Abstract
Malaria is a major global health problem for which an effective vaccine is required urgently. Prime-boost vaccination regimes involving plasmid DNA and recombinant modified vaccinia virus Ankara-encoding liver-stage malaria antigens have been shown to be powerfully immunogenic for T cells and capable of inducing partial protection against experimental malaria challenge in humans, manifested as a delay in time to patent parasitemia. Here, we report that substitution of plasmid DNA as the priming vector with a specific attenuated recombinant fowlpox virus, FP9, vaccine in such prime-boost regimes can elicit complete sterile protection that can last for 20 months. Protection at 20 months was associated with persisting memory but not effector T cell responses. The protective efficacy of various immunization regimes correlated with the magnitude of induced immune responses, supporting the strategy of maximizing durable T cell immunogenicity to develop more effective liver-stage vaccines against Plasmodium falciparum malaria.
Keywords: efficacy, vaccine, prime-boost, viral vector, immune correlate
Malaria caused by Plasmodium falciparum is a major global health problem, claiming the lives of up to an estimated 2.7 million children each year (1). This problem is increasing as parasite resistance to antimalarial drugs becomes more wide-spread and the mosquito vector develops resistance to insecticides. Thus, there is an urgent need for an effective vaccine against this pathogen.
Traditional approaches to vaccine development have concentrated on stimulating humoral immunity. However, there is considerable evidence from animal studies for the importance of cellular immunity in protection against several infectious diseases by depletion and adoptive transfer experiments. In murine malaria, CD8+ T cells have a key role in protection against liver-stage parasites (2). In humans, severe malaria is less likely in West African children expressing HLA-B53, suggesting a role for HLA class I-restricted T cells in immunity (3), and immunity in humans induced by irradiated sporozoites is associated with cellular responses (4).
A safe vaccine strategy capable of inducing high-level CD8+ T cell responses could be valuable for prophylactic and therapeutic immunization against several infectious diseases, including malaria. Although DNA vaccines showed early promise, when strong cytotoxic T lymphocyte responses and protective immunity against influenza was demonstrated in mice after i.m. DNA vaccination (5), DNA vaccination alone generally elicited only weak cellular immune responses in humans (6, 7). In a murine model of malaria immunization with preerythrocytic antigens of Plasmodium berghei, consisting of a single “priming” immunization with plasmid DNA followed by a single “boosting” immunization with a recombinant modified vaccinia virus Ankara (MVA) expressing the same antigen, complete protection is induced against P. berghei sporozoite challenge, and this protection is associated with very high levels of peptide-specific IFN-γ-secreting CD8+ T cells (8). This “prime-boost” vaccination strategy of DNA vaccination followed by a booster immunization with a nonreplicating recombinant orthopox virus such as MVA or NYVAC encoding the same antigen has also proved to be immunogenic for T lymphocytes in P. yoelii malaria (9), tuberculosis (10), Ebola (11), and simian immunodeficiency virus (12) in animal studies. DNA-MVA regimes have proved to be safe (13), immunogenic, and capable of inducing partial but not complete protection (14) against malaria sporozite infection in human clinical trials. Although a significant delay in time to malaria infection was observed in vaccinees who had been immunized with high-dose DNA and MVA vaccines, encoding the multiepitope (ME)-thrombospondin-related adhesion protein (TRAP) polyepitope-protein insert, no vaccinee showed sterile immunity. Indeed, a review of all malaria subunit vaccines trials conducted to date indicates that only the RTS,S vaccine has been reported to induce sterile immunity in more than one vaccinee. Protective efficacy with RTS,S has been shown to diminish markedly over 6 months in nonimmune volunteers (15), and immunity was found to be very short-lived in a Gambian field study (16).
Although initial prime-boost vaccinations against liver-stage malaria have been encouraging, improved immunization regimes using recombinant subunit vaccines that can lead to higher levels of protection are still required. Avipox viruses are capable of expressing antigens in mammalian cells and can induce a protective immune response against mammalian pathogens (17-19). This discovery has led to the development of recombinant fowlpox viruses as vaccines for use in mammals. Recombinant fowlpox viruses encoding tumor (20-22) and HIV antigens (23, 24) have been shown to elicit CD8+ T cell responses in animal models. FP9 is a highly attenuated form of fowlpox virus, derived from the WT fowlpox virus HP-1 by 438 serial passages (25) then plaque purification, the genome of which has been fully sequenced and found to harbor several deletions/insertions and gene modifications when compared with the sequence of WT fowlpox virus strains (26). We have found that a recombinant FP9 virus encoding the P. berghei circumsporozoite protein (PbCS) potently induces CD8+ T cell responses against PbCS and is more immunogenic for CD8 T cell induction than a commercially available fowlpox vaccine strain. When used in heterologous prime-boost vaccination regimes with a recombinant MVA encoding the same antigen, potent CD8+ T cell responses against the PbCS antigen and significant protection against a stringent challenge with P. berghei sporozoites are observed (27). Other recombinant poxviruses that have been used as candidate malaria vaccines, but not in heterologous prime-boost regimes, are NYVAC, a highly attenuated vaccinia virus (28), and the avipox vector ALVAC (canarypox) (29).
