Abstract
Purpose
To assess the feasibility of measuring symptomatic adverse events (AEs) in a multicenter clinical trial using the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).
Methods and Materials
Patients enrolled in Trial XXXX (XXXX) were asked to self-report 53 PRO-CTCAE items representing 30 symptomatic AEs at 6 time points (baseline; weekly x4 during treatment; 12-weeks post-treatment). Reporting was conducted via wireless tablet computers in clinic waiting areas. Compliance was defined as the proportion of visits when an expected PRO-CTCAE assessment was completed.
Results
Among 226 study sites participating in Trial XXXX, 100% completed 35-minute PRO-CTCAE training for clinical research associates (CRAs); 80 sites enrolled patients of which 34 (43%) required tablet computers to be provided. All 152 patients in Trial XXXX agreed to self-report using the PRO-CTCAE (median age 66; 47% female; 84% white). Median time for CRAs to learn the system was 60 minutes (range 30–240), and median time for CRAs to teach a patient to self-report was 10 minutes (range 2–60). Compliance was high, particularly during active treatment when patients self-reported at 86% of expected time points, although compliance was lower post-treatment (72%). Common reasons for non-compliance were institutional errors such as forgetting to provide computers to participants; patients missing clinic visits; internet connectivity; and patients feeling “too sick”.
Conclusions
Most patients enrolled in a multicenter chemoradiotherapy trial were willing and able to self-report symptomatic adverse events at visits using tablet computers. Minimal effort was required by local site staff to support this system. The observed causes of missing data may be obviated by allowing patients to self-report electronically between-visits, and by employing central compliance monitoring. These approaches are being incorporated into ongoing studies.
BACKGROUND
Adverse events (AEs) are reported in cancer clinical trials using the U.S. National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE).1 The CTCAE is a library of items representing 790 discrete AEs. Each AE is graded using a 5-point numerical scoring system, which is anchored to discrete clinical criteria.2 Approximately 10% of AEs in the CTCAE are symptoms (e.g., nausea, sensory neuropathy), which in trials have historically been reported by clinical investigators.3 However, there is empiric evidence that collection of this information directly from patients improves the reliability and precision of symptomatic AE detection in trials.4–7
Recently, the U.S. National Cancer Institute (NCI) developed a library of patient-reported outcome (PRO) items to supplement the CTCAE, called the PRO-CTCAE.8 The PRO-CTCAE was developed by systematically identifying AEs in the CTCAE that are amenable to patient self-report then creating PRO items for each of these using rigorous interdisciplinary methods;9 then by establishing the measurement properties of these items using qualitative10 and quantitative11 psychometric methods.
For each AE in the PRO-CTCAE, between 1 and 3 items are included to assess the frequency, severity, and/or interference with activities related to that AE (Supplemental Table S1). PRO-CTCAE can be administered to patients electronically using software hosted at the NCI which has undergone usability testing and refinement.9
Although it is established that PRO-CTCAE items are well understood by patients and accurately represent symptomatic AEs, the feasibility of implementation of PRO-CTCAE items in multicenter cancer clinical trials is not established. Specifically, the level of staff effort required to teach and remind patients to self-report using the PRO-CTCAE software and to manage these data, staff acceptance, and patient willingness and ability to longitudinally self-report during treatment are unknown. This information is essential for determining if it is practical to employ the PRO-CTCAE in future trials, and for providing information about barriers and strategies towards more broadly integrating the PRO-CTCAE into clinical trial workflow.
METHODS
Patients enrolled in the U.S. National Clinical Trials Network multicenter trial, Trial XXXX (XXXX) (ClinicalTrials.gov: XXXX) were invited to participate in a correlative study to evaluate the feasibility of utilizing the PRO-CTCAE within a clinical trial. The Trial XXXX protocol, including the embedded PRO-CTCAE correlative study, was approved by the Institutional Review Boards of all participating institutions, and all patients underwent informed consent.
