Abstract
Histone deacetylase assays were first developed in the 1970s, and subsequently refined in the 1990s with the cloning of HDAC enzymes. Most of these early assays, relying on traditional in vitro chemical methodologies, are still applicable today. More recently, however, cell-based HDAC assays that measure HDAC activities in physiological conditions are emerging. Also, there is a continuing development of assays that can measure an isolated HDAC in the absence of other HDAC activities. This chapter reviews some of the older established methods for assaying HDAC activities, as well as introduces more recently developed nontraditional assays.
1. INTRODUCTION
Lysine acetylation at the N-terminal of histones was initially described as a transcriptional regulatory mechanism in cells. In a nonmodified state, the highly positive N-terminal ends of histones interact with DNA, generating an obstacle for the binding of transcription factors and, perhaps, the recruitment of proteins necessary to read the “writing pattern” on nucleosomes to exert transcription. Acetylation of histones potentially neutralizes these positive charges promoting a relaxed nucleosome conformation, which allow the binding of transcription factors and other proteins. These acetyl modifications are introduced by a heterogeneous group of proteins named histone acetyltransferases (HATs), most of them exist in multiprotein complexes that can be selectively recruited to DNA upon exogenous or endogenous cellular stimuli (Lee & Workman, 2007). Contrary to the action of HATs, acetyl groups can be removed by another group of proteins collectively known as histone deacetylases (HDACs). The 18 potential HDACs identified in humans are divided in two families and four classes. The classical HDAC family of zinc-dependent enzymes is composed by classes I, II, and IV, and the class III NAD+-dependent enzymes belonging to the sirtuin family of HDACs. This chapter will deal with only the classical HDACs. The class I HDACs (HDAC1, 2, 3, and 8) are most closely related to the yeast deacetylase RPD3, and the class II HDACs are subdivided into class IIa (HDAC4, 5, 7, and 9) and class IIb (HDAC6 and 10). Both subclasses share homology with the yeast deacetylase HDA1. Finally, the last HDAC discovered, HDAC11, is in its own class IV and does not share homology with either RPD3 or HDA1 yeast deacetylases.
The role of HDACs was initially thought to be limited to their effects on histones. Later studies, however, revealed that HDACs encompass more complex regulatory functions dependent on their tissue expression, cellular compartment distribution, and stage of cellular differentiation (Glozak, Sengupta, Zhang, & Seto, 2005; Minucci & Pelicci, 2006). Although major advances have been made in understanding the role of specific HDACs in cell proliferation and survival, their role in the regulation of many other biological processes requires more intensive investigations. Additionally, HDAC enzymes, originally described as histone modifiers, were later demonstrated to modify a variety of other proteins unrelated to chromatin. The expanded role of HDACs over nonhistone substrates has been explored in numerous areas, including the modulation of proteins related to cell cycle, apoptosis, immune regulation, oncogenesis, metabolism, cellular differentiation, etc. The specific action and selectivity of individual HDACs over most of these biological processes is still not completely understood and, therefore, requires development of new and improved current HDAC assays.
This chapter discusses various functional assays to evaluate the activity of HDACs on histone and nonhistone targets. In addition to discussions of commonly used, established techniques, we present protocols of new techniques aimed toward dissecting functional characterization of specific HDACs, including use of comparative models such as parallel assays with HDAC knockout and/or knockdown cell lines.
2. GENERATION OF HDAC STABLE CELL LINES USING LENTIVIRUS
There are many ways to produce HDACs in vitro and in vivo for the purpose of assaying HDAC activities. In our hands, we have found that most HDACs expressed in stable mammalian cell lines are suitable for most HDAC assays. Generation of stable cell lines is a well-established technique and has been previously described in many publications. Among the methods described, lentiviral particles offer many advantages when compared to other methodologies, including those using plasmid vectors. Perhaps the most significant benefits are lentivirus’ exceptional ability to infect both replicating and nonreplicating cells and, more importantly, their capacity of genomic integration, which provides stable expression of the transduced gene. We have successfully used this method to generate a large number of cells expressing various HDACs and, as an example, present here the generation of a Flag-tagged HDAC11 stable cell line.
This protocol is conducted over a span of several days. Although the general instructions are standardized to work with adherent cells, the protocol can be modified to work with suspension cells. The lentiviral particles overexpressing Flag-HDAC11 were reported previously (Cheng, Lienlaf, Perez-Villarroel, et al., 2014), and one of the models of choice is the mouse macrophage RAW264.7 cells. Lentiviral particles are commercially available already packaged by different suppliers; however, this procedure can be added as a preliminary step to the protocol (Cribbs, Kennedy, Gregory, & Brennan, 2013; Wang & McManus, 2009).
We suggest using a control empty vector for each condition to ensure specificity of the target gene overexpression. Once a lentiviral stock with a suitable titer is obtained, transduce the lentiviral particles into the RAW264.7 cell line. We recommend using a wide range of MOIs (multiplicity of infection). MOI equals of number of viral particles per cell, for example, a MOI of 1 = infection with one viral genome per cell.