Here, we report on the use of recombinant FP9 and MVA vaccines encoding the preerythrocytic-stage malaria antigen TRAP and an ME string (together called ME-TRAP) in a series of phase I and IIa human clinical trials. The vaccines were administered intradermally (i.d.) as part of different prime-boost vaccination regimes involving FP9-, MVA-, and, in some cases, plasmid DNA-vectored vaccines expressing the same insert ME-TRAP. The most successful vaccination regime, involving two priming FP9 vaccinations followed by a single boosting MVA vaccination, induced complete protection in two subjects in a human malaria challenge model by using a parasite strain different from the vaccine antigen strain. One of these subjects remained protected on rechallenge both 6 and 20 months later with detectable circulating memory T cell responses. This longevity (>10 months) of protective efficacy is apparently unprecedented in human malaria vaccine studies, including those using the effective but impractical regime of repeated irradiated sporozoite immunization. The current leading vaccine candidate RTS,S/AS02, that induces protection mediated, probably in part by a humoral response, has been shown to afford some measurable protection up to 6 months in nonimmune volunteers but (15) was shown only to last 9 weeks in a large field efficacy study in The Gambia (16). Partial protection, as defined as a delay in the time to patent parasitemia in vaccinated subjects compared with unvaccinated controls, was also seen for two other prime-boost vaccination regimes studied here. These vaccine regimes were immunogenic for T cells but not Abs, and the level of the T cell response induced by the various regimes correlated with the delay in time to patency for that immunization group.
This work shows that vaccination regimes designed specifically to stimulate potent cellular immune responses can lead to cases of full protection of humans against challenge with an infectious pathogen.
Materials and Methods
Study Design. We have shown previously that small sequential studies can identify highly immunogenic and protective regimes with DNA and MVA vaccines. Here, we extend this approach that provides a rapid and cost-effective means of identifying malaria vaccines and regimes, which should progress to field studies. This study is a series of open-label phase I and IIa clinical trials that have evaluated the safety, immunogenicity, and efficacy of prime-boost vaccination regimes by using the candidate vaccines DNA-ME-TRAP, FP9-ME-TRAP, and MVA-ME-TRAP. The studies took place between July 2001 and April 2003. Table 1 details vaccination regimes by groups. Groups 1 and 2 were small dose-finding studies to assess safety and tolerability of the FP9 vaccine. Subjects from vaccination groups 3-8 were invited to take part in a linked malaria challenge study to assess the efficacy of each vaccine regime. The malaria challenge studies took place 2 weeks after the final vaccination for groups 3a and 5-8 and at 7 weeks after final vaccination for groups 3b and 4. One volunteer from group 3b was challenged at 6 months. Two volunteers from group 3a were challenged a second time after 6 months and one of these volunteers was challenged again at 20 months.
Table 1. Vaccination regimes by group showing number of subjects per group and the number of those challenged.
| No. challenged
|
||||
|---|---|---|---|---|
| Group | Vaccine regime | No. of subjects | 2 wk | 7 wk |
| 1 | ff* | 3 | 0 | 0 |
| 2 | FF* | 3 | 0 | 0 |
| 3a | FFM† | 6 | 5 | 0 |
| 3b | FFM† | 12 | 0 | 11 |
| 4 | MMM† | 5 | 0 | 4 |
| 5 | FM‡ | 5 | 5 | 0 |
| 6 | MF‡ | 5 | 5 | 0 |
| 7 | DDMF§ | 4 | 4 | 0 |
| 8 | DDFM§ | 3 | 3 | 0 |
| Total | — | 46 | 22 | 15 |
f, FP9 at 5 × 107 plaque-forming units i.d.; F = FP9 at 1 × 108 plaque-forming units i.d.; M, MVA at 1.5 × 108 plaque-forming units i.d.; D, DNA at 2 mg i.m.
A 3-week interval between first and second vaccine.
A 3-week interval between the first and second vaccines and a 4-week interval between the second and third vaccine.
A 4-week interval between vaccines.
A 3-week interval between the first, second, and third vaccines and a 4-week interval between the third and fourth vaccines.
Subjects. The protocols for the vaccination and challenge studies were approved by the Oxford Research Ethics Committee and the vaccines were used in clinical trials after review of applications to the U.K. Medicines and Healthcare Products Regulatory Agency (governing the use of medicinal products within the United Kingdom). Forty-six healthy, malaria-naive male or female volunteers, aged 18-65, were recruited from the area near to the clinical trial site (Oxford) for the vaccination studies. Additional subjects were recruited as controls for the malaria challenge studies. Recruitment was noncoercive, and all volunteers underwent a medical screening evaluation and gave written, informed consent to participate.
Vaccine Insert. All of the study vaccines encode the same insert, known as ME-TRAP, which has been described in detail (14, 30). In brief, the ME-TRAP construct includes CD8+ and CD4+ T cell epitopes from preerythrocytic P. falciparum antigens fused in-frame to the entire preerythrocytic antigen TRAP and encodes a polypeptide of 789 aa. P. falciparum TRAP (PfTRAP) was selected because it is a well characterized protective antigen (14) with a protective homolog in rodents (8).
DNA-ME-TRAP and MVA-ME-TRAP Vaccines. The vaccine insert was inserted into a DNA plasmid and the genome of MVA as described (14).
