Abstract
Mesenchymal stem cells are an attractive cell type for cytotherapy in wound healing. The authors recently developed a novel, adipose-tissue-derived, injectable extracellular matrix/stromal vascular fraction gel (ECM/SVF-gel) for stem cell therapy. This study was designed to assess the therapeutic effects of ECM/SVF-gel on wound healing and potential mechanisms. ECM/SVF-gel was prepared for use in nude mouse excisional wound healing model. An SVF cell suspension and phosphate-buffered saline injection served as the control. The expression levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and monocyte chemotactic protein-1 (MCP-1) in ECM/SVF-gel were analyzed at different time points. Angiogenesis (tube formation) assays of ECM/SVF-gel extracts were evaluated, and vessels density in skin was determined. The ECM/SVF-gel extract promoted tube formation in vitro and increased the expression of the angiogenic factors VEGF and bFGF compared with those in the control. The expression of the inflammatory chemoattractant MCP-1 was high in ECM/SVF-gel at the early stage and decreased sharply during the late stage of wound healing. The potent angiogenic effects exerted by ECM/SVF-gel may contribute to the improvement of wound healing, and these effects could be related to the enhanced inflammatory response in ECM/SVF-gel during the early stage of wound healing.
1. Introduction
Wound healing is a highly complex process that remains a major challenge in modern medicine. Among the factors contributing to these nonhealing conditions, impairment of cytokine production and reduced vascularization play crucial roles [1]. Mesenchymal stem cells- (MSCs-) based cytotherapy is an attractive approach in wound healing due to the differentiation potential, immunomodulating properties, and paracrine effects of MSCs [2–5]. Among the available MSCs, adipose-derived stem cells (ASCs) are promising candidates for cytotherapy because they can be easily harvested from adipose tissue and are abundant in number [6, 7]. However, isolation of ASCs still requires enzymatic digestion, which increases the risk of biological contamination. Additionally, in vitro culture and expansion of ASCs require specific laboratory experience and can take days to weeks. Moreover, in most studies, ASCs suspensions are injected separately without the protection of extracellular matrix (ECM) components, leading to rapid elimination of ASCs by the immune system and subsequent poor cell retention at recipient sites [8–10]. All of these factors weaken the therapeutic effects and applications of ASCs-based cytotherapy.
We have recently described a novel adipose-tissue-derived injectable extracellular matrix/stromal vascular fraction gel (ECM/SVF-gel) [11]. Taking advantage of shearing force (mechanical emulsification of adipose tissue by shifting between two 10 mL syringes connected by a female-to-female Luer-Lok connector) to selectively break mature adipocytes, the ECM/SVF-gel eliminates most of the lipids and other unwanted components (such as tumescent fluid) of Coleman fat. The shear force causes more damage to adipocytes than to stromal vascular fraction (SVF) cells components due to the large size and fragility of the cells, leaving only the extracellular matrix (ECM) and SVF cells and greatly improving ASCs density. According to our previous results, standard Coleman fat mechanically processed for 1 min in a 10 mL syringe at a flow rate of 10 mL/s will lead to maximum enrichment of viable ASCs and minimal ECM fragmentation. This procedure is considered optimal for producing ECM/SVF-gel.
Because ECM/SVF-gel is rich in ASCs, we speculated that ECM/SVF-gel may exert cytotherapeutic effects on wound healing. To test this hypothesis, preliminary studies were conducted in a mouse excisional wound healing model to assess the therapeutic value of ECM/SVF-gel for wound repair. First, angiogenic factors expression and angiogenic effect of ECM/SVF-gel extract in vitro were tested. Next, angiogenic factors secretion and angiogenic efficacy of the grafted ECM/SVF-gel in vivo were examined. Finally, the inflammation level of ECM/SVF-gel in vivo was evaluated.
2. Materials and Methods
2.1. Fat Harvesting and ECM/SVF-gel Preparation
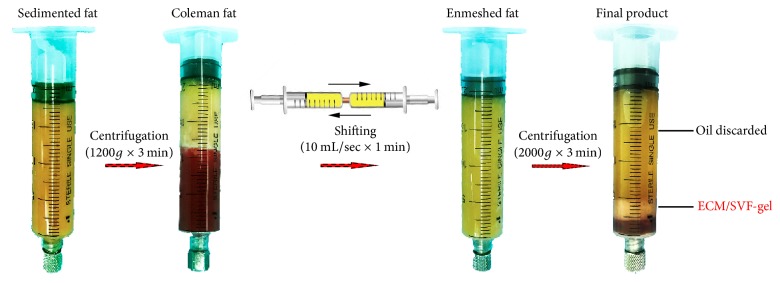
Human abdominal lipoaspirates were obtained from female abdominal lipoaspirates after informed consent and Nanfang Hospital ethics committee approval. ECM/SVF-gel was prepared as per the manufacturer's instruction [11]. Briefly, liposuction was performed with a 3 mm multiport cannula and the harvested fat was allowed to stand for 10 min in ice water. The liquid portion was then discarded and the fat layer was then centrifuged at 1200g for 3 min to generate Coleman fat. After centrifugation and discarding the liquid portion, the obtained Coleman fat was then mechanically emulsified by shifting between two 10 mL syringes connected by a female-to-female Luer-Lok connector with an internal diameter of 2.4 mm for 1 min (10 mL/sec). After processing, the fat turned into an emulsion and was then processed by centrifugation at 2000g for 3 min. Finally, the remaining substance under the oil layer was ECM/SVF-gel (Figure 1). For ASCs extraction, the ECM/SVF-gel was digested with 0.075% collagenase for 30 min on a shaker at 37°C. After centrifugation at 800g for 5 min, the cell pellets from ECM/SVF-gel were then filtered through a 100 μm mesh and SVF cells' number was then counted. The remaining SVF cells were cultured until 80% confluence. Passage 3 to Passage 5 ASCs were used in the following experiments.
Figure 1.
The fabrication of ECM/SVF-gel.
2.2. Scanning Electron Microscopy (SEM)
The surface characteristics of ECM/SVF-gel were determined by scanning electron microscopy (SEM) (Hitachi S-3000N; Hitachi Ltd., Tokyo, Japan). Samples were mounted on a carbon tape and coated with gold prior to imaging. An acceleration voltage of 20 kV was used and images were observed using a Canon digital camera (Canon Inc., Tokyo, Japan).