2.1 Initial Titration for Selecting Antibiotic Resistance
The vector carrying the transgene of interest must have an additional antibiotic resistance gene in order to facilitate the selection of positive transduced cells. In this particular case, we used a vector carrying puromycin resistance. Therefore, a preliminary step to test the minimal concentration of this antibiotic needed to kill the cells is necessary. Briefly, we seeded a 96-well plate with 1 × 104 RAW264.7 cells per well and tested, in triplicate, the following concentrations of puromycin; 0 (untreated), 0.1, 0.5, 1.0, 2.5, 5.0, 7.5, 10.0, 12.5, 15.0, 17.5, and 20.0 µg/mL. Change media every 48 h, keeping the aforementioned concentration of puromycin in each well. Usually, cellular death will be observed after 5–7 days of culture. The minimal concentration of puromycin needed to kill 100% of the cells will be the concentration to use in the selection of stable cell lines.
Day 1
-
1
Seed 1 × 104 cells per well in a 96-well plate using 120 µL of RPMI supplemented with 10% fetal bovine serum (FBS) and incubate overnight at 37°C in a humidified incubator in an atmosphere of 5% CO2 to allow complete adherence. Avoidance of antibiotics and fungicides is highly suggested. This recommended amount of cells will result in approximately 75% confluence. Some variation and further optimizations may be required when working with other cell types. We also suggest using all wells in the plate; this will avoid any loss of media due to evaporation
Day 2
-
2
Replace the media of each well with 120 µL of hexadimethrine bromide 2 mg/mL (Sigma™ Cat. # H9268). Incubate for 5 min. Some primary cells are sensitive to hexadimethrine bromide.
-
3
We suggest creating eight different triplicate dilutions of the lentiviral particles as follows: 0.0, 0.01, 0.05, 0.1, 0.5, 1.0, 5.0, and 10.0 MOI. At this point half of the plate will be in use, 3 × 8 for your target gene and 3 × 8 for your control vector. However, the entire plate must be filled with media to avoid evaporation. Incubate overnight at 37°C in a humidified incubator in an atmosphere of 5% CO2. Some specific cell lines may require shorter periods of incubation.
Day 3
-
4
Replace media with 120 µL of RPMI supplemented with 10% FBS and incubate for 48 h at 37°C in a humidified incubator in an atmosphere of 5% CO2.
Day 5
-
5
Replace media with 120 µL of RPMI supplemented with 10% FBS and 5.0 µg/mL of puromycin (as determined in the preliminary step). Incubate at 37°C in a humidified incubator in an atmosphere of 5% CO2 for 48 h. Carefully wash the cells with phosphate-buffered saline (PBS) and replace media with the selecting antibiotic every 48 h. During this period you will observe massive cellular death across the entire plate, and after 6–8 days you will observe single colonies in some wells. In general, wells containing more than one colony should be marked and avoided for expansion.
2.2 Establishment of Monoclonal Populations
-
6
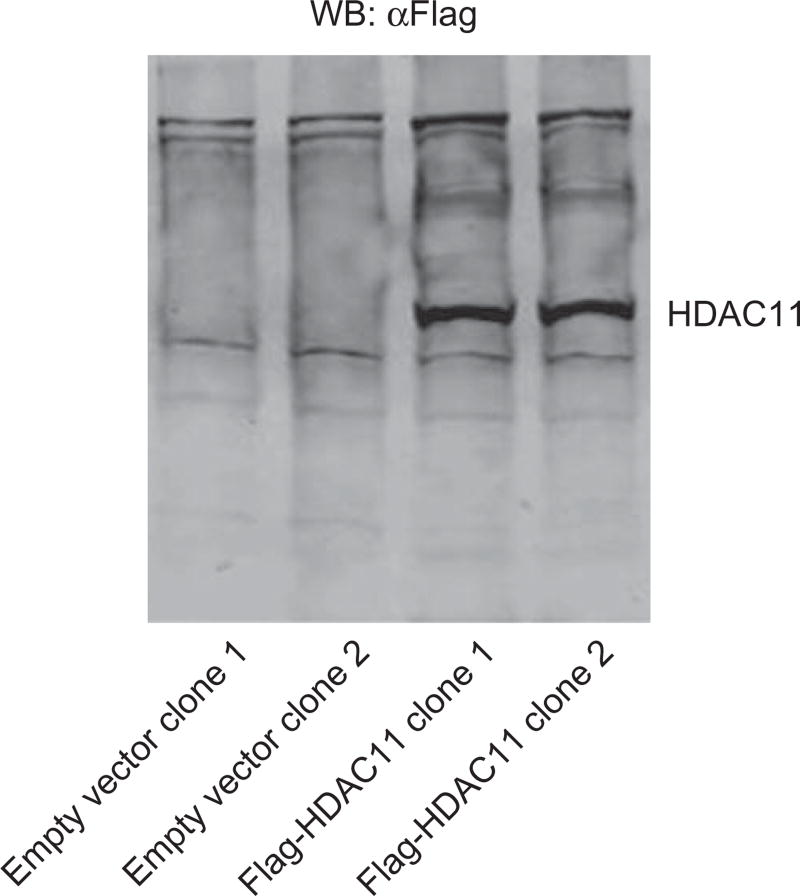
The following expansion step can be done in several ways. We prefer to expand wells with single colonies in the same plate until an eye-sight cluster is observed. Using a micropipette tip, scrape the cluster and transfer to a new 12-well plate; continue culturing the cells using the same conditions as described earlier. Test the expression of the protein after cellular confluence is achieved. Fig. 1 shows the expression of Flag-HDAC11 in two different monoclonal populations isolated using this method.
Fig. 1.