FP9-ME-TRAP Vaccine. FP9 is a highly attenuated strain of fowlpox virus derived from the WT fowlpox virus HP-1 by 438 serial passages (25) and then plaque purification (26). FP9 recombinants encoding ME-TRAP were constructed as follows. The ME-TRAP DNA sequence was ligated into the SmaI-cloning site of the fowlpox shuttle vector pEFL29, placing expression of this gene under the control of the vaccinia virus P7.5 promoter. The pEFL29 plasmid also encodes a copy of the β-galactosidase gene under the control of the FP4b fowlpox late promoter, allowing identification of recombinant viruses by X-gal staining as described for vaccinia virus (31). Recombinant viruses were prepared by in vitro recombination of the shuttle vector encoding ME-TRAP with the FP9 fowlpox strain in primary cultures of chicken embryo fibroblasts. Recombinant viruses were repeatedly plaque purified in chicken embryo fibroblast monolayers until homogenous. A stock of FP9 ME-TRAP was supplied to IDT (Rosslau, Germany) for production of the clinical lot under GMP conditions.
Immunization. All vaccines were stored in glass vials in a monitored and alarmed freezer at -20°C. Frozen vials were defrosted at room temperature for at least 30 min before vaccination and were used within 2 hours. The DNA vaccination was administered i.m. by using a 21-gauge needle with two injections of 1 mg/ml being administered, one into each arm. Both viral vector vaccines were administered i.d. by using a 27-gauge needle with subjects receiving the total dose as two to six injections (depending on vaccine titer) into one or both arms.
Immunogenicity Measures. The main immunological measure used to determine vaccine immunogenicity was the ex vivo IFN-γ ELISPOT response. This assay was performed at baseline, 7, 21-28, and 150-300 days after each vaccination. These were performed on fresh peripheral blood mononuclear cells (PBMCs) by using pools of 20-mer peptides that span the length of TRAP and overlap by 10 aa. The known epitopes in the ME string were also tested in pools. In brief, 400,000 PBMCs per well were plated directly onto the ELISPOT plate (MAIP S45, Millipore) in the presence of 25 μg/ml each peptide, and incubated for 18 hours. ELISPOT responses to TRAP peptides of the vaccine strain (T9/96) and the challenge strain (3D7) were assessed separately. The 57 T9/96 TRAP peptides were tested in four pools, and the 3D7 TRAP peptides were tested in six pools. The promiscuous HLA class II-binding peptides from bacillus Calmette-Guérin and tetanus toxoid were tested separately. Assays were performed in duplicate, and the results are given as arithmetic means with 1 SD.
Serum samples diluted 1:100 in 1% BSA/0.05% Tween-PBS were tested for the presence of IgG Abs by using a standard ELISA method, and 1 μg/ml whole TRAP (3D7) or 4 μg/ml NANP-repeat-containing (R32LR) recombinant proteins were kindly provided by GSK Biologicals (Rixensart, Belgium) as antigens. The NANP repeat is the immunodominant B cell epitope of the circumsporozoite protein from P. falciparum (32).
Cultured ELISPOT assays were performed as described in ref. 33. In brief, 1 × 106 cryopreserved PBMCs were incubated with 10 μg/ml peptides, with 10 units/ml Lymphocult (Biotest, Dreieich, Germany) added on days 3 and 7, for a period of 10 days, after which a standard IFN-γ ELISPOT was performed by using 2.5 × 105 initially plated cells and 25 μg/ml peptides per well.
Malaria Challenge. By using a method adapted from Chulay et al. (34), subjects were infected with malaria at Imperial College, London. In brief, five Anopheles stephensi mosquitoes, each with 102-104 sporozoites per salivary gland, were allowed to bite each subject, thus delivering 3D7 strain P. falciparum sporozoites. Challenges took place 13-49 days after the final vaccination. Monitoring took place twice daily by using Giemsa-stained thick blood films and quantitative PCR starting on day 6.5 until day 14 and then once daily until the end of the study period at day 21. Subjects were treated with chloroquine after the first confirmed positive blood film or at day 21 if no parasitemia was detected. In addition to vaccinated subjects, five unvaccinated subjects were infected with malaria in each challenge study. The mean times to patent parasitemia in control subjects in different challenge studies were very similar and not statistically significantly different and thus “pooled” results from all controls (n = 28) are used for statistical analysis. The pooling of results from controls adds greater statistical power for the analysis of results from small, sequential clinical trials. All controls developed malaria. Efficacy results for different vaccine regimes have been analyzed by using Kaplan-Meier survival curves and log rank tests comparing each vaccine regime with pooled controls.