Participants in Trial XXXX were randomly assigned to receive either liquid Manuka honey, Manuka honey lozenge, or placebo daily during radiation treatment. The primary endpoint of Trial XXXX was to assess the effects of Manuka honey on dysphagia at 4 weeks based on a numerical rating scale,12 and results of that analysis have been reported elsewhere.13
All participants in Trial XXXX were asked to self-report 53 PRO-CTCAE items representing 30 discrete toxicities (Supplemental Table S2) at baseline and weekly during the four weeks of active radiation treatment, and once post-treatment at week 12. These items were selected by the clinical trial investigators based on expected toxicities related to the trial therapy, as well as based on a set of previously identified symptoms that are prevalent among cancer patients undergoing treatment.14 These items were loaded into the web questionnaire platform for the PRO-CTCAE (Figure 1), which is hosted by the NCI. PRO-CTCAE items were available in English or Spanish.
Figure 1.
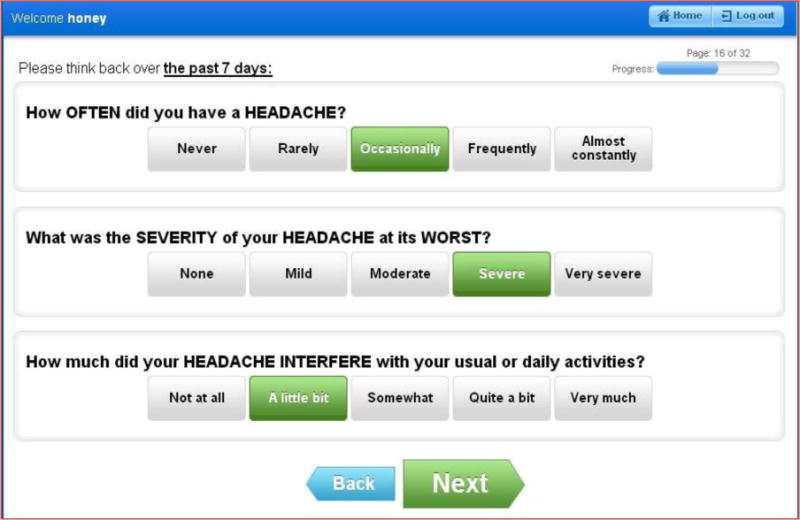
PRO-CTCAE patient questionnaire interface, used via iPad in waiting areas at study visits (software hosted at the NCI)
A central PRO-CTCAE data manager was responsible for training clinical research associates (CRAs) at all participating sites. This entailed a standardized 35-minute webinar which taught CRAs how to register patients into the PRO-CTCAE software system and how to educate patients to login and self-report adverse events using the system. The central data manager also offered refresher orientations as needed (e.g., for changes in CRA personnel), and was available for technical questions or problems experienced by sites.
Site CRAs educated participants in the clinical trial to complete PRO-CTCAE items via wireless tablet computers anytime between informed consent and the baseline visit. Then, at each specified PRO-CTCAE assessment time point, CRAs were instructed, per protocol, to approach participants at their clinic visits and provide the wireless tablet computer to complete the PRO-CTCAE items. A 72-hour window prior to the due date for each PRO-CTCAE assessment was allowed. If a participant did not complete the PRO-CTCAE within that time frame, an email alert was generated to the site CRA, who was instructed to contact the patient and attempt to obtain the PRO-CTCAE information and enter it into the system.
At each study visit, site investigators reported adverse events using criteria from the CTCAE via a standardized AE form, which is the typical approach used in cancer clinical trials15 and required by the trial protocol. PRO-CTCAE reports of adverse events were not shared with CRAs or site clinical investigators. Patients were educated not to rely on the PRO-CTCAE system as a mechanism to inform clinicians about their symptoms, and to communicate directly with their nurse or treating physician about symptoms of concern.
PRO-CTCAE compliance during active treatment was defined as the proportion of pre-specified PRO-CTCAE reporting time points (i.e., baseline visit and weekly ×4 visits) at which PRO-CTCAE assessments were completed by participants who were still alive and enrolled in the trial. Compliance was also evaluated at each pre-specified reporting time point individually, including at the post-treatment 12-week visit. Reasons for missed PRO-CTCAE assessments were collected using a standardized form.
A survey of site CRAs was conducted to understand the effort required to use the PRO-CTCAE system and to obtain feedback. In addition, 10 one-to-one interviews were conducted with randomly selected site CRAs after they had 6 months of experience with the system, to focus on issues identified in the surveys. Effort required of the central data manager was evaluated by tabulating time for site trainings and refreshers. A patient survey was added halfway through the trial to collect patient impressions of the PRO-CTCAE system.