2.3. Multilineage Differentiation Assay
Assays of adipogenic, osteogenic, and chondrogenic lineages were conducted in a control medium supplemented with one of the three formulae as previously described [12]. Cultured ASCs were stained with Oil-Red O, Alizarin Red S, and Alcian Blue to identify fat, bone, and cartilage cells, respectively.
2.4. Healing of Full-Thickness Wounds in Nude Mice
Female nude mice (4–6-week-old) were purchased from the Southern Medical University Laboratory Animal Center and maintained in microisolator cages at the Animal Experiment Center of Nanfang Hospital. All animal experiments were approved by Nanfang Hospital Institutional Animal Care and Use Committee and conducted according to the guidelines of the National Health and Medical Research Council (China). After general anesthesia, the mice were depilated and two 6 mm radius full-thickness excisional wounds were produced on the back of every mouse. A silicone ring was secured around the wound to prevent wound contraction. 54 mice were divided into ECM/SVF-gel treated group (injection of ECM/SVF-gel into the four quadrants, n = 18), SVF cell treated group (n = 18), and control group (injection of PBS, n = 18). A local injection of ECM/SVF-gel or an SVF cell suspension contains the same number of SVF cells (2 × 104). After treatment, a Tegaderm sterile dressing (3M Healthcare, St. Paul, MN) was used to cover the wounds and was replaced every other day. The wound area was photographed with a digital camera and quantified using ImageJ software (NIH, Bethesda, MD).
2.5. Histological Examination and Capillary Density Measuring
Skin and ECM/SVF-gel around the wounds were harvested at days 2, 4, 7, 10, and 14. One half of the samples were paraffin-embedded for hematoxylin and eosin (HE) staining and the rest were snap-frozen and preserved at −80°C for RNA analysis. For HE staining, paraffin-embedded samples were cut into 4 μm sections and stained with hematoxylin for 5 min and washed with Scott's solution three times.. Samples were treated with 1% HCl for 20 s and then rinsed with Scott's solution for 1 min. After incubation in 80% ethanol for 2 min, sections were stained with eosin for 2 min. Images were taken using a Nikon E200 microscope (Nikon Corp., Tokyo, Japan). Vascularization of skin was assessed by counting the capillaries in 8 fields of each HE section, performed by two blinded reviewers.
2.6. ECM/SVF-gel Extract Preparation
ECM/SVF-gel extract was prepared according to the manufacturer's instructions [13]. Briefly, in vivo ECM/SVF-gel samples from all 5 time points were harvested. The samples were cut into small pieces and mixed with an equal volume of Dulbecco's modified Eagle's medium. The mixture was incubated for 24 h at 37°C in a CO2 incubator. ECM/SVF-gel extracts were collected after the mixture was centrifuged at 12,000 rpm for 5 min and sterile-filtered through a 0.22 mm filter (Sarstedt, Nümbrecht, Germany). The extracts were stored at −20°C until use.
2.7. Cytokines Expression in ECM/SVF-gel Extract and Tube Formation Assay
Serum samples were collected from each mouse at all time points. Expressions of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and monocyte chemotactic protein-1 (MCP-1) in ECM/SVF-gel extracts or serum were determined by high sensitivity enzyme-linked immunosorbent assay kits (R&D Systems). For tube formation assay, human umbilical vein endothelial cells (HUVECs) were obtained from the Research Laboratory Collaboration Alliance of Nanfang Hospital (Guangzhou, China). Culture plate and the experimental equipment were precooled at −20°C before operation; Matrigel (Becton, Dickinson & Co., 356230, Franklin Lakes, NJ) was added to the culture plate and solidified by incubation at 37°C for 2 h before seeding cells. HUVECs (2 × 104 per well) mixed with 1 mL of ECM/SVF-gel extract from 7-day samples were then seeded in the culture plate. As a positive control, the HUVECs in the culture plate were treated with angiogenic growth factors (10 ng/mL vascular endothelial growth factor (VEGF; R&D Systems, MN, USA) and 1 ng/mL basic fibroblast growth factor (bFGF; Sigma)). As a control, HUVECs alone were cultured in normal culture medium. Angiogenesis (tube formation) was observed and photographed in high-powered fields (HPF) after 24 h.
2.8. Quantitative Real-Time Polymerase Chain Reaction (PCR)
RNA was isolated from skin and ECM/SVF-gel samples using TRIzol Reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA) according to the manufacturer's protocol. Reverse transcription was performed using a cDNA synthesis kit (Thermo Scientific, USA). Primer sequence for PPARγ was designed. Quantitative PCR was performed using FastStart Universal SYBR Green Master (Rox). PCR specificity was assessed by the Ct method using β-actin as an endogenous reference gene.
2.9. Statistical Analysis
Data were expressed as mean ± SD (standard deviation). Results were analyzed with one-way analyses of variance (SPSS, version 21; IBM Corporation, Armonk, NY, USA). Furthermore, an independent Student's t-test of two groups at a single time point was performed. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Structural Characteristics of ECM/SVF-gel and Fresh Adipose Tissues
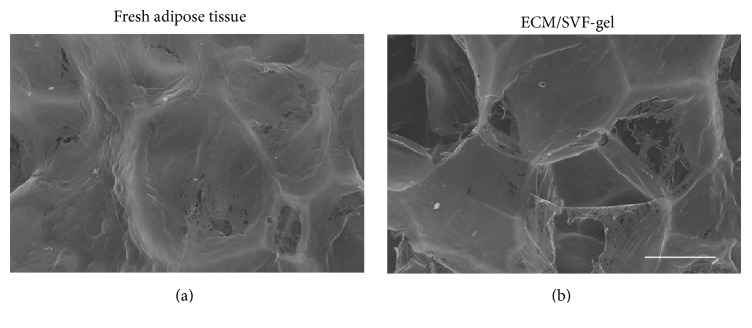
ECM/SVF-gel and fresh adipose tissues were evaluated by SEM. The control adipose tissues had a relatively well integrated ECM structure (Figure 2(a)). The ECM integrity in ECM/SVF-gel decreased slightly after mechanical processing (Figure 2(b)).
Figure 2.
SEM analysis of ECM/SVF-gel and fresh adipose tissues. SEM analysis revealed the structural characteristics of fresh adipose tissue sample (a) and ECM/SVF-gel (b). Scale bar = 50 μm.