Results
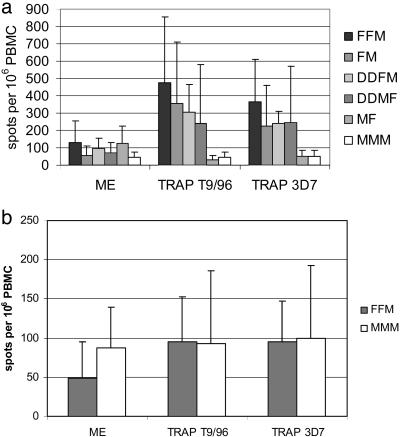
Prime-Boost Vaccination Regimes Are Immunogenic for T Lymphocytes. The insert in the vaccines used here, ME-TRAP, has been described in detail (14, 30) and includes a polyepitope string fused to the TRAP antigen from the T9/96 strain of P. falciparum; this TRAP sequence differs by 6% from the amino acid sequence of TRAP in the 3D7 strain of P. falciparum used for sporozoite challenge. The mean summed ex vivo IFN-γ responses to the ME string and to overlapping peptides representing each strain of TRAP, measured 7 days after the final vaccination for the different vaccine regimens, are shown (Fig. 1a). In general, this time point represents the highest T cell responses in these groups. For groups in which this is not the case, the peak response is seen 7 days after the penultimate vaccination (e.g., in the DDMF group the peak response is seen 7 days after the MVA vaccination; DDM plus 7, 1,166 specific fluorescence units (sfu) compared with DDMF plus 7, 242 sfu to T9/96 TRAP). This magnitude of response is similar to that seen after three priming DNA vaccinations followed by one boosting MVA vaccination (14). The responses seen after FP9 priming, either once or twice, followed by MVA boosting, are less strong (FFM, 475 sfu to T9/96 TRAP). In each vaccine regime group, the IFN-γ responses to TRAP were broadly cross-reactive between the vaccine strain (T9/96) and the different challenge strain (3D7). Homologous boosting did not increase T cell responses, and the individual vaccines used sequentially did not give IFN-γ responses that were statistically significantly different from the baseline; i.e., there was no boosting of the response after the second FP9 vaccination in the FFM group and no boosting after sequential MVA vaccinations in the MMM group.
Fig. 1.
Immune responses after vaccination. (a) Summed IFN-γ ELISPOT responses 7 days after final vaccination in each group. (b) Summed IFN-γ ELISPOT responses 7 weeks after final vaccination in groups 3b and 4. In all groups prevaccination responses were very low (33). One FFM subject and one MMM subject did not proceed to malaria challenge and are not included in this data set.
The ex vivo immune responses measured fell rapidly after the peak, and Fig. 1b shows the IFN-γ responses to both T9/96 and 3D7 strain peptides in the subjects from group 3b (11 FFM) and group 4 (4 MMM) on the day of challenge, 7 weeks after the final vaccination.
Prime-Boost Vaccination with These Vectors Does Not Induce a Detectable Ab Response. No induction of IgG Ab responses to the whole TRAP antigen or to the NANP repeats (included in the ME string) could be detected in subjects receiving these vaccine regimens. Subjects were tested before vaccination for a baseline reading and then 7 days after the final vaccination. The number of tested subjects from each regime was FFM (eight), MMM (three), DDMF (three), DDFM (two), FM (two), and MF (one). The two completely protected volunteers from group 3a were tested at day 0, 7 days after the final vaccination, and 7 days after the first challenge study, and no IgG Abs to the TRAP protein were detected.
The FFM Regime Results in Complete Protection Against Experimental Malaria Challenge in Some Subjects. Two of five subjects who went on to a malaria challenge conducted 14 days after their final vaccination from group 3a (FFM) were completely protected (subjects 132 and 137). These two subjects were entered, without further vaccinations, into a second malaria challenge 6 months later in which one subject (137) remained completely protected. This protected subject remained protected in an additional third challenge study carried out an additional 14 months later (20 months after his final vaccination). The two protected volunteers still had moderate ex vivo T cell responses at the time of second challenge, and these responses were broadly cross-reactive between the two TRAP strains (the results of subjects 132 and 137 were 225 and 154 sfu to T9/96 and 171 and 134 sfu to 3D7, respectively). However, at the time of the third challenge, subject 137 had no detectable ex vivo IFN-γ ELISPOT response, despite being fully protected. To measure circulating memory cells that are not detectable in ex vivo assays and that may represent central memory cells, we used a cultured ELISPOT assay that restimulates cells in vitro for 10 days before conducting the ELISPOT assay. Subject 137 had an elevated malaria-specific T cell response in cultured ELISPOT assays at the time of both challenges, 6 and 20 months after final vaccination. This response was cross-reactive between the two TRAP strains (400 and 700 sfu, respectively, to 3D7 and 360 and 370 sfu to T9/96).
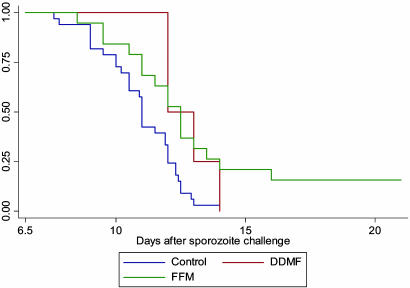
Analysis of all 17 subjects immunized with this FFM regime who underwent challenge (groups 3a and 3b combined) shows that, overall, compared with nonvaccinees, there is a highly significant delay in time to onset of parasitemia (P = 0.0013) as shown in Fig. 2. This regime leads to some fully protected individuals, whereas the previously reported DDDMM regimes did not (14). Although group 3a showed more fully protected individuals than group 3b, this difference is not statistically significant and, thus, may be a chance finding. Alternatively, this finding could reflect some early waning of protection as groups 3a and 3b were challenged, respectively, 2 and 7 weeks after the final vaccination.
Fig. 2.
Kaplan-Meier plot comparing time with patent parasitemia (by blood film) in FFM and DDMF-vaccinated subjects and in pooled controls from all challenge studies. Both vaccination regimes differ significantly from the nonvaccinated controls: P = 0.0013 for FFM and P = 0.0189 for DDMF. Subjects (18) received the FFM regime; five were challenged at 2 weeks, 11 were challenged at 7 weeks, and one was challenged at 6 months. Subjects (4) received the DDMF regime and were challenged after 2 weeks.
