Abstract
Background
The treatment options for non-muscle invasive bladder cancer (NMIBC) remain limited. BCG was the last major breakthrough in bladder cancer therapy almost 4 decades ago. There have been improvements in the understanding of immune therapies and cancer biology, leading to the development of novel agents. This has led to many clinical trials that are currently underway to find the next generation of therapies for NMIBC.
Method
We reviewed clinicaltrials.org and pubmed.gov to find the recently completed and ongoing clinical trials in NIMBC. Included in this review are clinical trials that are currently active and trials that were completed in and after 2014.
Result
Many trials with BCG naïve and BCG unresponsive/recurrent/refractory/failure NMIBC patients are either currently underway or have been recently completed. A wide variety of novel therapeutic agents are being investigated that range from cytotoxic agents to immunomodulatory agents to targeted molecular therapies. Other approaches include cancer vaccines, gene therapies, and chemoradiation potentiation agents. Novel drug delivery methods are also being tested.
Conclusion
This comprehensive update of current trials provides researchers an overview of the current clinical trial landscape for patients with NMIBC.
Keywords: Non-Muscle Invasive Bladder Cancer, BCG Naïve, BCG unresponsive, BCG refractory, BCG recurrent, BCG failure
Introduction
Urothelial carcinoma of the bladder is the fourth most common malignancy in men, with about 70% being non-muscle invasive bladder cancer (NMIBC). While NMIBC is associated with a higher than 88 percent survival rate over 5 years, up to 70 percent of NMIBC recur after initial treatment, with 10–20 percent progressing to muscle-invasive bladder cancer. [1] The high rate of recurrence with current therapies requires lifelong active surveillance, making bladder cancer the most expensive cancer to treat from diagnosis to death.[2]
The current standard of treatment for NMIBC is Transurethral Resection of Bladder Tumor (TURBT) with or without intravesical therapy, such as Mitomycin C (MMC) or Bacillus Calmette-Guerin (BCG), determined by the stage and grade of tumor. Intravesical BCG is the standard of care for patients with intermediate to high risk NMIBC. However, it is known that about 50% of patients fail BCG, significantly increasing the risk of progression and death. [3] Patients who fail BCG then require either surgical removal of the bladder with urinary diversion or chemotherapy and radiation, both of which have considerable morbidity. Furthermore, the recent termination of future BCG production at Sanofi Pasteur threaten global supply of BCG. [4] Given current therapies for bladder cancer have high rates of progression and recurrence, definitive therapy requires drastic change in quality of life, and the future potential for a BCG shortage, there are several reasons to urgently pursue new therapies in NMIBC patients. [5] Here we present a review of current or recently completed trials in NMIBC highlighting the many strategies being used to treat bladder cancer in both BCG naïve patients and patients who fail BCG.
Methods
This is a systematic review of currently active clinical trials and recently completed clinical trials (between 2014–2017) in NMIBC. We queried clinicaltrials.gov and pubmed.gov using the keywords ‘non-muscle invasive bladder cancer’ and ‘bladder cancer’ to search for the trials. The last review of the trials was performed on February 12, 2017. The trials were primarily categorized by the BCG status of the enrolled patients as self-described by the trials on clinicaltrials.org, which may differ from the emerging definition as described by Kamat et al.[6] Therefore, trials involving BCG refractory or BCG relapsing NMIBC have been grouped under ‘BCG unresponsive’. Further organization is based on therapy type – cytotoxic therapies, vaccines, gene therapy, immunomodulators, targeted therapy, and drug delivery. Within each therapy type, the trials were organized by the study status – completed, active with closed enrollment, and active with open enrollment.
BCG-Naïve
As mentioned previously, the standard-of-care for intermediate- and high-risk non-muscle invasive bladder cancer (NMIBC) includes transuretheral resection of bladder tumor (TURBT) followed by intravesical immunotherapy with BCG.[7] In general, while most patients are free of recurrence at 1 year with induction and maintenance BCG, as many as 75% develop a new tumor in 5 years, with a proportion progressing to muscle-invasive bladder cancer.[7] Many clinical trials are exploring new treatment options for these patients, and here we discuss the recently completed and currently ongoing trials in BCG naïve NIMBC patients (see table 1 and figure 1).
Table 1.
Summary of BCG-Naïve Clinical Trials
| Therapy Type | Agent | Study Type | Study Design | Patient Disease Status | Primary Outcome | Trial ID | Study Status |
|---|---|---|---|---|---|---|---|
| Cytotoxic Drugs | Epirubicin | Phase IV | Randomized Arm 1: Immediate intravesical instillation post TURBT Arm 2: TURBT only |
Stage and grade (not specified) | RR progression, death within 12 months-TURBT | NCT022 14602 |
Active, Not Recruiting |
| Mitomycin C | Phase III | Randomized: Arm A: BCG alone Arm B: BCG + MMC |
High Grade Ta, T1, or Tis | DFS over 5 years | NCT029 48543 |
Actively Recruiting | |
| Vaccine | recMAG E-A3 | Phase I | Non-randomized, single arm | High Grade Ta, T1, or Tis | Safety and Tolerability | NCT014 98172 |
Completed |
| TICE | Phase II | Non-randomized, single arm | High Grade Ta, T1, Tis | CR at 12 mo | NCT023 26168 |
Completed | |
| CAVATAK | Phase I | Non-randomized, Two groups Arm 1: CAVATAK Arm 2: CAVATAK + MMC |
Stage and grade (not specified) | Safety and Tolerability | NCT023 16171 |
Completed | |
| Immunomodulator | TMX-101 | Phase II | Non-randomized, single arm | High Grade Ta, T1, or Tis | CR by 5–7wks post-last tx | NCT017 31652 |
Completed |
| ALT-803 | Phase Ib/II | Randomized: Arm 1: BCG+ALT-803 Arm 2: BCG |
High Grade Ta, T1, or Tis | Safety, max. tolerated Dose, recommended dose with BCG | NCT021 38734 |
Actively Recruiting | |
| Drug Delivery | GemRIS | Phase Ib | Non-randomized, single arm | Low or intermediate risk | Safety, tolerability | NCT027 20367 |
Actively Recruiting |
CR = Complete Response, DFS = Disease Free Survival, RR = Recurrence Rate, MMC = Mitomycin C
Figure 1.
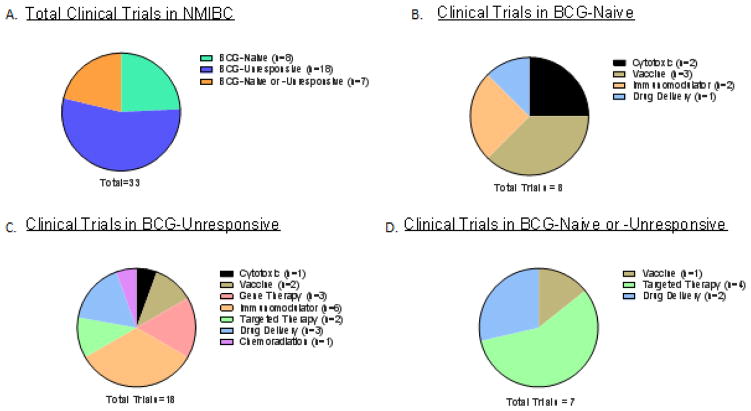
A) The total number of clinical trials in NMIBC reviewed in this article. B) Summary of recently completed or on-going clinical trials in BCG-Naïve patients. C) Summary of recently completed or on-going clinical trials in BCG-Unresponsive patients. D) Summary of recently completed or on-going clinical trials in patients with either BCG-Naïve or BCG-unresponsive NMIBC.
