Abstract
More than half of patients with X-linked lympho-proliferative disease, which is caused by a defect in the intracellular adapter protein SH2D1A, suffer from an extreme susceptibility to Epstein-Barr virus. One-third of these patients, however, develop dysgammaglobulenemia without an episode of severe mononucleosis. Here we show that in SH2D1A-/- mice, both primary and secondary responses of all Ig subclasses are severely impaired in response to specific antigens. Because germinal centers were absent in SH2D1A-/- mice upon primary immunization, and because SH2D1A was detectable in wt germinal center B cells, we examined whether SH2D1A-/- B cell functions were impaired. Using the adoptive cotransfer of B lymphocytes from hapten-primed SH2D1A-/- mice with CD4+ T cells from primed wt mice into irradiated wt mice provided evidence that signal transduction events controlled by SH2D1A are essential for B cell activities resulting in antigen specific IgG production. Defects in naïve SH2D1A-/- B cells became evident upon cotransfer with non-primed wt CD4+ cells into Rag2-/- recipients. Thus, both defective T and B cells exist in the absence of SH2D1A, which may explain the progressive dysgammaglobulinemia in a subset of X-linked lympho-proliferative disease patients without involvement of Epstein-Barr virus.
Keywords: Epstein-Barr virus, germinal center, immunoglobulin, SLAM/CD150
X-linked lymphoproliferative disease (XLP) is a primary immunodeficiency that, in more than half of patients, results in an extreme susceptibility to Epstein-Barr virus (EBV), leading to fatal infectious mononucleosis or B cell lymphoma (1-6). XLP patients who are unaffected by an EBV infection develop dysgammaglobulinemia or agammaglobulinemia even at a very young age (4, 7-10). SH2D1A, the XLP gene, encodes a single SH2-domain adapter, which is primarily expressed in T lymphocytes and natural killer cells (6, 11-20). SH2D1A binds to a tyrosine motif in the cytoplasmic tail of six CD150-related surface receptors (12, 21) and in turn recruits the protein tyrosine kinase Fyn (16, 17, 22-24). Because the influence of EBV on B cell responses greatly complicates analyses of dysgammaglobulinemia of XLP patients, we examined whether dysgammaglobulinemia occurs in a mouse in which the XLP gene SH2D1A (or SAP) has been disrupted (25-28). Upon infection with the parasites Leishmania major and Toxoplasma gondii, impaired T helper 2 responses were observed in SH2D1A-/- mice (25-27). Memory antibody responses to lymphocytic choriomeningitis virus (LCMV) were impaired, because SH2D1A-/- mice had a severe defect in maintaining anti-LCMV IgG levels (28).
Here we show that SH2D1A is essential for both T and B cell responses to soluble T dependent (T-D) antigens. That SH2D1A-controlled signaling is pivotal in primary Ig responses is evident from impaired IgM and IgG responses to LCMV and to the murine Gamma Herpes virus 68 (MHV68), as well as to well defined protein antigens and haptens. Furthermore, hapten-specific class switching of all Ig isotypes and germinal center (GC) formation is defective in SH2D1A-/- mice. Using adoptive transfers of CD4+ T cells together with B lymphocytes from SH2D1A-/- mice into Rag2-/- or wt recipients, we find that SH2D1A controls both T and B cell activities.
Materials and Methods
Mice. C57BL/6, BALB/c, and Rag2-/- mice were purchased from The Jackson Laboratory and were kept under specific pathogen-free conditions at the Beth Israel Deaconess Medical Center (BIDMC) Animal Facility. SH2D1A-/- C57BL/6 and SH2D1A-/- BALB/c mice were backcrossed seven times (25). All animal studies were approved by the BIDMC Institutional Animal Care and Use Committee.
Quantitation of Serum Ig by ELISA. Isotype-specific Igs were detected and quantitated by ELISA, as described (29).
4-Hydroxy-3-nitrophenylacetyl-Keyhole Lymphocyte Hemocyanin (NP-KLH)/2,4,6,-trinitrophenyl-KLH (TNP-KLH) Immunizations. Mice were injected i.p. with 300 μg of alum-precipitated NP-KLH (Biosearch). Imject Alum was purchased from Pierce. Mice were reinjected after 14-21 days with an i.p. injection of 100 μg of NP-KLH in PBS and killed 7 days later. For immunizations with TNP-KLH (Biosearch), 300 μg of alum-precipitated TNP-KLH plus pertussis toxin (300 ng per mouse) (Calbiochem) was used.
Infection with LCMV or MHV68. C57BL/6 SH2D1A-deficient mice and their wt littermates were infected i.p. with 2 × 104 plaque-forming units of the Armstrong strain (ARM) of LCMV-ARM, as described (25). In other experiments, C57BL/6 SH2D1A-deficient mice and controls were infected with MHV68 (30).
Histology and Immunofluorescence. Snap-frozen spleens in OCT media were cryosectioned and stained as described (29). Intracellular SH2D1A protein was detected by a combination of two antibodies: first, a rabbit anti-mouse-SH2D1A antibody (SH2D1A) was added (12). Subsequently, a Rhodamine-labeled donkey anti-rabbit IgG was used (Jackson ImmunoResearch).