3.2. Multilineage Differentiation of ASCs Isolated from ECM/SVF-gel
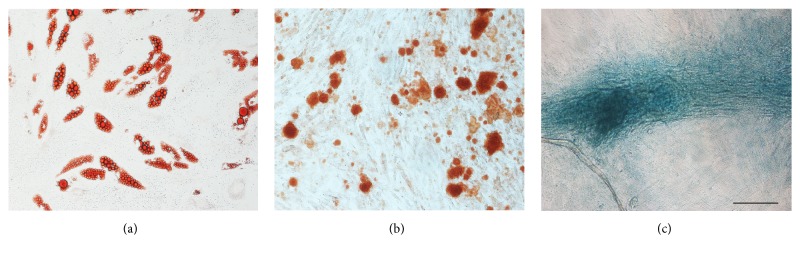
ASCs isolated from ECM/SVF-gel grew well in vitro. To verify multipotent differentiation, ASCs were incubated in a medium known to induce adipogenic, osteogenic, and chondrogenic lineages. Cells contained lipid droplets, exhibited matrix mineralization, expressed cartilage-specific proteoglycans, and were stained positively for Oil-Red O (Figure 3(a)), Alizarin Red S (Figure 3(b)), and Alcian Blue (Figure 3(c)), respectively.
Figure 3.
Multilineage differentiation of ASCs isolated from ECM/SVF-gel. Multilineage differentiation of ASCs isolated from ECM/SVF-gel underwent adipogenic (a), osteogenic (b), and cartilage differentiation (c). Scale bar = 200 μm.
3.3. ECM/SVF-gel Promoted Wound Healing
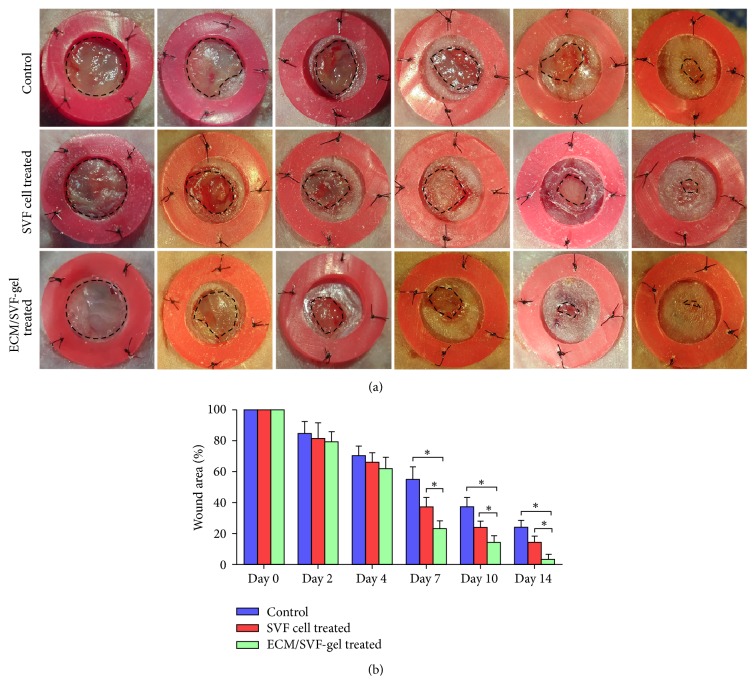
The therapeutic value of ECM/SVF-gel and SVF in wound healing was evaluated in a nude mouse excisional wound healing model. Healing of wounds was significantly more rapid with ECM/SVF-gel injection compared with that of the SVF cell suspension and the PBS control on day 7. On day 14, the ECM/SVF-gel achieved complete wound healing, whereas wounds in the groups treated with SVF cell suspension and PBS remained unhealed (Figure 4).
Figure 4.
Wound healing in nude model. 6 mm radius cutaneous wounds were created on dorsal areas of nude mice and treated with ECM/SVF-gel (2 × 104 cells, n = 18), SVF cells (2 × 104 cells suspended in 0.1 mL of PBS, n = 18), and PBS injection (n = 18), respectively. (a) All injections were made subdermally into the four quadrants of the wound. (b) Quantification of wound area at different time points (∗P < 0.05).
3.4. ECM/SVF-gel Extracts Promoted Vascularization In Vitro
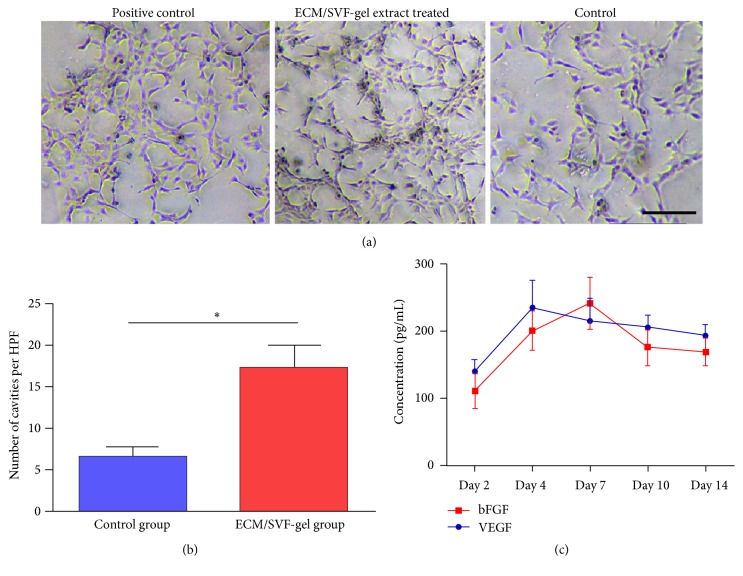
In an angiogenesis (tube formation) assay, treatment with ECM/SVF-gel extracts derived from day 7 for 24 h resulted in the formation of interconnected tubular structures of HUVECs, whereas HUVECs were unable to connect to the vascular net in the control group (Figures 5(a)-5(b)). VEGF and bFGF expression levels in ECM/SVF-gel extracts at different time points were measured by enzyme-linked immunosorbent assays. The results revealed high concentrations of VEGF and bFGF (Figure 5(c)).
Figure 5.