1. Cytotoxic Drugs
Epirubicin: Epirubicin is a derivative of doxorubicin that is commonly used as a chemotherapy agent in Europe and Japan post-TURBT, especially for patients with low and intermediate risk NMIBC with low risk of progression.[8–10] For intermediate and high-risk patients, BCG immunotherapy has been shown to be superior to epirubicin in prolonging the time to first recurrence, preventing distant metastasis, and improving overall survival, albeit at an increased risk of toxicity.[11] The current phase 4 trial is evaluating the efficacy of immediate intravesical instillation of epirubicin after TURBT in patients with primary or recurrent NMIBC by assessing the rates of recurrence, progression, and/or death from cancer over a 12-month period.
Mitomycin C with BCG: Mitomycin C is an antitumor agent that exerts its anti-tumor effects via DNA alkylation. [12] While intravesical BCG therapy is superior to MMC therapy in reducing rate of recurrence and tumor progression, a phase II trial has found the combination of MMC with BCG to be better than BCG therapy alone in improving recurrence-rates (42% vs. 58%). [13] The on-going phase III trial is evaluating the efficacy of the MMC and BCG combination intravesical therapy in high-risk NMIBC patients by assessing the disease free survival over 5 years.
2. Vaccine
recMAGE-A3 with AS15: MAGE-A3 is a cancer-testis antigen, expressed in various cancers including 40% of NMIBC.[14] Combining RecMAGE-A3 with an immunostimulant, AS15, has shown to induce a significant antigen-specific immune response and eliminate MAGE-A3 expressing tumors in an animal model.[15]In the recently completed phase I trial, intravesical BCG instillation with rec-MAGE-A3 + AS15 was found to be safe, tolerable, and effective in increasing vaccine specific T-cells locally in the bladder.[16]
Percutaneous BCG vaccination: Patients with a pre-existing positive tuberculin skin test have better recurrence-free survival rates than their counterparts with a negative skin test after intravesical BCG therapy.[17] Similar findings have also been reported in a murine orthotopic bladder cancer model.[17,18] The current phase 2 trial (PRIME trial/SWOG 160) is evaluating the efficacy of combining percutaneous BCG vaccine with the standard-of-care intravesical induction and maintenance BCG instillation in NMIBC patients by measuring the complete response rate at 12 months. Additionally, another strain of BCG, the Tokyo Strain, has been added to evaluate its efficacy in the event that TICE BCG is no longer available, as mentioned previously.
CAVATAK: CAVATAK, or Coxsackievirus A21 (CVA21), is an enterovirus which can be used as an oncolytic agent. It binds to CVA21 receptors, also known as intercellular adhesion molecule-1 (ICAM-1), that are upregulated on NMIBC cells, and it exerts its antitumor effects via the lytic viral cycle. [19,20] Interestingly, ICAM-1 expression is further enhanced in NMIBC when CVA21 is combined with Mitomycin C.[20] Hence, a recently completed phase I trial combined CVA21 with mitomycin C in pre-TURBT patients, which was tolerated well. [20]
3. Immunomodulator
TMX-101: TMX-101 is a formulation of imiquimod that is optimized for intravesical delivery. Imiquimod is a toll-like receptor 7-agonist (TLR), which is used as a topical chemotherapeutic agent for skin malignancies.[21] It has also been shown to have anti-tumor activity in bladder cancer via apoptosis and production of IL-6 and TNF-α cytokines.[21] Since TLR-7 is expressed on bladder cancer cells, imiquimod is believed to have efficacy in treating bladder cancer.[22] Its administration via intravesical instillation has also been found to be safe and tolerable.[23] In the recently completed phase 2 trial involving patients with carcinoma in situ (CIS), 2 out of 12 patients demonstrated negative cytology and biopsy results at 6 weeks following treatment. [24]
ALT-803: ALT-803 is a recombinant fusion protein with enhanced IL-15 biological activity, which is critical in the development, proliferation and activation of effector natural killer cells and CD-8+ T cells.[25,26] A preclinical study consisting of a carcinogen-induced NMIBC rat model demonstrates that the combination of intravesical BCG with ALT-803 reduces the tumor burden by 46% compared to control.[25] The ongoing phase I/II trial is evaluating the safety and efficacy of intravesical instillation of ALT-803 with BCG in high-risk NMIBC. Efficacy will be evaluated by assessing the complete response rate over a 4-year period.
4. Drug Delivery
GemRIS: GemRIS is a drug delivery system that allows for slow release of “enclosed” gemcitabine upon intravesical instillation over a 7-day period. Gemcitabine is part of the common chemotherapy regimen for invasive urothelial cancer in the neo-adjuvant and palliative settings.[27] Its intravesical administration has been shown to be safe and efficacious in improving recurrence-free survival, with multiple doses being superior to a single dose.[28] To enhance drug delivery over multiple days, the current phase 1b trial is evaluating the safety of intravesical gemcitabine delivery using GemRIS over 7days in low to intermediate risk NMIBC patients.
BCG-Unresponsive
BCG-unresponsive refers to BCG-refractory or BCG-relapsing disease. More specifically, BCG-refractory refers to presence of persistent high-grade cancer 6 months after the start of induction therapy, or cancers that have progressed by grade or stage 3 months after the start of the induction therapy.[7] On the other hand, “BCG-relapse” refers to patients who experience cancer recurrence after achieving a disease-free state at 6 months after treatment. [7] These are relatively new definitions and may not be reflected in trials started before 2015. The standard-of-care for these patients remains radical cystectomy, which has been shown to have significant disease-specific survival advantage if performed “early” (within 2 years of initial BCG treatment). [7,29] When cystectomy is not an option, Valrubicin is the only FDA approved intravesical agent available, albeit only for BCG-refractory CIS, with the disease-free response rate 16% at 12 months. [7] Therefore, many trials have emerged in this space (Figure 1 and table 2). Based on consensus among bladder cancer specialists and the FDA, adequate responses in BCG-unresponsive disease consist of an initial complete-response rate of 40–50% at 6 months and a durable response rate of at least 30% for 18 to 24 months. [30]
Table 2.