Adoptive Transfers. CD4+ T and B220+ B cells were purified from the spleen of primed or unprimed SH2D1A-/- BALB/c or wt BALB/c mice by using negative selection, as described (31). Cell purity was assessed by FACS analysis. Naïve CD4+ cells (5 × 106) from SH2D1A-/- and wt mice (>99% of the cells were CD4+, and <1% of the cells were B220+) were transferred into Rag2-/- mice together with 10 × 106 SH2D1A-/- or wt B cells (cell purity >99%, with <1% of CD4+ cells). Mice were then rested for 1 week before standard i.p. immunization with NP-KLH plus alum. For transfer experiments of hapten-carrier primed cells, KLH primed and boosted CD4+ cells (5 × 106 cells) of SH2D1A-/- and wt mice were transferred into irradiated wt recipients (600 rad) together with NP-hen egg lysozyme primed B cells (10 × 106) and 100 μg of NP-KLH.
Flow Cytometry. Red cell-depleted single-cell suspensions were stained with fluorescence- or biotin-conjugated antibodies, as described (25, 29). Cell sorting was performed on a FACSCalibur cytometer (Becton Dickinson).
Results
Impaired Primary and Secondary Ig Responses in SH2D1A-/- Mice. Because dysgammaglobulinemia is one of the major manifestations of XLP (7, 32), we assessed B cell functions and phenotypes in SH2D1A-/- mice in the absence of immunization. Whereas the basal serum concentrations of IgG1 were lower in SH2D1A-/- C57BL/6 mice than in age-matched wt C57BL/6 mice kept under specific pathogen-free conditions, the serum IgG2a concentration was consistently increased during 6 months (data not shown). Although B cell subsets in bone marrow, lymph nodes, and spleens of 10-week-old SH2D1A-/- and wt C57BL/6 mice were comparable to those of wt mice, the number of Transitional-2 B lymphocytes (CD21bright IgMbright B220+) in the lymph nodes of SH2D1A-/- mice was lower than in wt mice (Table 1, which is published as supporting information on the PNAS web site). An increase in the number of CD21-IgM-B220+ and CD21-CD23-B220+ B lymphocytes was also observed (Table 1). Taken together, our results indicate that minor B cell abnormalities are detectable in nonimmunized SH2D1A-/- mice.
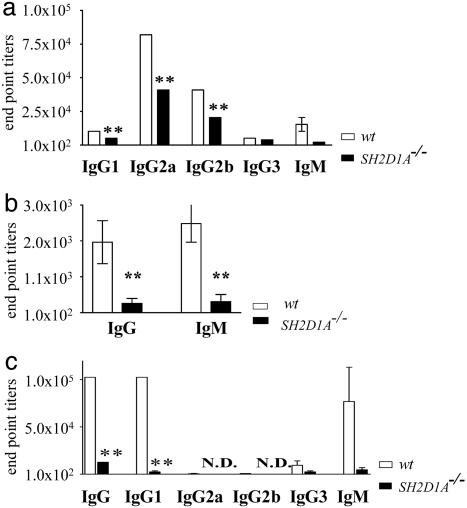
Because reduced primary antigen-specific IgM and IgG responses were detected after infection of SH2D1A-/- mice with LCMV at day 8 (Fig. 1a) or MHV68 (day 15) (Fig. 1b), we also determined responses to a better-defined antigen. To this end, we immunized mutant and wt mice with TNP-KLH precipitated by alum. Although this protocol primarily elicits a Th2 response and promotes IgG1 production, the serum titers of all TNP-KLH-specific IgG isotypes and IgM were severely reduced in SH2D1A-/- BALB/c mice even 5 days after NP-KLH immunization (Fig. 1c and data not shown). Similar results were obtained after immunization of SH2D1A-/- C57BL/6 mice with alum-precipitated TNP-KLH (data not shown).
Fig. 1.
Impaired primary antibody responses in SH2D1A-/- mice. (a) LCMV-specific antibody responses in SH2D1A-/- mice. SH2D1A-/- (filled bars) and wt (open bars) C57BL/6 mice were infected with LCMV. LCMV-specific IgM, IgG1, IgG2a, IgG2b, and IgG3 antibody end-point titers were determined at day 8 after infection, as indicated. y axis, end-point titers (**, P < 0.001; n = 3; n = number of mice tested per experiment). Results are representative of two independent experiments. (b) MHV68-specific antibody responses in SH2D1A-/- mice. SH2D1A-/- (filled bars) and wt (open bars) C57BL/6 mice were infected with MHV68. MHV68-specific IgM and IgG antibody end-point titers were determined at day 15 after infection, as indicated. y axis, end-point titers (**, P < 0.01; seven SH2D1A-/- and four wt mice were tested). (c) Primary antibody responses to T-D antigens in SH2D1A-/- BALB/c mice. Primary TNP-specific antibody titers in the serum of SH2D1A-/- BALB/c mice (n = 4) were determined 10 days after i.p. immunization with alum-precipitated TNP-KLH. TNP-specific IgG, IgM, IgG1, IgG2a, IgG2b, and IgG3 antibody titers of SH2D1A-/- (filled bars) and wt (open bars) mice were determined by ELISA. Serum dilutions start at 1:100; N.D., nondetectable titers (<1:100) (y axis = end-point titers) (**, P < 0.001; n = 4; n = number of mice tested per experiment). Results are representative of three independent experiments.
