Abstract
OBJECTIVES
To examine the effect of socioeconomic factors on survival in black and white patients with local or regional prostate cancer.
METHODS
All cases (n = 2046) of clinically localized prostate cancer diagnosed from 1990 to 2000 at the Henry Ford Health System and the Henry Ford Medical Group, equal access health centers, were included. Data on the stage, grade, age at diagnosis, socioeconomic status, treatment given, comorbidities, and vital statistics were gathered from the Henry Ford Medical Group tumor registry and computerized databases, pathologic reports, patient charts, Surveillance, Epidemiology, and End Results database, and the national death registry. The endpoints were the overall and cancer-specific survival. Survival was calculated using Cox proportional hazards regression models.
RESULTS
Of the 2046 cases, 1243 were white and 803 were black. Black patients were more likely to have lower incomes, a greater baseline prostate-specific antigen level, and greater comorbidities. They were also more likely to undergo radiotherapy and less likely to undergo radical prostatectomy. Univariate analysis, with white race as the baseline hazard, showed that black patients had significantly increased cancer-specific (hazard ratio [HR] 1.47, 95% confidence interval [CI] 1.01–2.13) and overall (HR 1.29, 95% CI 1.09–1.53) mortality. However, adjusting for insurance status and income on multivariate analysis revealed no significant differences in cancer-specific (HR 1.04, 95% CI 0.66–1.64) and overall (HR 0.96, 95% CI 0.78–1.18) survival.
CONCLUSIONS
In this cohort, socioeconomic factors were sufficient to explain the disparity in survival between white and black patients. Survival differences disappeared after adjusting for income status on multivariate analysis. UROLOGY 73: 624–630, 2009. © 2009 Elsevier Inc.
Prostate cancer affects an estimated 218 890 men annually and is the third leading cause of cancer death among men in the United States, claiming nearly 27 000 lives each year.1 Although the cancer death rates in the United States are declining, the incidence and mortality of prostate cancer in black men has remained disproportionately high. For all cancer sites combined, black men have a 15% greater incidence rate and 38% greater death rate than white men.1 From 1999 to 2003, black men were 1.5 times more likely to develop prostate cancer and 2.4 times as likely to die of this disease than white men.1 Racial differences in the disease characteristics are apparent as well. Compared with whites, blacks typically present with greater prostate-specific antigen (PSA) levels, higher grade tumors, and a more advanced clinical stage at diagnosis.2,3 The extent of these disparities and their implications for public health has led researchers to seek out the responsible factors.
In particular, previous studies have investigated differences in the access to healthcare, the prevalence of comorbid conditions, socioeconomic factors, knowledge of the disease, treatment, genetics, and disease aggressiveness.3–9 Because much of these works examined the interval to postprostatectomy PSA recurrence as the primary dependent variable, it is relatively unclear whether being black is, in and of itself, a risk factor in healthy men and/or a predictor of morbidity in the event of developing the disease. Moreover, the conclusions from previous studies have been controversial and conflicting, leaving integral questions unanswered with respect to our understanding and treatment of prostate cancer in black men. Should we consider prostate cancer in black men a biologically distinct disease entity? Do inherent, genetic factors predispose black men to develop and die of prostate cancer or the socioeconomic and demographic disparity is the primary cause of outcome disparities in blacks compared with whites? Finally, how does each of these factors contribute differently to the cancer incidence, mortality, and disease characteristics in black men?
Overall, previous research has not untangled the independent roles of racial differences and typically correlated socioeconomic differences in the incidence and morbidity associated with prostate cancer in black compared with white men. This study has taken up this important issue and sought to determine whether being black is an independent risk factor for prostate cancer-specific mortality and overall mortality, after controlling for differences in clinical characteristics, treatment group, and demographic/socioeconomic factors. In addition, we examined the relative influence of these covariates, identifying the factors most responsible for racial disparities in survival.