Summary of BCG-Unresponsive Clinical Trials
| Therapy Type | Agent | Study Type | Study Design | Patient Disease Status | Primary Outcome | Trial ID | Study Status |
|---|---|---|---|---|---|---|---|
| Cytotoxic | Cabazitaxel, (Cab) Gemcitabine (Gem) and Cisplatin (Cis) |
Phase I | Randomized: Arm 1: Gem + low Cab Arm 2: Gem + High Cab Arm 3: Gem + High Cab + low Cis Arm 4: Gem + High Cab + Mod Cis Arm 5: Gem + High Cab + High Cis |
High grade Ta, T1, or Tis | Safety, tolerability | NCT022 02772 |
Actively Recruiting |
| Vaccine | ALT-801 | Phase Ib/II | Non-randomized, single arm IV ALT-801 + IV Gemcitabine |
High Grade Ta, T1, or Tis | Safety, tolerability, CRR up to 13 weeks | NCT016 25260 |
Active, not recruiting |
| PANVAC | Phase II | Randomized: Arm 1: PANVAC + BCG Arm 2: BCG only |
High Grade Ta, T1, or Tis | DFS within 4–5 years | NCT020 15104 |
Actively Recruiting | |
| Gene Therapy | CG-0700 | Phase III | Non-Randomized -single arm | High Grade Ta, T1, or Tis | DCR at 18 months | NCT023 65818 |
Active, not recruiting |
| rAD-IFN/Syn3 | Phase III | Non-Randomized -single arm | High Grade Ta, T1, or Tis | EFS at 12 months | NCT027 73849 |
Actively Recruiting | |
| VPM1002BC | Phase I/II | Phase 1: Induction: 6 intravesical instillations in 6–12 weeks (dose de-escalation) Phase 2: Induction: as phase 1 Maintenance: 3 instillations at months 3, 6, and 12 |
High Grade Ta, T1, or Tis | Safety, tolerability, RFS at 60 weeks | NCT023 71447 |
Actively Recruiting | |
| Immunomodulator | Lenalidomide | Phase II | Non-randomized: Arm A: BCG + PO Lenalidomide Arm B: BCG only |
High Grade Ta, T1 or Tis | PFS, RFS, intact bladder survival at 12 months, | NCT013 73294 |
Active, not recruiting |
| Atezolizumab | Phase Ib/II | Arm 1: IV Atezolizumab Arm 2: IV Atezolizumab + BCG (BCG-unresponsive) Arm 3: Atezolizumab + BCG (BCG relapse) Arm 4: Atezolizumab + BCG (BCG-naïve) |
High Grade Ta, T1 or Tis | Safety, tolerability, CRR at 6 months | NCT027 92192 |
Actively Recruiting | |
| Atezolizumab | Phase II | Non-Randomized -single arm - IV Atezolizumab | High Grade Ta, T1 or Tis | EFS up to 18 months | NCT028 44816 |
Not yet open | |
| ALT-803 | Phase II/III | Non-Randomized: Single arm Arm 1: BCG+ALT-803 |
High Grade Ta, T1 or Tis | CRR at 6 months | NCT030 22825 |
Not yet open | |
| Pembrolizumab | Phase II | Non-Randomized -single arm - IV Pembrolizumab | High Grade Ta, T1 or Tis | CRR and DFS over 36 months, | NCT026 25961 |
Actively Recruiting | |
| Pembrolizumab | Phase I | Non-Randomized - single arm - Pre-Induction phase: Intravesical Pembrolizumab Induction phase: Intravesical Pembrolizumab + Intravesical BCGMaintenance phase: Intravesical Pembrolizumab + Intravesical BCG | High Grade Ta, T1 or Tis | Maximum Tolerated Dose | NCT028 08143 |
Not yet open | |
| Targeted Therapy | Vicinium (Opotuzumab Monatax) | Phase III | Non-Randomized - single arm Induction: 1x weekly for 6 weeks Maintenance: 1x weekly for up to 104 weeks | High Grade Ta, T1 or Tis | CRR up to 24 months | NCT024 49239 |
Actively Recruiting |
| BGJ398 | Phase I | Non-Randomized -single arm PO BGJ398 | High Grade Ta | TR within 7 weeks | NCT026 57486 |
Actively Recruiting | |
| Drug Delivery | ABI-009 (Nab-rapamycin) | Phase I/II | Non-Randomized - single arm | High Grade Ta, T1, or Tis | Safety, tolerability, CRR at 3 months | NCT020 09332 |
Actively Recruiting |
| Thermochemotherapy | Phase III | Non-Randomized - Single arm Synergo RITE + MMC | High Grade Ta, T1 | RFS at 12 months | NCT024 71495 |
Not yet open | |
| Albumin-bound Paclitaxel Nanoparticles | Phase III | Randomized: Arm 1: Nanoxel Arm 2: MMC |
High Grade Ta, T1 | RFS at 12 months | NCT029 82395 |
Actively Recruiting | |
| Chemoradiotion | Trimodality Therapy (Radition, cisplatin mitomycin C, 5-FU) | Phase II | Non-randomized -Single Arm - Radiation + Cisplatin - Radiation + MMC + 5-FU |
High Grade, Ta, T1 | Rate of freedom from radical cystectomy at 36 months | NCT009 81656 |
Actively Recruiting |
CRR = Complete Response Rate, DCR = Durable Complete Response, DFS = Disease-Free Survival, EFS = Event-Free Survival, PFS = Progression-Free Survival, RFS = Recurrence-free Survival, TR = Tumor Response
1. Cytotoxic Drugs
Cabazitaxel/Gemcitabine/Cisplatin: Multi-agent therapy is regularly used systemically in patients with metastatic disease. However, there is growing interest in using multiple agents intravesically, especially in patients who fail the BCG therapy. Since gemcitabine acts by inhibiting DNA synthesis and agents like docetaxel and MMC act via inhibition of cell division and DNA alkylation, respectively, sequential intravesical chemotherapy is used with gemcitabine given first followed by either docetaxel or MMC. In one series involving 45 patients with NMIBC recurrence after BCG therapy, the intravesical combination of gemcitabine with docetaxel showed disease-free rates of 54% at 1 year and 34% at 2 years.[31] Similarly, a multi-institutional retrospective study of high-risk NMIBC patients treated with sequential intravesical gemcitabine followed by MMC demonstrated recurrence-free survival rates of 50% at 12 months.[32] Given the encouraging outcomes, a phase I trial is underway to evaluate the safety of various combinations of chemotherapeutic agents, such as cabazitaxel, gemcitabine, and cisplatin when given intravesically in patients with BCG-unresponsive NMIBC.
2. Vaccine
ALT-801: ALT-801 is a fusion protein between IL-2 and a T cell receptor that recognizes surface peptide antigens derived from p53 when presented in the context of HLA-A2.[33] This facilitates a greater cell-mediated immune response by cytotoxic effector cells in a high expression p53 microenvironment by bringing together tumor cells and immune-effector cells.[33] The ongoing phase Ib/II trial is evaluating the safety, tolerability, and efficacy of systemic administration of ALT-801 with gemcitabine in patients who have failed BCG therapy. The efficacy will be evaluated by looking at complete response rate over the course of 13 weeks.