The DDMF Regime Shows Partial Efficacy and Is Associated with a Delay in the Onset of Patent Parasitemia. In an attempt to improve the immunogenicity and efficacy of our vaccination regimes, some subjects were vaccinated with triple regimes involving DNA, MVA, and FP9 (DDMF and DDFM). The DDMF vaccine regime partially protects subjects from malaria challenge (Fig. 2). This degree of protection is comparable with that shown in a previous study using a DNA-MVA prime-boost regime (14). The addition of FP9 to this vaccine regime does not improve the immunogenicity or degree of protection. DDFM is less immunogenic and shows no evidence of protection.
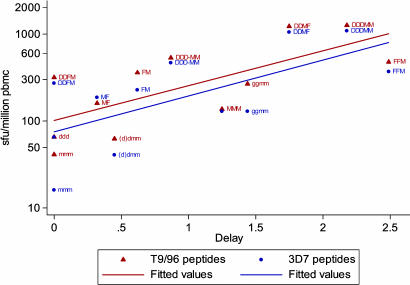
Immune Responses by Regime Correlate with Protective Efficacy. Various immunization regimes and three vectors have been assessed by using the ME-TRAP insert, and 66 subjects have been challenged. The ex vivo IFN-γ ELISPOT assay, which correlates with protection in mouse sporozoite challenge studies, was used as the primary read-out of immunogenicity in all of these studies. In an overall analysis, the mean level of this immune response in each different vaccination group correlates with the degree of protection seen in that group. The peak, the last (i.e., last vaccination plus 7 days), and the mean of these two ex vivo IFN-γ responses measured in the ELISPOT assay were plotted against the delay to parasitemia observed (values are given in Table 2, which is published as supporting information on the PNAS web site). Spearman's nonparametric analysis shows a positive correlation of protection with each immunogenicity value (i.e., peak immunogenicity, 7 days after final vaccination immunogenicity, and an average of these two values). Both peak T9/96 (correlation coefficient, 0.714; P = 0.0091) and peak 3D7 cellular immune responses (correlation coefficient, 0.659; P = 0.0198) and delay are plotted in Fig. 3. An estimated reduction in parasite burden in the liver was derived for all groups (Table 2). This reduction was calculated assuming an 8-fold replication rate of blood-stage parasites per 48-hour cycle. This replication rate was observed in early studies of malaria infection of nonimmune volunteers (35) and is lower than the rate recently estimated by quantitative analysis of PCR monitoring in the current volunteer studies (36). An 8-fold multiplication in 2 days implies that a 2-day delay in time to patent parasitemia corresponded to an ≈87.5% reduction in the number of viable parasites emerging from the liver on day 6.5 after the sporozoite challenge (34). Using the more precise estimate of growth rate obtained from sequential PCR density measurements (36), the regimes that have shown protection in this study (i.e., FFM and DDMF, cause a 92% and 84% reduction in parasite numbers, respectively, emerging from the liver).
Fig. 3.
The correlation between the level of the ex vivo IFN-γ ELISPOT response (the peak responses to both T9/96 and 3D7 peptides) for each group plotted against delay with ELISPOT values on a log scale. d = 1 mg of DNA ME-TRAP i.m.; g = 4 μg of DNA ME-TRAP given i.d. by gene gun; m = 5 × 107 plaque-forming units of MVA i.d.; D = 2 mg of DNA ME-TRAP i.m.; M = 15 × 107 plaque-forming units MVA i.d.; and F = 10 × 107 plaque-forming units FP9 i.d.. All vaccinations are given 3-4 weeks apart. Correlations of both peak T cell immune response to T9/96 strain peptides (correlation coefficient, 0.714; P = 0.0091) and 3D7 strain peptides (correlation coefficient, 0.659; P = 0.0198) with delay are significant.
Discussion
We describe subunit vaccines, designed specifically to induce a strong cell-mediated immune response, that can confer complete protection against an infectious disease in humans. This two-component vaccination approach, known as heterologous prime-boost immunization, could have wide applicability. Protection against many pathogens, both human and veterinary, depends on strong T cell responses, and it is a simple procedure to generate these recombinant poxvirus vectors expressing a range of antigens. Two of five subjects were completely protected against experimental malaria challenge by using a vaccination regime comprising two priming immunizations with an FP9 vaccine and a single boosting immunization with an MVA vaccine encoding the same insert when challenged after 2 weeks. The challenge used was stringent because the number of administered bites and the level of salivary gland infection are higher than in general field conditions. Additionally, the strain of parasite used in the challenge (3D7) was intentionally different than the vaccine strain (T9/96). The extent of sequence difference between these two strains for the TRAP antigen insert is greater than that generally found between African parasite strains (37).
