignyi | W* | IP | KJ550401 | KJ550886 | KJ551203 |
| 00220 | orion | W* | EP | AY588211.1 | AY382020.1 | |
|
| ||||||
| 02344 | otohimeae | W* | IP | KJ550545 | KJ550946 | KJ551226 |
| 02083 | pagoda | W* | IP | EU015729 | FJ868151 | FJ868136 |
| 02090 | parius | IP | KJ550406 | KJ550887 | KJ551121 | |
| 00223 | parvatus | W | IP | EU423429.1 | EU423355.1 | KJ551233 |
| 00225 | patricius | W* | EP | AY588212.1 | AY382021.1 | |
|
| ||||||
| 01811 | pennaceus | M | IP | KJ550235 | KJ550830 | KJ551084 |
| 00227 | pergrandis | IP | KJ549971 | KJ550697 | KJ550982 | |
| 00228 | perplexus | W* | EP | KJ549972 | AY382022.1 | KJ551344 |
| 02104 | pertusus | W* | IP | KJ550411 | KJ550888 | KJ551168 |
| 00230 | philippii | WA | KJ549974 | KJ550699 | KJ551235 | |
| 00388 | pictus | W* | SA | KJ550078 | KJ551044 | |
| 02107 | pineaui | W* | EA | KJ550413 | KJ550889 | |
|
| ||||||
| 00234 | planorbis | W | IP | KJ549975 | KJ550701 | KJ551284 |
| 02120 | plinthis | IP | KJ550422 | KJ550891 | KJ551200 | |
| 00235 | poormani | W* | EP | KJ549976 | AY382023.1 | KJ551342 |
| 02121 | praecellens | W* | IP | KJ550423 | KJ550892 | KJ551062 |
| 00239 | princeps | W | EP | KJ549977 | AF174192.1 | KJ551237 |
| 02129 | profundorum (aff.) | IP | KJ550427 | KJ550895 | KJ551191 | |
| 00240 | proximus | F | IP | KJ549978 | KJ550704 | KJ551285 |
| 00241 | pseudocuneolus | EA | AY588214.1 | KJ550705 | ||
|
| ||||||
| 02189 | pseudokimioi | IP | KJ550455 | KJ550909 | KJ551077 | |
|
| ||||||
| pseudonivifer | ||||||
|
| ||||||
| 02132 | pseudorbignyi | IP | GU131312 | GU131289 | GU131277 | |
| 01247 | pulcher | W* | EA | AY726477.1 | AY726477.1 | |
| 02139 | pulicarius | W | IP | KJ550431 | KJ550896 | KJ551107 |
| 00243 | puncticulatus | W | WA | KJ549980 | AY382024.1 | KJ551340 |
| 00244 | purpurascens | F | EP | KJ549981 | AF480308.1 | KJ551357 |
| 02140 | queenslandis | IP | KJ550432 | KJ550897 | KJ551188 | |
| 02142 | quercinus | W | IP | KJ550433 | KJ550898 | KJ551063 |
| 02148 | radiatus | W* | IP | KJ550437 | KJ550900 | KJ551133 |
| 02153 | rattus | W | IP | KJ550439 | KJ550901 | KJ551209 |
| 00250 | raulsilvai | EA | AY588215.1 | KJ550710 | ||
|
| ||||||
| 00253 | recurvus | W* | EP | KJ549985 | AY382025.1 | KJ551238 |
| 00251 | regius | W | WA | AY588216.1 | AF174197.1 | KJ551239 |
| 00252 | regonae | EA | AY588217.1 | KJ550711 | ||
|
| ||||||
| 00254 | retifer | M* | IP | KJ549986 | KJ550712 | KJ551293 |
| 00256 | richardbinghami | WA | KJ549988 | KJ550714 | KJ551001 | |
| 00393 | richeri | W* | IP | KJ550083 | KJ550781 | KJ551029 |
| 02158 | rolani | IP | JF718574 | KJ550903 | KJ551135 | |
| 02343 | roseorapum | W* | IP | KJ550544 | KJ550945 | KJ551225 |
| 00258 | salreiensis | EA | AY588218.1 | KJ550716 | ||
|
| ||||||
| 00259 | sandwichensis | IP | KJ550717 | KJ550985 | ||
| 00260 | sanguinolentus | W | IP | HQ852562.1 | KJ550718 | KJ551295 |
| 02163 | sazanka | W* | IP | KJ550444 | KJ550905 | KJ551197 |
| 00262 | serranegrae | EA | AY588219.1 | KJ550719 | ||
|
| ||||||
| 00263 | shikamai | W* | IP | KJ549989 | AF160720.1 | KJ550986 |
| 00264 | sieboldii | IP | KJ549990 | KJ550720 | KJ551301 | |
| 02166 | simonis | IP | KJ550445 | KJ550907 | KJ551181 | |
|
| ||||||
| smirna | ||||||
|
| ||||||
| 00265 | spectrum | IP | KJ549991 | KJ550721 | KJ550988 | |
| 00266 | sponsalis | W | IP | EU423437.1 | EU423364.1 | |
|
| ||||||
| 00268 | spurius | W | WA | AY588194.1 | AY382012.1 | KJ551348 |
| 00270 | stearnsii | W | WA | KJ549993 | KJ550724 | KJ551048 |
| 00271 | stercusmuscarum | F | IP | EU733518.1 | EU078941.1 | EU682294.1 |
| 00273 | striatellus | W* | IP | KJ549994 | KJ550725 | KJ551252 |
| 02199 | striatus | F | IP | KJ550459 | KJ550910 | KJ551098 |
| 02203 | striolatus | F | IP | KJ550460 | KJ550911 | KJ551109 |
| 02205 | stupa | IP | KJ550461 | KJ550912 | KJ551159 | |
| 02206 | sugimotonis | IP | KJ550462 | KJ550913 | KJ551076 | |
| 02214 | sulcatus | W* | IP | JF718583 | KJ550916 | KJ551141 |
| 02225 | sutanorcum | IP | KJ550472 | KJ550924 | KJ551111 | |
| 00281 | suturatus | W* | IP | KJ550730 | KJ551254 | |
| 00282 | tabidus | W* | EA | AY588224.1 | AY382028.1 | KJ551337 |
| 00283 | taeniatus | W* | IP | KJ549997 | KJ550731 | KJ551331 |
| 02232 | tenuistriatus | W | IP | KJ550476 | KJ550925 | KJ551091 |
| 01404 | teodorae | EA | AY726484.1 | AY726484.1 | ||
| 02246 | teramachii | W* | IP | KJ550487 | KJ550928 | KJ551204 |
| 00286 | terebra | W | IP | KJ549998 | KJ550734 | KJ551277 |
| 02255 | tessulatus | W | IP | KJ550495 | KJ550929 | KJ551117 |
| 02261 | textile | M | IP | KJ550497 | KJ550930 | KJ551134 |
| 02341 | thalassiarchus | IP | KJ550542 | KJ550943 | KJ551223 | |
| 02340 | thomae | IP | KJ550541 | KJ550942 | KJ551222 | |
|
| ||||||
| tiaratus | ||||||
|
| ||||||
| 00291 | tinianus | W* | SA | KJ550002 | KJ550738 | KJ550962 |
| 00292 | tornatus | W* | EP | KJ550003 | KJ550739 | KJ551325 |
| 02285 | tribblei | W* | IP | KJ550510 | KJ550933 | KJ551140 |
| 00294 | trochulus | W* | EA | AY588227.1 | KJ550741 | KJ551338 |
| 02286 | tulipa | F | IP | KJ550511 | KJ550934 | KJ551079 |
| 02288 | varius | W* | IP | KJ550512 | KJ550935 | KJ551126 |
| 02294 | vaubani | W* | IP | KJ550518 | KJ550936 | KJ551195 |
| 00298 | ventricosus | W | EA | KJ550006 | KJ550745 | KJ551370 |
| 00300 | venulatus | W* | EA | AY588208.1 | KJ550747 | |
|
| ||||||
| 02298 | vexillum | W | IP | KJ550521 | KJ550937 | KJ551106 |
| 00303 | victoriae | M | IP | KJ550008 | KJ550749 | KJ551372 |
| 00304 | villepinii | WA | KJ550009 | KJ550750 | KJ551313 | |
| 00305 | vimineus | IP | GU134378.1 | EU682306.1 | EU682297.1 | |
| 00306 | viola | IP | KJ550010 | KJ550751 | KJ551373 | |
| 00307 | violaceus | IP | AY588233.1 | AY382032.1 | KJ551343 | |
| 00308 | virgatus | W* | EP | KJ550011 | KJ550752 | KJ551374 |
| 02301 | virgo | W | IP | KJ550523 | KJ550938 | KJ551065 |
| 00311 | vittatus | W* | EP | KJ550012 | KJ550753 | KJ551324 |
| 02304 | voluminalis | W* | IP | KJ550525 | KJ550939 | KJ551114 |
| 01570 | xicoi | W* | EA | AY726492.1 | AY726492.1 | |
| 00313 | ximenes | EP | AY588235.1 | AY382033.1 | ||
|
| ||||||
| 00314 | zeylanicus | IP | KJ550013 | KJ550755 | KJ551278 | |
| 00315 | zonatus | W | IP | GU134383.1 | GU134362.1 | GU134366.1 |
| 00316 | zylmanae | WA | KJ550014 | KJ550756 | KJ551002 | |
| 02339 | Anticlinura_sp. | HQ401572 | HQ401660 | HQ401590 | ||
| 02330 | Bathytoma_neocaledonica | EU015653 | HQ401661 | HQ401591 | ||
| 02327 | Benthofascis_lozoueti | HQ401574 | HQ401593 | |||
| 02338 | Benthomangelia_cf._trophonoidea | EU015644 | HQ401663 | HQ401594 | ||
| 02331 | Borsonia_sp. | EU015737 | HQ401664 | HQ401595 | ||
| 02332 | Clathurella_nigrotincta | HQ401575 | HQ401666 | HQ401599 | ||
| 02333 | Etrema_cf._tenera | EU015691 | HQ401675 | HQ401608 | ||
| 02336 | Eucyclotoma_cymatodes | EU015678 | HQ401676 | HQ401610 | ||
| 02328 | Genota_mitriformis | HQ401576 | HQ401680 | HQ401614 | ||
| 02324 | Harpa_kajiyamai | EU685626 | HQ401683 | HQ401617 | ||
| 02334 | Lovellona_atramentosa | HQ401580 | HQ401692 | HQ401628 | ||
| 02329 | Microdrillia_cf._optima | EU015710 | HQ401696 | HQ401632 | ||
| 02335 | Mitromorpha_metula | EU015672 | HQ401697 | HQ401633 | ||
| 02326 | Terebra_textilis | EU015750 | EU685771 | EU685478 | ||
| 02337 | Thatcheria_mirabilis | EU015736 | FJ868138 | HQ401647 | ||
| 02325 | Turris_babylonia | EU015677 | HQ401715 | HQ401652 | ||
type of prey inferred from the radula.