Secondary IgG1, IgG2a, IgG2b, and IgG3 responses to TNP-KLH were severely impaired in SH2D1A-/- BALB/c and C57BL/6 mice (Fig. 6, which is published as supporting information on the PNAS web site, and data not shown). IgG, IgG1, and IgG2a serum titers were also significantly reduced in SH2D1A-/- BALB/c mice that were immunized with ovalbumin and Freund's adjuvant, conditions that do not favor Th2 responses (data not shown). No defect in T independent antibody responses was found (data not shown). Thus, SH2D1A-/- mice have a defective early IgM and IgG antigen-specific antibody response against viruses and T-D antigens.
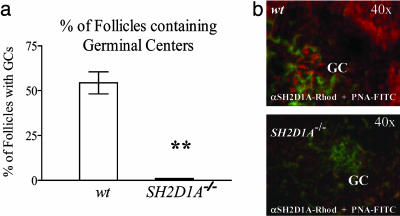
Requirement of SH2D1A for GC Formation. GCs, the anatomical sites of T-B cell cooperation (33-35), were almost completely absent in the spleen of SH2D1A-/- mice that had been immunized with NP-KLH/alum (Fig. 7, which is published as supporting information on the PNAS web site). Fewer than 2% of the splenic follicles contained GCs in immunized SH2D1A-deficient animals, whereas >50% of the follicles in identically treated wt mice contained one or more GC (Fig. 2a). Because of the almost complete absence of GC formations in immunized SH2D1A-/- mice and reports indicating the expression of SH2D1A by human GC B cells (13, 14, 20, 36, 37), splenic sections of immunized wt BALB/c mice were costained for SH2D1A. Fig. 2b shows that the SH2D1A protein is expressed by follicular GC B cells. Thus, an inability to form GCs correlates with defective primary IgM and IgG antibody responses in the absence of SH2D1A.
Fig. 2.
Follicular GC formation. (a) Reduced number of GC-containing follicles in the spleens of SH2D1A-/- mice. The number of GC-containing follicles was determined from at least three different consecutive stained cryosections taken from the spleens of the TNP-KLH mice, described in Fig. 1c. B cell follicles were identified by anti-CD45R/B220-phycoerythrin, and GCs were stained with PNA-FITC. y axis, percentage of follicles containing one or more GCs. Filled bar, SH2D1A-/- BALB/c; open bar, wt BALB/c(**, P < 0.001; n = 4; n = number of mice tested per experiment). Results are representative of three independent experiments. (b) Expression of the SH2D1A protein in follicular GCs. Cryosections of the spleen of wt BALB/c mice 12 days after immunization with NP-KLH were stained with immunofluorescent antibodies, as indicated. Fluorescence was recorded in a Nikon fluorescent microscope. To detect SH2D1A, acetone-fixed and permeabilized spleen sections were first stained with a rabbit anti-mouse anti-SH2D1A antibody (SH2D1A). In a second step, the sections were stained with Rhodamine-labeled donkey anti-rabbit IgG (red). Shown are FITC-labeled-PNA- (green) identified GCs. (Lower) One of the few GCs identified on spleens of SH2D1A-/- injected mice. Antibodies used for staining are indicated at the bottom; magnification is indicated at top right.