The cumulative incidence of post-baseline investigator-reported CTCAE grades and patient-reported PRO-CTCAE scores for each measured adverse event were tabulated by treatment arm (supportive care arm versus the combined Manuka honey arms).
RESULTS
Between February 2012 and October 2013, 163 patients enrolled in Trial XXXX, of which 3 were ineligible, 4 withdrew consent prior to treatment, and 4 opted not to receive treatment in the trial and were excluded from all analyses, yielding a total of 152 participants. Baseline characteristics were similar across the study arms (Table 1) with an overall median age of 66 years (range 37–85), 47% female, and 84% white.
Table 1.
Characteristics of the participants (N=152)
| Supportive Care (n=48) |
Liquid Honey (n=52) |
Lozenge Honey (n=52) |
|
|---|---|---|---|
| Age (years) | |||
| Median | 66 | 67 | 65 |
| Range | 45 – 85 | 37 – 83 | 47 – 83 |
| Gender | |||
| Male | 23 (48%) | 29 (56%) | 29 (56%) |
| Female | 25 (52%) | 23 (44%) | 23 (44%) |
| Race | |||
| American Indian or Alaskan Native | 0 (0%) | 1 (2%) | 1 (2%) |
| Asian | 2 (4%) | 1 (2%) | 1 (2%) |
| Black or African American | 7 (15%) | 5 (10%) | 7 (14%) |
| White | 39 (81%) | 45 (87%) | 43 (83%) |
| Use of IMRT | |||
| No | 20 (42%) | 23 (44%) | 29 (56%) |
| Yes | 28 (58%) | 29 (56%) | 23 (44%) |
| Percentage of Esophagus in Radiation Field | |||
| < 30% | 33 (63%) | 33 (64%) | 32 (62%) |
| ≥ 30% | 18(38%) | 19(37%) | 20 (39%) |
IMRT, Intensity Modulated Radiation Therapy
The study protocol was approved at 226 sites across the United States. CRAs from each of these sites underwent PRO-CTCAE orientation via a 35-minute webinar. Patients were actively enrolled into the trial at 80 of these sites, of which 34 (42%) required provision of tablet computers for PRO-CTCAE completion, while 46 sites (58%) had available waiting room computers.
During the trial, there were 715 scheduled clinic visits during active treatment at which participants were expected to complete a PRO-CTCE assessment (i.e., visits at which patients were still alive and enrolled in the trial). Of these, PRO-CTCAE assessments were completed at 618 (compliance rate of 86%, Figure 2). Compliance was lower at the post-treatment week 12 visit (72%), when application of protocol procedures was less stringent. Therefore, including all expected visits during active treatment and follow-up, patients self-reported at 715/849 (84%) time points.
Figure 2.
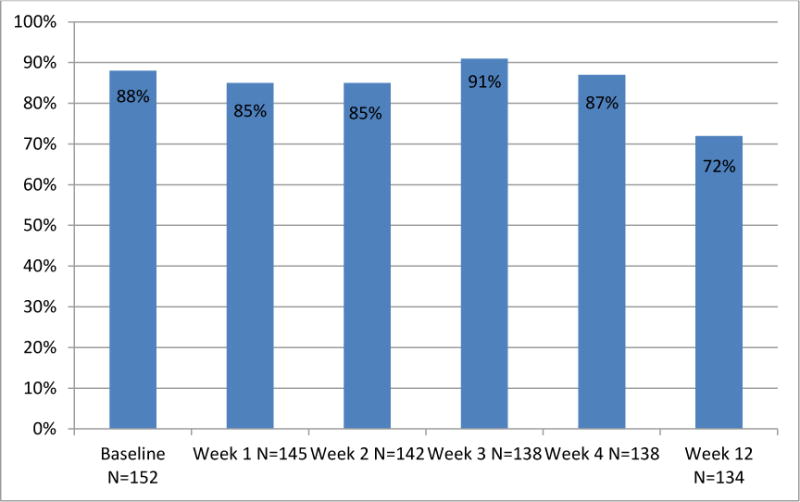
Proportion of study participants completing PRO-CTCAE Adverse Event self-reports at successive study visits.