Outgroups were chosen according to Puillandre et al. (2011a). To test the monophyly of the Conidae, representatives from closely related groups in the superfamily Conoidea were included: Benthofascis lozoueti (Conorbidae), Bathytoma neocaledonica, Borsonia sp., Genota mitriformis and Microdrillia cf. optima (Borsoniidae), Clathurella nigrotincta and Etrema cf. tenera (Clathurellidae), Mitromorpha metula and Lovellona atramentosa (Mitromorphidae), Anticlinura sp. and Benthomangelia cf. trophonoidea (Mangeliidae) and Eucyclotoma cymatodes and Thatcheria mirabilis (Raphitomidae). Less closely related genera were used as more distant outgroups: Turris babylonia (Turridae), and Terebra textilis (Terebridae). The non-conoidean Harpa kajiyamai (Harpidae) is the most distant outgroup.
2.2. DNA Extraction and Sequencing
Although all laboratories mentioned above utilized the same primer pairs [12S1/12S3 (Simon et al., 1991), 16Sar/16Sbr (Palumbi, 1996) and LCO1490/HCO2198 (Folmer et al., 1994)] and all amplification products were sequenced in both directions, our laboratories used a variety of DNA extraction protocols, amplification conditions and sequencing approaches to obtain sequences of regions of the mitochondrial 12S, 16S and COI genes. For brevity, only methodologies employed at the MNHN are described here. DNA was extracted using 6100 Nucleic Acid Prepstation system (Applied Biosystem), the Epmotion 5075 robot (Eppendorf) or DNeasy_96 Tissue kit (Qiagen) for smaller specimens, following the manufacturers' recommendations. All PCR reactions were performed in 25 μl, containing 3 ng of DNA, 1 reaction buffer, 2.5 mM MgCl2, 0.26 mM dNTP, 0.3 mM each primer, 5% DMSO, and 1.5 units of Qbiogene Q-Bio Taq. Amplification consisted of an initial denaturation step at 94°C for 4 min, followed by 35 cycles of denaturation at 94°C for 30 sec, annealing at 54°C for 12S gene, 52°C for 16S and 50°C for COI, followed by extension at 72°C for 1 min. The final extension was at 72°C for 5 min. PCR products were purified and sequenced by sequencing facilities (Genoscope and Eurofins). All genes were sequenced in both directions for increased accuracy. Specimens and sequences were deposited in GenBank (Table 2, Appendix A).
2.3. Phylogenetic Analyses
Sequences were manually (COI gene) or automatically aligned using Muscle 3.8.31 (Edgar, 2004) (16S and 12S genes). Preliminary analyses were performed for each gene separately using the Neighbor-Joining algorithm (with a K2P model) implemented in MEGA 4 (Tamura et al., 2007) to remove obviously misidentified or contaminated sequences from the dataset. One voucher (GU227112.1 and GU226998.1) identified as Conus sp. in GenBank actually corresponded to a member of the Raphitomidae, and eight others were obviously misidentified or contaminated (the sequence clustered with a non-phylogenetically related species: AF126172.1 was identified as C. monachus but clustered with C. radiatus; AF174157.1 was identified as C. circumactus but clustered with C. parius; AF036532.1 was identified as C. distans but clustered with C. bandanus; AF174169.1 was identified as C. frigidus but clustered with C. sanguinolentus; AJ717598.1 was identified as C. magus but clustered with C. furvus; AF174184.1 was identified as C. muriculatus but clustered with C. striatellus; AB044276.1 was identified as C. praecellens but clustered with C. boholensis and AY726487.1 was identified as C. ventricosus but clustered with C. venulatus). Additionally, the unique sequence labelled as C. centurio (AY382002.1) was also removed from the dataset, as it also corresponded to a misidentified specimen (M. Tenorio, pers. com.). Finally, 28 short COI sequences from GenBank (< 200bp) were also removed from the dataset; all corresponded to species represented by several other specimens in the final dataset. Because COI is generally more variable than 16S and 12S gene regions, COI is usually more valuable for specimen identification and distinction of closely related species. It was thus used to assign unidentified specimens from GenBank and to point at species-level issues. We analysed the COI dataset with ABGD (Puillandre et al., 2012b). This method relies on genetic distances only and seeks to identify in the distribution of genetic distances a gap that would correspond to a threshold between intra-specific and inter-specific distances. The defaults parameters provided on the web version of ABGD (version of March, 2014) were applied.
Each gene was analysed independently to check for incongruency between trees. The best model of evolution was selected for each gene and for each codon position of the COI gene using Modelgenerator V.85 (Keane et al., 2006) under the Hierarchical Likelihood Ratio Tests (with four discrete gamma categories): GTR+I+G was always identified as the best model, with I = 0.98, 0.85, 0.66, 0.58 and 0.49 and α = 0.66, 0.25, 0.24, 0.34 and 0.16 for the COI (first, second and third position of the codon), 16S and 12S genes respectively. Maximum Likelihood analyses (ML) were performed using RAxML 7.0.4 (Stamatakis, 2006), with a GAMMAI model for each gene. Three partitions were defined for the COI gene, corresponding to each position of the codon. RaxML analyses were performed on the Cipres Science Gateway (http://www.phylo.org/portal2/) using the RAxML-HPC2 on TG Tool. Accuracy of the results was assessed by bootstrapping (1000 replicates).
After visual inspection of the absence of supported incongruencies between the independent trees, a concatenated dataset was prepared by including only one representative of each species name represented in the independent gene datasets. When several specimens were available for a single named species, the preferred specimen had the highest number of genes and with, if possible, an available voucher. Three unnamed morphospecies and 16 species were represented by specimens sequenced for only one gene: they were excluded from the concatenated dataset. In several cases, specimens of a named species were found not to be monophyletic (see section 3). In all of these cases, the different specimens remained closely related and only one was included in the final dataset. Finally, 326 specimens (including 16 outgroups) were included in the concatenated dataset. ML analyses were performed as described before, with five partitions (three codon positions of the COI gene, 12S and 16S). Bayesian Analyses (BA) were performed running two parallel analyses in MrBayes (Huelsenbeck et al., 2001), consisting each of eight Markov chains of 200,000,000 generations each with a sampling frequency of one tree each thousand generations. The number of swaps was set to five, and the chain temperature at 0.02. Similarly to the ML approach, unlinked models (each with six substitution categories, a gamma-distributed rate variation across sites approximated in four discrete categories and a proportion of invariable sites) were applied for each partition. Convergence of each analysis was evaluated using Tracer 1.4.1 (Rambaut and Drummond, 2007), and analyses were terminated when ESS values were all superior to 200. A consensus tree was then calculated after omitting the first 25% trees as burn-in.
The COI gene is more variable than 16S or 12S, COI sequences were available for the largest number of morphospecies, and many of these were represented by several individuals. For these reasons, COI gene trees were used to explore the species-level α-taxonomy of cone snails.