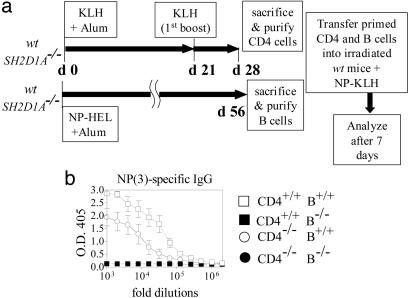
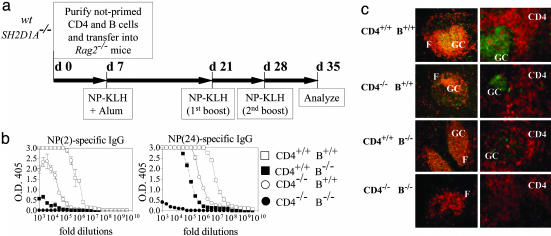
Defective B Cell Responses in the Absence of SH2D1A. The observation that early antibody responses of all isotypes (including IgM) are impaired in SH2D1A-deficient mice prompted us to focus on a potential contribution of SH2D1A-/- B cells to the impaired humoral responses in absence of SH2D1A. To this end, carrier-specific memory CD4+ T helper cells were generated by immunizations of SH2D1A-/- or wt mice with KLH (Fig. 3a). Hapten-specific memory B cells were generated by immunization with NP-hen egg lysozyme. Cotransfer of CD4-/- and B+/+ cells confirmed that a functional impairment of CD4-/- cells contributes to defective T-D responses by SH2D1A-/- mice (Fig. 3b), and a very limited NP-specific antibody response was evident upon antigen recall of recipients reconstituted with B-/- cells (Fig. 3b). Thus, the results of this hapten-carrier experiment show that primed SH2D1A-deficient B cells are unable to function efficiently in recipient mice even in the presence of wt primed T helper cells, as judged by T-D antigen responses. Although this apparent B cell defect was likely to be cell intrinsic, because the effect was determined upon adoptive transfer in a wt host, it could not be excluded that the T cell environment of the donor SH2D1A-/- mouse had permanently affected the donor memory B-/- cell. This prompted us to examine whether naive SH2D1A-/- B cells were functionally impaired by using adoptive transfers of naïve B and T cells. Four combinations of CD4+ and B cells were adoptively transferred into Rag2-/- recipient mice: [CD4+/+ B+/+], [CD4+/+ B-/-], [CD4-/-B+/+], and [CD4-/- B-/-] (outlined in Fig. 4a). Recipient animals were immunized with NP-KLH at day 7 after the transfer and were boosted twice with NP-KLH (Fig. 4a). Seven days after the last antigen challenge, the [CD4+/+ B+/+] chimeras had the highest-titer NP(2)-specific high-affinity antibodies (the number in brackets indicates the NP:BSA conjugation ratio), whereas the [CD4-/-B-/-] chimeras had the lowest titers (Fig. 4b Left). Consistent with an intrinsic B cell defect, [CD4+/+ B-/-] chimeras almost had no detectable NP(2)-specific IgG (Fig. 4b Left). As expected, Rag2-/- mice reconstituted with [CD4-/- B+/+] cells had intermediate levels of NP(2)-specific IgG (Fig. 4b Left). Although the low-affinity responses by the [CD4+/+ B-/-] chimeras were more robust than their high-affinity antibody response, they were still defective compared with those of the [CD4+/+ B+/+] chimeras (Fig. 4b Right). As judged by the total IgG serum concentrations (Fig. 8, which is published as supporting information on the PNAS web site), reconstitution of CD4+ and B cells was comparable in all four chimeras. To examine whether the impaired hapten-specific antibody production in the [CD4+/+ B-/-] and [CD4-/- B+/+] mice correlated with defects in the organization of GCs, the spleens of all four chimeras were stained with anti-CD4, anti-B220, and peanut agglutinin (PNA). Whereas chimeras that had received CD4+/+ and B+/+ cells had follicular GCs with normal follicular and T cell areas, [CD4-/- B-/-] chimeras had no GCs (Fig. 4c, CD4-/- B-/-). Importantly, the organization of GCs was disrupted in [CD4+/+ B-/-] chimeras, and large numbers of extrafollicular PNA+ cells were detected (Fig. 4c, CD4+/+ B-/-). Consistent with a defect in SH2D1A-/- T helper cells, [CD4-/- B+/+] chimeras had smaller follicular GCs and an increased relative number of extrafollicular PNA-positive cells (Fig. 4c, CD4-/- B+/+). Thus, the presence of extrafollicular GC B cells, which are known to produce low-affinity antibodies, might explain low-affinity hapten-specific responses detected in [CD4+/+ B-/-] and [CD4-/- B+/+] chimeras. It was conceivable that during the week after transfer into the Rag2-/- recipients, the wt and mutant cells multiplied at a different rate before immunization. To address this issue, a second set of adoptive transfer experiments into Rag2-/- recipient mice was done in which the recipient mice received a single immunization at time of cell transfer (outlined in Fig. 5a). At day 10 after the transfer, NP-specific IgM antibody responses (Fig. 5b) were defective in the [CD4+/+ B-/-] and [CD4-/- B+/+] chimeras (Fig. 5b Left). Total IgM serum levels were lower in chimeras reconstituted with B-/- cells, when compared with mice reconstituted with B+/+ cells (Fig. 9a, which is published as supporting information on the PNAS web site). Furthermore, high- and low-affinity NP-specific IgG antibody responses at day 10 (Fig. 5b Right; see also Fig. 9b) and at day 20 (data not shown) were markedly lower in [CD4+/+ B-/-] and [CD4-/- B+/+] chimeras than in [CD4+/+ B+/+] mice. The IgG concentrations in the serum of all recipients were comparable (Fig. 9c). Taken together, the early antibody responses to one injection of antigen given at the day of the adoptive transfer confirm that both B and CD4+ T cell defects contribute to severely impaired primary and secondary humoral immune responses in SH2D1A-/- mice.
Fig. 3.