MATERIAL AND METHODS
Data
The cohort of patients underwent treatment at Henry Ford Health System and the Henry Ford Medical Group (HFMG). The HFMG consists of 1100 primary and specialty care physicians in Southeastern Michigan. This medical group maintains extensive computerized databases that hold information on inpatient, outpatient, and emergency department encounters, procedures, demographics, laboratory results, and tumor registry data. The HFMG tumor registry employs a thorough case finding system and includes data on cancer stage, date of diagnosis, grade, and initial treatment. These tumor registry data are included in the National Cancer Institute’s Surveillance, Epidemiology, and End Results program. We used the HFMG tumor registry to identify all cases of local or regional prostate cancer (based on clinical examination, bone scan, and/or computed tomography findings) diagnosed from January 1, 1990 through December 31, 2000. We obtained data on stage, grade, and initial treatment (defined as treatment received within 6 months after prostate cancer diagnosis) from the HFMG tumor registry. The tumor grade was determined from the initial histologic examination of prostate biopsy by Henry Ford Health System pathologists was reported as well differentiated, Gleason score 2–4; moderately differentiated, Gleason score 5–7; or poorly differentiated, Gleason score 8–10. All patients who were neither black nor white were excluded.
A total of 2046 patients were categorized into 3 treatment groups: radical prostatectomy included patients who had undergone radical prostatectomy (alone or in combination with other treatment) within 6 months after the diagnosis; radiotherapy included patients who received radiotherapy with curative intent, and no radical prostatectomy within 6 months after diagnosis; and watchful waiting included all the remaining patients. An intent-to-treat analysis was performed. All patients who underwent pelvic lymphadenectomy remained in the radical prostatectomy group, regardless of whether they underwent prostatectomy.
The variables obtained from the HFMG computerized databases included the baseline PSA value (defined as the last PSA measurement before diagnosis available in the database), age at diagnosis, median household income (estimated by geocoding patient address and linking to 1990 U.S. Census data), insurance status (health maintenance organization [HMO] or fee for service), and race. Using Deyo’s modification10 of the method described by Charlson, we calculated a comorbidity score using all inpatient and outpatient diagnoses recorded before the prostate cancer diagnosis. We followed each member of the cohort through December 31, 2001, using hospital records and the State of Michigan death tapes, to identify prostate-specific and all-cause mortality. Patients were considered to have died of prostate cancer if prostate cancer was listed as the primary or contributing cause of death (ie, it appeared on any of the first 3 lines of the death certificate). In addition, the patient’s last contact was identified with the health system from the HFMG encounter databases. Patients were censored at the time of death, the last contact, or May 31, 2002, whichever came first. The mean follow-up was 77.5 months (median 79.1) for whites and 72.8 months (median 74) for blacks.
Statistical Analysis
The survival time to death from prostate cancer and all other causes was analyzed using Cox proportional hazards regression models from which we calculated hazard ratios (HR) and corresponding 95% confidence intervals (CIs). Comparisons between racial groups were made using the χ2 and Wilcoxon rank sum tests. The Statistical Analysis Systems procedures PROC FREQ PROC NPAR1WAY, and PROC PHREG were used for these analyses.11
RESULTS
The demographic and clinical characteristics of the 2046 men diagnosed with local or regional prostate cancer are listed in Table 1. Overall, 60.8% of the study population was white, and 39.2% was black. The age distribution was similar by race, with approximately 44% of the cohort >70 years. White patients tended to live in areas with a greater income: 55% in areas with median incomes >$40 000 compared with only 12% among blacks. Overall, 38% of the study population had insurance provided by an HMO, with significantly more blacks having HMO coverage compared with whites (42% and 35%, respectively; P = .004).
Table 1.