2.4. Character Evolution
The evolution of two characters was analysed by mapping their character states on the Bayesian phylogenetic tree obtained with the concatenated dataset: geographic distribution (five states: East Atlantic; East Pacific; Indo-Pacific; South Africa; West Atlantic) and prey type (four states: worms; fishes; molluscs; worms, fishes, shrimps and molluscs). The prey type was based on direct observation for 100 species, was inferred from the radula type for 103 species and remains unknown for 107 species (http://biology.burke.washington.edu/conus/). It should be noted that the vermivorous type may refer to preys from different phyla. However, among the 53 species for which the vermivorous diet was based on direct observation, only one species (C. leopardus) is known to mainly feed on a non-polychaete (enteropneust Ptychodera - Kohn, 1959) The evolution of the prey type was assessed with Mesquite V2.74 (Maddison and Maddison, 2009), using the option ‘tracing character history’ and the likelihood ancestral reconstruction method. The BBM (Bayesian Binary MCMC) method implemented in RASP (Yu et al., 2013, 2010) was used to reconstruct ancestral ranges for each node. To account for uncertainties, the 10,000 last trees obtained with the Bayesian analyses were loaded. Analyses were run with default parameters, except the number of cycles (set to 500,000) and the root distribution (set to “wide”).
3. Results and Discussion
3.1. Species-Level Phylogeny
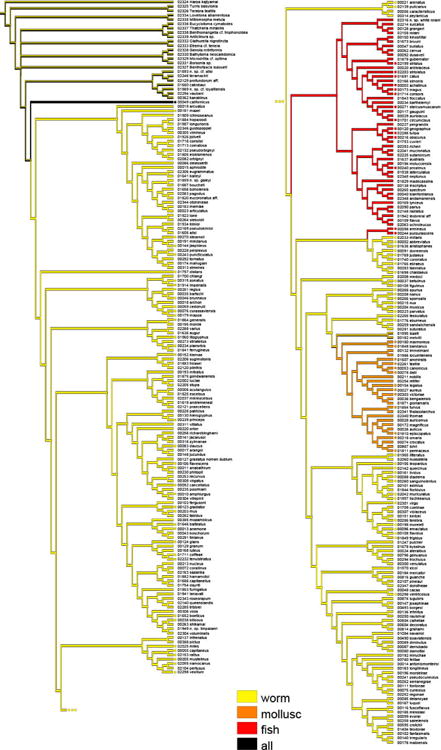
Final alignments included 658bp, 457bp and 553bp for the COI, 16S and 12S genes respectively. Single-gene analyses produced poorly resolved trees (Appendices B-D), with only a few clades supported. Trees constructed with the concatenated dataset also recovered these clades, albeit with higher support. However, single-gene trees are useful to identify unknown specimens and for evaluation of species-level taxonomy of cone snails.
The eight remaining unidentified Conus from GenBank (after one was discarded from the dataset because it was not a cone snail) were identified following a barcoding approach in which an unknown specimen is identified based on the identity of its closest neighbour in the tree (Austerlitz et al., 2009): one specimen which consisted of an egg capsule collected in the Philippines (Puillandre et al., 2009) matched C. australis; five other specimens (Cunha et al., 2008, 2005) belonged to the C. venulatus complex; another matched C. capitaneus (Dang et al., unpublished); and the last corresponded to C. tabidus (Cunha et al., 2005).
In most cases (213 of the 320 named species), DNA analyses were congruent with species delimitation based on shell characters (i.e. species with several specimens were found monophyletic, and species with a single specimen were found different from all the others). For the remaining species, DNA analyses were not found congruent with species delimitation based on morphological characters, and we examined four hypotheses that could explain this high number of discrepancies: 1. Specimens were not identified correctly. Although specimens with vouchers (or at least a picture) were examined by several experts to verify identification, a large proportion of the sequences (especially those from GenBank) did not have any voucher material and could not be evaluated. 2. The sequence obtained belongs to a contaminant. Several identical sequences independently obtained by different laboratories reduce the likelihood of contamination, but checking for contamination is more difficult when only a single specimen is available for a given named species. 3. The three analysed genes all belong to the maternally transmitted mitochondrial genome, and its evolutionary history is distinct from the species tree. In particular, the non-monophyly of a given morphospecies may be linked to the fact that the analysed gene(s) have not yet coalesced (Funk and Omland, 2003). 4. Lack of morphological variability (e.g. cryptic species) or, conversely, high within species morphological variability (e.g. linked to phenotypic plasticity) resulted in incorrectly delimited species, suggesting that the taxonomy needs to be revised.
In addition to the phylogenetic analyses, the ABGD method was also used to discuss the species complexes. In the vicinity of the barcode gap, the ABGD method constantly returns a partition in 343 primary species hypotheses (PSH). Because it is not the primary objective of this article, and because most species are represented by one or a few specimens only, we will not discuss in detail the ABGD results, but instead identify the problematic cases and suggest that they deserve more in-depth analyses. In numerous cases several species names were mixed in a single clade. For most of them (C. aulicus/C. episcopatus/C. magnificus, C. dalli/C. canonicus, C. frigidus/C. flavidus, C. jaspideus/C. mindanus, C. mucronatus/C. sutanorcum, C. muriculatus/C. floridulus, C. sulcatus complex, C. striatellus/C. planorbis/C. ferrugineus, C. ximenes/C. mahogani, C. loyaltiensis/C. kanakinus/C. vaubani, C. pennaceus/C. crocatus/C. lohri, C. bandanus/C. marmoreus, C. pagodus/C. aff. eucoronatus and C. tessulatus/C. eburneus/C. suturatus/C. sandwichensis) correlating these preliminary results with morphological, geographical or bathymetrical variation would require analyses of additional specimens. Nonetheless, in some cases we can propose preliminary hypotheses to interpret the results. C. arenatus occurs in two clades, one corresponding to the form aequipunctatus and the other being mixed with C. pulicarius; ABGD places these two lineages in two different PSH. In the case of C. lividus and C. sanguinolentus (only two specimens from GenBank, one for each name), specimens may have been incorrectly identified as C. lividus or C. sanguinolentus or the morphological criteria used to delimit these species are inappropriate. For members of the C. teramachii/C. smirna/C. aff. profundorum/C. n. sp. g complex (Fig. 1A), four clades are recognized: two restricted to New Caledonia (one including C. n. sp. g and the second containing specimens with C. profundorum-like shells), another to Madagascar (it would correspond to the form neotorquatus of C. teramachii), and one that occurs in the Philippines, Solomon Islands, Papua-New Guinea and New Caledonia (with C. smirna and C. teramachii-like shells). In this complex ABGD recognizes only three PSH, merging the C. profundorum-like shells and the Philippines/Solomon Islands/Papua-New Guinea/New Caledonia clade in a single PSH. Also, several species complexes were revealed that have been treated previously (C. sponsalis complex in Duda et al. (2008), C. orbignyi complex in Puillandre et al. (2011b), C. ventricosus complex in Cunha et al. (2005) and Duda and Rolan (2005) and C. venulatus complex in Cunha et al. (2005), Cunha et al. (2008) and Duda and Rolan (2005), but our results suggest that their taxonomy is not fully resolved yet, and that numerous cryptic species still need formal description.
Figure 1.
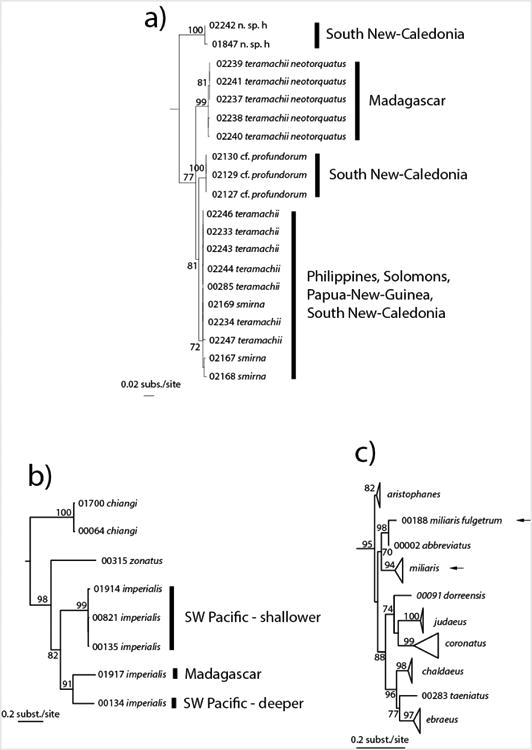
Three sub-parts of the COI Bayesian tree that illustrate discrepancies between COI diversity and morphological diversity. a) C. teramachii complex. b) Putative cryptic species in C. imperialis. c) C. miliaris complex (black arrows).