Among the 134 instances when patients did not self-report PRO-CTCAE at expected time points during treatment and follow up, 28 (21%) occurred because participants missed their clinic appointment; 28 (21%) due to staff errors including forgetting to provide computers to participants and lack of staff coverage during CRA vacations; and 11 (8%) occurred at a single site where staff misinterpreted the protocol and did not observe PRO-CTCAE procedures. There were 20 (15%) cases where technical problems prevented PRO-CTCAE completion (including computer malfunction and internet connectivity problems); 18 cases (13%) when patients were considered “too sick” to self-report; and 12 (9%) PRO-CTCAE reports that were provided by patients outside the required time frame for reporting at a given time point.
Based on a survey of the 70 site CRAs who covered accrual at the 80 sites during conduct of this trial (some CRAs cover more than one site), the median duration for CRAs to teach a patient how to report PRO-CTCAE data electronically was 10 minutes (range 2–60) (Table 2a). At each follow-up visit, administrative work for the PRO-CTCAE took an average of 10 minutes (0–60) while patient contact for the PRO-CTCAE took an average of 15 minutes (0–60). Most research staff found the software system easy to use (79%) and perceived no obstacles at their site for implementing the system (72%, Table 2b). Nonetheless, about one-third experienced some technical difficulties, most commonly attributed to slow internet connectivity (reported by 31% of CRAs, Table 2c).
TABLE 2.
Results of surveys of site clinical research associates (N=70) and patients (N=63)
|
a. Effort by site clinical research associates
| |||
|---|---|---|---|
| Activity | Median | Mean | Range |
| Time for CRA to learn PRO-CTCAE software | 60 min | 70 min | 30–240* |
| Time for CRA to teach PRO-CTCAE to one patient | 10 min | 16 min | 2–60 |
| Time per clinic visit for PRO-CTCAE administrative tasks | 10 min | 12 min | 0–60 |
| Time spent with each patient at clinic visits for PRO- CTCAE | 15 min | 17 min | 0–60 |
|
b. Ease of use by site clinical research associates
| |
| After training, PRO-CTCAE software was: | |
| - Easy to use | 52/66 (79%) |
| - Moderate to use | 13/66 (20%) |
| - Difficult to use | 1/66 (2%) |
|
| |
| Did you experience any of these obstacles to implementing PRO- CTCAE at your site: | |
| - No obstacles | 46/64 (72%) |
| - Staff resources inadequate | 8/64 (13%) |
| - Patient resistance | 12/64 (19%) |
| - Staff resistance | 2/64 (3%) |
|
c. Technical difficulties experienced by site clinical research associates
| |
| Proportion of CRAs who noted experiencing more than minimal technical difficulties | 23/55 (35%) |
|
| |
| Number of CRAs reporting any problems (non-mutually exclusive) with: | |
| - Connectivity/network problems/slow | 17/55 (31%) |
| - Lost passwords | 8/55 (15%) |
| - Software errors | 6/55 (11%) |
| - Other (firewall, hardware, screen problem) | 5/55 (10%) |
|
d. Patient impressions
| ||
|---|---|---|
| Agree | Disagree | |
| PRO-CTCAE questions were easy to understand | 55/60 (92%) | 5/60 (8%) |
| PRO-CTCAE software was easy to use | 51/60 (85%) | 9/60 (15%) |
| PRO-CTCAE improved discussions with my doctor/nurse | 45/59 (76%) | 14/59 (24%) |
| I would recommend PRO-CTCAE to other patients | 45/59 (76%) | 14/59 (24%) |
| PRO-CTCAE made me feel more in control of my own care | 40/58 (69%) | 18/58 (31%) |
Abbreviation: CRA, Clinical Research Assistant at individual study sites
1 CRA noted 240 min; 8 CRAs 120 min; all others <90 min
Abbreviation: CRA, Clinical Research Assistant at individual study sites
In depth one-on-one interviews with 10 randomly selected site CRAs identified slow network connectivity as the most substantial barrier to feasibility and survey compliance. Staff felt that PRO-CTCAE was more challenging for older, ill, and non-computer experienced participants, but was feasible in such patients with encouragement and adequate support.
Effort by the central data manager included 226 35-minute training webinars and 17 refresher training webinars, and 42 interactions with site CRAs to answer questions about PRO-CTCAE software and internet connectivity problems. Approximately 15% of full-time effort was dedicated to this role during the study.