Angiogenesis assay and angiogenic factors expression. (a) HUVECs in ECM/SVF-gel extract (7-day samples) treated group and in positive control group formed numerous interconnected tubular structures at 12 hours while HUVECs did not connect to the vascular net in the control group. Scale bar = 200 μm. (b) Quantitative analysis of tube formation. (c) Quantitative analysis of angiogenic factors in ECM/SVF-gel extract at different time points (∗P < 0.05).
3.5. ECM/SVF-gel Enhanced Vascularization In Vivo
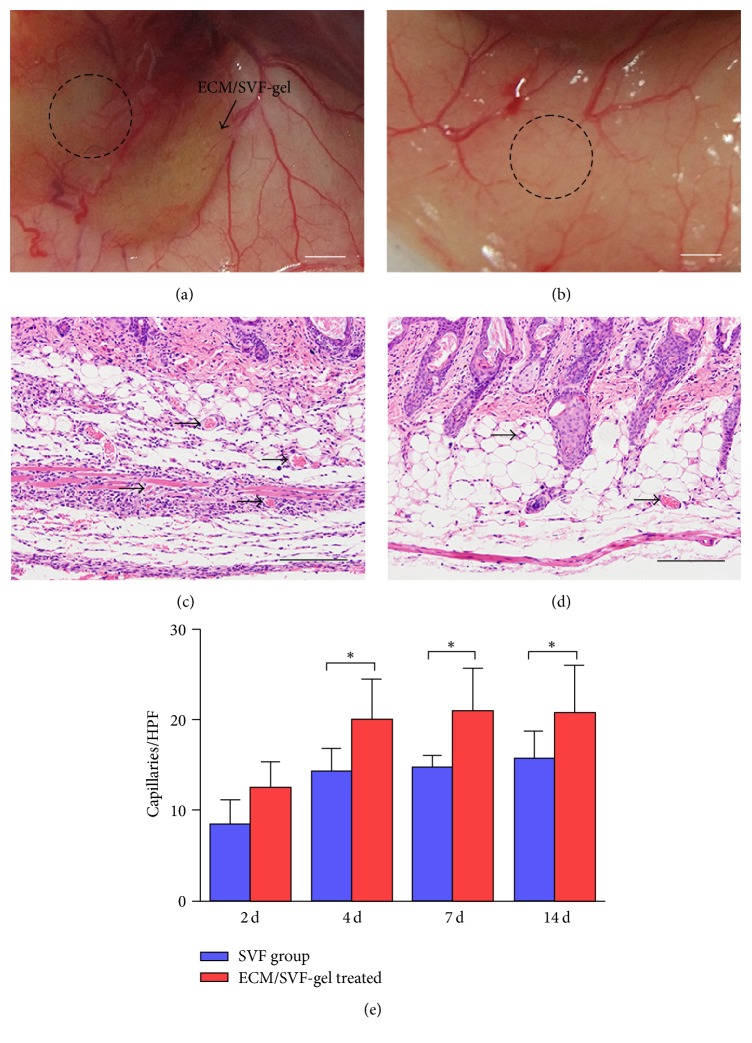
Macroscopic visualization of subcutaneous blood vessels around the wound was performed on day 30. In the ECM/SVF-gel group, around the wound, vessels and their fine branches extended into the ECM/SVF-gel, forming networks of blood vessels and nourishing the wound (Figure 6(a)). In wounds in the SVF group, vessels were limited to the skin surrounding the wounds (Figure 6(b)). Observation of vascularization in skin HE stained sections revealed that ECM/SVF-gel-induced promotion of vascularization (Figure 6(c)) was significantly greater than that in the SVF group (Figure 6(d)). Quantification of capillary densities in HE stained skin sections was then performed. Capillary density was significantly higher in the ECM/SVF-gel treated group than in the SVF treated group from day 4 to day 14 (Figure 6(e)).
Figure 6.
Vascularization and Immunofluorescence staining. Macroscopic visualization of subcutaneous blood vessels around the wound (dotted area) in ECM/SVF-gel treated group (a) and SVF cell treated group (b) at day 30. Scale bar = 2 mm. Observation of vessels (arrows) in skin HE stained sections in the ECM/SVF-gel treated group (c) and SVF cell treated group (d) at day 7. Scale bar = 200 μm. Quantification of capillary densities in HE stained skin sections (e) (∗P < 0.05).
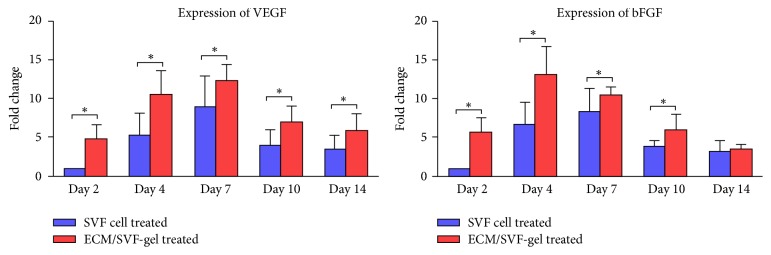
The RNA expression level of the angiogenic factor VEGF peaked on day 7 and was higher in the ECM/SVF-gel treated group than in the SVF treated group at all time points, whereas expression of the angiogenic factor bFGF peaked on day 4 in the ECM/SVF-gel treated group and was higher than that in the SVF treated group between days 2 and 10 (Figure 7).
Figure 7.
Expression of angiogenic factors in skin samples. VEGF and bFGF expression of skin samples in the ECM/SVF-gel treated group and SVF group at different time points (∗P < 0.05).
3.6. Inflammatory Reaction in ECM/SVF-gel
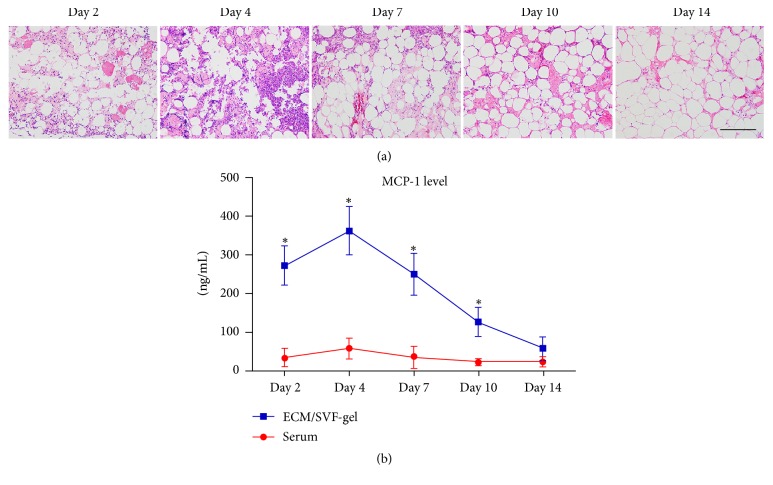
Obvious inflammatory cell infiltration was found in ECM/SVF-gel at the early stage and declined during the late stage of wound healing (Figure 8(a)). The expression of MCP-1 from ECM/SVF-gel extracts peaked on day 4 and decreased sharply thereafter, showing higher levels than that in serum from day 2 to day 10 (Figure 8(b)).