Sequences of specimens representing 11 species names were not monophyletic and included two (C. miliaris, C. glans, C. longurionis, C. mappa, C. quercinus, C. villepinii, C. generalis, C. regius) or three (C. australis, C. daucus, C. imperialis) lineages. All this lineages correspond to different PSH as defined by ABGD, the high genetic distances thus suggesting that they may belong to different species. In some cases, one of the lineages is geographically (e.g., C. longurionis) or bathymetrically (e.g., two of the C. imperialis lineages – Fig. 1B) distinct. In other cases, one is associated with a previously recognized subspecies or forms (e.g., granarius for one lineage of C. mappa, fulgetrum for C. miliaris – Fig. 1C, maldivus for C. generalis, abbotii for C. regius, gabryae for C. australis, boui for C. daucus, and fusctaus for C. imperialis). The two lineages of C. quercinus (one being identified as “aff quercinus”) were not found with the 12S and 16S genes. In several other cases, divergent lineages within a single morphospecies were revealed, although the corresponding morphospecies remained monophyletic, thus suggesting the presence of cryptic species (e.g., C. consors), some of which are associated with a previously described subspecies or form (e.g. archiepiscopus for C. textile). ABGD defines two PSH associatedwith the name C. consors and three with the name C. textile. Finally, in a few cases (e.g., C. recurvus and C. virgatus), two species names shared identical or very similar sequences, suggesting synonymy; ABGD places them in a single PSH. However, the low number of specimens sequenced for each species name prevents adequate evaluation of this hypothesis.
3.2. Phylogeny Above the Species Level
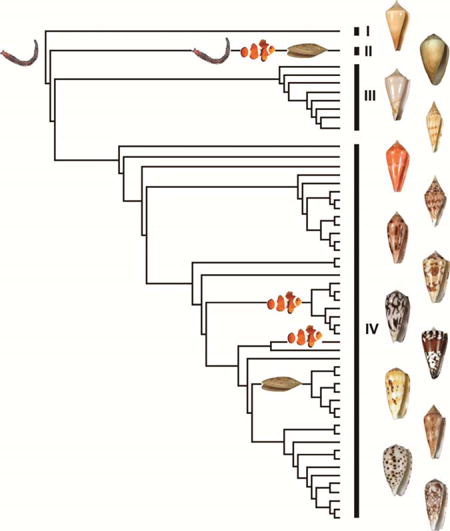
Analyses of the concatenated dataset revealed four main highly divergent clades (Fig 2, Table 3). Three of them correspond to previously reported lineages with molecular data: one with only one species (C. californicus), a second corresponding to the Small Major Clade (SMC – sensu (Duda and Kohn, 2005) and roughly to the Conilithinae (sensu Tucker and Tenorio, 2009), and a third, the most species-rich, corresponding to the Large Major Clade (LMC – sensu (Duda and Kohn, 2005) and roughly to the Conidae (sensu Tucker and Tenorio, 2009). A fourth main clade was found here for the first time with DNA characters. It roughly corresponds to Profundiconus sensu Tucker and Tenorio (2009) and includes a number of deep-water species from the Indo-Pacific that were not examined in previous molecular phylogenetic analyses. Profundiconus is sister-group to all the other Conidae, but this relationship is not supported. The inclusion of Profundiconus in Conidae thus remains doubtful, although the morphological characters would place it in cone snails. The recovery of this clade illustrates the fact that more complete taxon sampling can provide a much better view of the evolutionary history and taxonomic diversity of groups. Although our current phylogenetic treatment more than doubles the number of species examined, our analyses included less than 50% of the recognized cone snail species; inclusion of additional species and analyses of additional gene sequence regions will be instrumental in reconstructing the history of the Conidae and may reveal additional previously unrecognized groups.
Figure 2.
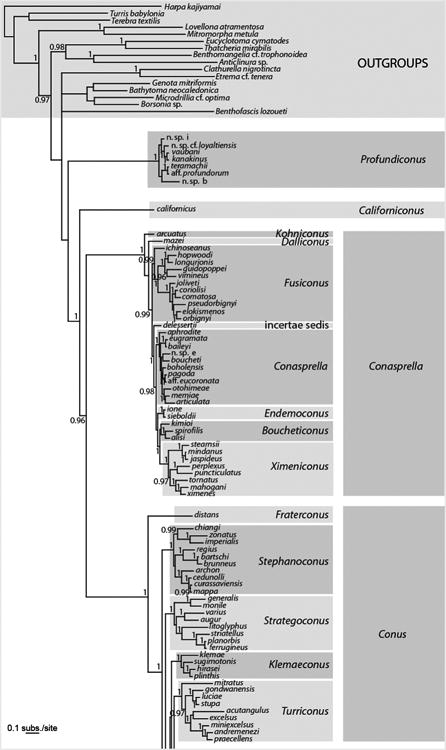
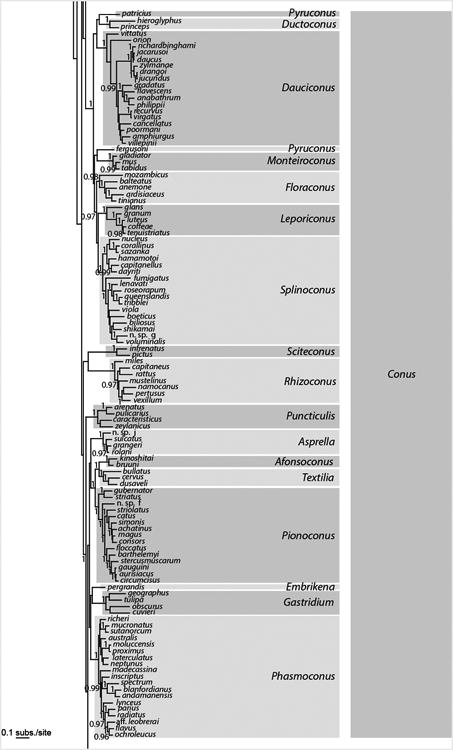
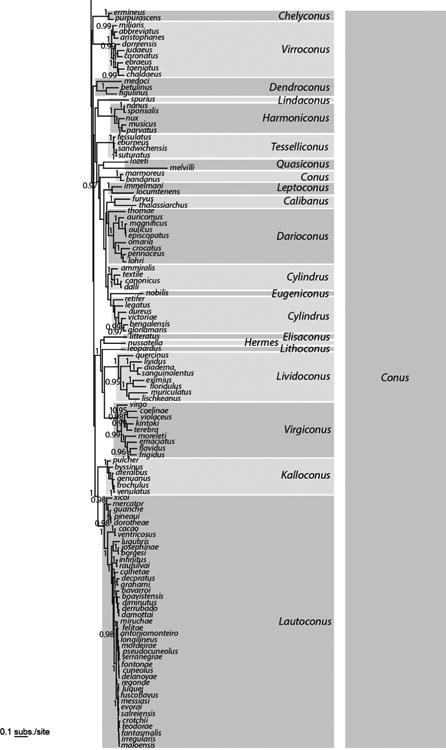
Bayesian tree based on a concatenation of the COI, 16S and 12S genes for the reduced dataset of 326 specimens. Posterior probabilities (> 0.95) are shown for each node. Genus and subgenus names follow the classification based on the phylogenetic tree and published in Puillandre et al. (in press).
Table 3.
Statistical support (Bayesian and Maximum likelihood analyses) for the clades associated to a genus or subgenus name in the new classification (Puillandre et al., in press).
| Group | Posterior Probabilities | Bootstraps |
|---|---|---|
| Profundiconus | 1 | 99 |
| Californiconus | na | na |
| Conasprella | 1 | 100 |
| Kohniconus | na | na |
| Dalliconus | na | na |
| Fusiconus | 0.99 | 53 |
| Conasprella | 1 | 83 |
| Endemoconus | 1 | 100 |
| Boucheticonus | 0.53 | 48 |
| Ximeniconus | 1 | 98 |
| Conus | 1 | - |
| Fraterconus | na | na |
| Stephanoconus | 1 | 99 |
| Strategoconus | 1 | 85 |
| Klemaeconus | 1 | 100 |
| Turriconus | 1 | 100 |
| Pyruconus (group 1) | na | na |
| Ductoconus | 1 | 100 |
| Dauciconus | 1 | 99 |
| Pyruconus (group 2) | na | na |
| Gladioconus | 1 | 100 |
| Floraconus | 0.98 | 96 |
| Leporiconus | 1 | 99 |
| Splinoconus | 1 | 100 |
| Sciteconus | 1 | 100 |
| Rhizoconus | 1 | 100 |
| Puncticulis | 1 | 100 |
| Asprella | 1 | 100 |
| Afonsoconus | 1 | 100 |
| Textilia | 1 | 97 |
| Pionoconus | 1 | 94 |
| Embrikena | na | na |
| Gastridium | 1 | 100 |
| Phasmoconus | 1 | 100 |
| Chelyconus | 1 | 100 |
| Virroconus | 1 | 100 |
| Dendroconus | 0.86 | 31 |
| Lindaconus | na | na |
| Harmoniconus | 1 | 100 |
| Tesselliconus | 1 | 100 |
| Quasiconus | 0.48 | - |
| Conus | 1 | 100 |
| Nataliconus | 1 | 94 |
| Calibanus | 1 | 100 |
| Darioconus | 0.72 | 48 |
| Cylindrer (group 1) | 1 | 97 |
| Eugeniconus | na | na |
| Cylindrer (group 2) | 0.74 | 70 |
| Elisaconus | na | na |
| Hermes | na | na |
| Lithoconus | na | na |
| Lividoconus | 1 | 98 |
| Virgiconus | 1 | 100 |
| Kalloconus | 1 | 100 |
| Lautoconus | 1 | 100 |
Within the SMC and LMC, reconstructed phylogenies show several well-resolved subclades that generally correspond to genus-level groups defined by Tucker and Tenorio (2009). However, most of the relationships among the subclades of the SMC and LMC were not resolved; this could be due to a lack of phylogenetic signal for the three mitochondrial genes analysed here and/or to a radiation process that led to multiple lineages originating in a short period of time. Nonetheless, some groupings can be noted, although in most cases only supported by the Bayesian analysis (Fig. 2). Within the SMC, all the species except for C. arcuatus and C. mazei clustered together (PP = 1, bootstrap = 34). C. distans is the sister-species of all other members of the LMC (PP = 1; this relationship was absent in the ML analysis). Half of the members of the LMC (from Puncticulis to the bottom of Fig. 2) occur within a well-supported clade (PP = 1; relationship not found with the ML analysis).
