Abstract
This report describes outpatient (OP) administration of clofarabine in older patients (≥ 60 years) with untreated acute myelogenous leukemia (AML). Overall, 112 patients underwent clofarabine induction. Clofarabine was administered to 35 OPs for a total of 72 OP cycles, with 81% of these cycles representing consolidation treatment. Median length of hospital stay was 0–6 days and 5–25 days across OP and inpatient (IP) cycles, respectively. The most common adverse events (AEs) were nausea, vomiting, diarrhea, febrile neutropenia, edema, hypokalemia and pneumonia. The overall frequency of treatment-emergent grade ≥ 3 AEs and serious AEs was generally not different with IP or OP administration of clofarabine. No deaths were reported within 30 days following OP or IP consolidation cycles. In the appropriately selected older patient, OP administration of clofarabine consolidation appears feasible, is as well tolerated as IP administration and has potential to contribute to the quality of life in elderly patients with AML.
Keywords: Clofarabine, acute myelogenous leukemia, outpatient administration
Introduction
The prognosis for acute myelogenous leukemia (AML) in elderly adults ≥ 60 years of age is generally poor [1–5]. The typical induction chemotherapy protocol in elderly patients (i.e. 7 days of treatment with cytarabine and 3 days of treatment with anthracycline [7 + 3 regimen]) [4–6] is associated with a relatively poor overall response rate [2,5], a low median overall survival [1–3,5] and a high induction mortality rate [1,7,8]. In addition to the overall poor prognosis, few strategies have been developed to maintain the quality of life (QOL) for these patients [9,10]. Studies in patients with a variety of cancers, including both hematologic malignancies and solid tumors, have evaluated the post-induction administration of chemotherapy on an outpatient (OP) basis [9–13]. In select patients and with careful monitoring, these studies have demonstrated that this treatment strategy can be implemented safely, and in some cases can result in a substantial reduction in hospital stays, shorter duration of febrile neutropenia and fewer nosocomial infections [9,10,14], which may be associated with an improvement in patients’ QOL [9].
Clofarabine (Clolar®; Genzyme Corporation) is a rationally designed, second-generation purine nucleoside analog (2-chloro-2-fluoro-deoxy-9-β-D-arabinofuranosyladenine) that has demonstrated efficacy in older patients with previously untreated AML, without the neurotoxicity seen with other purine nucleoside analogs [6,7,15]. The phase 2 CLASSIC II trial evaluated clofarabine in a prospectively well-defined population of patients 60 years of age with previously untreated AML [16]. This open-label, single-arm study showed an overall remission rate of 46% in all evaluable patients and remission rates of 39% for patients ≥70 years; 32% for patients with an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 2; 51% for patients with antecedent hematologic disorder (AHD); and 54% and 42% for patients who had an intermediate or unfavorable karyotype, respectively. The median duration of remission was 56 weeks, and median overall survival was 41 weeks for all patients. These results indicate that clofarabine has activity in the treatment of elderly patients with AML who have at least one unfavorable prognostic factor.
The purpose of this current post hoc analysis was to describe the experience of OP administration of clofarabine in the CLASSIC II trial. The length of hospitalization with successive cycles of inpatient (IP) and OP clofarabine administration was assessed, and an analysis of the safety profile was conducted.
Materials and methods
Patient eligibility
Patients eligible for CLASSIC II had previously untreated AML (de novo, secondary or with AHD) according to World Health Organization criteria, were ≥60 years old, had an ECOG PS of 0–2 and had at least one of the following four unfavorable prognostic factors: age ≥70 years, ECOG PS 2, presence of AHD, or intermediate or unfavorable karyotype. Other principal inclusion and exclusion criteria have been described previously [16].
Treatment and study design
A treatment cycle was defined as the first day of study drug administration (day 1) up to and including the day before the first day of the immediate next treatment cycle. Treatment cycles commenced after day 28, and no later than day 85, from day 1 of the immediate previous treatment cycle.
During induction (treatment cycle 1), patients received 30 mg/m2 clofarabine via 1-hour (hr) intravenous (IV) infusion daily for 5 days. Clofarabine administration was discontinued upon evidence of leukemic progression, which was defined as an increase in bone marrow or peripheral blood blast count of ≥50%, or the appearance of new extramedullary disease. Patients could receive a second treatment cycle (as re-induction), administered after day 28 of cycle 1, if they had residual leukemia but did not meet the criteria for leukemic progression. Subsequent cycles were given as consolidation (cycle 2 as consolidation and cycles 3–6) to patients with documented complete remission (CR) or CR with incomplete platelet recovery (CRp). The clofarabine dose was 20 mg/m2 via 1 hr IV infusion daily for 5 days during either re-induction or consolidation; either four or five consolidation cycles were allowed, depending on whether or not patients underwent re-induction in their second cycle of clofarabine administration, but the maximum number of cycles allowed was six.