A patient survey was distributed to 67 participants and completed by 63 (94%), with most reporting that questions were easy to understand, software was easy to use, and PRO-CTCAE use led to improved discussions with physicians and nurses (Table 2d).
The cumulative incidence of post-baseline investigator-reported CTCAE and patient-reported PRO-CTCAE ratings are shown in Table 3. Rate of symptomatic AEs based on CTCAE or PRO-CTCAE were similar between supportive care and Manuka honey arms, as Manuka honey does not appear to confer adverse symptoms. The incidence of AEs was higher with patient reporting than clinician reporting, but because this was a supportive care trial testing a relatively benign intervention, investigators were not oriented to report AEs related to chemoradiotherapy, while the PRO-CTCAE asks patients to report symptoms regardless of potential etiology. Patients reported different frequencies for different symptoms. Figure 3 shows longitudinal PRO-CTCAE trajectories during the trial of two common adverse events related to chemoradiotherapy in this population, dysphagia and radiation dermatitis, as examples of how PRO-CTCAE can elucidate the patient experience over time.
Table 3.
Cumulative incidence of adverse events post-baseline for patients enrolled in Trial XXXX, based on investigator-reported CTCAE and patient-reported PRO-CTCAE.
| Adverse Event | Any Level (>0) | High-Level* | ||||
|---|---|---|---|---|---|---|
| Control | Manuka§ | Control | Manuka§ | |||
| Anorexia | CTCAE: | 24% | 21% | 2% | 1% | |
| PRO-CTCAE: |
Severity Interference |
76% 54% |
89% 74% |
26% 20% |
27% 27% |
|
| Anxiety | CTCAE: | 9% | 6% | – | – | |
| PRO-CTCAE: |
Frequency Severity Interference |
74% 72% 50% |
90% 89% 59% |
22% 20% 15% |
27% 20% 18% |
|
| Concentration impairment | CTCAE: | 2% | 2% | – | – | |
| PRO-CTCAE: |
Severity Interference |
67% 48% |
70% 56% |
9% 9% |
4% 10% |
|
| Constipation | CTCAE: | 28% | 24% | – | 1% | |
| PRO-CTCAE: | Severity | 74% | 81% | 17% | 30% | |
| Cough | CTCAE: | 30% | 34% | – | 1% | |
| PRO-CTCAE: |
Severity Interference |
93% 61% |
94% 71% |
26% 20% |
18% 16% |
|
| Depression | CTCAE: | 2% | 4% | – | – | |
| PRO-CTCAE (sad feelings): |
Frequency Severity Interference |
74% 74% 48% |
87% 84% 60% |
11% 11% 11% |
18% 13% 13% |
|
| PRO-CTCAE (nothing can cheer you up): |
Frequency Severity Interference |
57% 54% 35% |
70% 61% 45% |
7% 9% 7% |
13% 10% 10% |
|
| Radiation dermatitis | CTCAE: | 26% | 22% | 2% | 1% | |
| PRO-CTCAE: | Severity | 54% | 61% | 9% | 10% | |
| Diarrhea | CTCAE: | 20% | 15% | – | 1% | |
| PRO-CTCAE: | Frequency | 61% | 61% | 15% | 12% | |
| Dry mouth | CTCAE: | 7% | 7% | – | – | |
| PRO-CTCAE: | Severity | 65% | 79% | 11% | 11% | |