PANVAC: PANVAC is a pox viral vector-based vaccine that contains tumor associated antigens - carcinoembryonic antigen (CEA) and mucin-1 (MUC-1), and three T cell co-stimulatory molecules - B7-1, intracellular adhesion molecule 1 (ICAM-1), and lymphocyte function-associated antigen 3 (LFA-3).[26] This vaccine has been shown to induce a CD4 and CD8 antigen specific response against the aforementioned tumor associated antigens.[34] Given that CEA and MUC-1 are over expressed in 76% and 93% of high grade bladder tumors, respectively, the ongoing trial is evaluating the 12 month recurrence-free survival of combining PANVAC with BCG induction therapy in patients who have failed initial BCG induction.[35,36]
3. Gene Therapy
CG-0700: CG0700 is a recombinant adenovirus that specifically targets the often deregulated retinoblastoma tumor suppressor (Rb) pathway, allowing for selective viral replication in tumor cells and local production of granulocyte macrophage-colony stimulating factor (GM-CSF). [26,37] The local production of GM-CSF helps in recruitment and maturation of myeloid cells and promotion of local anti-tumor activity.[26] Its intravesical instillation has been shown to be safe and tolerable, with a complete response rate of 48.6% at 10.4 months.[26] The ongoing phase III trial is evaluating the efficacy of CG-0700 in NMIBC patients who have failed BCG therapy by looking at durable complete response lasting at least 12 months.
rAd-IFN/Syn-3 (Instiladrin): rAd-IFN is a non-replicating recombinant adenovirus vector containing the human IFN alpha-2b (IFNα2b) gene. The addition of the novel excipient Syn-3 to Rad-IFNα markedly increases its transduction of urothelium and NMIBC, allowing for high and durable IFNα in urine.[38] This is believed to be critical given the short half-life of IFNα in urine when added to BCG and the combination’s ability to improve upon response rates seen with BCG alone in BCG-unresponsive patients.[7] The phase I and II trials involving rAd-IFN/Syn-3 have shown that it leads to detectable levels of IFN-α in urine. [38,39] In fact, recurrence-free survival was 35% at 12 months in the phase II trial. Expanding on these findings, the current phase III trial evaluates the efficacy of rAD-IFN/Syn-3 in BCG-unresponsive NMIBC patients by looking at the event-free survival at 12 months.
VPM1002BC: VPM1002BC is a live genetically modified Mycobacterium bovis BCG that expresses the bacterial toxin listeriolysin. Studies in mice have shown that the antigen-specific memory T-cell and T follicular helper T-cells associated with specific antibody responses were higher using this recombinant form of BCG, presumably through the pore-forming ability of the toxin that enhances antigen presentation.[40] The ongoing phase I/II trial is evaluating the safety, tolerability, and efficacy of intravesical instillation of VMP1002BC along with standard BCG therapy in patients with recurrent NMIBC. The efficacy will be evaluated by assessing recurrence-free survival over 60 weeks.
4. Immunomodulators
Lenalidomide: Lenalidomide is a thalidomide derivative with anti-neoplastic, antiangiogenic, and immunomodulatory properties.[41] It has been shown to enhance the response to BCG in a murine model of bladder cancer.[41] The ongoing phase II trial is evaluating the efficacy of oral Lenalidomide with BCG instillation in BCG failure NMIBC patients by assessing the 1-year progression-free survival.
Atezolizumab: Atezolizumab is an anti-Program Death – Ligand 1 (PD-L1) humanized monocolonal immunoglobulin G1 antibody, which targets PD-L1 on tumor cells. PD-L1 is an immune checkpoint that suppresses T-cell function by binding to either program death (PD-1) or B7-1 on activated T lymphocytes and other immune cells, preventing overstimulation of the immune system against self-antigens. [42,43] Tumor cells have adapted by overexpressing PD-L1 and use it to create a “molecular shield” to prevent effector cells from killing tumor cells.[44] Inhibiting this interaction using Atezolizumab has recently been shown to be efficacious in locally advanced or metastatic urothelial carcinoma, gaining it an FDA approval. [44,45] The on-going phase Ib/II trial is evaluating its safety, tolerability, pharmacokinetics, immunogenicity, and anti-tumor activity when given with and without intravesical BCG therapy in NMIBC patients who have failed BCG. Additionally, an upcoming, inter-group, non-randomized, phase 2 trial will be evaluating its efficacy in treating BCG-unresponsive NMIBC patients by looking at 18-month event-free survival.
ALT-803: As mentioned previously, it is a recombinant fusion protein with enhanced IL-15 biological activity, an important interleukin in the development, proliferation, and activation of NK cells and CD8 T cells.[25,26] The current phase II/III trial is evaluating the 6-month complete response rate of ALT-803 in patients with BCG-unresponsive NMIBC.
Pembrolizumab (MK-3475): Pembrolizumab is an antibody that targets program death protein-1 (PD-1) on T cells, preventing its interaction with the PD-L1 ligand, similarly to PD-L1 antibodies.[44] The ongoing phase II trial is evaluating its efficacy in BCG unresponsive NMIBC patients by looking at the complete response rate and disease free survival over the course of 36 months. A separate phase I trial will be evaluating the safety and maximum tolerated dose of intravesical pembrolizumab when combined with intravesical BCG regimen in patients with BCG-unresponsive NMIBC.
5. Targeted Therapy
Oportuzumab Monatox (OM): OM is a recombinant fusion protein of a humanized anti-epithelial cell adhesion molecule (EpCAM) antibody linked to Pseudomonoas exotoxin A.[46] The EpCAM surface antigen is usually overexpressed in higher grade tumors, including bladder cancer, allowing OM to specifically target tumor cells.[26,46] A phase II trial evaluated two OM dosing strategies in BCG-refractory CIS patients and found complete response rates of 26.7% and 15.6% at 6- and 12-month intervals, respectively.[46] Its efficacy is now being evaluated in a phase III trial of BCG-unresponsive NMIBC patients.
BGJ398- BGJ398 is a tyrosine kinase inhibitor targeting FGFR3, which is found to be activated in approximately 75% of all cases of NMIBC.[47] The targeting of activated FGFR3 in preclinical rat models of bladder cancer has shown efficacy in inhibiting tumor growth. [48] The safety and efficacy of oral administration of BGJ398 is now being evaluated in a phase I/II trial of BCG refractory NMIBC patients using a marker lesion response at 7 weeks with cystoscopy and cytology.
6. Drug Delivery
ABI-009: ABI-009 is an albumin-bound Rapamycin nanoparticle that allows the hydrophilic rapamycin to better penetrate deeper tissues. Rapamycin is an mTOR pathway inhibitor which has been shown to limit progression of NMIBC to MIBC when given intravesically in a murine model of bladder cancer.[49] Currently, an ongoing phase I/II clinical trial is evaluating 12-month complete response rate of ABI-009 in the setting of BCG-unresponsive NMIBC.
