Abstract
Introduction
Retroperitoneal soft tissue sarcomas (RPS) are rare tumors. Surgery is the mainstay of curative therapy, but local recurrence is common. No recommendations concerning the best management of recurring disease have been developed so far. Although every effort should be made to optimize the initial approach, recommendations to treat recurring RPS will be helpful to maximize disease control at recurrence.
Methods
An RPS transatlantic working group was established in 2013. The goals of the group were to share institutional experiences, build large multi-institutional case series, and develop consensus documents on the approach to this difficult disease. The outcome of this document applies to recurrent RPS that is nonvisceral in origin. Included are sarcomas of major veins, undifferentiated pleomorphic sarcoma of psoas, ureteric leiomyosarcoma (LMS). Excluded are desmoids-type fibromatosis, angiomyolipoma, gastrointestinal stromal tumors, sarcomas arising from the gut or its mesentery, uterine LMS, prostatic sarcoma, paratesticular/spermatic cord sarcoma, Ewing sarcoma, alveolar/embryonal rhabdomyosarcoma, sarcoma arising from teratoma, carcinosarcoma, sarcomatoid carcinoma, clear cell sarcoma, radiation-induced sarcoma, paraganglioma, and malignant pheochromocytoma.
Results
Recurrent RPS management was evaluated from diagnosis to follow-up. It is a rare and complex malignancy that is best managed by an experienced multidisciplinary team in a specialized referral center. The best chance of cure is at the time of primary presentation, but some patients may experience prolonged disease control also at recurrence, when the approach is optimized and follows the recommendations contained herein.
Conclusions
International collaboration is critical for adding to the present knowledge. A transatlantic prospective registry has been established.
INTRODUCTION
Retroperitoneal sarcomas (RPS) are rare tumors, with an expected incidence of .5–1 new case/100,000 inhabitants per year.1 Surgery is the mainstay of curative therapy and local control is critical for cure.2–13
Overall local/abdominal recurrence is common (≈ 50 % at 5 years overall) following resection of primary retroperitoneal sarcoma (RPS).2–13 A high proportion of recurrences occur late (after 5 years), mandating ongoing follow-up.13–15
Different histologic subtypes have different patterns and timing of recurrence following resection of primary RPS.2,3,16 The distribution of histological subtypes in recurrent RPS is distinct from that of primary RPS.2,3,16,17 Local recurrence, and/or its treatment, is the most common cause of death from RPS. In general, recurrent RPS is associated with a worse prognosis than primary RPS.3,11,18–20
A trans-Atlantic RPS working group (TARPSWG) was established in 2013. Included in the working group were North-American and European centers who accepted the invitation. The group has published a consensus approach to primary RPS and a large, multicenter, retrospective case series analysis on primary RPS.2,13 As a follow-up of the consensus approach to primary RPS, the group expanded substantially, including several more European and North American centers, and developed this consensus on recurrent RPS. A larger, multicenter, retrospective review on recurrent RPS is presently ongoing and a prospective registry, just finalized, is starting soon.
METHODS
An extensive literature search of published evidence was made and—given the paucity of available data—the TARPSWG developed guidelines on recurrent RPS mainly based on expert agreements. The working group initially met during the 2015 American Society of Clinical Oncology Annual Meeting in Chicago, IL. A document was drafted and circulated in the following months. The group then formally convened during the 2015 Connective Tissue Oncology Society Annual Meeting in Salt-Lake City, Utah. The document was finally approved at a meeting during the 2016 Society of Surgical Oncology Annual Meeting in Boston, Massachusetts. All group members reviewed and agreed to the present version of the recommendations.
The following statements apply to recurrent RPS that is nonvisceral in origin.
Included are “usual” recurrent retroperitoneal sarcoma (liposarcoma, leiomyosarcoma, solitary fibrous tumor, malignant peripheral nerve sheath tumors, synovial sarcoma, etc.)
Included are sarcomas of major veins (inferior vena cava, renal vein, ovarian/testicular vein), undifferentiated pleomorphic sarcoma of psoas, ureteric leiomyosarcoma (LMS).
Excluded are benign entities, such as desmoids and angiomyolipoma.
Excluded are gastrointestinal stromal tumors, visceral sarcomas, such as those arising from the gut or its mesentery, uterine LMS, prostatic sarcoma, paratesticular/spermatic cord sarcoma.
Excluded are Ewing sarcoma, alveolar/embryonal rhabdomyosarcoma, sarcoma arising from teratoma, carcinosarcoma, sarcomatoid carcinoma, clear cell sarcoma, radiation-induced sarcoma.
Excluded are paraganglioma and malignant pheochromocytoma.
RESULTS
Principles of recommended practice from diagnosis to follow-up are summarized in 42 statements. Each statement has been attributed a level of evidence according to the scale reported in Table 1.21
TABLE 1.