Evaluation of Flag-HDAC11 in murine RAW264.7 cells. RAW264.7 cells were transduced with lentiviral particles containing Flag-HDAC11 or empty vector. The expression of Flag-HDAC11 expression was evaluated after the establishment of monoclonal populations for each condition.
3. CONVENTIONAL HDAC ACTIVITY ASSAY
Several protocols have been described for the preparation of substrates for HDAC activity assays. One of the first procedures and the most commonly used for many years, involved incubation of immature chicken erythrocytes, calf thymus nuclei, or HeLa cells with 3H- or 14C-acetate followed by the isolation of radiolabeled, hyperacetylated histones (Carmen, Rundlett, & Grunstein, 1996; Hendzel, Delcuve, & Davie, 1991; Inoue & Fujimoto, 1969; Sun, Spencer, Chen, Li, & Davie, 2003). The main advantage in using biologically acetylated substrates is that they contain physiologically relevant, naturally acetylated sites. An alternative to in vivo labeling of histones is to use purified recombinant HAT to add 3H-acetate onto purified histones in vitro (Wade, Jones, Vermaak, & Wolffe, 1999). A benefit to using this method is that the histone substrates can be labeled with very high-specific activity. However, this method requires the preparation of high-quality HAT. Finally, a third method involves the chemical acetylation of histones with 3H- or 14C-acetic anhydride. This last method offers the advantage of obtaining very high-specific activity substrates, although it does present the problem of introducing nonspecific acetyl groups onto lysine residues.
The Guarente laboratory analyzed Sir2 deacetylase activity by high-pressure liquid chromatography (HPLC) using unlabeled, acetylated peptides corresponding to the N-termini of histones H3 or H4 (Imai, Armstrong, Kaeberlein, & Guarente, 2000). Later, a fluorogenic HDAC assay was developed that is well suited for high-throughput activity screening (Wegener, Wirsching, Riester, & Schwienhorst, 2003), including nonradioactive HDAC fluorescent activity assay and HDAC colorimetric assay kits that are commercially available.
In this section, we provide a straightforward protocol that uses a labeled peptide corresponding to the N-terminal of histone H4 as a substrate. This protocol offers a quick and very convenient approach for measuring HDAC activity, even though it is a method that could introduce substrates with nonnaturally acetylated sites. The original procedure was pioneered in the Schreiber lab (Taunton, Hassig, & Schreiber, 1996) and was modified in our laboratory. It is important to note that many HDACs are also capable of deacetylating nonhistone cellular proteins. Consequently, this protocol can be applied to studies of nonhistone proteins by synthesizing and labeling peptides corresponding to the nonhistone substrate of interest. The following protocols were originally published in 2004 (Rezai-Zadeh et al., 2004) and repeated here for completeness.
3.1 Labeling of H4 Peptide with [3H] for HDAC Assays
Synthesize or purchase a H4 peptide (SGRGKGGKGLGKGGAKR HRKVLR) corresponding to residues 2–24 of histone H4 with a free amine at the N-terminus and an amide at the C-terminus. Synthetic H4 peptide should be purified by HPLC to greater than 90% purity.
For radiolabeling of the synthetic H4 peptide, add 5 mCi of [3H]acetic acid (2–5 Ci/mmol in ethanol) to 0.4 mg of the H4 peptide. Add 10 µL of freshly prepared BOP solution (0.24 M BOP and 0.2 M triethylamine in acetonitrile) to the peptide/label mix and rock gently on a rocker at room temperature overnight.
Use a Microcon-SCX or similar spin column to purify the labeled peptide. Prewash column with 500 µL of 10 mM hydrochloric acid (HCl) in methanol once, and then with 500 µL of 10 mM HCl in 10% methanol. Spin down wash solutions from the column and discard.
Load 250 µL of the labeling mixture onto the prewashed column and spin column at 1200 × g for 1 min.
Wash column twice with 500 µL of 10 mM HCl in 10% methanol. Invert column and place in a new collection tube.
To elute the labeled peptide, apply 50 µL of 3 N HCl in 50% isopropanol to the column and spin at 14,000 × g for 15 s. This step can be repeated once to ensure complete elution of the labeled peptide.
- In a fume hood, dry the labeled peptide with the cap of the collection tube open or in a SpeedVac. Add 500 µL of dd H2O to dissolve the peptide. Aliquot dissolved radioactive peptide and store at −80°C. The following equation is used to estimate the purity of the radiolabeled peptide:
where purified counts per minute (CPM) refers to the CPM of the final purified peptide and the ethyl acetate-extractable CPM refers to the CPM of the same volume of the purified peptide diluted in dd H2O and extracted by ethyl acetate (see later). A successful acetylation reaction should yield a value not smaller than 1000.
3.2 HDAC Assay
For each reaction, the following reagents are mixed in a microcentrifuge tube: 40 µL of 5 × HDAC buffer (50 mM Tris–HCl [pH 8.0], 750 mM NaCl, and 50% glycerol), 20,000 cpm [3H]acetyl histone H4 peptide, the HDAC enzyme, and dd H2O to a total volume of 200 µL.
Incubate reactions at room temperature overnight.
Stop the reaction by the addition of 50 µL of stop solution (1 M HCl and 0.4 M acetic acid).
Extract released [3H]acetate by adding 400 µL of ethyl acetate to the stop reaction. Vortex the mixture briefly, and centrifuge at 14,000 × g for 3 min at room temperature to separate the phases.