Chemohyperthermia with MMC: Hyperthermia has been shown to improve penetration of the urothelium by chemotherapeutic agents, thereby improving the efficacy of agents such as MMC in treating NMIBC. The 10-year disease-free survival rate for this combination therapy has been found to be 53% compared to 15% for chemotherapy alone. [50] However, the combination has a significant side effect profile such as urinary urgency and frequency, pain, and bladder spasms. [51] Nevertheless, an ongoing phase III trial will be evaluating the efficacy of chemohyperthermia using MMC in patients with NMIBC who have failed BCG therapy.
Albumin-bound Paclitaxel Nanoparticles: Nanoxel is an albumin bound Paclitaxel nanoparticle (Nab-Paclitaxel) that has a fivefold solubility in aqueous environments with improved penetration of epithelial tumor cells via albumin receptor. [52] The phase 2 trial involving intravesical nab-paclitaxel treatment in patients with BCG failure NMIBC showed minimal toxicity and durable response rate of 35.7% at 1 year. [52] The current phase III trial aims to evaluate the efficacy of intravesical nab-paclitaxel in BCG failure NMIBC patients by looking at the 1 year recurrence-free rate.
7. Chemotherapy with Radiation
Trimodality Therapy (TMT): TMT refers to visually complete TURBT, followed by concurrent chemo-radiation involving agents such as 5-FU, mitomycin C, paclitaxel, cisplatin, and carboplatin. In muscle-invasive bladder cancer patients, it has been extensively evaluated in multiple non-randomized, prospective and retrospective trials showing similar overall survival as radical cystectomy.[53] Recent data indicates that TMT may also be efficacious in recurrent high grade T1 tumor, with University of Erlangen reporting an 88% complete response rate and a 19% progression rate in patients with T1G3 NMIBC tumors. [54] The current phase II trial (RTOG 0926) aims to evaluate the efficacy of TMT in NMIBC patients with persistent T1 disease who have failed BCG therapy. The efficacy will be evaluated by looking at the rate of freedom from radical cystectomy at 36 months. Of note, CIS is thought to be radio-resistant, which led to exclusion of patients with CIS from the trial. [55]
BCG-naïve or -unresponsive
The trials discussed below include both the BCG naïve and BCG-unresponsive patients, and they have either been recently completed or are ongoing (Figure 1 and table 3).
Table 3.
Summary of BCG-Naïve or-Unresponsive Clinical Trials
| Therapy Type | Agent | Study Type | Study Design | Patient Disease Status | Primary Outcome | Trial ID | Study Status |
|---|---|---|---|---|---|---|---|
| Targeted Therapy | Sunitinib | Phase II | Non-randomized, single arm: Intravesical BCG + PO Sunitinib | High grade Ta, T1, or Tis | CRR at 3 months | NCT007 94950 |
Active, not recruiting |
| Enzalutamide | Phase II | Non-randomized: Arm 1: Intermediate risk Arm 2: High risk |
Intermediate risk, high risk who undergo BCG treatment | RR within 12 months | NCT026 05863 |
Actively Recruiting | |
| Ethacrynic Acid | Phase I | Non-randomized, single arm | (Not listed) | Safety and tolerability | NCT028 52564 |
Actively Recruiting | |
| Tamoxifen | Phase II | Non-randomized, single arm, marker lesion | Low to intermediate Ta | Response of marker lesion over 4 year | NCT021 97897 |
Actively Recruiting | |
| Vaccine | HS-410 | Phase I/II | Phase I: non- randomized, single arm Phase Ii: Randomized, placebo-controlled Arm 1: HS-410 Low-Dose + BCG Arm 2: H2-410 High Dose + BCG Arm 3: Placebo + BCG Arm 4: If no BCG, will receive high dose HS-410 |
High Grade Ta, T1 or Tis | Phase I: Safety and tolerability Phase II: DFS over 12 months | NCT020 10203 |
Active, not recruiting |
| Drug Delivery | TC-3 Gel + MMC | Phase II | 3+3 Dose Escalation | Low and High Grade Ta, T1, or Tis | Rate of adverse events | NCT023 07487 |
Active, Not recruiting |
| TC-3 Gel + MMC | Phase I/II? | Randomized: Arm 1: 40mg MMC gel Arm 2: Standard of Care MMC w/water Arm 3: 80mg MMC gel |
Low Grade Ta | Ablative effects of pre-TURBT intravesical instillation, safety and tolerability over 24 months | NCT018 03295 |
Active, Not recruiting |
CRR = Complete Response Rate, DFS = Disease-Free Survival, RR = Recurrence Rate
1. Targeted Therapy
Sunitinib: Sunitinib is a multi-targeted receptor tyrosine kinase inhibitor that inhibits various key tyrosine kinases including vascular endothelial growth factor (VEGF) receptors-1,-2, and -3.[56] The anti-tumor effects of Sunitinib in advanced urothelial cancer is believed to be from inhibition of the VEGF axis.[56] In the ongoing phase II trial, oral sunitinib is combined with intravesical BCG instillation in high-risk NMIBC patients. The data from the study thus far shows that 72% of patients have a complete response at 3 months with 77% recurrence-free survival and 100% progression-free survival at 24 months.[57]
Enzalutamide: Enzalutamide is an androgen receptor antagonist that has been used in the treatment of metastatic castrate resistant prostate cancer. While the role of androgen in bladder tumorogenesis is unclear, it has been reported that androgen receptor activation correlates with promotion of tumor growth.[58] A preclinical study shows that enzalutamide can inhibit bladder cancer proliferation, migration, and invasion in AR+ cell lines.[58] Additionally, it can slow the growth of bladder tumors in AR+ xenografts.[58] The current non-randomized, phase 2 study aims to evaluate the efficacy of enzalutamide in preventing bladder cancer recurrence in patients with both AR+ or AR- NMIBC. The efficacy will be evaluated by looking at recurrence-rate over a 12-month period.
Ethacrynic Acid: Ethacrynic acid (EA) is a commonly used loop diuretic, but it has also been shown to be cytotoxic via inhibition of glutathione S-transferase (GST) and Wnt/β-catenin signaling. [59] Dysregulation of Wnt signaling is implicated in various tumors including bladder cancer, where it has been shown to be activated. [60] The current phase I trial aims to evaluate the safety and efficacy of Ethacrynic acid when given immediately before TURBT in patients with NMIBC.
Tamoxifen: Tamoxifen is a non-steroidal selective estrogen receptor modulator that is mainly used in the treatment of breast cancer. While normal urothelium has been shown to express estrogen receptor, expression of ERβ has been noted in up to 81% of bladder cancers, with increased levels of expression associated in higher-grade tumors.[61] A preclinical study involving nitrosamine induced bladder cancer in mice showed that tamoxifen treated mice had a reduced incidence of bladder cancer of 10–14% compared to 76% in the control group.[26,61] The ongoing phase II trial is evaluating the efficacy of oral tamoxifen citrate in patients with low to intermediate risk NMIBC by assessing the clinical response of a marker lesion over a 4-year period.