Level of Evidence (LOE) and Grade of Recommendation (GOR) adapted from the Infectious Diseases Society of American-United States Public Health Service Grading System
| I | Evidence from at least one large randomized control trial of good methodological quality (low potential for bias) or meta-analyses of well-conducted randomized trials without heterogeneity |
| II | Small randomized trials or large randomized trials with a suspicion of bias (lower methodological quality) or meta-analyses of such trials or of trials with demonstrated heterogeneity |
| III | Prospective cohort studies |
| IV | Retrospective cohort studies or case-control studies |
| V | Studies without control group, case reports, experts opinions |
| A | Strong evidence for efficacy with a substantial clinical benefit, strongly recommended |
| B | Strong or moderate evidence for efficacy but with a limited clinical benefit, generally recommended |
| C | Insufficient evidence for efficacy or benefit does not outweigh the risk or the disadvantages (adverse events, costs,), optional |
| D | Moderate evidence against efficacy or for adverse outcome, generally not recommended |
| E | Strong evidence against efficacy or for adverse outcome, never recommended |
-
1
Prior to undertaking a management decision, patients with recurrent RPS should be presented at a Multi-Disciplinary Case Conference with participation by surgical oncologists, medical oncologists, radiation oncologists, pathologists and radiologists with expertise in sarcoma (VA).
Pretreatment Assessment Imaging
-
2
All relevant imaging studies performed prior to resection of the primary RPS should be obtained and reviewed, as should all subsequent imaging studies, in particular the initial postoperative baseline imaging to determine whether prior resection was in fact grossly incomplete (R2) (VA).
-
3
Current extent of local and distant disease should be determined using oral and intravenous (IV) contrast enhanced computed tomography of the chest, abdomen and pelvis (CT-CAP) (IVA).22–24
-
4
Current imaging should be compared to all prior imaging to ascertain the extent and progression of recurrent disease, with attention to pattern of relapse (locoregional vs. peritoneal) and rate of progression (IVB).25
-
5
The risk of invasion into adjacent organ(s)/critical structure(s) with further progression should be evaluated, and taken into account when deciding on the best management approach (VB).
-
6
MRI may be a useful ancillary modality in selected cases to define extent of adjacent organ/structure involvement that is not clear on CT but is not required in most cases (IVB).26
MRI may be helpful in operative planning for pelvic tumors or tumors that abut/involve bone or psoas or oblique muscles or vertebral foramen.23 MRI also is an option in patients with IV contrast allergy or other serious contraindication to CT (IVB).23
-
7
PET scan is rarely indicated; it may be indicated if the extent of active abdominal disease is difficult to evaluate and/or there is uncertainty about different growth characteristics in multifocal lesions that would alter management (IVB).27,28
-
8Abdominal (nonhepatic parenchymal) recurrence should be categorized as
- locoregional (at the site of the primary RPS or within the ipsilateral RP);
- multifocal/contralateral RP;
- both (VB).
An example is shown in Fig. 1.
FIG. 1.

Contrast-enhanced computed tomography scan, axial view, of a right retroperitoneal ipsilateral recurrent well-differentiated liposarcoma (a); multifocal controlateral recurrent dedifferentiated liposarcoma (b); bilateral recurrend well-differentiated (on the right) and dedifferentiated (on the left) liposarcoma (c)
Pathology
-
9
Histopathology of the primary tumor should be reviewed by a pathologist specialized in the evaluation of soft tissue tumors; molecular subtyping should be performed where appropriate (e.g., Mdm2 in lipomatous tumors) (VA).
-
10
Percutaneous core biopsy confirmation of recurrence is often useful, for a number of reasons: 1) to provide a definitive diagnosis, because a variety of other entities can be mistaken for recurrence of the original primary RPS, for example desmoid fibromatosis (Fig. 2), radiation-associated osteosarcoma, or angiosarcoma in the bed of original LPS; 2) to guide selection of preoperative therapies, including potential targeted therapies; 3) as part of a translational research program or clinical trial; 4) because resection often is challenging and may be morbid and should not be undertaken without due cause (VB).
FIG. 2.
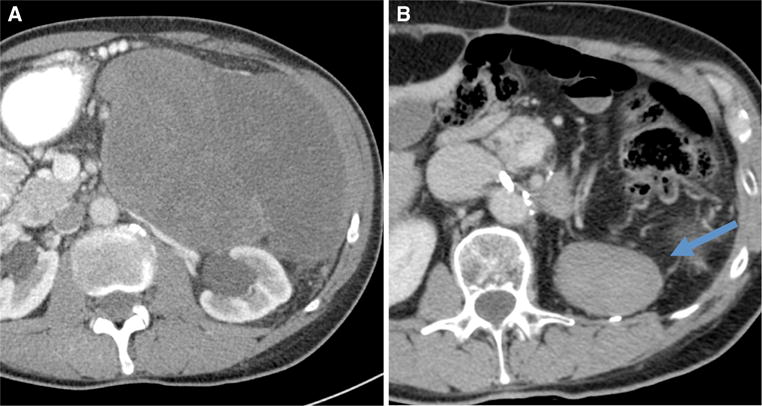
Contrast-enhanced computed tomography scan, axial view, of a primary left retroperitoneal dedifferentiated liposarcoma at presentation (a) and a suspected recurrence detected 2 years after resection of the primary tumor, wich turned out to be desmoid-type fibromatosis on percutaneous biopsy and final pathology (b)
Patient Evaluation
-
11
The patient’s symptoms, and pace of symptom progression, should be noted
The patient’s current performance status should be recorded. Physical examination should include notation of previous incisions, and the relationship of new tumor mass(es) to previous incisions, including port sites (VB).