| Dry skin | CTCAE: | 11% | 2% | – | – | |
| PRO-CTCAE: | Severity | 67% | 73% | 4% | 9% | |
| Dysgeusia | CTCAE: | 13% | 13% | – | – | |
| PRO-CTCAE: | Severity | 80% | 84% | 17% | 15% | |
| Dyspepsia | CTCAE: | 22% | 9% | – | – | |
| PRO-CTCAE: |
Frequency Severity |
76% 76% |
80% 80% |
33% 28% |
27% 18% |
|
| Dysphagia | CTCAE: | 37% | 38% | – | 2% | |
| PRO-CTCAE: | Severity | 76% | 86% | 33% | 23% | |
| Dyspnea | CTCAE: | 20% | 34% | 2% | 5% | |
| PRO-CTCAE: |
Severity Interference |
83% 65% |
90% 78% |
17% 15% |
19% 18% |
|
| Fatigue | CTCAE: | 61% | 50% | 7% | 1% | |
| PRO-CTCAE: |
Severity Interference |
98% 93% |
97% 93% |
37% 33% |
39% 44% |
|
| Headache | CTCAE: | 11% | 7% | – | – | |
| PRO-CTCAE: |
Frequency Severity Interference |
63% 61% 33% |
68% 65% 40% |
15% 13% 11% |
11% 5% 5% |
|
| Hiccups | CTCAE: | 2% | 5% | – | – | |
| PRO-CTCAE: |
Frequency Severity |
63% 57% |
65% 60% |
24% 11% |
13% 3% |
|
| Hoarseness | CTCAE: | 7% | 3% | – | – | |
| PRO-CTCAE: | Severity | 67% | 73% | 16% | 6% | |
| Insomnia | CTCAE: | 13% | 7% | – | 1% | |
| PRO-CTCAE: |
Severity Interference |
85% 67% |
84% 74% |
24% 13% |
22% 22% |
|
| Memory impairment | CTCAE: | 2% | 3% | – | – | |
| PRO-CTCAE: |
Severity Interference |
61% 46% |
65% 53% |
2% 2% |
4% 5% |
|
| Mucositis | CTCAE: | 4% | 7% | – | 1% | |
| PRO-CTCAE: |
Severity Interference |
35% 26% |
48% 33% |
7% 7% |
3% 3% |
|
| Nausea | CTCAE: | 37% | 34% | 2% | 1% | |
| PRO-CTCAE: |
Frequency Severity |
63% 63% |
79% 77% |
15% 7% |
16% 18% |
|
| Pain | CTCAE: | 26% | 23% | 2% | 2% | |
| PRO-CTCAE: |
Frequency Severity Interference |
78% 78% 65% |
87% 87% 71% |
37% 30% 26% |
37% 23% 28% |
|
| Pruritus | CTCAE: | 11% | 3% | – | – | |
| PRO-CTCAE: | Severity | 54% | 57% | 9% | 4% | |
| Rash maculo-papular | CTCAE: | 9% | 2% | – | – | |
| PRO-CTCAE: | Presence | 33% | 35% | – | – | |
| Skin hyperpigmentat ion | CTCAE: | 2% | 3% | – | – | |
| PRO-CTCAE: | Presence | 28% | 28% | – | – | |
| Urticaria | CTCAE: | – | – | – | – | |
| PRO-CTCAE: | Presence | 13% | 18% | – | – | |
| Voice alteration | CTCAE: | – | 6% | – | – | |
| PRO-CTCAE: | Presence | 50% | 48% | – | – | |
| Vomiting | CTCAE: | 17% | 10% | 2% | – | |
| PRO-CTCAE: |
Frequency Severity |
35% 35% |
51% 47% |
9% 9% |
4% 5% |
|
| Wheezing | CTCAE: | – | 4% | – | – | |
| PRO-CTCAE: | Severity | 54% | 65% | 9% | 11% | |
High-level clinician-reported adverse events are defined as CTCAE grade 3 or 4. High-level patient-reported adverse events are defined as PRO-CTCAE scores for severity items as severe or very severe; for frequency items as frequently or almost constantly; and for interference items as quite a bit or very much.
Manuka honey liquid and lozenge arms are collapsed into a single Manuka group for this table.
Figure 3.