Transfer 200 µL of the organic phase to a scintillation vial and measure CPM.
4. ASSESSMENT OF SELECTIVE INHIBITION OF HDACs USING HDAC KNOCKOUT CELLS
In the last two decades, HDACs have been discovered to be involved in numerous normal and abnormal physiological activities. For this reason, there is now a growing need to develop novel assays to study the enzymatic activities of HDACs under physiological and pathological conditions. Standard assays such as analysis of immunopurified products were not adequate, and newer techniques discussed here will aim toward addressing this need.
As previously mentioned, there is a growing interest to study the effect of individual HDACs on specific substrates. This knowledge will, in turn, increase the understanding of the regulatory capabilities of single HDACs on diverse biological processes. Currently, there is an urgent ongoing effort to design and produce new small molecules to inhibit HDAC enzymatic activity in a more selective fashion. However, the final validation of these potential ultraselective inhibitors requires specific tools and assays to accurately identify their selectivity and potency based on individual HDACs. Most of the commercially available assays rely on the evaluation of purified HDACs. However, in most cases these nonphysiological conditions do not mirror the actual effects encountered in cells. For example, it has been demonstrated that purified HDACs do not retain their full enzymatic activity, and in some situations they may even completely lose their activity. In order to avoid these problems, our group has developed a specific test to evaluate the selectivity of ultraselective HDAC inhibitors. This technique uses a comparative evaluation of total deacetylase activity in cells isolated from HDAC knockout and wild-type (WT) mice. For example, to evaluate the selectivity of a putative ultraselective HDAC6 inhibitor we used cells isolated from HDAC6−/− as well as WT mice. We prefer to isolate total T-cells from the mouse as they do not require extensive preparative procedures.
For the purposes of this chapter, we have only discussed T-cell isolation; however, this assay can be used to isolate other cells such as B-cells and myeloid cells. This protocol is an adaptation of the EasySep™ mouse T-cell isolation system from StemCell™ Technologies (Cat. # 19851). A number of steps have been modified to suit the particular experimental procedure described in this section, with the goal of using the isolated cells for subsequent HDAC assays. Briefly, the system contains a cocktail of monoclonal antibodies that bind cell surface antigens and positively label non-T-cells. A magnetic bead is conjugated to these antibodies and these cells are removed using a magnetic device. Left over cells in the suspension cocktail will contain all the T-cells (a negative selection technique). Generally, T-cell isolation is done via negative selection to avoid activation via CD3 signaling.
Splenocytes are extracted by mechanical dissociation of the spleen with 10 mL of RPMI media.
Cells are resuspended in 15 mL of PBS
Centrifuge cells at 1250 rpm for 5 min at room temperature.
Suction off supernatant.
Add 1 mL of PBS and thoroughly reconstitute splenocytes by pipetting two to three times. Add 9 mL to bring the total volume to 10 mL.
Repeat steps 3–5.
Count live splenocytes and reconstitute in PBS at 1 × 108 cells/mL in a 5 mL clear flow cytometry tube.
Add normal rat serum at 50 µL/mL of cells (blocking step).
Add Mouse T-Cell Isolation Cocktail at 25 µL/mL of cells. Mix well and incubate at room temperature for 10 min.
Vortex Streptavidin RapidSpheres for 30 s.
Add Streptavidin particles at 75 µL/mL of cells. Mix well and incubate at room temperature for 2.5 min.
Bring the suspension up to a total volume of 2.5 mL (<2 × 108 cells), 5 mL (<4 × 108 cells), or 10 mL (4–8 × 108 cells) with PBS and pipette up and down gently 2–3 times.
Place the tube (without cap) in the magnet for 2.5 min at room temperature.
Leave the tube in the magnet for 5 min; pour the liquid, which contains the T-cells, off into a 15 mL conical tube.
Proceed with the analysis of deacetylase activity in a multimode plate reader.
4.1 Quantification of Deacetylase Activity in Multiplate Readers
This protocol requires a 2 days procedure (Day 1: Seed cells, Day 2: Treat with compounds of interest and then analyze in a multimode plate reader). For this particular procedure we used the Cytation™ system from Biotek™.
Day 1
Using a 96-well plate, in a 86 µL volume plate purified HDAC6−/− and WT at 10,000 cells/well.
Centrifuge plate at 200 × g for 3 min to pull all the liquid to the bottom of the well in order to ensure none is remaining on the well sides.
Incubate plate overnight at 37°C 5% CO2 in cell incubator.
Day 2
Make all compound stocks for Tubastatin A—a selective HDAC6 inhibitor (Selleckchem Cat. # S8049) and a selective class I HDAC, Entinostat (MS-275) (Selleckhem Cat. # S8049) prepared as shown in Table 1.
- For each of the compound dilution tubes add as follows (Table 1A):
-
–In row A add 15 µL per well of tube 1
-
–In row B add 15 µL per well of tube 2
-
–In row C add 15 µL per well of tube 3
-
–In row D add 15 µL per well of tube 4
-
–In row E add 15 µL per well of tube 5
-
–In row F add 15 µL per well of tube 6
-
–In row G add 15 µL per well of tube 7
-
–In row H add 15 µL per well of tube 8
-
–
Mix at low speed in an orbital shaker for 30 s.
Thaw and prepare the HDAC-Glo™ (Promega, Cat. # G6422) media as indicated in Table 1B.