Similar to the results obtained by Puillandre et al. (2011a) with similar outgroups, monophyly of the cone snails (= Conidae sensu Bouchet et al., 2011 – see Table 1) is not supported, suggesting that more taxa, in particular within the closely related families (Borsoniidae, Clathurellidae, Conorbiidae), and additional genes with lower rates of evolution, should be analysed to fully resolve the relationships of cone snails and other Conoidea. The diversity pattern within Conidae remained unchanged from previous studies (e.g. Duda and Kohn, 2005; Tucker and Tenorio, 2009), with very disparate numbers of species between the main lineages. By far most cone snails (∼ 85%) are in the LMC.
Most, if not all, previously published molecular phylogenies are congruent with the phylogenetic results presented here; this does not come as a surprise as most of the specimens and sequences analysed in these studies were combined in our dataset. However, phylogenetic trees that were reconstructed with other gene regions (intron 9 CIS Kraus et al. (2011); and calmodulin exon+intron gene sequences, Duda and Palumbi (1999a) are also consistent with those produced here. All clades defined in these prior trees were recovered in our trees (taking into account that not all the same species were included in all studies). The inclusion of many more species compared to the previously published phylogenies, however, revealed many clades that were previously unrecognized either because members of these clades were not included in the previous analyses or because the inclusion of additional species and/or sequences improved the resolution of the tree. The phylogenetic analysis of the 329 cone snail species has been turned into a new classification for the family Conidae that now includes 4 genera and 71 subgenera (Puillandre et al., in press).
3.3. Evolution of Diet
Most cone snails feed on polychaete worms, and reconstruction of the evolution of their diets supports the hypothesis that the cone snail ancestor was vermivorous (Fig. 3). The form of its radular tooth (Kohn et al., 1999) and its position in the tree (Fig. 3) also support the evolution of the unusual diet of C. californicus—this species is able to feed on molluscs, worms, shrimps and fishes (Biggs et al., 2010)—from a worm-hunting ancestor. This is also likely in the few clades that specialize on fishes (members of Chelyconus, Phasmoconus, Gastridium and Pionoconus) and molluscs (most of the members of the subgenera Conus, Leptoconus, Calibanus, Darioconus, Cylindrer, and Eugeniconus). The capacity to feed on molluscs likely appeared only once, with a probable reversion to worm-hunting behaviour in C. nobilis (diet predicted from radular tooth characters).
Figure 3.
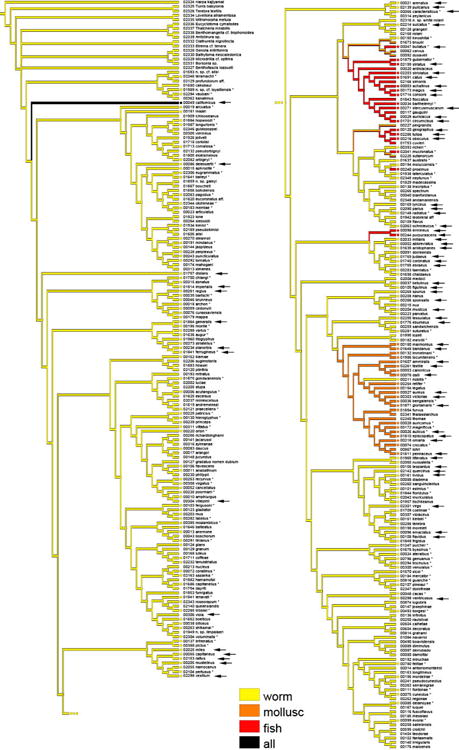
Mapping of the type of prey on the Bayesian tree based on a concatenation of the COI, 16S and 12S genes for the reduced dataset of 326 specimens. *: species for which at least one nucleotide sequence of conotoxin is registered in GenBank. 1: species for which the diet is know from direct observations. 2: species for which the diet has been inferred from the radula. ?: species for which the diet is unknown. When species for which the diet has been inferred from the radula are not taken into account for the ancestral state reconstruction, the clade delimited by the ligh grey box is inferred to include only mollusc-hunting species and the two clades delimited by the dark grey boxes are inferred to include only fish-hunting species.
Reconstruction of the evolution of the cone snail diet shows that the capacity to prey on fishes probably appeared several times during the evolution of the group. If we rely only on the species for which piscivory has been confirmed by direct observation, and not on the species for which the diet has been inferred from the radula (marked “2” in the Fig. 3), the piscivorous diet evolved only twice, in C. ermineus and C. purpurascens within Chelyconus, and in several species of the clade (Asprella, Afonsoconus, Textilia, Pionoconus, Embrikena, Gastridium, Phasmoconus), as represented by the two grey boxes in the Figure 3. However, the relationships between these two clades are not supported, and we thus cannot rule out that piscivory evolved only once. Similarly, several previous phylogenetic investigations of cone snails suggest that fish-eating arose multiple times during the evolution of this group, but many of the resultant trees from these studies lacked rigorous support to reject the hypothesis that fish-eating evolved only once (Duda et al., 2001, Fig 1-3, 5; Kraus et al., 2011, Fig. 2 and 3; but see Duda and Palumbi, 2004).
Figure 5.
3.4. Clade Specificity of Venom Peptides
In this section we relate an independent dataset – the major peptide toxins expressed in the venom of each species in Conidae – to the phylogeny based on standard mitochondrial marker genes shown in Fig. 2. At present, the range of species whose venom has been comprehensively analyzed is far more phylogenetically restricted than the species for which the mitochondrial markers are available (as shown by the asterisks in Fig. 3); consequently, it was thus not possible to directly map the evolution of the toxins on the tree, as done with the diet and biogeography. Nevertheless, it is clear even from the more limited dataset available that the major venom peptides expressed in a given species tightly correlate with the clade to which that particular species is assigned, based on the molecular data (Fig. 2). Consequently, venom peptides can be used as an independent dataset to confirm or refute the clades defined using mitochondrial genes.
We specifically tested this hypothesis with the fish-hunting clades. As discussed above, the phylogeny suggests that worm hunting was the ancestral state. One family of venom peptides that are well understood at the mechanistic level are the α-conotoxins, targeted to the nicotinic acetylcholine receptor, a molecular target that is key to prey capture. Blocking this receptor at the synapse between nerve and muscle results in the paralysis of potential prey. The major snake toxins in the venoms of cobra-related snake species, such as cobratoxin or α-bungaratoxin, similarly target the nicotinic acetylcholine receptor of their prey. In the shift from worm hunting to fish hunting, the peptides that belong to a particular family, the α-conotoxins, were clearly under selection to diverge from the ancestral worm-hunting nicotinic antagonists, and to target the very distinctive nicotinic acetylcholine receptor expressed in the skeletal muscle of all vertebrates. Thus, the members of the α-conotoxin family in worm-hunting cones mostly belong to a specific toxin gene subfamily called the α4/7 subfamily. These have the canonical sequence CCX4CX7C – the peptides in the gene superfamily, as defined from the similarity in the signal sequence, have 4 cysteine residues with diverse amino acids in betweenthem –. In the typical ancestral peptide there are 4 and 7AA respectively in the two inter-cysteine intervals. Appendix E shows examples of α4/7 subfamily peptides from two different clades of worm-hunting Conus snails, Puncticulus and Dendroconus; peptide sequences from two species in each clade are shown.