Administration of clofarabine was either IP or OP, at the discretion of the treating physician. The protocol recommended that patients receive hydration according to institutional guidelines. Daily prophylactic steroids before study drug administration were permissible but not mandated. The use of prophylactic antibacterial, antifungal and antiviral agents and treatment of fever and neutropenia were recommended according to each institution’ s guidelines. However, the use of nephrotoxic agents (e.g. vancomycin, amphotericin B) was avoided during clofarabine administration, to the extent possible.
End point definitions
Inpatient administration of study drug was defined as administration of study drug to patients who had been admitted to the hospital, whereas OP administration was defined as administration of study drug to patients who received treatment at a hospital or clinic but were not admitted to the hospital overnight. At the investigator’ s discretion, patients were either hospitalized or treated on an OP basis during the study. Separate determinations of hospital length of stay (LOS) were made for cycle 2 as re-induction, cycle 2 as consolidation and consolidation cycles 3–6. Length of stay, measured in days, included admission or readmission to the hospital for drug administration and/or complications of therapy.
Adverse events
Adverse events (AEs) were physician reported and were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE v3.0). The types of AEs experienced, and their incidence, severity, duration, causality and seriousness, were taken into account in determining the tolerability of clofarabine. After study treatment was discontinued, AEs continued to be reported for 45 days or until the patient received alternative therapy for AML. The safety profile of clofarabine was assessed by determining the incidence of treatment-emergent and treatment-related AEs and serious adverse events (SAEs) when the study drug was administered in either the IP or OP setting.
The institutional review board at each study site approved the study. Patients provided informed consent, and the terms of the study were in accordance with the Declaration of Helsinki.
Statistical analysis
Data results are expressed with descriptive statistics, as numbers, percentages, medians and ranges. The Fisher exact test was used to determine statistically significant differences at a level of 0.05. Adverse events were grouped according to their occurrence during IP or OP clofarabine cycles, in relation to the total number of treatment cycles and the relative incidence of each AE.
Results
Patient demographic and disease characteristics
All 112 patients enrolled in the CLASSIC II study were included in the post hoc analysis. Patient demographic and disease characteristics for the IP and OP groups across treatment cycles are summarized in Table I. Overall, the median age of patients in these two groups was similar. The proportion of patients with ECOG PS 0 was greater in the OP group than in the IP group in all consolidation cycles. The proportion of patients with ECOG PS 2 was about the same for IP and OP settings in cycle 2 as re-induction and appeared to be greater in the IP than in the OP setting in cycle 2 as consolidation, whereas all patients with this prognostic factor were allocated to an OP setting in consolidation cycles 3–6.
Table I.
Patient demographic and disease characteristics.
| Characteristic* | Cycle 1
|
Cycle 2 re-induction
|
Cycle 2 consolidation
|
Cycle 3
|
Cycle 4
|
Cycle 5
|
Cycle 6
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IP (n = 110) |
IP (n = 26) |
OP (n = 12) |
IP (n = 10) |
OP (n = 18) |
IP (n = 9) |
OP (n = 14) |
IP (n = 5) |
OP (n = 11) |
IP (n = 2) |
OP (n = 8) |
IP (n = 1) |
OP (n = 7) |
|
| Age, years | |||||||||||||
| Median | 71 | 72 | 69.5 | 70.5 | 67.5 | 71 | 70 | 70 | 67 | 76.5 | 70 | 72 | 70 |
| Range | 60–88 | 60–88 | 60–80 | 66–81 | 60–79 | 63–81 | 60–88 | 63–81 | 60–78 | 72–81 | 60–78 | 72–72 | 60–78 |
| Gender, % | |||||||||||||
| Female | 53.6 | 34.6 | 50 | 60 | 72.2 | 44.4 | 57.1 | 40 | 54.5 | 0 | 75 | 0 | 85.7 |
| ECOG PS, % | |||||||||||||
| 0 | 18.2 | 19.2 | 16.7 | 20 | 44.4 | 0 | 28.6 | 0 | 18.2 | 0 | 25 | 0 | 28.6 |
| 1 | 59.1 | 57.7 | 58.3 | 60 | 50 | 100 | 50 | 100 | 63.6 | 100 | 50 | 100 | 42.9 |
| 2 | 22.7 | 23.1 | 25 | 20 | 5.6 | 0 | 21.4 | 0 | 18.2 | 0 | 25 | 0 | 28.6 |
| With AHD, % | 6.4 | 7.7 | 8.3 | 0 | 11.1 | 0 | 7.1 | 0 | 9.1 | 0 | 12.5 | 0 | 14.3 |
IP, inpatient; OP, outpatient; ECOG PS, Eastern Cooperative Oncology Group performance status; AHD, antecedent hematologic disorder.
For each variable, if the patient had at least one assessment on or before the first clofarabine dose, then the latest of all measurements assessed before administration of the first clofarabine dose began is used for the baseline value.