Demographic and clinical characteristics stratified by race (n = 2046)
| Characteristic | White (n = 1243) | Black (n = 803) | Total (%) | P Value |
|---|---|---|---|---|
| Age at diagnosis (y) | .813 | |||
| <60 | 195 (16) | 112 (14) | 15 | |
| 60–69 | 508 (41) | 325 (40) | 41 | |
| 70–79 | 462 (37) | 312 (39) | 38 | |
| ≥80 | 78 (6) | 54 (7) | 6 | |
| Income ($) | <.001 | |||
| <10 000 | 18(1) | 122 (15) | 7 | |
| 10 000–20 000 | 72(6) | 284 (36) | 18 | |
| 20 000–40 000 | 449 (37) | 288 (37) | 37 | |
| >40 000 | 669 (55) | 95 (12) | 38 | |
| Insurance status | .004 | |||
| HMO | 439 (35) | 335 (42) | 38 | |
| Fee for service | 804 (65) | 468 (58) | 62 | |
| Baseline PSA* (ng/mL) | <.001 | |||
| <4 | 153 (12) | 44 (5) | 10 | |
| 4–9.9 | 518 (42) | 273 (34) | 39 | |
| >10 | 374 (30) | 282 (35) | 32 | |
| None | 198 (16) | 204 (25) | 20 | |
| Stage | .525 | |||
| Local | 1105 (89) | 721 (90) | 89 | |
| Regional | 138 (11) | 82 (10) | 11 | |
| Grade | .541 | |||
| I | 211(17) | 122 (15) | 16 | |
| II | 859 (69) | 563 (70) | 70 | |
| III | 173 (14) | 118(15) | 14 | |
| Comorbidity index† | .007 | |||
| ≤1 | 525 (42) | 326 (41) | 42 | |
| >1 but ≤2 | 193 (16) | 144 (18) | 16 | |
| >2 but ≤3 | 292 (23) | 148 (18) | 22 | |
| ≥3 | 233 (19) | 185 (23) | 20 | |
| Treatment group | <.001 | |||
| Conservative | 360 (29) | 239 (30) | 29 | |
| Radiotherapy | 353 (28) | 333 (41) | 34 | |
| Prostatectomy | 530 (43) | 231 (29) | 37 | |
| Mean ± SD follow-up time* (mo) | 77.5 ± 32.6 | 72.8 ± 31.4 | 0.004 |
HMO = health maintenance organization; PSA = prostate-specific antigen.
Data in parentheses are percentages.
Baseline PSA data missing for 198 whites (16%) and 204 blacks (25%); percentages shown for baseline PSA represent results for patients who had baseline PSA level.
Based on method of Deyo et al.10
There were differences across groups for certain clinical characteristics. Blacks were more likely to have a baseline PSA value >10 ng/mL (47% compared 36%; P < .001). However, more whites than blacks had baseline PSA information available. Among treatment groups, more whites underwent radical prostatectomy (43% compared with 29%), and more blacks tended to receive radiotherapy (41% compared with 28%; P < .001 for treatment differences). No significant racial differences were found in stage, grade, or comorbidities.
Table 2 lists the unadjusted effect estimates of prostate-specific and all-cause mortality for blacks and whites. The results would suggest that blacks are at an increased risk of prostate-specific mortality (HR 1.47, 95% CI 1.01–2.13) and all-cause mortality (HR 1.29, 95% CI 1.09–1.53). Table 2 also shows that after adjusting for income, HMO status, clinical characteristics (baseline PSA level, stage, grade, comorbidity, age), and treatment, any racial differences in prostate-specific mortality (HR 1.06, 95% CI 0.66–1.70) and all-cause mortality (HR 0.94, 95% CI 0.76–1.16) failed to reach statistical significance.
Table 2.