As shown in Appendix E, in one specific clade of fish-hunting cone snails (Pionoconus), the α-conotoxin family peptides that are highly expressed diverge systematically from the ancestral canonical sequence, and belong to a different subclass of α-conotoxins, the α3/5 toxin gene subfamily (canonical sequence: CCX3CX5C). However, in a different clade of fish-hunting cone snails (Chelyconus), the ancestral subfamily has also been altered, but the change is entirely different: an extra disulfide bond has been added (leading to peptides with 6 instead of 4 cysteines). Thus, all piscivorous species in Pionoconus express the α3/5 subfamily member as the major venom peptide for inhibiting the nicotinic acetylcholine receptor at the neuromuscular junction. However, in the piscivorous Chelyconus clade, it is the longer peptides with an extra disulfide linkage (known as αA-conotoxins) that have this physiological role. Thus, although the Bayesian analysis in Fig 2 does not statistically allow the unequivocal conclusion of independent origins of fish-hunting in the Pionoconus and Chelyconus clades, this is strongly supported by the type of venom-peptide expression data shown in Appendix E. The same divergence between venom peptides in Pionoconus and Chelyconus is found if the peptides targeted to voltage-gated K channels are examined.
Furthermore, the major nicotinic acetylcholine receptor antagonists in some highly specialized worm-hunting lineages, such as Stephanoconus (specialized to prey on amphinomid polychaetes), also diverge systematically from the canonical α4/7 subfamily, to peptides in the α4/3 subfamily (CCX4CX3C). In this case, the most highly expressed nicotinic antagonist targets a different nicotinic receptor subtype, presumably similar to the isoform expressed at the neuromuscular synapse of the amphinomed prey of species in the Stephanoconus clade.
3.5. Biogeography
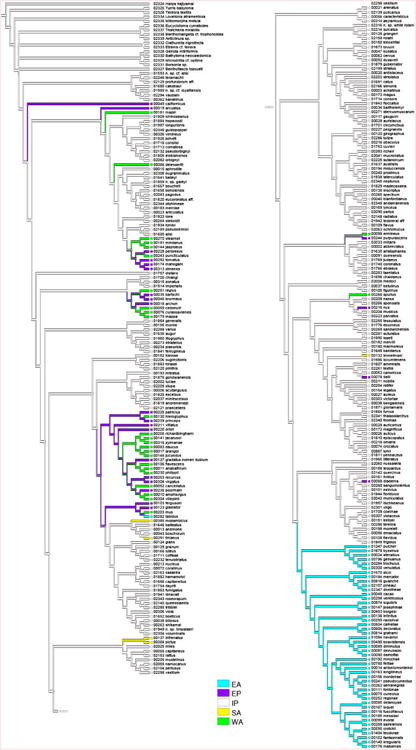
Mapping geographic distributions of species onto the reconstructed phylogeny requires more transition events than the evolution of the diet (Fig. 4). Based on the tree, most species occur in the Indo-Pacific (IP), which may be the ancestral source of the Conidae (frequency of occurrence of Indo-Pacific region at the node 1 – Fig. 4: 90.5%) and of Conus (node 2: 99.2%). However, the fossil record supports the view that the center of diversity of Conidae in the Eocene was the former Tethys region (Kohn, 1985), also the region of its oldest known fossils (Kohn, 1990). In total, 22 events of dispersals and 27 events of vicariance are inferred. Several of these events are relatively recent and involve species from the IP and EP, e.g., the clades containing the EP species C. nux, C. dalli and C. diadema, that suggest recent migration events across the East Pacific Barrier to establish these species in the EP. Several other clades included sets of species from both the EP and WA, e.g., the clade containing the piscivores C. purpurascens and C. ermineus, suggesting recent allopatric speciation events linked to vicariance of lineages associated with the emergence of Isthmus of Panama. In addition, in one case it is possible to reconstruct a scenario of consecutive speciation (and possible dispersion and/or vicariance) events to explain the origins of current IP, EP, WA and EA distributions of members of a clade: a first dispersion or vicariance event between the IP and EP led to the origin of C. fergusoni and C. gladiator in the EP, followed by another dispersion or vicariance event that gave rise to C. mus in the WA (possibly associated with the emergence of the Isthmus of Panama), which was then followed by separation of lineages in (or a migration event between) the WA and EA and ultimate origin of C. tabidus in the EA. C. tabidus is the only EA cone snail species on the tree that is restricted to the EA and does not occur in a clade with other EA species.
Figure 4.
Mapping of the geographic distribution (EA = East Atlantic; EP = East Pacific; IP = Indo-Pacific; SA = South Africa; WA = West Atlantic) on the Bayesian tree based on a concatenation of the COI, 16S and 12S genes for the reduced dataset of 326 specimens.
Overall, the number of suspected migration and vicariance events is low relative to the number of species included in the analysis. Indeed, few cone snail species occur in more than one of the main marine biogeographic provinces (e.g., C. ermineus occurs in the WA and EA and as stated above C. chaldaeus, C. ebraeus and C. tessulatus occur in both the IP and EP). The low levels of connectivity between these provinces is probably linked to large-scale historical-geological events, such as the existence of the East Pacific Barrier between the islands of the central Pacific and the offshore islands and coast of the Americas and the Mid-Atlantic Barrier that separates the Atlantic Ocean into western and eastern regions (Duda and Kohn, 2005) as well as physiological barriers that prevent migration through cold water barriers at higher latitudes.
The only previous analysis of the biogeographic history of cone snails (Duda and Kohn, 2005) inferred that the group contains two main groups, the SMC and LMC, that were largely restricted to the EP+WA and IP respectively and that this geographic separation likely promoted the divergence of the lineages that gave rise to these clades. That study was able to include only nine SMC species, and with increased taxonomic coverage, this pattern is no longer apparent. Most (70%) SMC species occur in the IP, while the others are evenly distributed in the EP and WA (Fig. 4). The IP SMC members are deep-water species, while most of the EP and WA members are not. Thus, bathymetric isolation, and not isolation in separate biogeographic provinces as inferred by Duda and Kohn (2005), may account for the separation of the SMC and LMC.
3.6. Speciation Patterns in Cone Snails
Allopatric patterns, either linked to a speciation event or to within-species differentiation that has not led to speciation, occur throughout Conidae (e.g. Duda and Lee, 2009a; Duda and Rolan, 2005; Puillandre et al., 2011b). The likely propensity of such populations to evolve different venoms (Duda and Lee, 2009b; Duda et al., 2009) that may be linked to prey shifts, make cone snails a promising model to also explore the effects of non-geographic factors on the diversification of the group. Prey shifts after speciation could induce strong positive selection on venom properties and the evolution of new toxins more adapted to new prey (Duda et al., 2008), in agreement with the hypothesis proposed for snakes (Barlow et al., 2009; Kordis and Gubensek, 2000; Lynch, 2007) and scorpions (Kozminsky-Atias et al., 2008). Duda & Lee (2009b) also proposed that ecological release, occurring when an isolated population is under relaxed selective pressure (e.g. from a predator-prey arms race), may lead to the appearance of new toxins, even without prey shift, in C. miliaris. However, the available data on conotoxins remain too scarce (species with an asterisks in Fig. 3) to reconstruct the evolution of the conotoxins from the phylogenetic tree presented here and to eventually identify shifts in venom composition between closely related species that could be linked to prey shift or ecological release (but see pararagraph 3.4.). Only 71 species of cone snails are represented by at least one nucleotide sequence of conotoxin in GenBank (Puillandre et al., 2012a), and for most of them the conotoxin sampling is not saturated, as revealed by recent next-gen sequencing (Terrat et al., 2011; Violette et al., 2012), precluding a robust comparison of venom composition at a large-scale.
Because our analysis revealed only a few diet shifts, one could argue that this could explain only few speciation events in cone snails. However, we limited prey categories to only the three major types (molluscs, worms and fishes), and important shifts likely occur at finer taxonomic levels of prey. Actually, closely related sympatric Conus species of cone snails typically exhibit different feeding specializations, as shown before (e.g. (Kohn and Nybakken, 1975; Kohn, 2001, 1959), and additional comparative analyses may provide stronger evidence linking prey shift to speciation events in some cases.
3.7. Conclusion
Molecular phylogenetic analysis has confirmed that cone snails constitute a largely heterogeneous group in spite of overall morphological homogeneity that justified their inclusion until recently in a single genus. Speciation in cone snails results from different evolutionary processes, since several models of speciation, either linked to geography or ecology, may apply to the group. This propensity to speciate following several evolutionary processes would be one of the key factors to explain why cone snails are one of the most diverse groups of marine invertebrates. We also argue that the pharmacological diversity of the peptides found in the venom gland of the cone snails could be underestimated, since most of the studies of the last three decades focused on species that belong to only a few lineages (Puillandre et al., 2012a), and several lineages remain largely understudied (or even not studied at all – e.g. Profundiconus). The newly defined, highly divergent lineages of cone snails may represent novel biological strategies not found in the limited set of cone snail lineages analyzed so far. One indication of this is the high diversity of conotoxins found in C. californicus (only half of the subfamilies found in C. californicus are also found in Conus species – Biggs et al., 2010), this would imply that conotoxin study is only in its infancy, suggesting a promising future for the discovery of new conotoxins and new therapeutic applications.
Supplementary Material
Highlights.