This is only the second type of subunit vaccine for malaria to induce complete protective efficacy in some volunteers with the entire group of vaccinees showing significant evidence of protection compared with challenge controls. In contrast to the current most advanced malaria vaccine candidate, RTS,S/AS02A, the protection induced by these viral vector vaccines is likely to be entirely cell-mediated because no Ab responses to TRAP or to the NANP repeats from the ME string were detected. RTS,S/AS02A induces less strong effector T cell responses but very high Ab levels. Although RTS,S/AS02 protects a larger proportion of vaccinees than the vectored vaccines used here, no protection has been demonstrated past 6 months (15) and was relatively short-lived in Gambian and Mozambique field trials (16, 38). The FFM vaccinee that was repeatedly protected represents the longest time that vaccine-induced protection has lasted in malaria challenge studies, in this case, 20 months. In contrast, the longest protection demonstrated after vaccination with irradiated sporozoites is 42 weeks (39). This subject received no other vaccinations during this time but did participate in three challenge studies that might have boosted his cell-mediated immunity. However, ELISPOT analysis of large numbers of challenge subjects has shown little or no induction of T cell responses to ME-TRAP by sporozoite challenge and this procedure does not induce measurable protection against re-challenge. At the time of the third challenge study, this subject had no demonstrable ex vivo IFN-γ ELISPOT response but had a strong immune response as measured in a cultured ELISPOT assay. This responding cell population in cultured ELISPOT assays is likely to represent central memory T cells (40). Interestingly, responses measured by a cultured ELISPOT assay to another preerythrocytic P. falciparum antigen are associated with protection against infection and disease in a recent field study (41). Although we cannot rule out some boosting of the memory T cell response by exposure to parasites in the challenge studies, any such effect, i.e., boosting of vaccine-induced immunity by natural infection, would likely be of value in field settings in endemic areas.
It has been very difficult to identify correlates of protection in malaria vaccine studies. In analyses of small numbers of challenge subjects, it has been suggested that antisporozoite opsonophagocytic Abs or cellular responses to the circumsporozoite protein may correlate with induced protection, but these analyses were confounded by use of different adjuvants within the analyzed group (42, 43). Here, we show, in a large number of vaccinated challenge subjects, that the level of the ex vivo IFN-γ response correlates with protective efficacy across the various ME-TRAP vaccine immunization regimes studied. This observation will help design future vaccination approaches and help identify those that warrant further investigation in field studies where these strong cell-mediated responses may translate into a greater degree of protection in semiimmune individuals. Indeed the FFM regime identified here has very recently been shown to be safe and immunogenic in a phase I study in west Africa (44) and phase IIb efficacy studies are planned.
We have previously shown that a vaccine regime consisting of DNA as a priming agent and MVA as a boosting agent can induce very high levels of malaria-specific T cells (14) and that this response is associated with partial protection against malaria challenge. Here, we found that the regime DDMF induces a T cell response of a similar magnitude and is also associated with partial protection against malaria challenge. Vaccination regimes by using three vectors did not improve immunogenicity or protective efficacy. The DDFM regime was less immunogenic than DDM and was not protective possibly, we speculate, because some degree of immunity to the FP9 vector may have impaired boosting by MVA. Interestingly, similar studies in mice also failed to demonstrate increased immunogenicity of triple vector regimes (27). However, in contrast to the mouse model, the order of vaccination appears critical in human studies: when MVA was used as the prime, there was no boosting when a subsequent FP9 was administered. In mouse P. berghei studies, both FM and MF regimes induce very high immune responses (27). However, FP9 did moderately boost the immune response after two DNA vaccinations (see Fig. 1a).
The ex vivo IFN-γ responses to the DNA-MVA vaccine regimes are greater than those of the FP9-MVA regime and yet only the latter could induce complete protection in some subjects. We present elsewhere (33) a more detailed analysis of immune responses in these subjects showing that vaccine regimes using DNA priming predominantly stimulated CD4+ T cells whereas vaccine regimes with FP9 as the priming agent induced significantly more CD8+ T cells in addition to the CD4+ T cells. This finding suggests, in keeping with much previous indirect evidence, that induced CD8+ T cell responses may be of particular value in vaccination against liver-stage malaria.
In summary, we have shown that the recombinant poxviruses FP9 and MVA used in prime-boost regimes are safe and immunogenic for both CD8 and CD4 T cells, and they generate promising levels of T cell-mediated, protective efficacy in human volunteer studies of malaria. It remains to be determined how well these candidate vaccines and poxviruses expressing other malaria antigens (45) will protect against infection and disease in endemic areas.
Supplementary Material
Acknowledgments
We thank Angela Hunt-Cooke and Simon Correa for slide reading, Lynn Andrews for nursing assistance, and the volunteers for participating. This work was supported by the Wellcome Trust. D.P.W. is a Wellcome Trust Research Fellow, S.M.L. and M.A.S. are supported by the Biotechnology and Biological Sciences Research Council. J.M.V. is in part supported by a grant from the Finnish Academy. A.V.S.H. is a Wellcome Trust Principal Research Fellow.
Author contributions: S.D., J.M.V., R.S., S.C.G., and A.V.S.H. designed research; D.P.W., S.D., J.M.V., T.B., S.K., S.M.L., S.J.M., I.P., L.A., R.F.A., P.B., G.B., R.S., M.A.S., S.A.G., and A.V.S.H. performed research; D.P.W., L.A., R.F.A., P.B., G.B., R.S., S.A.G., and A.V.S.H. analyzed data; and D.P.W., S.G., and A.V.S.H. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MVA, modified vaccinia virus Ankara; ME, multiepitope; TRAP, thrombospondin-related adhesion protein; PBMC, peripheral blood mononuclear cell; sfu, specific fluorescence units; i.d., intradermally.