Add 15 µL of HDAC-Glo media mix to each well.
Centrifuge plate 200 g for 3 min to pull all liquid into well so that none remains on the well sides.
Table 1.
HDAC-GLO Deacetylase Analysis. (A) Descriptive Calculation and Explanation of Compound Dilutions to Achieve a 100 nm–25 µm Treatment Range. Sample Plate Layout (96-Well Format) for Each Compound in Triplicates [Boxes 1, 2, 3 for Tubastatin A (Tuba A) and 4, 5, 6 for Entinostat]. (B) Descriptive Calculations for HDAC-GLO Media Mix Preparation
| (A) Compound stock: Make enough for 10 wells for each compound (Note: for each compound prepare the stock in tube 1 of the 8-well strip). Stock concentration for each compound should be adjusted to 25 µM | |||||
|---|---|---|---|---|---|
| Add media to tubes 2–8 (as calculated in 2nd and 5th columns) and to dilute the stock as indicated | |||||
| Tuba A | Entinostat | ||||
| 1 | 25 µM stock | 1 | 25 µM stock | ||
| 2 | 90 µL media | 60 µL from tube 1 | 2 | 90 µL media | 60 µL from tube 1 |
| 3 | 75 µL media | 75 µL from tube 2 | 3 | 75 µL media | 75 µL from tube 2 |
| 4 | 75 µL media | 75 µL from tube 3 | 4 | 75 µL media | 75 µL from tube 3 |
| 5 | 135 µL media | 15 µL from tube 2 | 5 | 135 µL media | 15 µL from tube 2 |
| 6 | 135 µL media | 15 µL from tube 3 | 6 | 135 µL media | 15 µL from tube 3 |
| 7 | 135 µL media | 15 µL from tube 5 | 7 | 135 µL media | 15 µL from tube 5 |
| 8 | DMSO | 2.5 µL DMSO in 147.5 µL media | 8 | Triton X | 1.5 µL of Triton X in 148.5µL media |
| (B) Final 96-Well Plate Layout (in Triplicates) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tuba A | Entinostat | Additional Compounds |
Additional Compounds |
|||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| A | 25,000 | 25,000 | 25,000 | 25,000 | ||||||||
| B | 10,000 | 10,000 | 10,000 | 10,000 | ||||||||
| C | 5000 | 5000 | 5000 | 5000 | ||||||||
| D | 2500 | 2500 | 2500 | 2500 | ||||||||
| E | 1000 | 1000 | 1000 | 1000 | ||||||||
| F | 500 | 500 | 500 | 500 | ||||||||
| G | 100 | 100 | 100 | 100 | ||||||||
| H | DMSO control (Neg) | Triton X control (Pos) | Untreated control | |||||||||
Preparation of HDAC-GLO media mix. 1-Premix HDAC-GLO substrate + reagent (included in the Promega kit) and aliquot into 1500 µL volume.
To add 5 µL of developer (included in Promega kit) in 1500 µL premix HDAC-GLO solution.
Begin reading immediately on the Cytation multimode plate reader (assay protocol runs for 1 h and 15 min). Fig. 2 shows T-cells isolated from HDAC6 knockout and WT mice that were treated with either Entinostat or Tubastatin A. As seen in Fig. 2A, the percentage of deacetylation does not change between the samples because Entinostat does not inhibit HDAC6. However, Fig. 2B indicated that Tubastatin A has selectivity toward HDAC6 since a higher level of deacetylase activity is seen in the WTT-cells when compared to the HDAC6 knockout cells. Methods for identifying deacetylated proteins are described in Section 6.
Fig. 2.
Quantification of deacetylase activity using HDAC-GLO assay. T-cells isolated from either HDAC6 knockout or wild-type mice were treated with varying concentrations of Entinostat (A) or Tubastatin A (B) and deacetylase activity was measured by the addition of HDAC-GLO reagent and detected by Cytation multimode plate reader.
5. HDAC CHROMATIN IMMUNOPRECIPITATION ASSAY
Related to deacetylase enzymatic activity assays, another important functional assay to study HDACs is chromatin immunoprecipitation (ChIP). Although one shared characteristic among all HDACs is the lack of classical sequence-specific DNA-binding domains, it is still possible to examine the recruitment of HDACs to DNA via ChIP. HDACs physically interact tightly with histones and with proteins that do contain DNA-binding domains. Here, we describe a protocol to identify the recruitment of HDAC11 to the IL-10 promoter in antigen-presenting cells (APCs). A detailed protocol for the isolation of these cells can be found in previous publications from our group.
5.1 Cross-Linking
-
1
Approximately 20 × 106 APC cells from C57BL/6 and HDAC11 knockout mice per condition will be optimal.
-
2
Cells are collected in 50-mL conical tubes and washed twice with 10 mL 1 × PBS.
-
3
Remove PBS by centrifuging the samples at 1500 × g for 5 min at room temperature.
-
4
Gently reconstitute cells in 20 mL of cross-linking buffer (2.7 mL 37% formaldehyde added into 97.3 mL 1 × PBS, pH 7.2, to reach a final concentration of 1% formaldehyde in a final volume of 100 mL). Set up the sample tubes on a tube-rocker or orbital rotator and incubate for 10 min at room temperature. Both chromatin shearing efficiency and immunoprecipitation (IP) efficiency can be affected by the formaldehyde concentration and cross-linking time. In this protocol, we are using 1% formaldehyde and 10 min incubation time for RAW264.7 cells (mouse APC cell line). Cross-linking time and formaldehyde percentage should be optimized for other cell types (Lee, Johnstone, & Young, 2006; Nelson, Denisenko, & Bomsztyk, 2006).