-
12
Renal function and nutritional status must be assessed (VB).
Review of Previous Treatment
-
13
The operative note describing the resection of the primary tumor should be obtained and reviewed, as should notes describing any subsequent operative or other interventional procedures (VB).
-
14
Timing from previous surgery should be noted, as well as factors that precluded macroscopically complete resection; if not described, these should be elicited through personal communication with the referring surgeon (VB).
-
15
Previous pathology reports, and if possible any tissue slides/blocks, from previous resection(s) should be obtained and reviewed (VB).
-
16The nature of previous surgery should be categorized as
- Macroscopically complete (en bloc resection).
- Macroscopically incomplete (gross residual disease as noted on operative report or on immediate postoperative cross-sectional imaging, Fig. 3)
- Piecemeal and/or associated with tumor rupture or morcellation (VB).
-
17
Details of any previously administered radiotherapy or systemic therapy should be reviewed (VA).
FIG. 3.
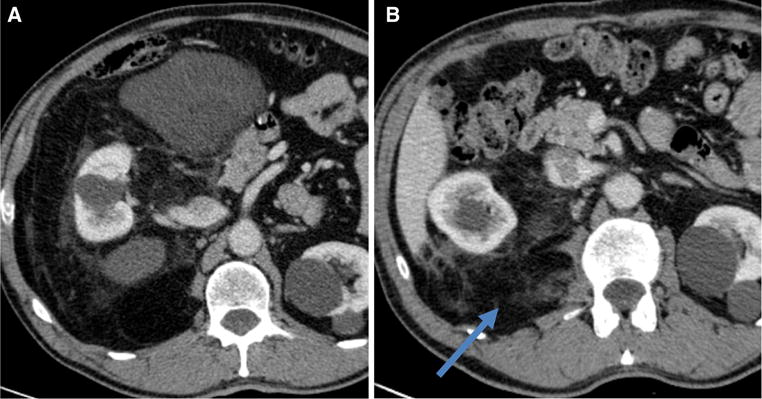
Contrast-enhanced computed tomography scan, axial view, of a primary right retroperitoneal dedifferentiated liposarcoma at presentation (a) and a persistent perirenal disease detected at the first postoperative assessment after resection of the primary tumor (b)
Patient Selection for Resection of Recurrence
-
18
While complete gross resection can be viewed as the only curative-intent option in patients with recurrent RPS, the chance of long term disease-free survival is limited and this must be recognized in multidisciplinary evaluation and planning of management for the individual patient (IVA).3,29
The decision to pursue curative resection is multifactorial and often nuanced. A nomogram to assist in this decision is presently lacking, the available ones mainly built to predict the risk of primary disease.30 Variables to be considered are discussed below.
Abdominal Recurrence
-
19In the case of isolated locoregional recurrence, especially if the previous resection was incomplete, the goal of resection should be curative, and the extent of resection should be as required to achieve complete gross resection (IVB).31–34
-
19.1For local recurrence of WD-LPS within the field of previous resection(s), the surgeon may rationally wait for an increase in tumor size in order to space out the interval between operations (IVB).35
-
19.1
-
20
Multifocal intra-abdominal disease is difficult to resect completely, will almost certainly recur again, and carries a poor prognosis particularly with resection alone. The prognosis following resection worsens with increasing number of intraperitoneal deposits.34 Any resection should be with the goal of avoiding complications of progression and preserving function (i.e., limited resection) or with palliative intent as discussed below. Because the oncologic benefit of surgery is likely to be limited and the risk of morbidity may be substantial, a very careful approach to patient selection for surgical intervention is required (IVA).32–36
-
21
A history of previous piecemeal resection/tumor rupture indicates a strong potential for multifocal peritoneal recurrence and curative-intent resection is generally not appropriate; resection should be with the goals of avoiding complications of progression and preserving function (limited resection); a possible exception is a previous flank/lumbar approach, where piecemeal resection may not have been associated with peritoneal contamination (IVB).4,5
-
22
Histopathologic subtype should factor into the decision to pursue re-resection, because outcomes differ (e.g., WD-LPS would be favored for re-resection) (IVA).3,29–36
-
23
A period of observation and reimaging may help to select more favorable candidates for resection, particularly for asymptomatic WD-LPS (rate of growth of less than 1 cm per month is associated with better duration of disease control) (IVB).
-
24
Long-term survival may be achieved in well-selected patients (e.g. no history of tumor rupture, low grade, long DFI, solitary local recurrence), however there is a very limited chance of cure and this must be considered when offering aggressive therapy 33,36 (IVB).