- A molecular phylogeny of the cone snails is proposed.
- The phylogeny is based on 329 species and three genes
- Four major highly divergent clades are defined.
- Diet shifts and large-scale phylogeography of cone snails are inferred.
Acknowledgments
The PANGLAO 2004 Marine Biodiversity Project was funded by the Total Foundation and the French Ministry of Foreign Affairs; The PANGLAO 2005 cruise on board M/V DA-BFAR associated the USC, MNHN (co-PI Philippe Bouchet) and the Philippines Bureau of Fisheries and Aquatic Research (BFAR; co-PI Ludivina Labe); the MNHN-IRD-PNI Santo 2006 expedition was made possible by grants, among others, from the Total Foundation and the Stavros Niarchos Foundation; the AURORA 2007 cruise was made possible through a grant from the Lounsbery Foundation; The Miriky and Atimo Vatae expeditions were funded by the Total Foundation, Prince Albert II of Monaco Foundation, and Stavros Niarchos Foundation, and conducted by MNHN and Pro-Natura International (PNI) as part of their “Our Planet Reviewed” programme; the Coral Sea and Solomon Islands cruises took place on board R/V Alis deployed from Nouméa by the Institut de Recherche pour le Développement (IRD), and Bertrand Richer de Forges and Sarah Samadi were cruise leaders for the Solomons, Coral Sea and Vanuatu expeditions. U.S. National Science Foundation Grant 0316338 supported the contributions of AJK, TFD, and CPM. Ellen Strong, Marie-Catherine Boisselier and Sarah Samadi are thanked for their role in molecular sampling during these expeditions. This work was supported the Service de Systématique Moléculaire (UMS 2700 CNRS-MNHN), the network “Bibliothèque du Vivant” funded by the CNRS, the Muséum National d'Histoire Naturelle, the INRA and the CEA (Centre National de Séquençage) and the NIH program project grant (GM48677), as well as partial support from the ICBG grant (1U01TW008163) from Fogarty (NIH). The phylogenetic analyses were performed on the MNHN cluster (UMS 2700 CNRS-MNHN). The authors also thank Barbara Buge, Virginie Héros, and Julien Brisset for curation of the voucher specimens in the MNHN and Eric Monnier, Loïc Limpalaër and Manuel Tenorio who helped in identifying the specimens.
Appendices.
Appendix A
List of specimens analysed. Sequences of different genes published by the same author and identified with the same species name were considered to correspond to the same specimen (only when only one sequence per species was in GenBank).
Appendix B
Maximum likelihood tree based on COI sequences. Bootstraps values > 80 are shown for each node.
Appendix C
Maximum likelihood tree based on 16S sequences. Bootstraps values > 80 are shown for each node.
Appendix D
Maximum likelihood tree based on 12S sequences. Bootstraps values > 80 are shown for each node.
Appendix E
Venom peptides in the a-conotoxin family in five clades.
References
- Austerlitz F, David O, Schaeffer B, Bleakley K, Olteanu M, Leblois R, Veuille M, Laredo C. DNA barcode analysis: a comparison of phylogenetic and statistical classification methods. BMC Bioinformatics. 2009;10:S10. doi: 10.1186/1471-2105-10-S14-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay PK, Stevenson BJ, Ownby JP, Cady MT, Watkins M, Olivera BM. The mitochondrial genome of Conus textile, coxI-coxII intergenic sequences and conoidean evolution. Mol Phylogenet Evol. 2008;46:215–223. doi: 10.1016/j.ympev.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow A, Pook CE, Harrison RA, Wüster W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc R Soc B Biol Sci. 2009;276:2443–2449. doi: 10.1098/rspb.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biass D, Dutertre S, Gerbault A, Menou JL, Offord R, Favreau P, Stöcklin R. Comparative proteomic study of the venom of the piscivorous cone snail Conus consors. J Proteomics. 2009;72:210–218. doi: 10.1016/j.jprot.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Biggs JS, Watkins M, Puillandre N, Ownby JP, Lopez-Vera E, Christensen S, Moreno KJ, Bernaldez J, Licea-Navarro A, Showers Corneli P, Olivera BM. Evolution of Conus peptide toxins: analysis of Conus californicus Reeve, 1844. Mol Phylogenet Evol. 2010;56:1–12. doi: 10.1016/j.ympev.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchet P, Kantor Y, Sysoev A, Puillandre N. A new operational classification of the Conoidea (Gastropoda) J Molluscan Stud. 2011;77:273–308. [Google Scholar]
- Bouchet P, Rocroi JP. Classification and nomenclator of Gastropod families. Malacologia. 2005;47:1–397. [Google Scholar]
- Cunha RL, Castilho R, Ruber L, Zardoya R. Patterns of cladogenesis in the venomous marine gastropod genus Conus from the Cape Verde Islands. Syst Biol. 2005;54:634–650. doi: 10.1080/106351591007471. [DOI] [PubMed] [Google Scholar]
- Cunha RL, Tenorio MJ, Afonso C, Castilho R, Zardoya R. Replaying the tape: recurring biogeographical patterns in Cape Verde Conus after 12 million years. Mol Ecol. 2008;17:885–901. doi: 10.1111/j.1365-294X.2007.03618.x. [DOI] [PubMed] [Google Scholar]
- Duda TFJ, Bolin MB, Meyer C, Kohn AJ. Hidden diversity in a hyperdiverse gastropod genus: discovery of previously unidentified members of a Conus species complex. Mol Phylogenet Evol. 2008;49:867–876. doi: 10.1016/j.ympev.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Duda TFJ, Kohn AJ. Species-level phylogeography and evolutionary history of the hyperdiverse marine gastropod genus Conus. Mol Phylogenet Evol. 2005;34:257–272. doi: 10.1016/j.ympev.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Duda TFJ, Kohn AJ, Matheny AM. Cryptic Species Differentiated in Conus ebraeus, a Widespread Tropical Marine Gastropod. Biol Bull. 2009;217:292–305. doi: 10.1086/BBLv217n3p292. [DOI] [PubMed] [Google Scholar]
- Duda TFJ, Kohn AJ, Palumbi SR. Origins of diverse feeding ecologies within Conus, a genus of venomous marine gastropods. Biol J Linn Soc. 2001;73:391–409. [Google Scholar]
- Duda TFJ, Lee T. Isolation and population divergence of a widespread Indo-West Pacific marine gastropod at Easter Island. Mar Biol. 2009a;156:1193–1202. [Google Scholar]
- Duda TFJ, Lee T. Ecological release and venom evolution of a predatory marine Snail at Easter Island. PLoS ONE. 2009b;4:e5558. doi: 10.1371/journal.pone.0005558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda TFJ, Lessios HA. Connectivity of populations within and between major biogeographic regions of the tropical Pacific in Conus ebraeus, a widespread marine gastropod. Coral Reefs. 2009;28:651–656. [Google Scholar]
- Duda TFJ, Palumbi SR. Developmental shifts and species selection in gastropods. Proc Natl Acad Sci. 1999a;96:10272–10277. doi: 10.1073/pnas.96.18.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda TFJ, Palumbi SR. Molecular genetics of ecological diversification: duplication and rapid evolution of toxin genes of the venomous gastropod Conus. Proc Natl Acad Sci. 1999b;96:6820–6823. doi: 10.1073/pnas.96.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda TFJ, Palumbi SR. Gene expression and feeding ecology: evolution of piscivory in the venomous gastropod genus Conus. Proc R Soc B. 2004;271:1165–1174. doi: 10.1098/rspb.2004.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda TFJ, Rolan E. Explosive radiation of Cape Verde Conus, a marine species flock. Mol Ecol. 2005;14:267–272. doi: 10.1111/j.1365-294X.2004.02397.x. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espino SL, Kohn AJ, Villanueva JA, Heralde FM, Showers Corneli P, Concepcion GP, Olivera BM, Santos AD. Feeding behavior, phylogeny, and toxinology of Conus furvus Reeve, 1843 (Gastropoda: Neogastropoda: Conidae) Nautilus. 2008;122:143–150. [Google Scholar]
- Espiritu DJD, Watkins M, Dia-Monje V, Cartier GE, Cruz LE, Olivera BM. Venomous cone snails: molecular phylogeny and the generation of toxin diversity. Toxicon. 2001;39:1899–1916. doi: 10.1016/s0041-0101(01)00175-1. [DOI] [PubMed] [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- Funk DJ, Omland KE. Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annu Rev Ecol Evol Syst. 2003;34:397–423. [Google Scholar]