2. Vaccine
HS-410: HS-410 is an intra-dermally delivered vaccine derived from irradiated cancer cells that are genetically engineered to secrete gp96 heat shock protein. Gp96-cancer antigen complexes have been identified as efficient stimulators of CD8 cytotoxic T cell production via tumor antigen cross-presentation on MHC class I.[62] The ongoing phase I and II trials aim to evaluate the safety and efficacy of HS-410 with standard intravesical BCG in BCG-naïve patients with intermediate to high risk NMIBC. At the recent SUO Annual Meeting in 2016, the investigators of this trial reported on 1-year recurrence-free survival across all arms to be 84.6% with no difference between BCG alone and BCG with HS-410 arms. However, antigen-specific responses were only seen in patients who received HS-410.
3. Drug Delivery
TC-3 hydrogel: TC-3 gel is a reverse thermal biodegradable gel that exists as a free-flowing solution at room temperature and viscous hydrogel at body temperature. Its ability to stay in the gel form at body temperature allows it to serve as a sustained release drug reservoir inside the bladder by only dissolving upon contact with the urine, improving drug contact duration.[26] An ongoing phase 2 trial is evaluating the safety of intravesical instillation of escalating doses of TC-3 gel with mitomycin C in NMIBC patients. A separate, ongoing trial is evaluating the safety and efficacy of mitomycin C instillation using TC-3 in low risk, recurrent NMIBC patients pre-TURBT. The efficacy will be evaluated by assessing the ablative effect of the above treatment over 24 months.
Conclusion
In this review, we discussed the on-going and recently completed clinical trials involving the BCG naïve, BCG refractory/recurrent, and BCG naïve or refractory NMIBC. NMIBC remains a very challenging disease to treat, requiring extensive follow-up after diagnosis and initial treatment. The patients who are in even more peril are those who have failed BCG therapy, as they have a very high chance of progression. Given this high rate of recurrence and progression, especially in the face of the potential BCG vaccine shortage, the need to explore new avenues in treating NMIBC has never been more urgent. Evident by this review, many novel treatments ranging from targeting specific mutations or gene amplifications to gene therapy to immunotherapy are currently being tested in clinical trials in the setting of NMIBC. As our understanding of bladder cancer continues to evolve and new therapeutic approaches are developed, it will be crucial to utilize molecular subtyping in designing future studies and predicting treatment response in patients, as evident by some recent work in MIBC. [63] Additionally, going forward, it will be critical to standardize the clinical outcomes across trials, as emphasized and described by Kamat et al. [6] The ongoing efforts in this field are very encouraging, and the outlook for finding more effective therapies look very promising.
Highlights.
Article reviews and summarizes the recently completed or ongoing clinical trials in NMIBC
Total of 33 trials were reviewed, which included 8 trials that were specific to BCG-naïve patients, 18 trials that were specific for BCG-unresponsive patients, and 7 trials that included both BCG-Naïve or -Unresponsive
The following therapeutic approaches are being used in the trials: cytotoxic agents, vaccines, gene therapy, immunonodulators, targeted therapy agents, novel drug delivery mechanisms, and chemoradiation
Acknowledgments
FUNDING: This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, and other private donors. For a complete list, visit the foundation website at http://www.fnih.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaufman DS, Shipley WU, Feldman AS, Jemal A, Siegal R, Ward E, et al. Bladder cancer. Lancet (London, England) 2009;374:239–49. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 2.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–30. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 3.Packiam VT, Johnson SC, Steinberg GD. Non-muscle-invasive bladder cancer: Intravesical treatments beyond Bacille Calmette-Guérin. Cancer. 2017;123:390–400. doi: 10.1002/cncr.30392. [DOI] [PubMed] [Google Scholar]
- 4.Important Updates Regarding Sanofi Pasteur’s BCG Announcement. Am Urol Assoc. 2016 http://www.auanet.org/press-media/2016-BGC-announcement.cfm.
- 5.Veeratterapillay R, Heer R, Johnson MI, Persad R, Bach C. High-Risk Non-Muscle-Invasive Bladder Cancer-Therapy Options During Intravesical BCG Shortage. Curr Urol Rep. 2016;17:68. doi: 10.1007/s11934-016-0625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamat AM, Sylvester RJ, Böhle A, Palou J, Lamm DL, Brausi M, et al. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: Recommendations from the International Bladder Cancer Group. J Clin Oncol. 2016;34:1935–44. doi: 10.1200/JCO.2015.64.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamat A, Colombel M, Sundi D, Lamm D, Boehle A, Brausi M, et al. BCG-unresponsive non-muscle invasive bladder cancer: recommendations from the IBCG. Nat Rev Urol. 2017;14:244–55. doi: 10.1038/nrurol.2017.16. [DOI] [PubMed] [Google Scholar]
- 8.Hendricksen K, Cornel EB, de Reijke TM, Arentsen HC, Chawla S, Witjes JA. Phase 2 study of adjuvant intravesical instillations of apaziquone for high risk nonmuscle invasive bladder cancer. J Urol. 2012;187:1195–9. doi: 10.1016/j.juro.2011.11.101. [DOI] [PubMed] [Google Scholar]
- 9.Malmström P-U, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An Individual Patient Data Meta-Analysis of the Long-Term Outcome of Randomised Studies Comparing Intravesical Mitomycin C versus Bacillus Calmette-Guérin for Non–Muscle-Invasive Bladder Cancer. Eur Urol. 2009;56:247–56. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 10.Urdaneta G, Solsona E, Palou J. Intravesical Chemotherapy and BCG for the Treatment of Bladder Cancer: Evidence and Opinion. n.d doi: 10.1016/j.eursup.2008.04.006. [DOI] [Google Scholar]
- 11.Sylvester RJ, Oosterlinck W, van der Meijden APM. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186–90. doi: 10.1097/01.ju.0000125486.92260.b2. quiz 2435. [DOI] [PubMed] [Google Scholar]
- 12.Verweij J, Pinedo H. Mitomycin C: Mechanism of Action, Usefulness, and Limitations. 1990:5–15. [PubMed] [Google Scholar]
- 13.Hayne D, Stockler M, McCombie SP, Chalasani V, Long A, Martin A, et al. BCG+MMC trial: adding mitomycin C to BCG as adjuvant intravesical therapy for high-risk, non-muscle-invasive bladder cancer: a randomised phase III trial (ANZUP 1301) BMC Cancer. 2015;15:432. doi: 10.1186/s12885-015-1431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derré L, Cesson V, Lucca I, Cerantola Y, Valerio M, Fritschi U. Intravesical Bacillus Calmette Guerin combined with a cancer- vaccine increases local T-cell responses in non-muscle-invasive bladder cancer patients Statement of translational relevance Despite a gold standard treatment with Bacillus Calmette Guérin. BC n.d doi: 10.1158/1078-0432.CCR-16-1189. [DOI] [PubMed] [Google Scholar]
- 15.Gérard C, Baudson N, Ory T, Louahed J, van der Bruggen P, Traversari C, et al. Tumor Mouse Model Confirms MAGE-A3 Cancer Immunotherapeutic As an Efficient Inducer of Long-Lasting Anti-Tumoral Responses. PLoS One. 2014;9:e94883. doi: 10.1371/journal.pone.0094883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derré L, Cesson V, Lucca I, Cerantola Y, Valerio M, Fritschi U, et al. Intravesical Bacillus Calmette Guerin combined with a cancer-vaccine increases local T-cell responses in non-muscle-invasive bladder cancer patients. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1189. [DOI] [PubMed] [Google Scholar]