-
25
Risk of mortality and serious morbidity after radical resection of recurrent RPS are significant; good performance status is important to recovery.
In general, with each serial resection, the oncologic outcome deteriorates, and the chance of mortality and serious morbidity are magnified (IVB).32 The often indolent course of recurrent disease (particularly LPS), even in the presence of a bulky mass, should be considered when major morbidity is expected following resection and the patient is asymptomatic (IVB).35
Distant Recurrence
-
26
Selected patients with limited oligo-metastatic recurrence of RPS may have prolonged survival following metastasectomy (e.g., LMS) (IVB).37
-
27
In general, synchronous abdominal and distant recurrence should not be resected, and the patient should be considered for systemic therapy as part of a multimodality approach based on tumor histology/molecular subtyping (IVB).37
Preoperative Therapy
-
28For patients being considered for resection of recurrent RPS, neoadjuvant therapy should be considered (VB).
-
28.1Cytotoxic and/or targeted systemic therapies may be of benefit in downsizing recurrent disease to improve resectability, especially in the case of LMS, DD-LPS, UPS, SFT, and synovial sarcoma, and also may facilitate assessment of tumor biology/prognosis, especially when a high grade RPS has recurred after a short disease-free interval, and when resection of locally recurrent disease is anticipated to be morbid (VB).
-
28.2Preoperative XRT should be considered, particularly if no previous XRT has been administered and the recurrence is isolated, although its value in improving disease control has not been studied, and toxicity may be magnified in the setting of prior resection (VB).
-
28.1
-
29
Response to treatment varies by histological subtype of RPS and the management plan should be developed in recognition of this and in conjunction with a multidisciplinary care team (VB).
Preoperative treatment of recurrent RPS should ideally be given within the context of a clinical trial.
Resection
-
30The technical challenge of resection is typically compounded following curative-intent en bloc resection of primary RPS (VA).
-
30.1Loss of the original planes increases the difficulty of determining the extent of disease and the optimal extent of resection (VA).
-
30.2Distortion of anatomical relationships due to prior resection can lead to injury of important structures (e.g., IVC, duodenum, femoral nerve) (VA).
-
30.3Vascular involvement by recurrent disease may necessitate vessel resection and reconstruction; this should be done in a planned manner and as part of curative-intent resection, at a specialized center (VA).
-
30.1
-
31
The use of intraoperative frozen sections on marginal tissues as a guide to extent of resection is generally not advised. Frozen-section analysis of soft-tissue tumors is frequently problematic, and particularly so in the setting of reoperative surgery and previous receipt of adjuvant therapies (VA). Result of frozen-section analysis may be misleading. In some specific scenarios, such as LMS of a major vein, frozen-section analysis of a vascular margin may be useful if additional tissue at the relevant site can be removed (VB).
-
32
Technical and non-technical peri-operative considerations are as enunciated in the TARPSWG consensus statement on management of primary RPS (IVA).13,38
Postoperative Systemic Therapy and Other Locoregional Therapies
-
33
There is no proven role for prophylactic systemic therapy after complete resection of recurrent RPS (IVC).39,40
- 34
-
35
There is no proven role for intraperitoneal chemotherapy, but it could be considered for highly selected patients and histologies (IVD).44–49 Ideally intraperitoneal therapy for these specific histologies should be administered within the context of a clinical trial. For usual histological subtypes, the results of debulking surgery and intraperitoneal chemotherapy for multifocal intraperitoneal sarcoma have been uniformly poor with prohibitive morbidity.
-
36
There is no proven role for regional hyperthermia (IIID).50
-
37
After resection of recurrent RPS, patients should be followed with regular cross-sectional imaging, given the high risk for further relapse (IVB).3,29,31–36
Treatment of Patients Who are Not Eligible for Curative Resection
- 38
-
39
Patients should be considered for palliative procedures as symptoms arise. Early referral for symptom management is desirable (VA).
-
40
XRT may be of palliative benefit, in particular with pain control related to nerve compression or infiltration (VB).
-
41
In select patients, survival with recurrent RPS may be prolonged (e.g., WD-LPS). The potential to live many years despite the ongoing presence of recurrent disease should be recognized and adequately communicated to the patient (VB).
-
42
R2 resection may be indicated for symptom control in highly selected patients, particularly if WD-LPS; in contrast R2 resection for more aggressive histologies may increase the risk for peritoneal dissemination/sarcomatosis (VB).
CONCLUSIONS
The available evidence on the approach to recurrent RPS is clearly limited. This is the largest consensus document on this topic and follows the one on primary RPS. An effort to merge and formally report the retrospective experience of the participating institutions, which backs its development, is presently ongoing. The two documents will help to harmonize the approach to this difficult disease. Another major effort to enter all eligible patients onto an international collaborative prospective registry is being finalized and will be active soon. We anticipate that this registry will be the basis of much further understanding of this difficult disease and will serve as a platform for collaborative translational research.