IP versus OP administration of clofarabine
Overall, 112 patients received a total of 235 clofarabine treatment cycles. The median number of clofarabine cycles administered was 2 (range, 1–6 cycles), and eight patients (7.1%) received six cycles of treatment. Clofarabine was administered on an OP basis to 35 of 112 patients (31.2%) for a total of 72 OP cycles. Patients enrolled in the trial received a median of one OP cycle (range, 1–5 cycles). All 112 patients received treatment in the first induction cycle (cycle 1); in this cycle, IP administration occurred in 110 of 112 patients (98.2%) and OP administration in two of 112 patients (1.8%). A total of 66 of 112 patients (58.9%) began a second cycle of clofarabine: 38 of 66 patients (57.6%) as re-induction and 28 of 66 patients (42.4%) as consolidation. Of the 66 patients who initiated a second cycle of clofarabine, 33 patients (50.0%) received at least one OP cycle. During consolidation, 58 of 85 (68.2%) cycles were delivered in an OP setting in a total of 28 patients. The percentage of patients treated in the OP setting generally increased with administration of subsequent consolidation cycles.
Length of hospital stay for inpatients and outpatients
The initial treatment with clofarabine (induction cycle 1) was predominantly administered in the hospital setting (110 of 112 patients [98.2%]). Hospitalization was also maintained for most patients with documented progression of leukemia who required a second induction cycle (68.0% of patients). However, after cycle 2 as re-induction and as consolidation, there was a shift toward OP administration of further consolidation cycles of clofarabine treatment (median number of consolidation cycles was 1 [range, 1–6 cycles]). The proportion of patients receiving OP drug administration increased with each successive cycle and ranged from 14 of 23 patients (60.9%) in cycle 3 to seven of eight patients (87.5%) in cycle 6. The LOS for individual patients and the median LOS at each cycle for IP and OP groups are shown in Fig. 1. As the proportion of patients treated in the OP setting increased beyond cycle 2, the median LOS decreased.
Figure 1.
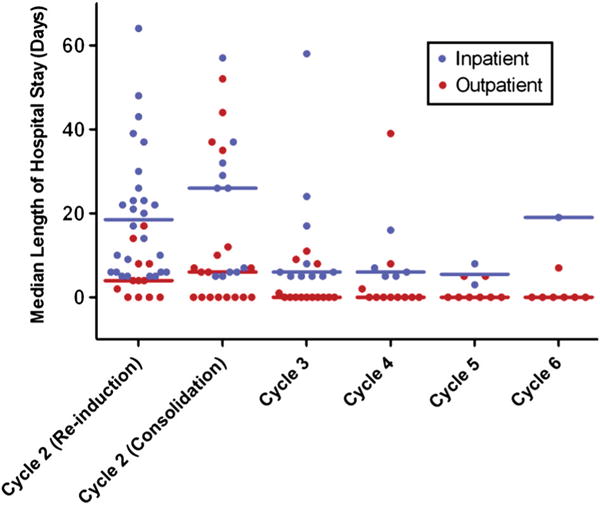
Length of stay (LOS) following inpatient or outpatient administration of clofarabine. Median LOS (indicated for each group by the horizontal bars) was determined for the intent-to-treat (n = 112) population; median LOS for inpatients includes the number of days of drug administration. Cycles 3 through 6 are consolidation cycles.
Among all patients in cycle 2, the median LOS decreased was 26 days (range, 4–66 days), and the median LOS was lower for cycles 2–6 than for cycle 1, regardless of whether cycle 2 was re-induction or consolidation treatment (9 [range, 0–64] and 7 [range, 0–57] days, respectively); LOS in cycles 3, 4, 5 and 6 was 3 [range, 0–58], 0 [range, 0–39], 0 [range, 0–13] and 0 [range, 0–19] days, respectively.
Safety and tolerability
All but two patients who received clofarabine in the OP setting tolerated the administration. One patient was hospitalized after 2 days of OP administration in cycle 2 as consolidation because of rash. All 5 days of subsequent cycles (cycles 3–6) in this patient were administered in the OP setting. The second patient who required hospitalization during clofarabine OP administration was admitted after the second dose of cycle 3 because of increasing transaminase levels. This patient also received all 5 days of the subsequent cycle (cycle 4) in the OP setting. Both patients achieved a complete response.
Adverse events
Except for IP cycle 4, treatment-emergent AEs occurred in 100% of inpatients and outpatients in all cycles (Table II). There were no significant differences in the frequency of treatment-emergent AEs between groups within any of the cycles of drug administration. Overall, nausea was the most common treatment-emergent AE (Table II). The most common treatment-emergent AEs in IP cycle 1 (induction) were nausea (69.1%), diarrhea (61.8%), febrile neutropenia (50.9%), edema (47.3%), vomiting (46.4%) and rash (40.0%). Pneumonia occurred most frequently in IP cycle 1 and IP cycle 2 as re-induction than in other cycles of drug administration.
Table II.