Figure 8.
HE staining of ECM/SVF-gel and MCP-1 expression. (a) Inflammatory cell infiltration was observed obviously in ECM/SVF-gel at an early stage and declined at a late stage of wound healing. Scale bar = 200 μm. (b) MCP-1 expression in ECM/SVF-gel and serum at different time points (∗P < 0.05).
4. Discussion
Adipose ECM/SVF-gel, a novel injectable consisting of adipose native ECM and functional ASCs, was applied in this study to optimize cytotherapy in wound healing. The results revealed that the ECM/SVF-gel treated group showed more rapid wound closure than did the SVF group. The enhanced expression of angiogenic factors in ECM/SVF-gel and a rich vascular network around the wound area indicated that the faster healing of wounds in the ECM/SVF-gel group could be attributed to increased vascularization. Vascularization of the wound bed is known to play a significant role in the wound healing process [13, 14]. Recent cytotherapy strategies using MSCs have aimed at increasing vascularization to achieve wound healing due to their paracrine effects and differentiating potential [15, 16]. Despite demonstrating clear therapeutic potential, limitations still remain regarding the clinical application of MSCs, primarily because of their low therapeutic efficacy and biosafety concerns [17, 18]. Moreover, in most studies of cytotherapy for cutaneous wound healing, MSCs are subdermally injected into or around the wound area. Although this strategy has been shown to promote wound healing, the ultimate therapeutic potential of MSCs appears to be limited because of poor cell retention and reduced cell potency at the wound site [19, 20]. In previous decades, studies have attempted to optimize MSC-based therapeutics either by enhancing the expression of trophic factors or through prevention of MSC death at the recipient site. Overexpression of trophic factors through transgenic methods or by exposure to potency-enhancing factors has been used successfully in animal models [21–23]. Despite the success of some transgenic strategies, the tumorigenic potential of MSCs due to insertional mutations resulting from gene transfer is still a major safety concern for clinical translation. Various synthetic materials have been developed to provide carrier scaffolds that mimic ECM properties to enhance cytotherapy [24–26]. The advantages of synthetic materials and scaffolds mainly rely on the technical possibility that chemical and physical properties (e.g., porosity and surface characteristics) can be specifically designed [27]. However, native ECM exhibits biocompatibility, biodegradability, and inherent biological functions that could make it more suitable for a range of cytotherapy applications [28, 29].
Wound healing is a dynamic and complex process involving hemostasis, inflammation, proliferation, and remodeling [30, 31]. Multiple studies have indicated that inflammatory cells are an important source of the overall proangiogenic stimulus in the healing process [32–34]. Tissue injury causes a rapid acute inflammatory response designed to clear microbes, dying cells, and debris. In response to specific chemoattractants, such as fragments of ECM protein, transforming growth factor β, and MCP-1, a large number of monocytes infiltrate the wound site and become activated macrophages, which produce high levels of cytokines, such as platelet-derived growth factor (PDGF), VEGF, and bFGF [35, 36]. In response to increased levels of cytokines, vascularization occurs, providing oxygen and nutrients to sustain high levels of cellular activity and promote reepithelialization of wounds. In this study, significant expression of the inflammatory chemoattractant MCP-1 and obvious inflammatory cells infiltration can be found in ECM/SVF-gel during the early stage of wound healing, and inflammation levels decrease markedly during the late stage. The intense inflammatory response of ECM/SVF-gel in the early stage is likely to contribute to the secretion of angiogenic factors and subsequent vascularization in the wound area. Actually, the implanted ECM/SVF-gel around the wound served only as a stimulus that recruited inflammatory cells from the recipient mouse. Then, the recruited cells became a source and secreted a large amount of angiogenic factors (VEGF and bFGF) that promoted the vascularization and healing of the wound. This process is much similar to the remodeling of grafted adipose tissue in vivo, during which numerous host inflammatory cells infiltrated in the graft and scavenged oil drop in necrotizing zone [37]. Moreover, the angiogenesis of the adipose tissue after transplantation mainly originated from the recipient environment and most of the regeneration cells and secreted growth factors that take part in adipogenesis under adipose tissue inflammation condition come from recipient circulation [38, 39].
Studies have suggested that persistent high levels of inflammation cause fibrosis and impair wound healing, whereas a reduction in inflammation during the tissue remodeling period in wound healing can reduce scar formation and improve skin wound healing outcomes [40–42]. Thus, the rapidly decreased inflammation reaction during the late stages of wound healing may serve as a protective factor, as demonstrated in this study. However, the mechanism mediating this special inflammation pattern in ECM/SVF-gel in vivo still remains unknown. Studies have revealed that platelets are exposed to collagen and other extracellular matrix components when blood components extravasate into the site of injury. This exposure causes the platelets not only to facilitate the formation of a hemostatic plug but also to secrete several mediators of wound healing, such as PDGF, that recruit neutrophils and macrophages to the wound site [43, 44]. Since ECM/SVF-gel contains concentrated ECM components, it may likely serve as a stimulus for the robust inflammation response during the early stages of wound healing. In cytotherapeutic strategies, MSCs have recently been shown to have various immunomodulatory effects on host immune cells in both wound healing and transplant biology [45–48]. To release immunosuppressive factors, MSCs can downregulate the inflammatory response and move the wound past the state of persistent inflammation, conferring the cells with immune privilege and making them an attractive cell type for the treatment of chronic wounds [49–51]. The density of ASCs in ECM/SVF-gel is significantly higher than that in Coleman fat and control fat [11], which may partly explain the rapid decline of inflammation in ECM/SVF-gel at the late stage of wound healing.