Bio-banking of fresh/frozen tissue should be encouraged to facilitate future research. Evaluations of long-term function and quality-of-life following therapy for recurrent RPS are lacking. Ideally quality-of-life should be assessed preoperatively, as well as postoperatively. The poor prognosis of recurrent RPS highlights the importance of optimal management of primary RPS.1
Trans-Atlantic RPS Working Group
Jan Ahlen, Department of Surgery, Karolinska Hospital, Stockholm, Sweden
Nita Ahuja, Department of Surgery, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, The Johns Hopkins University School of Medicine, Baltimore, MD
Robert Antbacka, Department of Surgery, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT Sanjay Bagaria, Department of Surgery, Mayo Clinic Florida, Jacksonville, FL
Jean-Yves Blay, Department of Cancer Medicine, Centre Leon Berard, Lyon, France
Sylvie Bonvalot, Department of Surgery, Institute Curie, Paris, France
Dario Callegaro, Department of Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy Robert J. Canter, Division of Surgical Oncology, Department of Surgery, UC Davis School of Medicine, Sacramento, CA
Kenneth Cardona, Division of Surgical Oncology, Department of Surgery, Winship Cancer Institute, Emory University, Atlanta, GA
Paolo G. Casali, Department of Cancer Medicine - Fondazione IRCCS Istituto Nazionale dei Tumori - Milan, Italy
Chiara Colombo, Department of Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
Angelo P. Dei Tos, Department of Pathology, Treviso General Hospital - Treviso, Italy
Antonino De Paoli, Department of Radiation Oncology, Centro di Riferimento Oncologico, Aviano, Italy
Anant Desai, Department of Surgery, Queen Elizabeth Hospital, Birmingham, United Kingdom
Brendan C. Dickson, Departments of Pathology and Laboratory Medicine, Mount Sinai Hospital, Toronto, Canada
Fritz C. Eilber, Department of Surgery, University of California - Los Angeles, Division of Surgical Oncology, Los Angeles, CA
Marco Fiore, Department of Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
Cristopher D. Fletcher, Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA
Samuel J. Ford, Department of Surgery, Queen Elizabeth Hospital, Birmingham, United Kingdom
Hans J. Gelderblom, Department of Clinical Oncology, Leiden University Medical Center, Leiden, The Netherlands
Ricardo Gonzalez, Department of Surgery, Moffitt Cancer Center and Research Institute, Tampa, FL
Giovanni Grignani, Department of Cancer Medicine, Istituto Candiolo - Turin, Italy
Valerie Grignol, Department of Surgery, Division of Surgical Oncology, Ohio State University Medical Center, Columbus, OH
Alessandro Gronchi, Department of Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
Rick L. Haas, Department of Radiation Oncology, Netherlands Cancer Institute, Amsterdam, The Netherlands
Andrew J. Hayes, Department of Surgery, Royal Marsden Hospital NHS Foundation Trust, London, United Kingdom
Wolfgang Hartmann, Department of Pathology, Gerhard-Domagk-Institute of Pathology University Hospital Münster, Germany
Thomas Henzler, Institute of Clinical Radiology and Nuclear Medicine, University Medical Center Mannheim, Heidelberg University, Mannheim, Germany
Peter Hohenberger, Department of Surgical Oncology & Thoracic Surgery, Mannheim, Germany
Antoine Italiano, Department of Cancer Medicine, Institute Bergonie, Bordeaux, France
Jens Jakob, Department of Surgical Oncology & Thoracic Surgery, Mannheim, Germany
Robin L. Jones, Department of Cancer Medicine, Royal Marsden Hospital, London, United Kingdom
Ian Judson, Department of Cancer Medicine, Royal Marsden Hospital, London, United Kingdom
John M. Kane, 3rd, Department of Surgical Oncology, Roswell Park Cancer Institute, Buffalo, NY
Guy Lahat, Department of Surgery, Sourasky Medical Center, Tel Aviv, Israel
Andrea J. MacNeill, Department of Surgery, Vancouver General Hospital, University of British Columbia, Vancouver, Canada
Roberta Maestro, Department of Experimental Oncology, Centro di Riferimento Oncologico, Aviano, Italy
Christina Messiou, Department of Radiology, Royal Marsden Hospital NHS Foundation Trust, London, United Kingdom
Pierre Meeus, Department of Surgery, Centre Leon Berard, Lyon, France
Rosalba Miceli, Department of Biostatistics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
John T. Mullen, Department of Surgery, Massachusetts General Hospital, Boston, MA
Carolyn Nessim, Department of Surgery, The Ottawa Hospital, Ottawa, Canada
Elisabetta Pennacchioli, Department of Surgery, European Institute of Oncology, Milan, Italy
Vinu G. Pillarisetty, Department of Surgery, University of Washington, Seattle, WA
Raphael E. Pollock, Department of Surgery, Division of Surgical Oncology, Ohio State University Medical Center, Columbus, OH