Treatment-emergent adverse events after inpatient and outpatient administration of clofarabine*.
| Cycle 1induction
|
Cycle 2 re-induction
|
Cycle 2 consolidation
|
Cycle 3
|
Cycle 4
|
Cycle 5
|
Cycle 6
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IP (n = 110) |
IP (n = 26) |
OP (n = 12) |
IP (n = 10) | OP (n = 18) |
IP (n = 9) |
OP (n = 14) |
IP (n = 5) |
OP (n = 11) |
IP (n = 2) |
OP (n = 8) |
IP (n = 1) |
OP (n = 7) |
|
| Any emergent AE†, n (%) | 110 (100) | 26 (100) | 12 (100) | 10 (100) | 18 (100) | 9 (100) | 14 (100) | 4 (80.0) | 11 (100) | 2 (100) | 8 (100) | 1 (100) | 7 (100) |
| ALT increased | 19 (17.3) | 3 (11.5) | 2 (16.7) | 1 (10.0) | 6 (33.3) | 1 (11.1) | 1 (7.1) | 0.0 | 1 (9.1) | 0.0 | 1 (12.5) | 0.0 | 0.0 |
| AST increased | 17 (15.5) | 3 (11.5) | 2 (16.7) | 1 (10.0) | 7 (38.9) | 1 (11.1) | 1 (7.1) | 0.0 | 1 (9.1) | 0.0 | 1 (12.5) | 0.0 | 0.0 |
| Diarrhea | 68 (61.8) | 5 (19.2) | 3 (25.0) | 4 (40.0) | 7 (38.9) | 1 (11.1) | 4 (28.6) | 1 (20.0) | 2 (18.2) | 0.0 | 0.0 | 0.0 | 0.0 |
| Dyspnea | 27 (24.5) | 5 (19.2) | 2 (16.7) | 0.0 | 2 (11.1) | 0.0 | 2 (14.3) | 2 (40.0) | 1 (9.1) | 0.0 | 0.0 | 0.0 | 0.0 |
| Edema | 52 (47.3) | 10 (38.5) | 3 (25.0) | 5 (50.0) | 6 (33.3) | 2 (22.2) | 2 (14.3) | 1 (20.0) | 1 (9.1) | 0.0 | 0.0 | 1 (100) | 1 (14.3) |
| Fatigue | 25 (22.7) | 6 (23.1) | 4 (33.3) | 4 (40.0) | 7 (38.9) | 2 (22.2) | 3 (21.4) | 1 (20.0) | 4 (36.4) | 0.0 | 2 (25.0) | 1 (100) | 2 (28.6) |
| Febrile neutropenia | 56 (50.9) | 8 (30.8) | 6 (50.0) | 3 (30.0) | 3 (16.7) | 1 (11.1) | 2 (14.3) | 0.0 | 2 (18.2) | 1 (50.0) | 2 (25.0) | 0.0 | 1 (14.3) |
| Headache | 36 (32.7) | 3 (11.5) | 2 (16.7) | 4 (40.0) | 7 (38.9) | 2 (22.2) | 6 (42.9) | 2 (40.0) | 5 (45.5) | 0.0 | 3 (37.5) | 0.0 | 2 (28.6) |
| Hypokalemia | 33 (30.0) | 7 (26.9) | 2 (16.7) | 4 (40.0) | 3 (16.7) | 3 (33.3) | 1 (7.1) | 1 (20.0) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Hypomagnesemia | 12 (10.9) | 2 (7.7) | 0.0 | 2 (20.0) | 0.0 | 2 (22.2) | 0.0 | 2 (40.0) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Insomnia | 40 (36.4) | 3 (11.5) | 3 (25.0) | 5 (50.0) | 6 (33.3) | 1 (11.1) | 5 (35.7) | 1 (20.0) | 4 (36.4) | 1 (50.0) | 3 (37.5) | 0.0 | 1 (14.3) |
| Nausea | 76 (69.1) | 14 (53.8) | 6 (50.0) | 9 (90.0) | 12 (66.7) | 5 (55.6) | 7 (50.0) | 2 (40.0) | 4 (36.4) | 0.0 | 3 (37.5) | 0.0 | 3 (42.9) |
| Neutropenia | 13 (11.8) | 5 (19.2) | 1 (8.3) | 1 (10.0) | 3 (16.7) | 3 (33.3) | 2 (14.3) | 0.0 | 3 (27.3) | 0.0 | 2 (25.0) | 0.0 | 2 (28.6) |
| Pneumonia | 22 (20.0) | 6 (23.1) | 0.0 | 1 (10.0) | 2 (11.1) | 1 (11.1) | 0.0 | 1 (20.0) | 1 (9.1) | 0.0 | 0.0 | 0.0 | 0.0 |
| Rash | 44 (40.0) | 6 (23.1) | 1 (8.3) | 2 (20.0) | 5 (27.8) | 1 (11.1) | 6 (42.9) | 0.0 | 3 (27.3) | 0.0 | 2 (25.0) | 0.0 | 1 (14.3) |
| Vomiting | 51 (46.4) | 7 (26.9) | 6 (50.0) | 5 (50.0) | 11 (61.1) | 3 (33.3) | 6 (42.9) | 0.0 | 1 (9.1) | 0.0 | 1 (12.5) | 0.0 | 2 (28.6) |
IP, inpatient; OP, outpatient; AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
AEs, regardless of relationship to study drug, reported by at least two patients and with an incidence ≥30% in at least one cycle.