Crude “unadjusted” and “adjusted” effect of demographic and clinical characteristics on prostate cancer-specific and all-cause mortality (n = 2046)
| Characteristic | Prostate Cancer-Specific Mortality HR (95% CI)
|
All-Cause Mortality HR (95% CI)
|
||||
|---|---|---|---|---|---|---|
| Deaths (n) | Unadjusted | Adjusted | Deaths (n) | Unadjusted | Adjusted | |
| Race | ||||||
| Black | 59 (53) | 1.47 (1.01–2.13) | 1.06 (0.66–1.70) | 304 (57) | 1.29 (1.09–1.53) | 0.94 (0.76–1.16) |
| White | 52 (47) | 1.0 | 1.0 | 232 (43) | 1.0 | 1.0 |
| Age at diagnosis (y) | ||||||
| <60 | 7 (6) | 1.0 | 1.0 | 27 (5) | 1.0 | 1.0 |
| 60–69 | 31 (28) | 1.48 (0.65–3.36) | 0.94 (0.41–2.16) | 155 (29) | 1.91 (1.27–2.87) | 1.34 (0.88–2.03) |
| 70–79 | 54 (49) | 3.01 (1.37–6.62) | 1.81 (0.79–4.12) | 270 (50) | 3.88 (2.61–5.77) | 2.30 (1.52–3.46) |
| ≥80 | 19 (17) | 8.32 (3.49–19.8) | 2.96 (1.17–7.48) | 84 (16) | 9.73 (6.30–15.02) | 4.14 (2.61–6.56) |
| Income | ||||||
| <10 000 | 15 (14) | 1.0 | 1.0 | 61 (12) | 1.0 | 1.0 |
| 10 000–20 000 | 27 (24) | 0.63 (0.34–1.18) | 0.57 (0.3–1.09) | 117 (22) | 0.66 (0.49–0.90) | 0.72 (0.52–0.98) |
| 20 000–$40 000 | 39 (35) | 0.42 (0.23–0.76) | 0.53 (0.28–1.02) | 197 (37) | 0.51 (0.38–0.68) | 0.64 (0.47–0.87) |
| >40 000 | 30 (27) | 0.30 (0.16–0.56) | 0.47 (0.23–0.95) | 151 (29) | 0.36 (0.27–0.49) | 0.50 (0.36–0.71) |
| Insurance status | ||||||
| HMO | 21 (19) | 0.37 (0.23–0.60) | 0.46 (0.28–0.74) | 137 (26) | 0.55 (0.46–0.67) | 0.65 (0.53–0.80) |
| Fee for service | 90 (81) | 1.0 | 1.0 | 399 (74) | 1.0 | 1.0 |
| Baseline PSA (ng/mL) | ||||||
| <4 | 8 (7) | 1.0 | 1.0 | 48 (9) | 1.0 | 1.0 |
| 4–9.9 | 20 (18) | 0.60 (0.26–1.35) | 0.71 (0.31–1.62) | 119 (22) | 0.60 (0.43–0.84) | 0.67 (0.48–0.95) |
| >10 | 45 (41) | 1.60 (0.76–3.4) | 1.11 (0.51–2.41) | 217 (40) | 1.29 (0.94–1.77) | 1.12 (0.81–1.54) |
| None | 38 (34) | 1.92 (0.90–4.12) | 1.88 (0.84–4.21) | 152 (28) | 1.25 (0.90–1.73) | 1.32 (0.93–1.86) |
| Stage | ||||||
| Local | 87 (78) | 1.0 | 1,0 | 471 (88) | 1.0 | 1.0 |
| Regional | 24 (22) | 2.10 (1.33–3.30) | 2.35 (1.40–3.93) | 62 (12) | 1.03 (0.79–1.33) | 1.45 (1.08–1.93) |
| Grade | ||||||
| I | 10 (9) | 1.0 | 1.0 | 95 (18) | 1.0 | 1.0 |
| II | 53 (48) | 1.38 (0.70–2.72) | 1.62 (0.82–3.24) | 319 (60) | 0.89 (0.71–1.12) | 1.01 (0.80–1.28) |
| III | 48 (43) | 6.56 (3.32–12.99) | 6.08 (3.01–12.28) | 122 (23) | 1.82 (1.39–2.38) | 1.65 (1.25–2.19) |
| Comorbidity index* | ||||||