Vittorio Quagliuolo, Department of Surgery, Humanitas Cancer Center, Rozzano, Italy
Stefano Radaelli, Department of Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
Chandrajit P. Raut, Department of Surgery, Division of Surgical Oncology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA
Piotr Rutkowski, Department of Soft Tissue/Bone Sarcoma and Melanoma, Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland
Sergio Sandrucci, Department of Surgery, S Giovanni Battista Hospital, University of Turin, Turin, Italy
Yvonne M. Schrage, Department of Surgery, Leiden University Medical Center, Leiden, The Netherlands
Jason K. Sicklick, Division of Surgical Oncology, Department of Surgery, University of California, Moores Cancer Center, San Diego, CA
Myles J. Smith, Department of Surgery, Royal Marsden Hospital NHS Foundation Trust, London, United Kingdom
Silvia Stacchiotti, Department of Cancer Medicine, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
Eberhardt Stoeckle, Department of Surgery, Institute Bergonie, Bordeaux, France
Dirk C. Strauss, Department of Surgery, Royal Marsden Hospital NHS Foundation Trust, London, United Kingdom
Carol J. Swallow, Department of Surgery, University of Toronto, Canada
William Tseng, Department of Surgery, University of Southern California, Keck School of Medicine, Los Angeles, CA
Eva Wardelmann, Department of Pathology, Gerhard-Domagk-Institute of Pathology University Hospital Munster, Germany
Frits van Coevorden, Department of Surgical Oncology, Netherlands Cancer Institute, Amsterdam, The Netherlands
Winan J. van Houdt, Department of Surgical Oncology, Netherlands Cancer Institute, Amsterdam, The Netherlands
Nabil Wasif, Department of Surgery, Mayo Clinic Arizona, Phoenix, AZ
References
- 1.Cormier JN, Gronchi A, Pollock RE. Soft tissue sarcomas. In: Brunicardi F, Andersen D, Billiar T, Dunn D, Hunter J, Matthews J, Pollock RE, editors. Schwartz’s principles of surgery. Vol. 2015. McGraw Hill; New York: pp. 1465–94. [Google Scholar]
- 2.Gronchi A, Strauss DC, Miceli R, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): A report on 1007 patients from the multiinstitutional collaborative RPS Working Group. Ann Surg. 2016;263:1002–9. doi: 10.1097/SLA.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 3.Gronchi A, Miceli R, Allard MA, et al. Personalizing the approach to retroperitoneal soft tissue sarcoma: histology-specific patterns of failure and post-relapse outcome after primary extended resection. Ann Surg Oncol. 2015;22(5):1447–54. doi: 10.1245/s10434-014-4130-7. [DOI] [PubMed] [Google Scholar]
- 4.Toulmonde M, Bonvalot S, Meeus P, et al. Retroperitoneal sarcomas: patterns of care at diagnosis, prognostic factors and focus on main histological subtypes: a multicenter analysis of the French Sarcoma Group. Ann Oncol. 2014;25:735–42. doi: 10.1093/annonc/mdt577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–7. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 6.Gronchi A, Lo Vullo S, Fiore M, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:24–30. doi: 10.1200/JCO.2008.17.8871. [DOI] [PubMed] [Google Scholar]
- 7.Hassan I, Park SZ, Donohue JH, et al. Operative management of primary retroperitoneal sarcomas: a reappraisal of an institutional experience. Ann Surg. 2004;239:244–50. doi: 10.1097/01.sla.0000108670.31446.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359–68. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Strauss DC, Hayes AJ, Thway K, et al. Surgical management of primary retroperitoneal sarcoma. Br J Surg. 2010;97:698–706. doi: 10.1002/bjs.6994. [DOI] [PubMed] [Google Scholar]
- 10.Lehnert T, Cardona S, Hinz U, et al. Primary and locally recurrent retroperitoneal soft-tissue sarcoma: local control and survival. Eur J Surg Oncol. 2009;35:986–93. doi: 10.1016/j.ejso.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gronchi A, Miceli R, Colombo C, et al. Frontline extended surgery is associated with improved survival in retroperitoneal low-intermediate grade soft tissue sarcomas. Ann Oncol. 2012;23(4):1067–73. doi: 10.1093/annonc/mdr323. [DOI] [PubMed] [Google Scholar]
- 13.Management of primary retroperitoneal sarcoma (RPS) in the adult: a consensus approach from the Trans-Atlantic RPS Working Group. Ann Surg Oncol. 2015;22:256–63. doi: 10.1245/s10434-014-3965-2. [DOI] [PubMed] [Google Scholar]
- 14.Heslin MJ, Lewis JJ, Nadler E, et al. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol. 1997;15:2832–9. doi: 10.1200/JCO.1997.15.8.2832. [DOI] [PubMed] [Google Scholar]