- Huelsenbeck JP, Ronquist F, Hall B. MrBayes: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Kauferstein S, Huys I, Kuch U, Melaun C, Tytgat J, Mebs D. Novel conopeptides of the I-superfamily occur in several clades of cone snails. Toxicon. 2004;44:539–548. doi: 10.1016/j.toxicon.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kauferstein S, Porth C, Kendel Y, Wunder C, Nicke A, Kordis D, Favreau P, Koua D, Stöcklin R, Mebs D. Venomic study on cone snails (Conus spp) from South Africa. Toxicon. 2011;57:28–34. doi: 10.1016/j.toxicon.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Keane TM, Creevey CJ, Pentony MM, Naughton TJ, McInerney JO. Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol Biol. 2006;6:1–17. doi: 10.1186/1471-2148-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AJ. The ecology of Conus in Hawaii. Ecol Monogr. 1959;29:47–90. [Google Scholar]
- Kohn AJ. Type specimens and identity of the described species of Conus. 4. The species described by Hwass, Bruguière and Olivi in 1792. J Linn Soc Lond Zool. 1968;47:431–503. [Google Scholar]
- Kohn AJ. Evolutionary ecology of Conus on Indo-Pacific coral reefs. Fifth International Cora l Reef Congress. 1985:139–144. [Google Scholar]
- Kohn AJ. Tempo and mode of evolution in Conidae. Malacologia. 1990;32:55–67. [Google Scholar]
- Kohn AJ. Maximal species richness in Conus: diversity, diet and habitat on reefs of northeast Papua New Guinea. Coral Reefs. 2001;20:25–38. [Google Scholar]
- Kohn AJ. Egg size, life history, and tropical marine gastropod biogeography. Am Malacol Bull. 2012;30:163–174. [Google Scholar]
- Kohn AJ, Nishi M, Pernet B. Snail spears and scimitars: a character analysis of Conus radular teeth. J Molluscan Stud. 1999;65:461–481. [Google Scholar]
- Kohn AJ, Nybakken JW. Ecology of Conus on eastern Indian Ocean fringing reefs: Diversity of species and resource utilization. Mar Biol. 1975;29:211–234. [Google Scholar]
- Kordis D, Gubensek F. Adaptive evolution of animal toxin multigene families. Gene. 2000;261:43–52. doi: 10.1016/s0378-1119(00)00490-x. [DOI] [PubMed] [Google Scholar]
- Kozminsky-Atias A, Bar-Shalom A, Mishmar D, Zilberberg N. Assembling an arsenal, the scorpion way. BMC Evol Biol. 2008;8:333. doi: 10.1186/1471-2148-8-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus NJ, Showers Corneli P, Watkins M, Bandyopadhyay PK, Seger J, Olivera BM. Against expectation: a short sequence with high signal elucidates cone snail phylogeny. Mol Phylogenet Evol. 2011;58:383–389. doi: 10.1016/j.ympev.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus NJ, Watkins M, Bandyopadhyay PK, Seger J, Olivera BM, Showers Corneli P. A very short, functionally constrained sequence diagnoses cone snails In several Conasprella clades. Mol Phylogenet Evol. 2012;65:335–338. doi: 10.1016/j.ympev.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluisma AO, Milash BA, Moore B, Olivera BM, Bandyopadhyay PK. Novel venom peptides from the cone snail Conus pulicarius discovered through next-generation sequencing of its venom duct transcriptome. Mar Genomics. 2012;5:43–51. doi: 10.1016/j.margen.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch VJ. Inventing an arsenal: adaptive evolution and neofunctionalization of snake venom phospholipase A2 genes. BMC Evol Biol. 2007;7:2. doi: 10.1186/1471-2148-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Maddison D. Mesquite: a modular system for evolutionary analysis Version 2.01. 2009 Available from http://mesquiteproject.org.
- Monje VD, Ward R, Olivera BM, Cruz LJ. 16 S mitochondrial ribosomal RNA gene sequences: a comparison of seven Conus species. Philipp J Sci. 1999;128:225–237. [Google Scholar]
- Nam HH, Corneli PS, Watkins M, Olivera B, Bandyopadhyay P. Multiple genes elucidate the evolution of venomous snail-hunting Conus species. Mol Phylogenet Evol. 2009;53:645–652. doi: 10.1016/j.ympev.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Olivera BM. Conus peptides: biodiversity-based discovery and exogenomics. J Biol Chem. 2006;281:31173–31177. doi: 10.1074/jbc.R600020200. [DOI] [PubMed] [Google Scholar]
- Palumbi S. Nucleic Acids II: the polymerase chain reaction. In: Hillis D, Moritz C, Mable BK, editors. Molecular Systematics. Sinauer Associates; Sunderland (MA): 1996. pp. 205–247. [Google Scholar]
- Pereira CM, Rosado J, Seabra SG, Pina-Martins F, Paulo OS, Fonseca PJ. Conus pennaceus: a phylogenetic analysis of the Mozambican molluscan complex. Afr J Mar Sci. 2010;32:591–599. [Google Scholar]
- Puillandre N, Duda TF, Jr, Meyer CP, Olivera BM, Bouchet P. One, four or 757 100 genera? A new classification of the cone snails. J Molluscan Stud. doi: 10.1093/mollus/eyu055. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puillandre N, Kantor Y, Sysoev A, Couloux A, Meyer C, Rawlings T, Todd JA, Bouchet P. The dragon tamed? A molecular phylogeny of the Conoidea (Mollusca, Gastropoda) J Molluscan Stud. 2011a;77:259–272. [Google Scholar]
- Puillandre N, Koua D, Favreau P, Olivera BM, Stöcklin R. Molecular phylogeny, classification and evolution of conopeptides. J Mol Evol. 2012a;74:297–309. doi: 10.1007/s00239-012-9507-2. [DOI] [PubMed] [Google Scholar]
- Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol. 2012b;21:1864–1877. doi: 10.1111/j.1365-294X.2011.05239.x. [DOI] [PubMed] [Google Scholar]
- Puillandre N, Meyer CP, Bouchet P, Olivera BM. Genetic divergence and geographical variation in the deep-water Conus orbignyi complex (Mollusca: Conoidea) Zool Scr. 2011b;40:350–363. doi: 10.1111/j.1463-6409.2011.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puillandre N, Samadi S, Boisselier MC, Sysoev AV, Kantor YI, Cruaud C, Couloux A, Bouchet P. Starting to unravel the toxoglossan knot: molecular phylogeny of the “turrids” (Neogastropoda: Conoidea) Mol Phylogenet Evol. 2008;47:1122–1134. doi: 10.1016/j.ympev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Puillandre N, Strong E, Bouchet P, Boisselier MC, Couloux A, Samadi S. Identifying gastropod spawn from DNA barcodes: possible but not yet practicable. Mol Ecol Resour. 2009;9:1311–1321. doi: 10.1111/j.1755-0998.2009.02576.x. [DOI] [PubMed] [Google Scholar]
- Puillandre N, Watkins M, Olivera BM. Evolution of Conus peptide genes: duplication and positive selection in the A-superfamily. J Mol Evol. 2010;70:190–202. doi: 10.1007/s00239-010-9321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ. Tracer v1.4. 2007 Available from http://beast.bio.ed.ac.uk/Tracer.
- Simon C, Franke A, Martin A. The polymerase chain reaction: DNA extraction and amplification. In: Hewitt GM, Johnson AWB, Young JPW, editors. Molecular Techniques in Taxonomy. Springer-Verlag; New York: 1991. pp. 329–355. [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stanley SM. Macroevolution: pattern and process. Freeman; San Francisco: 1979. [Google Scholar]
- Stanley SM. Predation defeats competition on the seafloor. Paleobiology. 2008;34:1–21. [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Taylor JD, Kantor YI, Sysoev AV. Foregut anatomy, feedings mechanisms and classification of the Conoidea (= Toxoglossa)(Gastropoda) Bull Nat Hist Mus Lond Zool. 1993;59:125–170. [Google Scholar]
- Terrat Y, Biass D, Dutertre S, Favreau P, Remm M, Stöcklin R, Piquemal D, Ducancel F. High-resolution picture of a venom gland transcriptome: Case study with the marine snail Conus consors. Toxicon. 2011;59:34–46. doi: 10.1016/j.toxicon.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Tucker JK, Tenorio MJ. Systematic classification of Recent and fossil conoidean gastropods. Conchbooks; Hackenheim, Germany: 2009. [Google Scholar]
- Violette A, Leonardi A, Piquemal D, Terrat Y, Biass D, Dutertre S, Noguier F, Ducancel F, Stöcklin R, Križaj I, Favreau P. Recruitment of glycosyl hydrolase proteins in a cone snail venomous arsenal: further insights into biomolecular features of Conus venoms. Mar Drugs. 2012;10:258–280. doi: 10.3390/md10020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ST, Duda TFJ. Did tectonic activity stimulate oligo-miocene specition in the indo-west Pacific? Evolution. 2008;62:1618–1634. doi: 10.1111/j.1558-5646.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- Yu Y, Harris AJ, He XJ. S-DIVA (Statistical Dispersal-Vicariance Analysis): a tool for inferring biogeographic histories. Mol Phylogenet Evol. 2010;56:848–850. doi: 10.1016/j.ympev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Yu Y, Harris AJ, He XJ. RASP (Reconstruct Ancestral State in Phylogenies) 2.1 beta. 2013 doi: 10.1016/j.ympev.2015.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