Adipocytes account for more than 90% of the volume of adipose tissue, whereas SVF cells and the native adipose ECM component account for only 10% of the volume [52]. In our study, preparation of ECM/SVF-gel equated to selectively eliminating mature adipocytes and maximizing enrichment of SVF cells and native adipose ECM. There is an abundance of evidence confirming the beneficial effects of native ECM on tissue-resident stem cells. In our study, SEM analysis revealed that ECM/SVF-gel contained a large amount of ECM components, and quantification in a previous study revealed that there were no significant differences in ECM integrity between ECM/SVF-gel and fresh adipose tissues [11]. Native ECM provides a physical bioscaffold for cellular support and retains the natural state [53–55], which may prevent rapid migration and unexpected stem cell behaviors [53, 56]. The ECM is known to serve as a natural reservoir for growth factors, and various potent angiogenic factors reside within the ECM scaffolds [57]. Moreover, the ECM is in a state of continuous remodeling catalyzed by matrix metalloproteinases in vivo [53, 58]. In vivo degradation of the ECM may release growth factors from the scaffold and produce degradation products (peptides), and these peptides have chemoattractant effects on endothelial cells both in vivo and in vitro [57]. These protective effects of the ECM component in ECM/SVF-gel may also contribute to the enhanced angiogenic response and final vascularization during the wound healing process. Besides providing structural support to the cells, the ECM also serves as a dynamic microenvironment that plays an important role in modulating resident cell behaviors and functions [59, 60]. Specifically, the elasticity [61, 62], nanotopography [63, 64], protein composition, and mechanical strain [65, 66] inherent to the ECM are all independent factors that regulate MSC function. Compared with the SVF suspension, the ECM scaffold in the ECM/SVF-gel accommodates ASCs within their natural niche, thereby providing a beneficial environment for attached ASCs to maintain optimal cell survival and potency.
Recent studies have described improvements in wound healing with autologous fat graft treatment, mainly depending on regenerative capacity and paracrine secretion ability provided by native ASCs [67–70]. Despite the wealth of evidence supporting their efficacy in wound healing, the volume occupied by adipocytes within the grafted fat significantly decreased the density of ASCs and the ECM, which may limit the therapeutic effects of the treatment. In this case, by enriching ASCs and the ECM, ECM/SVF-gel may maximize the therapeutic effect, offering an effective and safe option for wound healing.
5. Conclusion
In this study, we preliminarily verify the potential mechanisms underlying the efficacy of ECM/SVF-gel therapy on wound healing in a murine model. The results revealed that the therapeutic effect of ECM/SVF-gel is mainly attributed to the secretion of angiogenic factors and promotion of vascularization, and this process could be related to the intense inflammatory response in ECM/SVF-gel during the early stage of wound healing. Besides, the protective effects of the ECM component on attached ASCs in ECM/SVF-gel may also contribute to the enhanced angiogenic response and final wound healing process.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81471881, 81372083), Key Clinical Specialty Discipline Construction Program, Health Collaborative Innovation Major Projects of Guangzhou (7414275040815), Natural Science Foundation of Guangdong Province of China (2014A030310155), Entry Point Project of Guangdong Province of China (PY2014N036), Innovative Project of Guangdong Province of China (2014KQNCX046), and Administrator Foundation of Nanfang Hospital (2014B009).
Conflicts of Interest
The authors have no financial interest to declare in relation to the content of this article.
Authors' Contributions
Mingliang Sun and Yunfan He contributed equally and should be regarded as co-first authors.
References
- 1.Falanga V. Wound healing and its impairment in the diabetic foot. The Lancet. 2005;366(9498):1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 2.Lee S. H., Lee J. H., Cho K. H. Effects of human adipose-derived stem cells on cutaneous wound healing in nude mice. Annals of Dermatology. 2011;23(2):150–155. doi: 10.5021/ad.2011.23.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duscher D., Barrera J., Wong V. W., et al. Stem cells in wound healing: the future of regenerative medicine a mini-review. Gerontology. 2015 doi: 10.1159/000381877. [DOI] [PubMed] [Google Scholar]
- 4.Isakson M., de Blacam C., Whelan D., McArdle A., Clover A. J. Mesenchymal stem cells and cutaneous wound healing: current evidence and future potential. Stem Cells International. 2015;2015:12. doi: 10.1155/2015/831095.831095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirby G. T. S., Mills S. J., Cowin A. J., Smith L. E. Stem cells for cutaneous wound healing. BioMed Research International. 2015;2015 doi: 10.1155/2015/285869.285869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longaker M. T., Aston S. J., Baker D. C., Rohrich R. J. Fat transfer in 2014. Plastic and Reconstructive Surgery. 2014;133:1305–1307. doi: 10.1097/PRS.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 7.Chung M. T., Paik K. J., Atashroo D. A., et al. Studies in fat grafting. Plastic and Reconstructive Surgery. 2014;134:29–38. doi: 10.1097/PRS.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araña M., Gavira J. J., Peña E., et al. Epicardial delivery of collagen patches with adipose-derived stem cells in rat and minipig models of chronic myocardial infarction. Biomaterials. 2014;35(1):143–151. doi: 10.1016/j.biomaterials.2013.09.083. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura K., Aoi N., Suga H., et al. Ectopic fibrogenesis induced by transplantation of adipose-derived progenitor cell suspension immediately after lipoinjection. Transplantation. 2008;85(12):1868–1869. doi: 10.1097/TP.0b013e3181775136. [DOI] [PubMed] [Google Scholar]
- 10.Cheng N.-C., Wang S., Young T.-H. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. 2012;33(6):1748–1758. doi: 10.1016/j.biomaterials.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 11.Yao Y., Dong Z., Liao Y., et al. Adipose Extracellular Matrix/Stromal Vascular Fraction Gel: A Novel Adipose Tissue-Derived Injectable for Stem Cell Therapy. Plastic and Reconstructive Surgery. 2017;139(4):867–879. doi: 10.1097/PRS.0000000000003214. [DOI] [PubMed] [Google Scholar]