-
5
Centrifuge samples at 1500 × g for 5 min at room temperature and remove the supernatant
-
6
Gently reconstituted cells in 20 mL of stop buffer (10 mL 1.25 M glycine added into 90 mL 1 × PBS, pH 7.2, to reach a 0.125 M glycine final concentration in a 100 mL final volume). Freshly prepare cross-linking buffer and stop buffer before each experiment, and add protease inhibitor cocktail tablets immediately before use. Incubate for 10 min on a tube-rocker at room temperature.
-
7
Centrifuge the samples at 1500 × g for 5 min at 4°C. Discard the supernatant.
-
8
Reconstitute cells in 20 mL of PBS, gently invert tubes for several times, and spin at 1500 × g for 5 min at 4°C. Discard the supernatant.
-
9
Repeat step 8 if needed. Aliquot cells before centrifugation.
-
10
Proceed with chromatin preparation. Cell pellets from step 9 can be stored at −80°C until further use.
5.2 Chromatin Preparation
-
11
Remove sample tubes containing frozen cell pellets from −80°C and allow them to thaw on ice.
-
12
Prepare Dounce homogenizer (7 mL), set on ice.
-
13
Reconstitute each pellet of 20 × 106 cells in 2 mL of lysis buffer (50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.8, 3 mM MgCl2, 20 mM KCl, 0.25% Triton X-100, 0.5% IGEPAL). Gently pipette up and down until completely homogenized. Incubate on ice for 10 min.
-
14
Transfer cells to the Dounce homogenizer and apply 25 strokes using the loose pestle. Transfer the nuclei to a new 15-mL conical tube and centrifuge at 1500 × g for 5 min at 4°C, then discard supernatant. The Dounce homogenizer has to be set on ice at all times. It should be rinsed with cold PBS three to five times between different samples. The number of strokes may vary and should be optimized for different cell types or cell lines used. In order to optimize for a particular cell line or type, take an aliquot of cells and stain with Tripan Blue and check under the microscope. Adjust stroke times until over 90% of the cells are stained but have kept round nuclei. Decrease the number of strokes if broken nuclei are observed.
-
15
Reconstitute nuclei pellet with 2 mL of wash buffer. Gently pipette up and down until completely homogenized. Incubate on ice for 10 min.
-
16
Pellet the nuclei by centrifuge at 1500 × g for 5 min at 4°C. Discard the supernatant.
-
17
Add 600 µL sonication buffer to each tube, gently pipette up and down until homogenized. Divide the sample by transferring 300 µL nuclei sample to each 1.5 mL Eppendorf tube and proceed to Section 5.3.
5.3 Sample Sonication
-
18
For this specific protocol we used a Bioruptor™ XL from Diagenode. However, any bath sonicator with timing programming will be suitable. The water bath was precooled to 4°C with crushed ice before each sonication cycle and a thin layer of crushed ice was kept in the water bath for each cycle to avoid rapid temperature increase during sonication.
-
19
For each sonication cycle, use a program with eight pulses of 30 s followed by 30 s of resting time at 20 kHz frequency (300 W). To obtain 250–500 base pair DNA fragments for the mouse peritoneal neutrophils, approximately eight cycles are required for each sample. However, this parameter needs to be adjusted for each cell type. Centrifuge the samples at 16,000 × g for 10 min at 4°C. Transfer the supernatant to a new 1.5-mL Eppendorf tube (approximately 600 µL of supernatant). Sonication efficiency varies according to different cell types and equipment. It is recommended to optimize this step by a pilot experiment using a number of different sonication cycles on different cell types or cell lines. For some types of sonicators, it is recommended to check the water bath temperature and replace ice after each cycle, or the water bath could be kept on a heat adjust platform to maintain a 4°C environment.
-
20
Take an aliquot (20 µL) from each sample and proceed to Section 5.4. The rest of the samples can either be stored at −80°C or continue with IP.
5.4 Shear Sample
-
21
To each tube, add 20 µL sample aliquot, 47.2 µL H2O, and 2.8 µL of 5 M NaCl in order to reach a final concentration of 200 mM.
-
22
Incubate at 65°C for 4 h or overnight.
-
23
Add RNAse A to a final concentration of 20 µg/mL to each sample aliquot; incubate at 37°C for 30 min.
-
24
Add Proteinase K to a final concentration of 100 µg/mL to each sample aliquot; incubate at 42°C for 2 h.
-
25
Add 200 µL of phenol/chloroform/isoamyl alcohol, pH 6.7, to each sample aliquot, vortex for 15 s and spin down at 16,000 × g for 5 min.
-
26
Transfer 15 µL of the supernatant from the top layer; add 3 µL of 5 × DNA loading dye with light xylene cyanol only.
-
27
Run samples on 1.5% agarose gel at 40 V for 1.5 h.
5.5 Chromatin Immunoprecipitation
-
28
For the preclearance add 30 µL of Protein A Agarose beads to each sample, incubate on a tube rotator for 1 h at 4°C.
-
29
Centrifuge the samples at 6000 × g for 5 min at 4°C.