AEs that did not resolve were considered to continue through the patient ’ s last cycle of treatment; if study drug was received as both IP and OP in the same cycle, that cycle was considered as an OP cycle.
The overall frequency of treatment-emergent grade ≥3 AEs was not significantly different for IP (100 of 110 patients [90.9%]) and OP (32 of 35 patients [91.4%]) administration cycles (Table III). The frequency of treatment-emergent grade ≥3 AEs in IP cycle 2 as consolidation (40%) was significantly less (p = 0.034) than that in OP cycle 2 as consolidation (83.3%), but such differences were not identified at other cycles. Overall, febrile neutropenia was the most common treatment-emergent grade ≥3 AE. Infections, including pneumonia and enterococcal or staphylococcal bacteremia, occurred most frequently in IP cycle 1 and IP cycle 2 as re-induction, as compared with other cycles of drug administration.
Table III.
Grade ≥3 adverse events after inpatient and outpatient administration of clofarabine*.
| Cycle 1 induction
|
Cycle 2 re-induction
|
Cycle 2 consolidation
|
Cycle 3
|
Cycle 4
|
Cycle 5
|
Cycle 6
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IP (n = 110) |
IP (n = 26) |
OP (n = 12) |
IP (n = 10) |
OP (n = 18) |
IP (n = 9) |
OP (n = 14) |
IP (n = 5) |
OP (n = 11) |
IP (n = 2) |
OP (n = 8) |
IP (n = 1) |
OP (n = 7) |
|
| Any emergent grade ≥3 AE†, n (%) | 98 (89.1) | 22 (84.6) | 10 (83.3) | 4 (40.0) ** | 15 (83.3) | 4 (44.4) | 11 (78.6) | 2 (40.0) | 6 (54.5) | 1 (50.0) | 4 (50.0) 1 (100) | 3 (42.9) | |
| ALT increased | 7 (6.4) | 2 (7.7) | 0.0 | 0.0 | 2 (11.1) | 1 (11.1) | 0.0 | 0.0 | 1 (9.1) | 1 (50.0) | 1 (12.5) | 0.0 | 0.0 |
| AST increased | 8 (7.3) | 2 (7.7) | 1 (8.3) | 1 (10.0) | 4 (22.2) | 0.0 | 0.0 | 0.0 | 1 (9.1) | 0.0 | 1 (12.5) | 0.0 | 0.0 |
| Cellulitis | 4 (3.6) | 2 (7.7) | 2 (16.7) | 0.0 | 1 (5.6) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Decreased appetite | 6 (5.5) | 0.0 | 0.0 | 0.0 | 2 (11.1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Fatigue | 4 (3.6) | 0.0 | 3 (25.0) | 1 (10.0) | 2 (11.1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Febrile neutropenia | 56 (50.9) | 8 (30.8) | 6 (50.0) | 3 (30.0) | 3 (16.7) | 1 (11.1) | 2 (14.3) | 0.0 | 2 (18.2) | 1 (50.0) | 2 (25.0) | 0.0 | 1 (14.3) |
| Hypertension | 12 (10.9) | 4 (15.4) | 0.0 | 1 (10.0) | 2 (11.1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Hypokalemia | 16 (14.5) | 3 (11.5) | 1 (8.3) | 1 (10.0) | 2 (11.1) | 1 (11.1) | 1 (7.1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Hypoxia | 7 (6.4) | 3 (11.5) | 0.0 | 0.0 | 1 (5.6) | 3 (33.3) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neutropenia | 11 (10.0) | 5 (19.2) | 1 (8.3) | 1 (10.0) | 3 (16.7) | 3 (33.3) | 2 (14.3) | 0.0 | 3 (27.3) | 0.0 | 2 (25.0) | 0.0 | 2 (28.6) |
| Nausea | 4 (3.6) | 0.0 | 1 (8.3) | 0.0 | 2 (11.1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Pneumonia | 16 (14.5) | 6 (23.1) | 0.0 | 1 (10.0) | 2 (11.1) | 1 (11.1) | 0.0 | 1 (20.0) | 1 (9.1) | 0.0 | 0.0 | 0.0 | 0.0 |
| Rash generalized | 4 (3.6) | 0.0 | 0.0 | 0.0 | 2 (11.1) | 0.0 | 1 (7.1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sepsis | 3 (2.7) | 1 (3.8) | 0.0 | 2 (20.0) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Staphylococcal bacteremia | 7 (6.4) | 0.0 | 0.0 | 0.0 | 2 (11.1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1 (12.5) | 0.0 | 0.0 |
| Thrombocytopenia | 11 (10.0) | 2 (7.7) | 0.0 | 0.0 | 4 (22.2) | 2 (22.2) | 2 (14.3) | 0.0 | 1 (9.1) | 0.0 | 2 (25.0) | 0.0 | 1 (14.3) |
| Urinary tract infection | 3 (2.7) | 0.0 | 0.0 | 0.0 | 2 (11.1) | 1 (11.1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
IP, inpatient; OP, outpatient; AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Grade 3 AEs, regardless of relationship to study drug, reported by at least two patients and with an incidence ≥ 10% in at least one cycle.