- 12.He Y., Dong Z., Xie G., Zhou T., Lu F. The combination of tissue dissection and external volume expansion generates large volumes of adipose tissue. Plastic and Reconstructive Surgery. 2017;139(4):888e–899e. doi: 10.1097/PRS.0000000000003212. [DOI] [PubMed] [Google Scholar]
- 13.Lu Z., Yuan Y., Gao J., Lu F. Adipose tissue extract promotes adipose tissue regeneration in an adipose tissue engineering chamber model. Cell and Tissue Research. 2016;364(2):289–298. doi: 10.1007/s00441-015-2322-5. [DOI] [PubMed] [Google Scholar]
- 14.Pan L., Tang J., Liu H., Cheng B. Sympathetic nerves: How do they affect angiogenesis, particularly during wound healing of soft tissues? Clinical Hemorheology and Microcirculation. 2016;62(2):181–191. doi: 10.3233/CH-152019. [DOI] [PubMed] [Google Scholar]
- 15.Alev C., Ii M., Asahara T. Endothelial progenitor cells: a novel tool for the therapy of ischemic diseases. Antioxidants and Redox Signaling. 2011;15(4):949–965. doi: 10.1089/ars.2010.3872. [DOI] [PubMed] [Google Scholar]
- 16.Otero-Viñas M., Falanga V. Mesenchymal stem cells in chronic wounds: the spectrum from basic to advanced therapy. Advances in Wound Care. 2016;5(4):149–163. doi: 10.1089/wound.2015.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terrovitis J. V., Smith R. R., Marbán E. Assessment and optimization of cell engraftment after transplantation into the heart. Circulation Research. 2010;106(3):479–494. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christman K. L., Lee R. J. Biomaterials for the treatment of myocardial infarction. Journal of the American College of Cardiology. 2006;48(5):907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Wagner J., Kean T., Young R., Dennis J. E., Caplan A. I. Optimizing mesenchymal stem cell-based therapeutics. Current Opinion in Biotechnology. 2009;20(5):531–536. doi: 10.1016/j.copbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Lee D. E., Ayoub N., Agrawal D. K. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Research & Therapy. 2016;7:p. 37. doi: 10.1186/s13287-016-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Z., Hu X., Zhu C., Wang D., Zheng X., Liu Q. Overexpression of CNTF in mesenchymal stem cells reduces demyelination and induces clinical recovery in experimental autoimmune encephalomyelitis mice. Journal of Neuroimmunology. 2009;206(1-2):58–69. doi: 10.1016/j.jneuroim.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Ishii M., Numaguchi Y., Okumura K., et al. Mesenchymal stem cell-based gene therapy with prostacyclin synthase enhanced neovascularization in hindlimb ischemia. Atherosclerosis. 2009;206(1):109–118. doi: 10.1016/j.atherosclerosis.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Zonari A., Martins T. M., Paula A. C., et al. Polyhydroxybutyrate-co-hydroxyvalerate structures loaded with adipose stem cells promote skin healing with reduced scarring. Acta Biomaterialia. 2015;17:170–181. doi: 10.1016/j.actbio.2015.01.043. [DOI] [PubMed] [Google Scholar]
- 24.Liu X., Lin T., Fang J., et al. In vivo wound healing and antibacterial performances of electrospun nanofibre membranes. Journal of Biomedical Materials Research—Part A. 2010;94(2):499–508. doi: 10.1002/jbm.a.32718. [DOI] [PubMed] [Google Scholar]
- 25.Vocetkova K., Buzgo M., Sovkova V., Bezdekova D., Kneppo P., Amler E. Nanofibrous polycaprolactone scaffolds with adhered platelets stimulate proliferation of skin cells. Cell Proliferation. 2016;49(5):568–578. doi: 10.1111/cpr.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Y., Lu F. Development of synthetic and natural materials for tissue engineering applications using adipose stem cells. Stem Cells International. 2016;2016:12. doi: 10.1155/2016/5786257.5786257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FitzGerald J. F., Kumar A. S. Biologic versus synthetic mesh reinforcement: what are the pros and cons? Clinics in Colon and Rectal Surgery. 2014;27(4):140–148. doi: 10.1055/s-0034-1394155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Meng H., Liu Y., Lee B. P. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. The Scientific World Journal. 2015;2015:10. doi: 10.1155/2015/685690.685690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosseinzadeh E., Davarpanah M., Hassanzadeh N. N., Tavakoli S. A. Fabrication of a hard tissue replacement using natural hydroxyapatite derived from bovine bones by thermal decomposition method. International Journal of Organ Transplantation Medicine. 2014;5(1):23–31. [PMC free article] [PubMed] [Google Scholar]
- 30.Ennis W. J., Sui A., Bartholomew A. Stem cells and healing: impact on inflammation. Advances in Wound Care. 2013;2(7):369–378. doi: 10.1089/wound.2013.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singer A. J., Clark R. A. F. Cutaneous wound healing. The New England Journal of Medicine. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 32.Goren I., Allmann N., Yogev N., et al. A transgenic mouse model of inducible macrophage depletion: Effects of diphtheria toxin-driven lysozyme m-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. American Journal of Pathology. 2009;175(1):132–147. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh T. J., DiPietro L. A. Inflammation and wound healing: the role of the macrophage. Expert Reviews in Molecular Medicine. 2011;13, article e23 doi: 10.1017/s1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucas T., Waisman A., Ranjan R., et al. Differential roles of macrophages in diverse phases of skin repair. Journal of Immunology. 2010;184(7):3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 35.Li J., Chen J., Kirsner R. Pathophysiology of acute wound healing. Clinics in Dermatology. 2007;25(1):9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Zhao R., Liang H., Clarke E., Jackson C., Xue M. Inflammation in chronic wounds. International Journal of Molecular Sciences. 2016;17(12, article 2085) doi: 10.3390/ijms17122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doi K., Ogata F., Eto H., et al. Differential Contributions of Graft-Derived and Host-Derived Cells in Tissue Regeneration/Remodeling after Fat Grafting. Plastic and Reconstructive Surgery. 2015;135(6):1607–1617. doi: 10.1097/PRS.0000000000001292. [DOI] [PubMed] [Google Scholar]
- 38.Dong Z., Peng Z., Chang Q., et al. The angiogenic and adipogenic modes of adipose tissue after free fat grafting. Plastic and Reconstructive Surgery. 2015;135(3):556–567. doi: 10.1097/PRS.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 39.Lilja H. E., Morrison W. A., Han X.-L., et al. An adipoinductive role of inflammation in adipose tissue engineering: Key factors in the early development of engineered soft tissues. Stem Cells and Development. 2013;22(10):1602–1613. doi: 10.1089/scd.2012.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stramer B. M., Mori R., Martin P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. Journal of Investigative Dermatology. 2007;127(5):1009–1017. doi: 10.1038/sj.jid.5700811. [DOI] [PubMed] [Google Scholar]