Reduced hapten-specific antibody responses after cotransfers of primed SH2D1A-/- CD4+ T cells with wt B cells or primed wt CD4+ T cells with SH2D1A-/- B cells into irradiated wt recipient mice. (a) Outline of the experiment. CD4+ T cells were purified from the spleens of SH2D1A-/- BALB/cor wt BALB/c mice that had been immunized with KLH attached to alum on day 0 (d 0) and boosted on day 21. B cells were purified from the spleens of SH2D1A-/- BALB/c or wt BALB/c mice that had been immunized 56 days earlier with NP-hen egg lysozyme (Materials and Methods). The purified CD4+ (5 × 106/recipient) and B cells (10 × 106/recipient) were then cotransferred into irradiated wt BALB/c recipients. At the same time, 100 μg of NP-KLH was injected. Four combinations of CD4 and B cells were used to reconstitute the irradiated recipients: [CD4+/+ B+/+]; [CD4+/+ B-/-]; [CD4-/- B+/+] and [CD4-/-B-/-], in which [-/-] represented SH2D1A-/- and [+/+], wt. Reconstituted animals were killed and analyzed 7 days after transfer. (b) Hapten-specific antibody responses. High-affinity NP-specific IgG antibody titers in the serum of recipient mice (n = 4) were determined, as described in Materials and Methods (n = number of mice tested per experiment). Results are representative of two independent experiments. Results of ELISAs are shown as follows: y axis, OD 405 units; x axis, fold dilutions. -/-, cells derived from SH2D1A-/- mice; +/+, cells derived from wt mice; open squares, mice reconstituted with CD4+/+ B+/+ cells; filled squares, mice reconstituted with CD4+/+B-/- cells; open circles, mice reconstituted with CD4-/- B+/+ cells; filled circles, mice reconstituted with CD4-/- B-/- cells.
Fig. 4.
Defective hapten-specific antibody responses after cotransfer of naïve SH2D1A-/- B cells with naïve wt CD4+ cells. (a) Outline of the experiment. CD4+ cells (5 × 106) together with 10 × 106 B cells from unprimed SH2D1A-/- BALB/cor wt BALB/c mice were transferred into Rag2-/- mice at day 0 (d 0). Four combinations of CD4 and B cells were used to reconstitute the Rag2-/- recipients: [CD4+/+ B+/+], [CD4+/+ B-/-], [CD4-/- B+/+], and [CD4-/- B-/-], in which [-/-] represents SH2D1A-/- and [+/+], wt. At day 7 (d 7), mice were immunized with NP-KLH in alum. Reconstituted RAG2-/- mice were then boosted twice with NP-KLH (at d 21 and d 28), and serum antibody levels responses were determined at d 35. (b) Analysis of hapten-specific antibody responses. High-[NP(2)-specific, Left] and low-affinity [NP(24)-specific, Right] IgG antibody titers in the serum of recipient mice (n = 4) were determined as described in Materials and Methods (n = number of mice tested per experiment). Results are representative of three independent experiments. Results of ELISAs are shown (y axis, OD 405 units; x axis, fold dilutions). -/-, cells derived from SH2D1A-/- mice; +/+, cells derived from wt mice; open squares, mice reconstituted with CD4+/+ B+/+ cells; filled squares, mice reconstituted with CD4+/+ B-/- cells; open circles, mice reconstituted with CD4-/- B+/+ cells; filled circles, mice reconstituted with CD4-/- B-/- cells. (c) Cryosections prepared from the spleens of mice reconstituted with naïve SH2D1A-/- or wt B and CD4 cells (as indicated on the left), 7 days after the last immunization with NP-KLH. Sections were stained with immunofluorescent antibodies, and fluorescence was recorded in a Nikon fluorescent microscope. Anti-CD45R/B220-phycoerythrin (PE) (red) (Left) detects follicle areas (F), anti-CD4-PE (red) (Right) detects T cell areas (CD4), and PNA-FITC (green) (Left and Right) identifies GC.
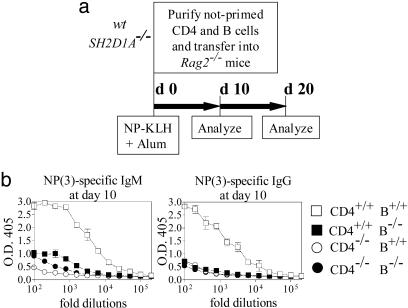
Fig. 5.
Defective early hapten-specific antibody responses after cotransfer of naïve SH2D1A-/- B cells with naïve wt CD4+ cells. (a) Outline of the experiment. CD4+ cells (5 × 106) together with 10 × 106 B cells from unprimed SH2D1A-/- BALB/cor wt BALB/c mice were transferred into Rag2-/- mice and immunized with NP-KLH in alum at day 0 (d 0). Four combinations of CD4 and B cells were used to reconstitute the Rag2-/- recipients: [CD4+/+ B+/+], [CD4+/+ B-/-], [CD4-/-B+/+], and [CD4-/- B-/-], in which [-/-] represented SH2D1A-/- and [+/+], wt. Serum antibody levels responses were determined at day 10 (d 10), and mice were killed at day 20 (d 20) for analysis. (b) Analysis of IgM and IgG hapten-specific antibody responses at day 10. High-affinity [NP(3)-specific] IgM (Left) and IgG (Right), NP-specific antibody titers in the serum of recipient mice (n = 3) were determined as described in Materials and Methods (n = number of mice tested per experiment). Low-affinity [NP(24)-specific] IgM and IgG NP-specific antibody titers are shown in Fig. 9b. Results are representative of two independent experiments. Results of ELISAs are shown (y axis, OD 405 units; x axis, fold dilutions). -/-, cells derived from SH2D1A-/- mice; +/+, cells derived from wt mice; open squares, mice reconstituted with CD4+/+ B+/+ cells; filled squares, mice reconstituted with CD4+/+ B-/- cells; open circles, mice reconstituted with CD4-/- B+/+ cells; filled circles, mice reconstituted with CD4-/- B-/- cells.