AEs that did not resolve were considered to continue through the patient ’ s last cycle of treatment; if study drug was received as both IP and OP in the same cycle, that cycle was considered as an OP cycle.
p = 0.034 versus OP cycle 2 consolidation.
The frequency of treatment-emergent SAEs in IP cycle 2 as re-induction (42.3%) was significantly less (p = 0.034) than that in OP 2 cycle as re-induction (83.3%) (Table IV), but such differences were not identified at other cycles. The most common treatment-emergent SAEs among inpatients in all cycles of drug administration were febrile neutropenia (17 of 110 patients [15.5%]), pneumonia (15 of 110 patients [13.6%]) and acute renal failure and sepsis (both six of 110 patients [5.5%]); among outpatients in all cycles these were febrile neutropenia (13 of 35 patients [37.1%]), dehydration (three of 35 patients [8.6%]) and pneumonia, various other infections, muscle weakness, vomiting, fatigue and acute renal failure (each two of 35 patients [5.7%]). There were no deaths within 30 days following IP or OP consolidation cycles.
Table IV.
Serious adverse events after inpatient and outpatient administration of clofarabine *.
| Cycle 1 Induction
|
Cycle 2 re-induction
|
Cycle 2 consolidation
|
Cycle 3
|
Cycle 4
|
Cycle 5
|
Cycle 6
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IP (n = 110) |
IP (n = 26) |
OP (n = 12) |
IP (n = 10) |
OP (n = 18) |
IP (n = 9) |
OP (n = 14) |
IP (n = 5) |
OP (n = 11) |
IP (n = 2) |
OP (n = 8) |
IP (n = 1) |
OP (n = 7) |
|
| Any emergent SAE†, n (%) | 42 (38.2) | 11 (42.3) ** | 10 (83.3) | 4 (40.0) | 11 (61.1) | 1 (11.1) | 4 (28.6) | 1 (20.0) | 4 (36.4) | 1 (50.0) | 2 (25.0) | 1 (100) | 2 (28.6) |
| Cellulitis | 1 (0.9) | 1 (3.8) | 2 (16.7) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Dehydration | 1 (0.9) | 0.0 | 0.0 | 0.0 | 3 (16.7) | 1 (11.1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Enterococcal bacteremia | 0.0 | 2 (7.7) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Febrile neutropenia | 12 (10.9) | 3 (11.5) | 6 (50.0) | 1 (10.0) | 1 (5.6) | 0.0 | 2 (14.3) | 0.0 | 2 (18.2) | 1 (50.0) | 2 (25.0) | 0.0 | 1 (14.3) |
| Pneumonia | 10 (9.1) | 4 (15.4) | 0.0 | 0.0 | 1 (5.6) | 1 (11.1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sepsis | 3 (2.7) | 1 (3.8) | 0.0 | 2 (20.0) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Staphylococcal bacteremia | 1 (0.9) | 0.0 | 0.0 | 0.0 | 2 (11.1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Urinary tract infection | 2 (1.8) | 0.0 | 0.0 | 0.0 | 2 (11.1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
IP, inpatient; OP, outpatient; SAE, serious adverse event.
SAEs, regardless of relationship to study drug, reported by at least two patients and with an incidence ≥5% in at least one cycle.
SAEs that did not resolve were considered to continue through the patient ’ s last cycle of treatment; if study drug was received as both IP and OP in the same cycle, that cycle was considered as an OP cycle.
p = 0.034 versus OP cycle 2 re-induction.
Discussion
In this post hoc analysis of the CLASSIC II trial in elderly patients with previously untreated AML and at least one unfavorable prognostic factor, patients received their consolidation treatment with clofarabine in both IP and OP settings. Conditions for the administration of clofarabine were designed to maximize the safety and effectiveness of therapy and thus increase the likelihood that patients could safely receive OP clofarabine administration. Only patients who achieved remission after induction or re-induction therapy, and who thus could be expected to do well, were among those selected to receive consolidation treatment cycles. Additionally, the lower dose of clofarabine used for consolidation therapy (i.e. compared with induction therapy) was expected to enhance the tolerability of treatment [17]. Tolerability may also have been enhanced because the tumor burden is typically less during the consolidation phase of treatment than it is during induction therapy. Aside from these factors, which were part of the study design, patients were selected for IP or OP clofarabine administration on the basis of physician preference. Although the specific criteria used by the treating physician were not identified, it is likely that the decision of whether to assign patients to IP or OP therapy may have been influenced by how well patients tolerated their initial course of treatment.