- 15.Canter RJ, Qin LX, Ferrone CR, et al. Why do patients with low-grade soft tissue sarcoma die? Ann Surg Oncol. 2008;15(12):3550–60. doi: 10.1245/s10434-008-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan MC, Brennan MF, Kuk D, et al. Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann Surg. 2016;263:593–600. doi: 10.1097/SLA.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer S, Antonescu CR, Riedel E, et al. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358–70. doi: 10.1097/01.sla.0000086542.11899.38. discussion 370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gronchi A, Casali PG, Fiore M, et al. Retroperitoneal soft tissue sarcomas: patterns of recurrence in 167 patients treated at a single institution. Cancer. 2004;100:2448–55. doi: 10.1002/cncr.20269. [DOI] [PubMed] [Google Scholar]
- 19.Anaya DA, Lahat G, Wang X, et al. Postoperative nomogram for survival of patients with retroperitoneal sarcoma treated with curative intent. Ann Oncol. 2010;21:397–402. doi: 10.1093/annonc/mdp298. [DOI] [PubMed] [Google Scholar]
- 20.Gyorki DE, Brennan MF. Management of recurrent retroperitoneal sarcoma. J Surg Oncol. 2014;109:53–9. doi: 10.1002/jso.23463. [DOI] [PubMed] [Google Scholar]
- 21.Dykewicz CA. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001;33:139–44. doi: 10.1086/321805. [DOI] [PubMed] [Google Scholar]
- 22.Lahat G, Madewell JE, Anaya DA, et al. Computed tomography scan-driven selection of treatment for retroperitoneal liposarcoma histologic subtypes. Cancer. 2009;115:1081–90. doi: 10.1002/cncr.24045. [DOI] [PubMed] [Google Scholar]
- 23.Morosi C, Stacchiotti S, Marchianò A, et al. Correlation between radiological assessment and histopathological diagnosis in retroperitoneal tumors: analysis of 291 consecutive patients at a tertiary reference sarcoma center. Eur J Surg Oncol. 2014 doi: 10.1016/j.ejso.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Tzeng CW, Smith JK, Heslin MJ. Soft tissue sarcoma: preoperative and postoperative imaging for staging. Surg Oncol Clin N Am. 2007;16:389–402. doi: 10.1016/j.soc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Tseng WW, Madewell JE, Wei W, et al. Locoregional disease patterns in well-differentiated and dedifferentiated retroperitoneal liposarcoma: implications for the extent of resection? Ann Surg Oncol. 2014;21:2136–43. doi: 10.1245/s10434-014-3643-4. [DOI] [PubMed] [Google Scholar]
- 26.Shiraev T, Pasricha SS, Choong P, et al. Retroperitoneal sarcomas: a review of disease spectrum, radiological features, characterisation and management. J Med Imaging Radiat Oncol. 2013;57:687–700. doi: 10.1111/1754-9485.12123. [DOI] [PubMed] [Google Scholar]
- 27.Niccoli-Asabella A, Altini C, Notaristefano A, et al. A retrospective study comparing contrast-enhanced computed tomography with 18F-FDG-PET/CT in the early follow-up of patients with retroperitoneal sarcomas. Nucl Med Commun. 2013;34:32–9. doi: 10.1097/MNM.0b013e32835ae545. [DOI] [PubMed] [Google Scholar]
- 28.Alford S, Choong P, Chander S, et al. Value of PET scan in patients with retroperitoneal sarcoma treated with preoperative radiotherapy. Eur J Surg Oncol. 2012;38:176–80. doi: 10.1016/j.ejso.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Grobmyer SR, Wilson JP, Apel B, et al. Recurrent retroperitoneal sarcoma: impact of biology and therapy on outcomes. J Am Coll Surg. 2010;210:602–8–608–10. doi: 10.1016/j.jamcollsurg.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Gronchi A, Miceli R, Shurell E, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center datasets. J Clin Oncol. 2013;31:1649–55. doi: 10.1200/JCO.2012.44.3747. [DOI] [PubMed] [Google Scholar]
- 31.van Dalen T, Hoekstra HJ, van Geel AN, et al. Locoregional recurrence of retroperitoneal soft tissue sarcoma: second chance of cure for selected patients. Eur J Surg Oncol. 2001;27:564–8. doi: 10.1053/ejso.2001.1166. [DOI] [PubMed] [Google Scholar]
- 32.Neuhaus SJ, Barry P, Clark MA, et al. Surgical management of primary and recurrent retroperitoneal liposarcoma. Br J Surg. 2005;92:246–52. doi: 10.1002/bjs.4802. [DOI] [PubMed] [Google Scholar]
- 33.Lochan R, French JJ, Manas DM. Surgery for retroperitoneal soft tissue sarcomas: aggressive re-resection of recurrent disease is possible. Ann R Coll Surg Engl. 2011;93:39–43. doi: 10.1308/003588410X12771863936729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anaya DA, Lahat G, Liu J, et al. Multifocality in retroperitoneal sarcoma: a prognostic factor critical to surgical decision-making. Ann Surg. 2009;249:137–42. doi: 10.1097/SLA.0b013e3181928f2f. [DOI] [PubMed] [Google Scholar]
- 35.Park JO, Qin LX, Prete FP, et al. Predicting outcome by growth rate of locally recurrent retroperitoneal liposarcoma: the one centimeter per month rule. Ann Surg. 2009;250:977–82. doi: 10.1097/sla.0b013e3181b2468b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang JY, Kong SH, Ahn HS, et al. Prognostic factors for reoperation of recurrent retroperitoneal sarcoma: the role of clinicopathological factors other than histologic grade. J Surg Oncol. 2015;111:165–72. doi: 10.1002/jso.23783. [DOI] [PubMed] [Google Scholar]