- 17.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat Rev Urol. 2014;11:153–62. doi: 10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 18.Biot C, Rentsch CA, Gsponer JR, Birkhäuser FD, Jusforgues-Saklani H, Lemaître F, et al. Preexisting BCG-Specific T Cells Improve Intravesical Immunotherapy for Bladder Cancer. Sci Transl Med. 2012:4. doi: 10.1126/scitranslmed.3003586. [DOI] [PubMed] [Google Scholar]
- 19.Skelding KA, Barry RD, Shafren DR. Enhanced oncolysis mediated by Coxsackievirus A21 in combination with doxorubicin hydrochloride. Invest New Drugs. 2012;30:568–81. doi: 10.1007/s10637-010-9614-0. [DOI] [PubMed] [Google Scholar]
- 20.Pandha HS, Annels NE, Simpson G, Mostafid H, Harrington KJ, Melcher A, et al. Phase I/II Canon Study: Oncolytic Immunotherapy for the treatment of non-muslce invasive bladder (NMIBC) cancer using intravesical Coxsackievirus A21. J Clin Oncol. 2016;34:2016. [Google Scholar]
- 21.Arends TJH, Lammers RJM, Falke J, van der Heijden AG, Rustighini I, Pozzi R, et al. Pharmacokinetic, Pharmacodynamic, and Activity Evaluation of TMX-101 in a Multicenter Phase 1 Study in Patients With Papillary Non-Muscle-Invasive Bladder Cancer. Clin Genitourin Cancer. 2015;13:204–209. e2. doi: 10.1016/j.clgc.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Smith EB, Schwartz M, Kawamoto H, You X, Hwang D, Liu H, et al. Antitumor effects of imidazoquinolines in urothelial cell carcinoma of the bladder. J Urol. 2007;177:2347–51. doi: 10.1016/j.juro.2007.01.112. [DOI] [PubMed] [Google Scholar]
- 23.Falke J, Lammers RJM, Arentsen HC, Ravic M, Pozzi R, Cornel EB, et al. Results of a phase 1 dose escalation study of intravesical TMX-101 in patients with nonmuscle invasive bladder cancer. J Urol. 2013;189:2077–82. doi: 10.1016/j.juro.2012.11.150. [DOI] [PubMed] [Google Scholar]
- 24.Donin NM, Chamie K, Lenis AT, Pantuck AJ, Reddy M, Kivlin D, et al. A phase 2 study of TMX-101, intravesical imiquimod, for the treatment of carcinoma in situ bladder cancer. Urol Oncol Semin Orig Investig. 2016 doi: 10.1016/j.urolonc.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Gomes-Giacoia E, Miyake M, Goodison S, Sriharan A, Zhang G, You L, et al. Intravesical ALT-803 and BCG treatment reduces tumor burden in a carcinogen induced bladder cancer rat model; a role for cytokine production and NK cell expansion. PLoS One. 2014;9:e96705. doi: 10.1371/journal.pone.0096705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehm BE, Svatek RS. Novel Therapeutic Approaches for Recurrent Nonmuscle Invasive Bladder Cancer. Urol Clin North Am. 2015;42:159–68. doi: 10.1016/j.ucl.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Jackson Benjamin, Griffiths TR, Leyshon Mellon JK. Intravesical Therapy for Bladder Cancer. 2015 doi: 10.1007/978-0-85729-482-1. [DOI] [Google Scholar]
- 28.Shelley MD, Jones G, Cleves A, Wilt TJ, Mason MD, Kynaston HG. Intravesical gemcitabine therapy for non-muscle invasive bladder cancer (NMIBC): a systematic review. BJU Int. 2012;109:496–505. doi: 10.1111/j.1464-410X.2011.10880.x. [DOI] [PubMed] [Google Scholar]
- 29.HERR HW, SOGANI PC. DOES EARLY CYSTECTOMY IMPROVE THE SURVIVAL OF PATIENTS WITH HIGH RISK SUPERFICIAL BLADDER TUMORS? J Urol. 2001;166:1296–9. doi: 10.1016/S0022-5347(05)65756-4. [DOI] [PubMed] [Google Scholar]
- 30.Jarow JP, Lerner SP, Kluetz PG, Liu K, Sridhara R, Bajorin D, et al. Clinical Trial Design for the Development of New Therapies for Nonmuscle-invasive Bladder Cancer: Report of a Food and Drug Administration and American Urological Association Public Workshop. Urology. 2014;83:262–5. doi: 10.1016/j.urology.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 31.Velaer KN, Steinberg RL, Thomas LJ, O’Donnell MA, Nepple KG. Experience with Sequential Intravesical Gemcitabine and Docetaxel as Salvage Therapy for Non-Muscle Invasive Bladder Cancer. Curr Urol Rep. 2016;17:38. doi: 10.1007/s11934-016-0594-2. [DOI] [PubMed] [Google Scholar]
- 32.Lightfoot AJ, Breyer BN, Rosevear HM, Erickson BA, Konety BR, O’Donnell MA. Multi-institutional analysis of sequential intravesical gemcitabine and mitomycin C chemotherapy for non-muscle invasive bladder cancer. Urol Oncol. 2014;32:35, e15-9. doi: 10.1016/j.urolonc.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen J, Zhu X, Liu B, You L, Kong L, Lee H, et al. Targeting activity of a TCR/IL-2 fusion protein against established tumors. Cancer Immunol Immunother. 2008;57:1781–94. doi: 10.1007/s00262-008-0504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gulley JL, Arlen PM, Tsang K-Y, Yokokawa J, Palena C, Poole DJ, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–9. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldenberg M. IMMUNOCYTOCHEMICAL DETECTION OF. n.d:1546–53. doi: 10.1002/1097-0142(197809)42:3+<1546::aid-cncr2820420829>3.0.co;2-d. [DOI] [PubMed]
- 36.Cardillo M, Castagna G, Memeo L, De Bernardinis E, Di Silverio F. Epidermal growth factor receptor, MUC-1 and MUC-2 in bladder cancer. J Exp Clin Cancer Res. 2000;19:225–33. [PubMed] [Google Scholar]
- 37.Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu I, Izumi K, et al. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 2012;109:1716–26. doi: 10.1111/j.1464-410X.2011.10706.x. [DOI] [PubMed] [Google Scholar]
- 38.Dinney CPN, Fisher MB, Navai N, O’Donnell MA, Cutler D, Abraham A, et al. Phase I Trial of Intravesical Recombinant Adenovirus Mediated Interferon-α2b Formulated in Syn3 for Bacillus Calmette-Guérin Failures in Nonmuscle Invasive Bladder Cancer. J Urol. 2016;190:850–6. doi: 10.1016/j.juro.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canter* Daniel, Park Elkins, Boorjian Stephen, Ogan Kenneth, Shore Neal, Bivalacqua Trinity, Bochner Bernard, Downs Tracy, Gomella Leonard, Grubb Robert, III, Inman Brant, Kamat Ashish, Karsh Larry, Krupski Tracey, Lerner Seth, Lotan Yair, Matthew Milowsky CD. Randomized Phase II trial of intravesical adenoviral mediated interferon-α gene therapy with the excipient Syn3 (rAd-IFNα/Syn3) in patients with BCG refractory or relapsing high grade (HG) non muscle invasive bladder cancer (NMIBC) J Urol. 2015;190:850–6. [Google Scholar]
- 40.Vogelzang A, Perdomo C, Zedler U, Kuhlmann S, Hurwitz R, Gengenbacher M, et al. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guérin ΔureC::hly vaccine’s superior protection against tuberculosis. J Infect Dis. 2014;210:1928–37. doi: 10.1093/infdis/jiu347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jinesh GG, Lee EK, Tran J, Kamat AM. Lenalidomide augments the efficacy of bacillus Calmette-Guerin (BCG) immunotherapy in vivo. Urol Oncol Semin Orig Investig. 2013;31:1676–82. doi: 10.1016/j.urolonc.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421–7. doi: 10.1038/bjc.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L, Han X, Ishida Y, Agata Y, Shibahara K, Honjo T, et al. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384–91. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atezolizumab for Urothelial Carcinoma. USA Food Drug Adm. 2016 https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm501878.htm.