Discussion
Because dysgammaglobulinemia develops in a subset of XLP patients without fatal infectious mononucleosis, we hypothesized that progressively impaired humoral responses might occur in SH2D1A-deficient individuals and mice, as a consequence of normal immune insults. Without specific immunization of SH2D1A-/- mice, the levels of serum IgG1 are consistently lower than in age-matched wt mice, whereas serum IgG2a is increased (data not shown), and serum IgE is almost undetectable (25, 26). Upon exposure to hapten, protein, or viral antigens (LCMV and MHV68), both primary and secondary humoral responses, however, are severely impaired in SH2D1A-/- mice. Concomitantly, GC formation and hapten-specific Ig class switching in SH2D1A-/- mice are impaired. Staining of GC with an anti-SH2D1A antibody shows that the adapter protein is expressed in a subset of B cells that are present in the GC. Indeed, using the adoptive transfer of B lymphocytes from unprimed SH2D1A-deficient mice together with either SH2D1A- deficient or wt CD4+ cells into Rag2-/- recipients, we demonstrate that both SH2D1A-/- B and SH2D1A-/- CD4+ T cells contribute to defective antigen-specific IgM and IgG production. Not unexpectedly, these defects are exacerbated upon adoptively transferring antigen-primed B and T cells. Thus, SH2D1A controls both CD4+ T cell and B cell functions that are implicated in T-D humoral immune responses.
We observed a reduction in primary antibody responses after infection of SH2D1A-/- mice with two unrelated viruses, LCMV and MHV68. Late humoral responses to infection with LCMV (28) or MHV68 (data not shown) are more severely affected in SH2D1A-/- mice, because these animals fail to generate long-lived virus-specific plasma cells and memory B cells (28, 38). Whereas the failure of naive and memory SH2D1A-/- CD4+ cells to produce IL-4 (refs. 25 and 26 and data not shown) could in part explain these defective early and late responses, the underlying cause of the low levels of antigen-specific IgM responses required further investigation. Obvious T cell-dependent factors that could have contributed to the SH2D1A-/- phenotype, such as up-regulation of CD40L or secretion of IL-21 (39), were not impaired in SH2D1A-/- mice (data not shown). We therefore focused first on examining the possibility of a B cell defect. Our adoptive transfer experiments indeed demonstrate that SH2D1A is essential for B cell activities that partake in T cell-dependent IgG and IgM production. The presence of endogenous wt B cells in the irradiated wt recipients could readily explain why Crotty et al. (28) did not detect a defect in SH2D1A-/- B cells. Because no endogenous B cells exist in the Rag2-/- recipients used in the current experiments, the defect in SH2D1A-/- B cells could be observed.
The finding that SH2D1A is expressed in a B cell subset is consistent with the results of the adoptive transfer experiments. SH2D1A has also been detected in human GC B cells and B cell tumors (13, 19, 20). EBV-positive Burkitt's lymphoma lines, which resemble B cells at the GC stage of differentiation, are mostly SH2D1A-positive (18). Moreover, a contraction of the CD19+ CD27+ B cell memory compartment has been observed in XLP patients (40). Taken together, the data strongly suggest that expression of SH2D1A by GC or memory B cells controls early and memory antibody responses. In the absence of SH2D1A, the contribution of a small B cell subset to the process of GC formation is likely to be affected. Because GC B cells express high levels of the CD95/Fas molecule (33), it is tempting to speculate that the absence of SH2D1A might result in exaggerated cell death and inefficient GC formation in SH2D1A-/- mice. This notion was indirectly supported by the observation that the CD150/SH2D1A receptor adapter complex modulates CD95-mediated apoptosis (20). In general, antibody production by mature B cells outside the GC is intact, and T-independent responses are normal in the absence of SH2D1A (data not shown).
Conclusion
The present study unequivocally demonstrates that dysgammaglobulinemia in SH2D1A-deficient mice takes place in the absence of a viral infection. This observation is consistent with dysgammaglobulinemia in XLP patients in the absence of a detectable infection with EBV or any other virus previously observed by us and others (4, 7-10). Because SH2D1A controls signal transduction pathways in both T and B cells initiated by at least six cell-surface receptors belonging to the CD150 family of costimulatory adhesion molecules, a dissection of the contribution of each of these receptors to humoral responses to T-D antigens will be required for an understanding of the molecular underpinnings of these biochemical events.