References
- 1.World Health Organization (2000) Tech. Rep. Ser. i-v 892, 1-74. [PubMed] [Google Scholar]
- 2.Doolan, D. L. & Hoffman, S. L. (2000) J. Immunol. 165, 1453-1462. [DOI] [PubMed] [Google Scholar]
- 3.Hill, A. V., Allsopp, C. E., Kwiatkowski, D., Anstey, N. M., Twumasi, P., Rowe, P. A., Bennett, S., Brewster, D., McMichael, A. J. & Greenwood, B. M. (1991) Nature 352, 595-600. [DOI] [PubMed] [Google Scholar]
- 4.Herrington, D., Davis, J., Nardin, E., Beier, M., Cortese, J., Eddy, H., Losonsky, G., Hollingdale, M., Sztein, M., Levine, M., et al. (1991) Am. J. Trop. Med. Hyg. 45, 539-547. [DOI] [PubMed] [Google Scholar]
- 5.Ulmer, J. B., Donnelly, J. J., Parker, S. E., Rhodes, G. H., Felgner, P. L., Dwarki, V. J., Gromkowski, S. H., Deck, R. R., DeWitt, C. M., Friedman, A., et al. (1993) Science 259, 1745-1749. [DOI] [PubMed] [Google Scholar]
- 6.Wang, R., Doolan, D. L., Le, T. P., Hedstrom, R. C., Coonan, K. M., Charoenvit, Y., Jones, T. R., Hobart, P., Margalith, M., Ng, J., et al. (1998) Science 282, 476-480. [DOI] [PubMed] [Google Scholar]
- 7.Wang, R., Epstein, J., Baraceros, F. M., Gorak, E. J., Charoenvit, Y., Carucci, D. J., Hedstrom, R. C., Rahardjo, N., Gay, T., Hobart, P., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 10817-10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider, J., Gilbert, S. C., Blanchard, T. J., Hanke, T., Robson, K. J., Hannan, C. M., Becker, M., Sinden, R., Smith, G. L. & Hill, A. V. (1998) Nat. Med. 4, 397-402. [DOI] [PubMed] [Google Scholar]
- 9.Sedegah, M., Jones, T. R., Kaur, M., Hedstrom, R., Hobart, P., Tine, J. A. & Hoffman, S. L. (1998) Proc. Natl. Acad. Sci. USA 95, 7648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McShane, H., Brookes, R., Gilbert, S. C. & Hill, A. V. (2001) Infect. Immun. 69, 681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan, N. J., Sanchez, A., Rollin, P. E., Yang, Z. Y. & Nabel, G. J. (2000) Nature 408, 605-609. [DOI] [PubMed] [Google Scholar]
- 12.Hanke, T., Samuel, R. V., Blanchard, T. J., Neumann, V. C., Allen, T. M., Boyson, J. E., Sharpe, S. A., Cook, N., Smith, G. L., Watkins, D. I., et al. (1999) J. Virol. 73, 7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moorthy, V. S., McConkey, S., Roberts, M., Gothard, P., Arulanantham, N., Degano, P., Schneider, J., Hannan, C., Roy, M., Gilbert, S. C., et al. (2003) Vaccine 21, 2004-2011. [DOI] [PubMed] [Google Scholar]
- 14.McConkey, S. J., Reece, W. H., Moorthy, V. S., Webster, D., Dunachie, S., Butcher, G., Vuola, J. M., Blanchard, T. J., Gothard, P., Watkins, K., et al. (2003) Nat. Med. 9, 729-735. [DOI] [PubMed] [Google Scholar]
- 15.Stoute, J. A., Kester, K. E., Krzych, U., Wellde, B. T., Hall, T., White, K., Glenn, G., Ockenhouse, C. F., Garcon, N., Schwenk, R., et al. (1998) J. Infect. Dis. 178, 1139-1144. [DOI] [PubMed] [Google Scholar]
- 16.Bojang, K. A., Milligan, P. J., Pinder, M., Vigneron, L., Alloueche, A., Kester, K. E., Ballou, W. R., Conway, D. J., Reece, W. H., Gothard, P., et al. (2001) Lancet 358, 1927-1934. [DOI] [PubMed] [Google Scholar]
- 17.Taylor, J. & Paoletti, E. (1988) Vaccine 6, 466-468. [DOI] [PubMed] [Google Scholar]
- 18.Taylor, J., Weinberg, R., Kawaoka, Y., Webster, R. G. & Paoletti, E. (1988) Vaccine 6, 504-508. [DOI] [PubMed] [Google Scholar]
- 19.Taylor, J., Weinberg, R., Languet, B., Desmettre, P. & Paoletti, E. (1988) Vaccine 6, 497-503. [DOI] [PubMed] [Google Scholar]
- 20.Restifo, N. P., Minev, B. R., Taggarse, A. S., McFarland, B. J., Wang, M. & Irvine, K. R. (1994) Folia Biol. (Prague) 40, 74-88. [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, M., Bronte, V., Chen, P. W., Gritz, L., Panicali, D., Rosenberg, S. A. & Restifo, N. P. (1995) J. Immunol. 154, 4685-4692. [PMC free article] [PubMed] [Google Scholar]
- 22.Grosenbach, D. W., Barrientos, J. C., Schlom, J. & Hodge, J. W. (2001) Cancer Res. 61, 4497-4505. [PubMed] [Google Scholar]