- 46.Kowalski M, Guindon J, Brazas L, Moore C, Entwistle J, Cizeau J, et al. A phase II study of oportuzumab monatox: an immunotoxin therapy for patients with noninvasive urothelial carcinoma in situ previously treated with bacillus Calmette-Guérin. J Urol. 2012;188:1712–8. doi: 10.1016/j.juro.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 47.Lindgren D, Liedberg F, Andersson a, Chebil G, Gudjonsson S, Borg a, et al. Molecular characterization of early-stage bladder carcinomas by expression profiles, FGFR3 mutation status, and loss of 9q. Oncogene. 2006;25:2685–96. doi: 10.1038/sj.onc.1209249. [DOI] [PubMed] [Google Scholar]
- 48.Guagnano V, Furet P, Spanka C, Bordas V, Le Douget M, Stamm C, et al. Discovery of 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamino]-pyrimidin-4-yl}-1-methyl-urea (NVP-BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J Med Chem. 2011;54:7066–83. doi: 10.1021/jm2006222. [DOI] [PubMed] [Google Scholar]
- 49.Seager CM, Puzio-Kuter AM, Patel T, Jain S, Cordon-Cardo C, Mc Kiernan J, et al. Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer. Cancer Prev Res (Phila) 2009;2:1008–14. doi: 10.1158/1940-6207.CAPR-09-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colombo R, Salonia A, Leib Z, Pavone-Macaluso M, Engelstein D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC) BJU Int. 2011;107:912–8. doi: 10.1111/j.1464-410X.2010.09654.x. [DOI] [PubMed] [Google Scholar]
- 51.Kiss B, Schneider S, Thalmann GN, Roth B. Is thermochemotherapy with the Synergo system a viable treatment option in patients with recurrent non-muscle-invasive bladder cancer? Int J Urol. 2015;22:158–62. doi: 10.1111/iju.12639. [DOI] [PubMed] [Google Scholar]
- 52.McKiernan JM, Holder DD, Ghandour RA, Barlow LJ, Ahn JJ, Kates M, et al. Phase II trial of intravesical nanoparticle albumin bound paclitaxel for the treatment of nonmuscle invasive urothelial carcinoma of the bladder after bacillus calmette-gu??rin treatment failure. J Urol. 2014;192:1633–8. doi: 10.1016/j.juro.2014.06.084. [DOI] [PubMed] [Google Scholar]
- 53.Premo C, Apolo AB, Agarwal PK, Citrin DE. Trimodality therapy in bladder cancer: who, what, and when? Urol Clin North Am. 2015;42:169–80. vii. doi: 10.1016/j.ucl.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss C, Wolze C, Engehausen DG, Ott OJ, Krause FS, Schrott K-M, et al. Radiochemotherapy after transurethral resection for high-risk T1 bladder cancer: an alternative to intravesical therapy or early cystectomy? J Clin Oncol. 2006;24:2318–24. doi: 10.1200/JCO.2006.05.8149. [DOI] [PubMed] [Google Scholar]
- 55.Tang DH, Chang SS. Management of carcinoma in situ of the bladder: best practice and recent developments. Ther Adv Urol. 2015;7:351–64. doi: 10.1177/1756287215599694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gallagher DJ, Milowsky MI, Gerst SR, Ishill N, Riches J, Regazzi A, et al. Phase II study of sunitinib in patients with metastatic urothelial cancer. J Clin Oncol. 2010;28:1373–9. doi: 10.1200/JCO.2009.25.3922. [DOI] [PubMed] [Google Scholar]
- 57.Helfand AM, Lee CT, Hafez K, Hussain M, Liebert M, Daignault S, et al. Phase II clinical trial of intravesical bacillus Calmette-Guerin (BCG) followed by sunitinib for the treatment of high-risk nonmuscle-invasive bladder cancer (NMIBC) J Clin Oncol. 2015;(Suppl 7) [Google Scholar]
- 58.Kawahara T, Ide H, Kashiwagi E, El-Shishtawy KA, Li Y, Reis LO, et al. Enzalutamide inhibits androgen receptor–positive bladder cancer cell growth. Urol Oncol Semin Orig Investig. 2016;34:432.e15–432.e23. doi: 10.1016/j.urolonc.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 59.Lu D, Liu JX, Endo T, Zhou H, Yao S, Willert K, et al. Ethacrynic acid exhibits selective toxicity to chronic lymphocytic leukemia cells by inhibition of the Wnt/beta-catenin pathway. PLoS One. 2009;4:e8294. doi: 10.1371/journal.pone.0008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Majid S, Saini S, Dahiya R. Wnt signaling pathways in urological cancers: past decades and still growing. Mol Cancer. 2012;11:7. doi: 10.1186/1476-4598-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Godoy G, Gakis G, Smith CL, Fahmy O. Effects of Androgen and Estrogen Receptor Signaling Pathways on Bladder Cancer Initiation and Progression. Bl Cancer (Amsterdam, Netherlands) 2016;2:127–37. doi: 10.3233/BLC-160052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strbo N, Garcia-Soto A, Schreiber TH, Podack ER. Secreted heat shock protein gp96-Ig: Next-generation vaccines for cancer and infectious diseases. Immunol Res. 2013;57:311–25. doi: 10.1007/s12026-013-8468-x. [DOI] [PubMed] [Google Scholar]
- 63.Seiler R, Ashab HAD, Erho N, van Rhijn BWG, Winters B, Douglas J, et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur Urol. 2017 doi: 10.1016/j.eururo.2017.03.030. [DOI] [PubMed] [Google Scholar]