Supplementary Material
Acknowledgments
We thank Drs. Stephen Laroux and Duncan Howie for critically reviewing the manuscript; Drs. Klaus Rajewsky and Stefano Casola for advice; Drs. Michael Grusby and Andrea Wurster for help with the IL-21 PCR; Drs. Khuong B. Nguyen and Gary C. Pien for help with LCMV infections; and Drs. John Kearney; Herbert C. Morse III; Max D. Cooper; and Goetz Ehrhardt for sharing PCR analysis data. M.M. is a Special Fellow of the Leukemia and Lymphoma Society. This work was supported by National Institutes of Health Grant AI-35714 (to C.T.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: XLP, X-linked lymphoproliferative disease; EBV, Epstein-Barr virus; LCMV, lymphocytic choriomeningitis virus; T-D, T dependent; MHV68, murine Gamma Herpes virus 68; KLH, keyhole lymphocyte hemocyanin; TNP, 2,4,6,-trinitrophenyl; NP, 4-hydroxy-3-nitrophenylacetyl; GC, germinal center; PNA, peanut agglutinin.
References
- 1.Purtilo, D. T., Cassel, C. K., Yang, J. P. & Harper, R. (1975) Lancet 1, 935-940. [DOI] [PubMed] [Google Scholar]
- 2.Purtilo, D. T., Yang, Y. P., Allegra, S., DeFlorio, D., Hutt, L. M., Soltani, M. & Vawter, G. (1977) Am. J. Med. 62, 225-233. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton, J. K., Paquin, L. A., Sullivan, J. L., Maurer, H. S., Cruzi, F. G., Provisor, A. J., Steuber, C. P., Hawkins, E., Yawn, D., Cornet, J. A., et al. (1980) J. Pediatr. 96, 669-673. [DOI] [PubMed] [Google Scholar]
- 4.Seemayer, T. A., Gross, T. G., Egeler, R. M., Pirruccello, S. J., Davis, J. R., Kelly, C. M., Okano, M., Lanyi, A. & Sumegi, J. (1995) Pediatr. Res. 38, 471-478. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan, J. L. & Woda, B. A. (1989) Immunodefic. Rev. 1, 325-347. [PubMed] [Google Scholar]
- 6.Morra, M., Howie, D., Grande, M. S., Sayos, J., Wang, N., Wu, C., Engel, P. & Terhorst, C. (2001) Annu. Rev. Immunol. 19, 657-682. [DOI] [PubMed] [Google Scholar]
- 7.Grierson, H. L., Skare, J., Hawk, J., Pauza, M. & Purtilo D. T. (1991) Am. J. Med. Genet. 40, 294-297. [DOI] [PubMed] [Google Scholar]
- 8.Gilmour, K. C., Cranston, T., Jones, A., Davies, E.-G., Goldblatt, D., Thrasher, A., Kinnon, C., Nichols, K. E. & Gaspar, H. B. (2000) Eur. J. Immunol. 30, 1691-1697. [DOI] [PubMed] [Google Scholar]
- 9.Morra, M., Silander, O., Calpe, S., Choi, M., Oettgen, H., Myers, L., Etzioni, A., Buckley, R. & Terhorst, C. (2001) Blood 98, 1321-1325. [DOI] [PubMed] [Google Scholar]
- 10.Aghamohammadi, A., Kanegane, H., Moein, M., Farhoudi, A., Pourpak, Z., Movahedi, M., Gharagozlou, M., Zargar, A. A. & Miyawaki, T. (2003) Int. J. Hematol. 78, 45-47. [DOI] [PubMed] [Google Scholar]
- 11.Coffey, A. J., Brooksbank, R.A., Brandau, O., Oohashi, T., Howell, G. R., Bye, J. M., Cahn, A. P., Durham, J., Heath, P., Wray, P., et al. (1998) Nat. Genet. 20, 129-135. [DOI] [PubMed] [Google Scholar]
- 12.Sayos, J., Wu, C., Morra, M., Wang, N., Zhang, X., Allen, D., van Schaik, S., Notarangelo, L., Geha, R., Roncarolo, M. G., et al. (1998) Nature 395, 462-469. [DOI] [PubMed] [Google Scholar]
- 13.Nichols, K. E., Harkin, D. P., Levitz, S., Krainer, M., Kolquist, K. A., Genovese, C., Bernard, A., Ferguson, M., Zuo, L., Snyder, E., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 13765-13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sidorenko, S. P. & Clark, E. A. (2003) Nat. Immunol. 4, 19-24. [DOI] [PubMed] [Google Scholar]
- 15.Veillette, A. & Latour, S. (2003) Curr. Opin. Immunol. 15, 277-285. [DOI] [PubMed] [Google Scholar]
- 16.Engel, P., Eck, M. J. & Terhorst., C. (2003) Nat. Rev. Immunol. 3, 813-821. [DOI] [PubMed] [Google Scholar]
- 17.Latour, S. & Veillette, A. (2003) Immunol. Rev. 192, 212-224. [DOI] [PubMed] [Google Scholar]
- 18.Kis, L. L., Nagy, N., Klein, G. & Klein, E. (2003) Int. J. Cancer 104, 658-661. [DOI] [PubMed] [Google Scholar]