-
30
Transfer the supernatant to a new 1.5-mL Eppendorf tube. Take an aliquot of 50 µL from each sample as input. Input samples can be stored at −80°C before reverse cross-linking.
-
31
Dilute 50 µL of each sample after preclearance with 450 µL dilution buffer I (50 mM HEPES, pH 7.8, 140 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), pH 8.0, 1% Triton X-100), to a final volume of 500 µL, one dilution per antibody, into a 1.5-mL Eppendorf tube.
-
32
During the first dilution of each sample, add a mixture of 5 µL (5 µg) Rabbit-anti-HDAC11 antibody from Sigma™ and 20 µL (4 µg) Rabbit-anti-HDAC11 antibody from BioVision™. In the second dilution tube of each sample, add 9 µg normal Rabbit IgG-B.
-
33
Set the sample tubes on a tube rotator overnight, at 4°C, and cover the tubes with aluminum foil to avoid light exposure. Add 50 µL of Protein A Agarose beads to each sample; incubate on a tube rotator for 4 h at 4°C. We recommend using a mixture of two different HDAC11 antibodies that have been confirmed with good IP efficiency on macrophages (Cheng et al., 2014). It is recommended to optimize IP antibody use for each different cell type or cell line used.
5.6 Sample Wash and Elution
-
34
After IP, centrifuge the samples at 6000 × g for 5 min at 4°C and discard the supernatant.
-
35Wash the pellet with 1 mL of each buffer in the following order:
-
–Twice with dilution buffer I.
-
–Twice with dilution buffer II (50 mM HEPES, pH 7.8, 500 mM NaCl, 1 mM EDTA, pH 8.0, 1% Triton X-100).
-
–Twice with lithium chloride (LiCl) buffer (20 mM Tris–HCl, pH 8.0, 250 mM LiCl, 1 mM EDTA, pH 8.0, 0.5% Triton X-100).
-
–Twice with Tris-EDTA buffer (TE) buffer (10 mM Tris–HCl, pH 8.0, 1 mM EDTA, pH 8.0).For each wash, centrifuge at 6000 × g for 5 min at 4°C.
-
–
-
36
Discard the supernatant from the last wash. Add 100 µL of elution buffer to each sample and incubate at 65°C for 15 min. The immune-complexes will be in the soluble fraction.
-
37
Centrifuge the samples at maximum speed for 20 s. Transfer supernatant to a new 1.5-mL Eppendorf tube.
-
38
Remove the input sample tubes from −80°C and thaw on ice. Add 50 µL of elution buffer (50 mM Tris–HCl, pH 8.0, 1 mM EDTA, pH 8.0, 1% sodium dodecyl sulfate) to each input tube to final volume of 100 µL.
-
39
Add 4.16 µL 5 M NaCl (200 mM final concentration) to each IP and input sample tubes. Reverse cross-linking at 65°C for 6 h or overnight.
-
40
Add RNAse A to a final concentration of 20 µg/mL to each sample and incubate at 37°C for 30 min.
-
41
Add Proteinase K to a final concentration of 100 µg/mL to each sample and incubate at 42°C for 2 h.
-
42
Use Qiagen™ DNA purification kit to purify each DNA sample.
To obtain a higher amount of DNA, researchers can elute DNA twice from each column; use 50 µL elution buffer each time to obtain a higher yield of DNA.
-
43
Use 2 µL DNA for each qRT-PCR reaction. This is done in triplicate for each reaction, and the purified DNA must be kept in TE buffer, pH 8.0.
It is recommended that the primer efficiency and melting temperature be determined before running PCR on the ChIP samples.
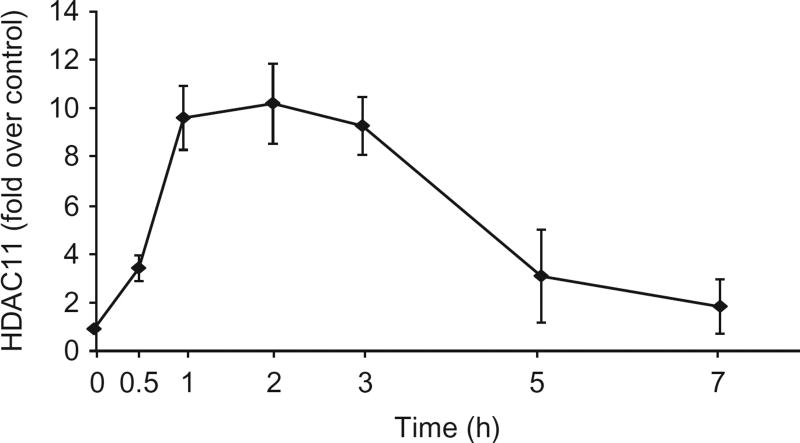
This protocol has been used by our lab and collaborators (Cheng et al., 2014; Villagra, Cheng, Wang, et al., 2009). A prototypical experimental result is shown in Fig. 3, where we evaluated the recruitment of HDAC11 to the IL-10 promoter after stimulation of mouse macrophages with lipopolysaccharide (LPS).
Fig. 3.
HDAC11 is recruited to the IL-10 gene promoter. Macrophages were treated with LPS (1.0 µg/mL), and then harvested at baseline (time 0) or at 0.5, 1, 2, 3, 5, and 7 h after treatment. Cells were then subjected to ChIP analysis using antibodies against HDAC11. Quantitative real-time PCR analysis was performed in the region between −87 and −7 of the IL-10 gene promoter.