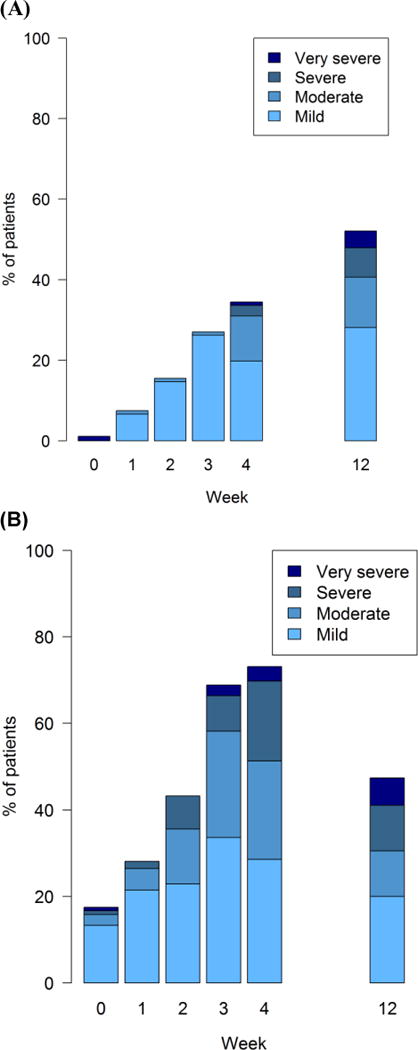
Longitudinal PRO-CTCAE trajectories for radiation dermatitis severity (Panel A) and dysphagia severity (Panel B) at successive visits during the clinical trial, showing the distribution of scores at each time point for all arms combined. Each score number reflects ascending severity of PRO-CTCAE severity response criteria (mild, moderate, severe, very severe).
DISCUSSION
Most patients enrolled in a multicenter chemoradiotherapy clinical trial for lung cancer were willing and able to self-report their own symptomatic adverse events at clinic visits using tablet computers. Minimal effort was required by local site staff and by a central data coordinator to support use of the PRO-CTCAE. Most missing data was attributable to patients not attending scheduled clinic visits, to staff errors, and to technical problems with internet connectivity.
Reasons for missing data in this study are informative for designing future PRO-CTCAE implementation strategies aimed at improving response rates. These findings also inform use of other types of electronic PRO data collection in clinical trials. First, because this trial depended on PRO-CTCAE reports being completed by patients at their clinic visits, if patients missed visits for any reason (e.g., illness, vacation, logistics), the PRO-CTCAE data could not be captured. An alternative strategy is to collect this information using an approach that does not depend on visit attendance, such as between-visit reporting via the web or an automated telephone system. This approach is currently being employed in follow-up PRO-CTCAE feasibility assessments. Second, the central data manager was not allowed to directly interact with patients in this trial because of the structure of the protocol. As a result, the central data manager could not contact patients who missed reports for backup data collection. Site CRAs were depended upon for this function without central monitoring; an approach that our results suggest yielded missing data. In ongoing follow-up PRO-CTCAE studies, an approach is being used in which the central data manager can contact patients directly for reminders and backup data collection. Third, the current PRO-CTCAE software depends on a continuous active internet connection throughout questionnaire completion. Internet connectivity was a common barrier and frustration for CRAs and patients in this study, suggesting the potential value of a downloadable application for the PRO-CTCAE software. Finally, in the analysis for this study we were not able to accurately assess the timing of PRO-CTCAE reports with treatment cycle timing and delays, because of the lack of a software interface between the PRO-CTCAE system and the trial’s clinical data management system. In the future, such interfaces would enhance the ability to analyze relationships between patient-reported toxicities and the timing of treatments.
Necessary additional resources to support use of the PRO-CTCAE in this study included software hosting, maintenance, secure data storage, and user technical support. These roles were performed by the NCI’s Center for Biomedical Informatics and Information Technology (CBIIT). However, in the future this role might be performed by entities that conduct trials such as cooperative groups, pharmaceutical companies, and their contracted technology vendors.
Limitations of this trial included assessment in a single disease, lung cancer, which historically has had lower levels of PRO questionnaire compliance than other cancer populations.16–18 Notably, the PRO-CTCAE is currently being assessed in other disease contexts, and even given this limitation, levels of compliance were relatively high. An additional limitation was that there was not an imbalance of toxicities between arms in this trial because Manuka honey does not cause adverse effects. Therefore, the capacity of the PRO-CTCAE to delineate toxicities between treatment arms could not be assessed. This is the focus of other ongoing studies. However, the analysis of PRO-CTCAE in this trial demonstrates the value of PRO-CTCAE for describing the relative prevalence of different symptomatic adverse events, and longitudinal trajectories of adverse events.
In conclusion, this study describes an approach for collecting and reporting patient-reported adverse events in clinical research, provides initial evidence of feasibility, and lends insights about approaches to potentially optimize response rates.
Supplementary Material
SUMMARY.
The Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) was developed by the National Cancer Institute to enable patient-reporting of toxicities in clinical research. To assess feasibility of implementation, PRO-CTCAE was integrated into an NRG Oncology trial. During treatment, patients reported via tablet computers at 86% of visits. Reasons for missing reports included staff errors, missed appointments, and internet connectivity. Strategies to address these reasons are being assessed in ongoing studies.
Acknowledgments
This project was supported by grants U10CA180822, U10CA180868, U10CA21661, U10CA37422, HHSN261200800043C, and HHSN261201000063C from the National Cancer Institute (NCI).
Conflicts of Interest: Dr. Mendoza reports funding to his institution from National Cancer Institute, outside the submitted work. Dr. Pugh reports grants to her institution from National Cancer Institute, during the conduct of the study and grants to her institution from PCORI, outside the submitted work. Dr. Rimner reports grants from Varian Medical Systems and Boehringer Ingelheim, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior presentations:
Berk LB, Deshmukh S, Fogh SE, Roof K, Yacoub S, Gergel TJ, Stephans K, Rimner A, DeNittis AS, Pablo J, Rineer JM, Chakravarti A, Bruner DW. Randomized Phase II Trial of Best Supportive Care, Manuka Honey Liquid and Manuka Honey Lozenges for Prevention of Radiation Esophagitis During Chemotherapy and Radiotherapy for Lung Cancer. Presented at the 2014 Annual Meeting of the American Society for Radiation Oncology (ASTRO). San Francisco, CA; September 2014.
Basch E, Pugh SL, Dueck AC, Mitchell SA, Berk LB, Rogak LJ, Gatewood M, Reeve BB, Mendoza TR, O’Mara AM, Denicoff A, Minasian L, Fogh SE, Roof K, Moore JK, Gergel TJ, Stephans K, Rimner A, DeNittis A, Bruner DW. Feasibility of Patient Reporting of Symptomatic Adverse Events via the PRO-CTCAE in a Radiation Therapy Oncology Group (RTOG) Cooperative Group Clinical Trial. Presented at the 21st Annual Conference of the International Society for Quality of Life Research (ISOQOL). Berlin, Germany; October 2014.
References
- 1.National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. (NIH publication # 09-7473).Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published May 29, 2009; Revised Version 4.03 June 14, 2010. Available at http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (last accessed March 16, 2015)
- 2.National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. caBIG Knowledge Center, CTCAE FAQ. Available at http://evs.nci.nih.gov/ftp1/CTCAE/Archive/CTCAE_4.01_2009-07-14_FAQ.doc (last accessed March 16, 2015)
- 3.XXXX
- 4.XXXX
- 5.XXXX
- 6.Fromme EK, Eilers KM, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22(17):3485–90. doi: 10.1200/JCO.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Pakhomov SV, Jacobsen SJ, Chute CG, Roger VL. Agreement between patient-reported symptoms and their documentation in the medical record. Am J Manag Care. 2008;14(8):530–9. [PMC free article] [PubMed] [Google Scholar]
- 8.XXXX
- 9.XXXX
- 10.XXXX
- 11.XXXX
- 12.Jensen MP, Turner JA, Romano JM, et al. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–162. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 13.XXXX
- 14.XXXX
- 15.National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. (H publication # 09-7473).Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published May 29, 2009; Revised Version 4.03 June 14, 2010. Available at http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (last accessed March 16, 2015)
- 16.Tanvetyanon T, Soares HP, Djulbegovic B, Jacobsen PB, Bepler G. A systematic review of quality of life associated with standard chemotherapy regimens for advanced non-small cell lung cancer. J Thorac Oncol. 2007 Dec;2(12):1091–7. doi: 10.1097/JTO.0b013e31815cff64. [DOI] [PubMed] [Google Scholar]
- 17.Belani CP, Pereira JR, von Pawel J, et al. TAX 326 study group Effect of chemotherapy for advanced non-small cell lung cancer on patients’ quality of life. A randomized controlled trial. Lung Cancer. 2006 Aug;53(2):231–9. doi: 10.1016/j.lungcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Helbekkmo N, Strøm HH, Sundstrøm SH, Aasebø U, Von Plessen C, Bremnes RM, Norwegian Lung Cancer Study Group Chemotherapy and quality of life in NSCLC PS 2 patients. Acta Oncol. 2009;48(7):1019–25. doi: 10.1080/02841860902795240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