- 19.Feldhahn, N., Schwering, I., Lee, S., Wartenberg, M., Klein, F., Wang, H., Zhou, G., Wang, S. M., Rowley, J. D., Hescheler, J., et al. (2002) J. Exp. Med. 196, 1291-1305. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Mikhalap, S. V., Shlapatska, L. M., Berdova, A. G., Law, C. L., Clark, E. A. & Sidorenko, S. P. (1999) J. Immunol. 162, 5719-5727. [PubMed] [Google Scholar]
- 21.Poy, F., Yaffe, M. B., Sayos, J., Saxena, K., Morra, M., Sumegi, J., Cantley, L. C., Terhorst, C. & Eck, M. J. (1999) Mol. Cell 4, 555-561. [DOI] [PubMed] [Google Scholar]
- 22.Chan, B., Lanyi, A., Song, H. K., Griesbach, J., Simarro-Grande, M., Poy, F., Howie, D., Sumegi, J., Terhorst, C. & Eck, M. J. (2003) Nat. Cell Biol. 5, 155-160. [DOI] [PubMed] [Google Scholar]
- 23.Latour, S., Roncagalli, R., Chen, R., Bakinowski, M., Shi, X., Schwartzberg, P. L., Davidson, D. & Veillette, A. (2003) Nat. Cell Biol. 5, 149-154. [DOI] [PubMed] [Google Scholar]
- 24.Li, C., Iosef, C., Jia, C. Y., Han, V. K. & Li, S. S. (2003) J. Biol. Chem. 278, 3852-3859. [DOI] [PubMed] [Google Scholar]
- 25.Wu, C., Nguyen, K. B., Pien, G. C., Wang, N., Gullo, C., Howie, D., Sosa, M. R., Edwards, M. J., Borrow, P., et al. (2001) Nat. Immunol. 2, 410-414. [DOI] [PubMed] [Google Scholar]
- 26.Czar, M. J., Kersh, E. N., Mijares, L. A., Lanier, G., Lewis, J., Yap, G., Chen A., Sher, A., Duckett, C. S., Ahmed, R., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 7449-7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin, L., Al-Alem, U., Liang, J., Tong, W. M., Li, C., Badiali, M., Medard, J. J., Sumegi, J., Wang, Z. Q. & Romeo, G. (2003) J. Med. Virol. 71, 446-455. [DOI] [PubMed] [Google Scholar]
- 28.Crotty, S., Kersh, E. N., Cannons, J., Schwartzberg, P. L. & Ahmed, R. (2003) Nature 421, 282-287. [DOI] [PubMed] [Google Scholar]
- 29.Barrington, R. A., Pozdnyakova, O., Zafari, M. R., Benjamin, C. D. & Carroll, M. C. (2002) J. Exp. Med. 196, 1189-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simas, J. P. & Efstathiou, S. (1998) Trends Microbiol. 6, 276-282. [DOI] [PubMed] [Google Scholar]
- 31.de Jong, Y. P., Abadia-Molina, A. C., Satoskar, A. R., Clarke, K., Rietdijk, S. T., Faubion, W. A., Mizoguchi, E., Metz, C. N., Alsahli, M., ten Hove, T., et al. (2001) Nat. Immunol. 2, 1061-1066. [DOI] [PubMed] [Google Scholar]
- 32.Purtilo, D. T., Grierson, H. L., Ochs, H. & Skare, J. (1989) Am. J. Med. 87, 421-424. [DOI] [PubMed] [Google Scholar]
- 33.Tarlinton, D. M. & Smith, K. G. (2000) Immunol. Today 21, 436-441. [DOI] [PubMed] [Google Scholar]
- 34.McHeyzer-Williams, M. G. & Ahmed, R. (1999) Curr. Opin. Immunol. 11, 172-179. [DOI] [PubMed] [Google Scholar]
- 35.Calame, K. L., Lin, K. I. & Tunyaplin, C. (2003) Annu. Rev. Immunol. 21, 205-230. [DOI] [PubMed] [Google Scholar]
- 36.Shlapatska, L. M., Mikhalap, S. V., Berdova, A. G., Zelensky, O. M., Yun, T. J., Nichols K. E., Clark, E. A. & Sidorenko, S. P. (2001) J. Immunol. 166, 5480-5487. [DOI] [PubMed] [Google Scholar]
- 37.Nagy, N., Maeda, A., Bandobashi, K., Kis, L. L., Nishikawa, J., Trivedi, P., Faggioni, A., Klein, G. & Klein, E. (2002) Int. J. Cancer 100, 433-440. [DOI] [PubMed] [Google Scholar]
- 38.Hron, J. D., Caplan, L., Gerth, A. J., Schwartzberg, P. L. & Peng, S. L. (2004) J. Exp. Med. 200, 261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozaki, K., Spolski, R., Feng, C. G., Qi, C. F., Cheng, J., Sher, A., Morse, H. C., III, Liu, C., Schwartzberg, P. L. & Leonard, W. J. (2002) Science 298, 1630-1634. [DOI] [PubMed] [Google Scholar]
- 40.Malbran, A., Belmonte, L., Ruibal-Ares, B., Bare, P., Massud, I., Parodi, C., Felippo, M., Hodinka, R., Haines, K., Nichols, K. E., et al. (2003) Blood 6, 6-13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.