| ≤1 | 40 (36) | 1.0 | 1.0 | 167 (31) | 1.0 | 1.0 |
| >1 but ≤2 | 18 (16) | 1.21 (0.70–2.12) | 1.34 (0.76–2.36) | 88 (16) | 1.44 (1.11–1.86) | 1.55 (1.19–2.02) |
| >2 but ≤3 | 19 (17) | 0.98 (0.57–1.70) | 1.17 (0.67–2.04) | 107 (20) | 1.35 (1.06–1.73) | 1.47 (1.15–1.89) |
| ≥3 | 34 (31) | 2.39 (1.51–3.78) | 2.34 (1.45–3.75) | 174 (32) | 3.07 (2.48–3.81) | 2.97 (2.38–3.72) |
| Treatment group | ||||||
| Conservative | 49 (44) | 1.0 | 1.0 | 218 (41) | 1.0 | 1.0 |
| Radiotherapy | 39 (35) | 0.57 (0.37–0.86) | 0.60 (0.38–0.94) | 214 (40) | 0.69 (0.57–0.83) | 0.80 (0.65–0.98) |
| Prostatectomy | 23 (21) | 0.28 (0.17–0.87) | 0.39 (0.21–0.72) | 104 (19) | 0.27 (0.21–0.34) | 0.47 (0.35–0.62) |
To further explore the potential effect of race on mortality outcomes, we estimated 3 separate multivariate models (Table 3). After adjusting for clinical characteristics only (baseline PSA level, stage, grade, comorbidity, age), race becomes insignificant in explaining mortality. Adjusting for treatment, insurance, or income similarly reduced any apparent affects of race. Figure 1A,B illustrates the racial differences in survival of patients for overall and prostate cancer-specific mortality. In each case, survival was better for whites; however, after adjusting for treatment, age, comorbidity, diagnosis year, income, and grade, the racial discrepancy was greatly reduced or eliminated. Figure 1C depicts the effect of income on the outcomes in both the low and the higher income group in relation to race, showing higher income group have a relatively better outcome in both the racial cohorts.
Table 3.
Association between race and prostate-specific and overall mortality, adjusting separately for potential confounding variables
| Characteristic | Prostate-Specific Mortality
|
All-Cause Mortality
|
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Crude | ||||
| Black | 1.47 | 1.01–2.13 | 1.29 | 1.09–0.53 |
| White | 1.0 | — | 1.0 | — |
| Adjusted | ||||
| For clinical characteristics (baseline PSA, stage, grade, comorbidity, age) | ||||
| Black | 1.36 | 0.93–1.99 | 1.22 | 1.02–1.45 |
| White | 1.0 | — | 1.0 | — |
| For treatment group (conservative, radiotherapy, prostatectomy) | ||||
| Black | 1.34 | 0.92–1.96 | 1.15 | 0.96–1.36 |
| White | 1.0 | — | 1.0 | — |
| For insurance status and income | ||||
| Black | 1.04 | 0.66–1.64 | 0.96 | 0.78–1.18 |
| White | 1.0 | — | 1.0 | — |
Figure 1.