- 23.Kent, S. J., Zhao, A., Best, S. J., Chandler, J. D., Boyle, D. B. & Ramshaw, I. A. (1998) J. Virol. 72, 10180-10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kent, S. J., Zhao, A., Dale, C. J., Land, S., Boyle, D. B. & Ramshaw, I. A. (2000) Vaccine 18, 2250-2256. [DOI] [PubMed] [Google Scholar]
- 25.Mayr, A. & Malicki, K. (1966) Zentralbl. Veterinarmed. B 13, 1-13. [PubMed] [Google Scholar]
- 26.Laidlaw, S. M. & Skinner, M. A. (2004) J. Gen. Virol. 85, 305-322. [DOI] [PubMed] [Google Scholar]
- 27.Anderson, R., Hannan, C., Gilbert, S., Laidlaw, S., Sheu, E., Korten, S., Sinden, R., Skinner, M. & Hill, A. (2004) J. Immunol. 172, 3094-3100. [DOI] [PubMed] [Google Scholar]
- 28.Ockenhouse, C. F., Sun, P. F., Lanar, D. E., Wellde, B. T., Hall, B. T., Kester, K., Stoute, J. A., Magill, A., Krzych, U., Farley, L., et al. (1998) J. Infect. Dis. 177, 1664-1673. [DOI] [PubMed] [Google Scholar]
- 29.Rogers, W. O., Baird, J. K., Kumar, A., Tine, J. A., Weiss, W., Aguiar, J. C., Gowda, K., Gwadz, R., Kumar, S., Gold, M. & Hoffman, S. L. (2001) Infect. Immun. 69, 5565-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert, S. C., Plebanski, M., Harris, S. J., Allsopp, C. E., Thomas, R., Layton, G. T. & Hill, A. V. (1997) Nat. Biotechnol. 15, 1280-1284. [DOI] [PubMed] [Google Scholar]
- 31.Chakrabarti, S., Brechling, K. & Moss, B. (1985) Mol. Cell. Biol. 5, 3403-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nussenzweig, V. & Nussenzweig, R. (1986) Ciba Found. Symp. 119, 150-163. [DOI] [PubMed] [Google Scholar]
- 33.Vuola. J, M., Keating, S., Webster, D. P., Berthoud, T., Dunachie, S., Gilbert, S. C., Hill, A. V. S. (2005) J. Immunol. 174, 449-455. [DOI] [PubMed] [Google Scholar]
- 34.Chulay, J. D., Schneider, I., Cosgriff, T. M., Hoffman, S. L., Ballou, W. R., Quakyi, I. A., Carter, R., Trosper, J. H. & Hockmeyer, W. T. (1986) Am. J. Trop. Med. Hyg. 35, 66-68. [DOI] [PubMed] [Google Scholar]
- 35.Simpson, J. A., Aarons, L., Collins, W. E., Jeffery, G. M. & White, N. J. (2002) Parasitology 124, 247-263. [DOI] [PubMed] [Google Scholar]
- 36.Bejon, P., Andrews, L., Andersen, E. F., Dunachie, S, Webster, D., Walther, M., Gilbert, S.C., Peto, T., Hill, A.V.S. (2005) J. Infect. Dis., in press. [DOI] [PubMed]
- 37.Robson, K. J., Hall, J. R., Davies, L. C., Crisanti, A., Hill, A. V. & Wellems, T. E. (1990) Proc. R. Soc. London Ser. B 242, 205-216. [DOI] [PubMed] [Google Scholar]
- 38.Alonso, P. L., Sacarlal, J., Aponte, J. J., Leach, A., Macete, E., Milman, J., Mandomando, I., Spiessens, B., Guinovart, C., Espasa, M., et al. (2004) Lancet 364, 1411-1420. [DOI] [PubMed] [Google Scholar]
- 39.Hoffman, S. L., Goh, L. M., Luke, T. C., Schneider, I., Le, T. P., Doolan, D. L., Sacci, J., de la Vega, P., Dowler, M., Paul, C., et al. (2002) J. Infect. Dis. 185, 1155-1164. [DOI] [PubMed] [Google Scholar]
- 40.Godkin, A. J., Thomas, H. C. & Openshaw, P. J. (2002) J. Immunol. 169, 2210-2214. [DOI] [PubMed] [Google Scholar]
- 41.Reece, W. H., Pinder, M., Gothard, P. K., Milligan, P., Bojang, K., Doherty, T., Plebanski, M., Akinwunmi, P., Everaere, S., Watkins, K. R., et al. (2004) Nat. Med. 10, 406-410. [DOI] [PubMed] [Google Scholar]
- 42.Schwenk, R., Asher, L. V., Chalom, I., Lanar, D., Sun, P., White, K., Keil, D., Kester, K. E., Stoute, J., Heppner, D. G. & Krzych, U. (2003) Parasite Immunol. 25, 17-25. [DOI] [PubMed] [Google Scholar]
- 43.Sun, P., Schwenk, R., White, K., Stoute, J. A., Cohen, J., Ballou, W. R., Voss, G., Kester, K. E., Heppner, D. G. & Krzych, U. (2003) J. Immunol. 171, 6961-6967. [DOI] [PubMed] [Google Scholar]
- 44.Moorthy, V. S., Imoukhuede, E. B., Keating, S., Pinder, M., Webster, D., Skinner, M. A., Gilbert, S. C., Walraven, G. & Hill, A. V. (2004) J. Infect. Dis. 189, 2213-2219. [DOI] [PubMed] [Google Scholar]
- 45.Prieur, E., Gilbert, S. C., Schneider, J., Moore, A. C., Sheu, E. G., Goonetilleke, N., Robson, K. J. & Hill, A. V. (2004) Proc. Natl. Acad. Sci. USA 101, 290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.