- 37.Toulmonde M, Bonvalot S, Ray-Coquard I, et al. Retroperitoneal sarcomas: patterns of care in advanced stages, prognostic factors and focus on main histological subtypes: a multicenter analysis of the French Sarcoma Group. Ann Oncol. 2014;25:730–4. doi: 10.1093/annonc/mdt576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonvalot S, Raut CP, Pollock RE, et al. Technical considerations in surgery for retroperitoneal sarcomas: position paper from E-Surge, a master class in sarcoma surgery, and EORTC-STBSG. Ann Surg Oncol. 2012;19:2981–91. doi: 10.1245/s10434-012-2342-2. [DOI] [PubMed] [Google Scholar]
- 39.Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic metaanalysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–81. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 40.Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol. 2012;13:1045–54. doi: 10.1016/S1470-2045(12)70346-7. [DOI] [PubMed] [Google Scholar]
- 41.Smith MJ, Ridgway PF, Catton CN, et al. Combined management of retroperitoneal sarcoma with dose intensification radiotherapy and resection: long-term results of a prospective trial. Radiother Oncol. 2014;110:165–71. doi: 10.1016/j.radonc.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 42.Tseng WH, Martinez SR, Do L, et al. Lack of survival benefit following adjuvant radiation in patients with retroperitoneal sarcoma: a SEER analysis. J Surg Res. 2011;168:e173–80. doi: 10.1016/j.jss.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Roeder F, Ulrich A, Habl G, et al. Clinical phase I/II trial to investigate preoperative dose-escalated intensity-modulated radiation therapy (IMRT) and intraoperative radiation therapy (IORT) in patients with retroperitoneal soft tissue sarcoma: interim analysis. BMC Cancer. 2014;14:617-2407-14-617. doi: 10.1186/1471-2407-14-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayes-Jordan A, Green HL, Lin H, et al. Complete cytoreduction and HIPEC improves survival in desmoplastic small round cell tumor. Ann Surg Oncol. 2014;21:220–4. doi: 10.1245/s10434-013-3269-y. [DOI] [PubMed] [Google Scholar]
- 45.Hayes-Jordan A. Cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy in DSRCT: progress and pitfalls. Curr Oncol Rep. 2015;17:38-015-0461-1. doi: 10.1007/s11912-015-0461-1. [DOI] [PubMed] [Google Scholar]
- 46.Sugarbaker P, Ihemelandu C, Bijelic L. Cytoreductive surgery and HIPEC as a treatment option for laparoscopic resection of uterine leiomyosarcoma with morcellation: early results. Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-4960-y. [DOI] [PubMed] [Google Scholar]
- 47.Baratti D, Pennacchioli E, Kusamura S, et al. Peritoneal sarcomatosis: is there a subset of patients who may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy? Ann Surg Oncol. 2010;17:3220–8. doi: 10.1245/s10434-010-1178-x. [DOI] [PubMed] [Google Scholar]
- 48.Bonvalot S, Cavalcanti A, Le Pechoux C, et al. Randomized trial of cytoreduction followed by intraperitoneal chemotherapy versus cytoreduction alone in patients with peritoneal sarcomatosis. Eur J Surg Oncol. 2005;31:917–23. doi: 10.1016/j.ejso.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Rossi CR, Deraco M, De Simone M, et al. Hyperthermic intraperitoneal intraoperative chemotherapy after cytoreductive surgery for the treatment of abdominal sarcomatosis: clinical outcome and prognostic factors in 60 consecutive patients. Cancer. 2004;100:1943–50. doi: 10.1002/cncr.20192. [DOI] [PubMed] [Google Scholar]
- 50.Angele MK, Albertsmeier M, Prix NJ, Hohenberger P, et al. Effectiveness of regional hyperthermia with chemotherapy for high-risk retroperitoneal and abdominal soft-tissue sarcoma after complete surgical resection: a subgroup analysis of a randomized phase-III multicenter study. Ann Surg. 2014;260(5):749–54. doi: 10.1097/SLA.0000000000000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanfilippo R, Bertulli R, Marrari A, et al. High-dose continuous-infusion ifosfamide in advanced well-differentiated/dedifferentiated liposarcoma. Clin Sarcoma Res. 2014;4(1):16. doi: 10.1186/2045-3329-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin-Liberal J, Alam S, Constantinidou A, et al. Clinical activity and tolerability of a 14-day infusional Ifosfamide schedule in soft-tissue sarcoma. Sarcoma. 2013;2013:868973. doi: 10.1155/2013/868973.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radaelli S, Stacchiotti S, Casali PG, et al. Emerging therapies for adult soft tissue sarcoma. Expert Rev Anticancer Ther. 20146:14. 689–704. doi: 10.1586/14737140.2014.885840. [DOI] [PubMed] [Google Scholar]