In this group of patients, clofarabine consolidation therapy appeared to be administered safely in the OP setting. The median LOS after successive OP treatment cycles was generally low. The results presented here suggest that, compared with that of inpatients, the median LOS is shorter in patients undergoing OP administration of clofarabine. In support of this suggestion, as the proportion of outpatients generally increased in successive treatment cycles, the median LOS decreased. However, because fewer patients than anticipated underwent OP consolidation cycles (i.e. the median number of OP consolidation cycles was 1) and because patients alternated between IP and OP groups at the discretion of the treating physician, it was difficult to definitively establish the difference in LOS between the two groups.
The safety analysis presented here showed that the types of AEs and the incidence rates of the most common AEs in the IP and OP settings were similar (nausea and febrile neutropenia were the most common AEs reported in both settings), indicating that the transfer of consolidation cycles to the OP setting does not present a safety concern in the treatment of elderly patients with AML who have at least one unfavorable prognostic factor. Overall, on the basis of these results, physicians could consider an OP setting for clofarabine administration after initial IP induction. Additionally, a recent publication by Dressel et al. supported this approach and suggested a means for outpatient management of clofarabine administration in adult patients with AML [18].
Gardin et al. used a successful post-remission induction strategy that included OP administration of idarubicin and daunorubicin in patients ≥65 years of age with AML after standard intensive remission induction in an IP setting [11]. This study prospectively compared an intensive consolidation course of treatment administered in hospitalized patients to a more prolonged OP consolidation. Outpatient administration, as compared to intensive IP consolidation, was associated with a significantly greater odds ratio in favor of overall survival for patients with complete remission (p = 0.04), longer disease-free survival (p = 0.05), significantly shorter rehospitalization duration (p < 0.001) and fewer red blood cell units and platelet transfusions [11]. Hence, at least in terms of LOS, these results are consistent with the findings reported here.
Infections are potentially a significant concern for patients with AML during post-consolidation leukopenia. It is interesting to note that the literature contains evidence of a lower incidence of septicemia with OP consolidation than with IP administration [10]. Furthermore, prophylactic antibiotic therapy has been used successfully to assist in the management of patients undergoing treatment for AML on an OP basis, thereby presumably lowering the potential for acquiring infections [10,14,19].
Notably, pneumonia, an AE which might be predicted to be a complication of therapy (from exposure to viral or fungal pathogens), was not seen with increased frequency in consolidation cycles in the present study. Although there were modest increases in the frequency of staphylococcal bacteremia in OP cycles, these differences were not statistically significant. There were seven cases of sepsis classified as SAEs, but all of these occurred in IP cycles.
The cost of care for patients with acute leukemia is an ongoing concern in the current health-system environment. Reduction of hospitalization will surely be associated with significant cost savings for such patients [9,20]. These savings may be compounded by reductions in nosocomial infections and their associated morbidities and costs. Although the present analysis does not directly address cost of care, given that outpatient clofarabine was not associated with increased complications, it seems likely that these patients, compared to those who were hospitalized, received equally effective care at a lower cost.
Further studies are needed to prospectively determine the relative safety profiles and LOS for OP and IP cycles of clofarabine administration. A limitation of this study is the low number of patients in the IP group in later consolidation cycles, a factor that makes it difficult to compare the relative safety profiles of the two groups. The small sample size also precluded univariate/multivariate analyses to determine the patient characteristics that could predict which patients are more likely to do well in an OP setting. However, patients who tolerated IP induction therapy (i.e. did not need re-induction) are likely candidates for subsequent (consolidation) cycles in the OP setting, a possibility that could be taken into account in the design of follow-up studies. In addition to an increase in the size of the patient cohort, clear guidelines for patient selection, education and monitoring also need to be established in future studies. Since this was a non-randomized, observational post hoc analysis, it was not possible to draw a definitive conclusion as to whether OP administration was in any way superior to IP administration of clofarabine. Another drawback was that this study was not prospectively designed to assess QOL or cost-effectiveness outcomes.
The results of the present analysis, which point to the feasibility of the OP administration of clofarabine in elderly patients with AML who have at least one unfavorable prognostic factor, are consistent with the current trend toward the OP administration of chemotherapeutic agents. Administration of clofarabine in the OP setting is associated with a safety profile similar to that seen with IP administration. With proper patient selection, education and monitoring for AEs, OP administration of clofarabine has the potential to contribute to improvement in the QOL of elderly patients undergoing treatment for AML.