6. IDENTIFICATION OF SPECIFIC HDAC SUBSTRATES
Identification of specific protein substrates for particular HDACs can be achieved by several methods. Among them, the characterization of acetyl residues by mass spectrometry has proven to be a reliable approach, in particular, when the discovery of unknown substrates is the main goal (Inoue & Fujimoto, 1969; Joshi, Greco, Guise, et al., 2013). Nevertheless, this technique may not be the first choice when analyzing a limited number of preidentified proteins. In that situation, the identification of acetyl proteins using variant-specific antibodies will provide a faster and an inexpensive approach. Unfortunately, antibodies targeting specific acetylated proteins are only available for a small number of targets, making this option not always feasible. A third option, also inexpensive and faster than mass spectrometry is a two-step assay involving the IP of all acetylated proteins and then an immunoblot against the protein(s) of interest. In this section, we describe a protocol for the identification of HDAC6 protein substrates comparing cellular extracts from HDAC6 knockdown (HDAC6KD) and control human WM164 melanoma cells.
Approximately 1 × 106 from HDAC6KD and control WM164 human melanoma cells are needed as starting material.
Cells were collected in 50-mL conical tubes and washed twice with 10 mL 1 × PBS.
Remove the PBS by centrifuging the samples at 1500 × g for 5 min at 4°C.
Gently reconstitute cells in 400 µL of immunoprecipitation lysis buffer (IPLB) (50 mM Tris [pH 8.0], 150 mM NaCl, 0.5% igepal, 5 mM MgCl2, 10% glycerol) containing 1 × protease inhibitor cocktail (Roche). Centrifuge at 10,000 × g for 15 min at 4°C.
Add 100 µL of protein G-agarose (Millipore #16-266) and incubate for 2 h at 4°C in an orbital rotator. Centrifuge at 6000 × g for 5 min at 4°C. Save 50 µL of the supernatant as an input sample.
Add 350 µL of IPLB to the precleared sample (700 µL total). Add 5 µg of anti-acetyl lysine antibody (Millipore #05-515) and incubate overnight at 4°C in an orbital rotator.
Add anti-acetyl lysine antibody, clone 4G12, agarose conjugate beads (Millipore #16-272) and incubate for 4 h at 4°C in an orbital rotator. Centrifuge at 6000 × g for 5 min at 4°C.
Remove supernatant and wash twice with 700 µL of cold IPLB.
Add 6 × loading buffer and proceed to analyze in parallel the immunoprecipitated fractions from HDAC6KD and control cells (nontarget) by immunoblotting against Hsp90 (Fig. 4). In parallel, we have shown Jak2 as control, a protein not being deacetylated by HDAC6.
Fig. 4.
(A) Nontarget and HDAC6KD WM164 melanoma cells were subjected to immunoblot using antibodies against HDAC6, acetylated α-tubulin, α-tubulin, Hsp90, and JAK2. Total acetylated proteins were immunoprecipitated from nontarget and HDAC6KD WM164 cells. The immunoprecipitated fraction was then assayed for the presence of Hsp90 (B) and JAK2 (C).
Acknowledgments
This work was supported in part by NIH Grants R01CA169210 (E.S.), R01CA187040 (E.S.), and R21CA184612 (A.V.).
ABBREVIATIONS
- APCs
antigen-presenting cells
- ChIP
chromatin immunoprecipitation
- CPM
counts per minute
- EDTA
ethylenediaminetetraacetic acid
- FBS
fetal bovine serum
- HAT
histone acetyltransferase
- HCl
hydrochloric acid
- HDAC
histone deacetylase
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HPLC
high-performance liquid chromatography
- IP
immunoprecipitation
- IPLB
immunoprecipitation lysis buffer
- KCl
potassium chloride
- LiCl
lithium chloride
- LPS
lipopolysaccharide
- M
molar
- mg
milligram
- µg
microgram
- MgCl2
magnesium chloride
- mL
milliliter
- mM
millimolar
- NaCl
sodium chloride
- PBS
phosphate-buffered saline
- qRT-PCR
quantitative real-time polymerase chain reaction
- RCF
(g) relative centrifugal force
- rpm
revolutions per minute
- TE
Tris-EDTA buffer
- µL
microliter
References
- Carmen AA, Rundlett SE, Grunstein M. HDA1 and HDA3 are components of a yeast histone deacetylase (HDA) complex. The Journal of Biological Chemistry. 1996;271(26):15837–15844. doi: 10.1074/jbc.271.26.15837. [DOI] [PubMed] [Google Scholar]
- Cheng F, Lienlaf M, Perez-Villarroel P, et al. Divergent roles of histone deacetylase 6 (HDAC6) and histone deacetylase 11 (HDAC11) on the transcriptional regulation of IL10 in antigen presenting cells. Molecular Immunology. 2014;60(1):44–53. doi: 10.1016/j.molimm.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs AP, Kennedy A, Gregory B, Brennan FM. Simplified production and concentration of lentiviral vectors to achieve high transduction in primary human T cells. BMC Biotechnology. 2013;13:98. doi: 10.1186/1472-6750-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Delcuve GP, Davie JR. Histone deacetylase is a component of the internal nuclear matrix. The Journal of Biological Chemistry. 1991;266(32):21936–21942. [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]