- 41.Peranteau W. H., Zhang L., Muvarak N., et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. Journal of Investigative Dermatology. 2008;128(7):1852–1860. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 42.Mori R., Shaw T. J., Martin P. Molecular mechanisms linking wound inflammation and fibrosis: Knockdown of osteopontin leads to rapid repair and reduced scarring. Journal of Experimental Medicine. 2008;205(1):43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graham S., Leonidou A., Lester M., Heliotis M., Mantalaris A., Tsiridis E. Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opinion on Investigational Drugs. 2009;18(11):1633–1654. doi: 10.1517/13543780903241607. [DOI] [PubMed] [Google Scholar]
- 44.Burnouf T., Goubran H. A., Chen T.-M., Ou K.-L., El-Ekiaby M., Radosevic M. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Reviews. 2013;27(2):77–89. doi: 10.1016/j.blre.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Bahrami B., Hosseini A., Talei A. R., Ghaderi A., Razmkhah M. Adipose derived stem cells exert immunomodulatory effects on natural killer cells in breast cancer. Cell Journal. 2017;19:137–145. [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao N., Liu Y., Liang H., Jiang X. Bone marrow-derived mesenchymal stem cells reduce immune reaction in a mouse model of allergic rhinitis. American Journal of Translational Research. 2016;8(12):5628–5636. [PMC free article] [PubMed] [Google Scholar]
- 47.Davies L. C., Heldring N., Kadri N., Le Blanc K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells. 2016;35(3):766–776. doi: 10.1002/stem.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee M. W., Ryu S., Kim D. S., et al. Strategies to improve the immunosuppressive properties of human mesenchymal stem cells. Stem Cell Research & Therapy. 2015;6, article 179 doi: 10.1186/s13287-015-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis. 2014;10(1):29–37. doi: 10.4161/org.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satija N. K., Singh V. K., Verma Y. K., et al. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. Journal of Cellular and Molecular Medicine. 2009;13(11-12):4385–4402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kokai L. E., Marra K., Rubin J. P. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Translational Research. 2014;163(4):399–408. doi: 10.1016/j.trsl.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Yoshimura K., Suga H., Eto H. Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regenerative Medicine. 2009;4(2):265–273. doi: 10.2217/17460751.4.2.265. [DOI] [PubMed] [Google Scholar]
- 53.Vorotnikova E., McIntosh D., Dewilde A., et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biology. 2010;29(8):690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Choi J. S., Yang H.-J., Kim B. S., et al. Fabrication of porous extracellular matrix scaffolds from human adipose tissue. Tissue Engineering Part C: Methods. 2010;16(3):387–396. doi: 10.1089/ten.tec.2009.0276. [DOI] [PubMed] [Google Scholar]
- 55.Sano H., Orbay H., Terashi H., Hyakusoku H., Ogawa R. Acellular adipose matrix as a natural scaffold for tissue engineering. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2014;67(1):99–106. doi: 10.1016/j.bjps.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Park I.-S., Chung P.-S., Ahn J. C. Enhanced angiogenic effect of adipose-derived stromal cell spheroid with low-level light therapy in hind limb ischemia mice. Biomaterials. 2014;35(34):9280–9289. doi: 10.1016/j.biomaterials.2014.07.061. [DOI] [PubMed] [Google Scholar]
- 57.Hodde J. P., Record R. D., Liang H. A., Badylak S. F. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8(1):11–24. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- 58.Li F., Li W., Johnson S. A., Ingram D. A., Yoder M. C., Badylak S. F. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium. 2004;11(3-4):199–206. doi: 10.1080/10623320490512390. [DOI] [PubMed] [Google Scholar]
- 59.Daley W. P., Peters S. B., Larsen M. Extracellular matrix dynamics in development and regenerative medicine. Journal of Cell Science. 2008;121(3):255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 60.Guilak F., Cohen D. M., Estes B. T., Gimble J. M., Liedtke W., Chen C. S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lo C.-M., Wang H.-B., Dembo M., Wang Y.-L. Cell movement is guided by the rigidity of the substrate. Biophysical Journal. 2000;79(1):144–152. doi: 10.1016/s0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engler A. J., Sen S., Sweeney H. L., Discher D. E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 63.Yang M. T., Fu J., Wang Y.-K., Desai R. A., Chen C. S. Assaying stem cell mechanobiology on microfabricated elastomeric substrates with geometrically modulated rigidity. Nature Protocols. 2011;6(2):187–213. doi: 10.1038/nprot.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu J., Wang Y.-K., Yang M. T., et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nature Methods. 2010;7(9):733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saha S., Ji L., De Pablo J. J., Palecek S. P. TGFβ/activin/nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophysical Journal. 2008;94(10):4123–4133. doi: 10.1529/biophysj.107.119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flanagan L. A., Rebaza L. M., Derzic S., Schwartz P. H., Monuki E. S. Regulation of human neural precursor cells by laminin and integrins. Journal of Neuroscience Research. 2006;83(5):845–856. doi: 10.1002/jnr.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marangi G. F., Pallara T., Cagli B., et al. Treatment of early-stage pressure ulcers by using autologous adipose tissue grafts. Plastic Surgery International. 2014;2014:6. doi: 10.1155/2014/817283.817283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klinger M., Caviggioli F., Vinci V., Salval A., Villani F. Treatment of chronic posttraumatic ulcers using autologous fat graft. Plastic and Reconstructive Surgery. 2010;126(3) doi: 10.1097/PRS.0b013e3181e3b585. [DOI] [PubMed] [Google Scholar]
- 69.Rigotti G., Marchi A., Galiè M., et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plastic and Reconstructive Surgery. 2007;119(5):1409–1424. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 70.Condé-Green A., Marano A. A., Lee E. S., et al. Fat grafting and adipose-derived regenerative cells in burn wound healing and scarring. Plastic and Reconstructive Surgery. 2016;137(1):302–312. doi: 10.1097/prs.0000000000001918. [DOI] [PubMed] [Google Scholar]