Acknowledgments
The development of this manuscript was supported by Genzyme Corporation. The authors would like to thank MedLogix Communications for editorial support and Angela Partisano (Genzyme Corporation) for help with coordinating the revisions of this manuscript. Additionally, the authors would like to thank all of the CLASSIC II investigators for their contributions to the study.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Büchner T, Berdel WE, Haferlach C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia:a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol. 2009;27:61–69. doi: 10.1200/JCO.2007.15.4245. [DOI] [PubMed] [Google Scholar]
- 3.Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25:1908–1915. doi: 10.1200/JCO.2006.10.2731. [DOI] [PubMed] [Google Scholar]
- 4.Löwenberg B, Suciu S, Archimbaud E, et al. Mitoxantrone versus daunorubicin in induction-consolidation chemotherapy—the value of low-dose cytarabine for maintenance of remission, and an assessment of prognostic factors in acute myeloid leukemia in the elderly: final report. European Organization for the Research and Treatment of Cancer and the Dutch-Belgian Hemato-Oncology Cooperative Hovon Group. J Clin Oncol. 1998;16:872–881. doi: 10.1200/JCO.1998.16.3.872. [DOI] [PubMed] [Google Scholar]
- 5.Rowe JM, Neuberg D, Friedenberg W, et al. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: a trial by the Eastern Cooperative Oncology Group. Blood. 2004;103:479–485. doi: 10.1182/blood-2003-05-1686. [DOI] [PubMed] [Google Scholar]
- 6.Larson ML, Venugopal P. Clofarabine:a new treatment option for patients with acute myeloid leukemia. Expert Opin Pharmacother. 2009;10:1353–1357. doi: 10.1517/14656560902997990. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian HM, Jeha S, Gandhi V, et al. Clofarabine: past, present, and future. Leuk Lymphoma. 2007;48:1922–1930. doi: 10.1080/10428190701545644. [DOI] [PubMed] [Google Scholar]
- 8.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 9.Cox KM, Goel S, O’Connell RL, et al. A randomized crossover trial comparing inpatient and outpatient administration of high dose cisplatin. Intern Med J. 2010 Feb 26; doi: 10.1111/j.1445-5994.2010.02201.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Halim TY, Song KW, Barnett MJ, et al. Positive impact of selective outpatient management of high-risk acute myelogenous leukemia on the incidence of septicemia. Ann Oncol. 2007;18:1246–1252. doi: 10.1093/annonc/mdm112. [DOI] [PubMed] [Google Scholar]
- 11.Gardin C, Turlure P, Fagot T, et al. Postremission treatment of elderly patients with acute myeloid leukemia in first complete remission after intensive induction chemotherapy: results of the multicenter randomized Acute Leukemia French Association (ALFA) 9803 trial. Blood. 2007;109:5129–5135. doi: 10.1182/blood-2007-02-069666. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Almaguer D, Ruiz-Argüelles GJ, Ruiz-Argüelles A, et al. Hematopoietic stem cell allografts using a non-myeloablative conditioning regimen can be safely performed on an outpatient basis: report of four cases. Bone Marrow Transplant. 2000;25:131–133. doi: 10.1038/sj.bmt.1702100. [DOI] [PubMed] [Google Scholar]
- 13.Yamazaki K, Boku N, Shibamoto K, et al. The role of the outpatient clinic in chemotherapy for patients with unresectable or recurrent gastric cancer. Jpn J Clin Oncol. 2007;37:96–101. doi: 10.1093/jjco/hyl145. [DOI] [PubMed] [Google Scholar]
- 14.Gillis S, Dann EJ, Rund D. Selective discharge of patients with acute myeloid leukemia during chemotherapy-induced neutropenia. Am J Hematol. 1996;51:26–31. doi: 10.1002/(SICI)1096-8652(199601)51:1<26::AID-AJH5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhenchuk A, Lotfi K, Juliusson G, et al. Mechanisms of anticancer action and pharmacology of clofarabine. Biochem Pharmacol. 2009;78:1351–1359. doi: 10.1016/j.bcp.2009.06.094. [DOI] [PubMed] [Google Scholar]
- 16.Kantarjian HM, Erba HP, Claxton D, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28:549–555. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 17.Burnett AK, Russell NH, Kell J, et al. European development of clofarabine as treatment for older patients with acute myeloid leukemia considered unsuitable for intensive chemotherapy. J Clin Oncol. 2010;28:2389–2395. doi: 10.1200/JCO.2009.26.4242. [DOI] [PubMed] [Google Scholar]
- 18.Dressel A, Kwari M, McGreal AM. Nursing considerations for optimal outpatient management of adult patients with leukemia treated with clofarabine. Clin J Oncol Nurs. 2011;15:E13–E23. doi: 10.1188/11.CJON.E13-E23. [DOI] [PubMed] [Google Scholar]
- 19.Girmenia C, Alimena G, Latagliata R, et al. Out-patient management of acute myeloid leukemia after consolidation chemotherapy. Role of a hematologic emergency unit. Haematologica. 1999;84:814–819. [PubMed] [Google Scholar]
- 20.Rose PG, Lappas PT. Analysis of the cost effectiveness of concurrent cisplatin-based chemoradiation in cervical cancer: implications from five randomized trials. Gynecol Oncol. 2000;78:3–6. doi: 10.1006/gyno.2000.5810. [DOI] [PubMed] [Google Scholar]