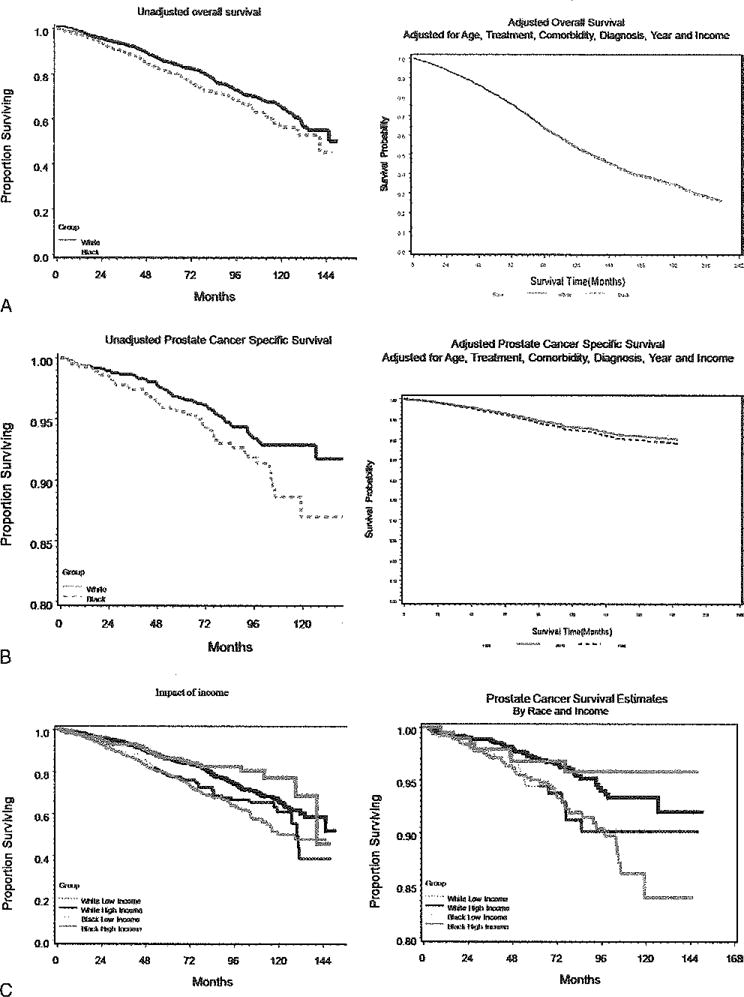
Kaplan-Meier curved showing (A) overall survival estimates unadjusted and adjusted for age, treatment, comorbidity, diagnosis year, income, and cancer grade, (B) prostate cancer-specific survival estimates unadjusted and adjusted for age, treatment, comorbidity, diagnosis year, income, and cancer grade, and (C) overall and prostate cancer-specific survival estimates in relation to race and income.
COMMENT
Consistent with large amounts of data from previous work and other centers, the present study found an increased risk of prostate-specific and all-cause mortality among blacks compared with white. However, multivariate adjustment for various correlated factors made these racial differences, essentially, disappear. Accounting for clinical characteristics, treatment, income, and insurance status (a proxy for access to care) was especially important. Moreover, although many of these variables were significant predictors of survival, we found that adjustments for insurance and income, alone, diminished the effect of race.
Our results concur with those of another recent study of prostatectomy outcomes showing that socioeconomic variables might explain the mortality,12 although the remaining data have been mixed on the association of race with prostate-cancer mortality, particularly when other clinical and treatment variables are taken into account.4,13–16 Thus, race might play a role in the initial clinical characteristics17,18 or in treatment choices19–21; however, given a particular treatment, survival might not differ by race,13 because it did not in this study. An additional potential reason for conflicting and otherwise ambiguous results in these data is that a possible disease-stage shift is diminishing the effect of ethnicity on prostate cancer-free survival,22 especially in equal access healthcare systems in which PSA screening is universally available.
Our study had some limitations. Chief among these was the nonrandomized treatment assignment that resulted horn the retrospective nature of this investigation. Consequently, there might be residual confounding caused by unmeasured selection bias in our data. For example, it is known that men who choose watchful waiting tend to be older, have a lower PSA level, and lower stage and grade at diagnosis.23 However, by limiting the cohort to an equal access healthcare center (ie, HFMG), we control for the potential effect health insurance might have had on the stage of disease presentation and access to PSA screening. Similarly, we controlled for many variables known to influence treatment and outcomes, but we recognize there could be residual confounding. With a Cox proportional hazards model, we assumed that the independent variables varied proportionately to the baseline hazard. However, if some interaction occurred between the predictor variables, or if the baseline hazard was unique for each treatment type, we might have misspecified the multivariate model, resulting in over-or underestimated effect sizes. Second, the follow-up period was rather short for meaningful analyses of the factors contributing to prostate cancer mortality, especially for more recently diagnosed patients, and longer follow-up is required by the substantial lead time introduced by PSA screening. Third, we used the median income in the zip code of residence as a surrogate for patients’ socioeconomic status. Although some investigators agree with this assumption, others have argued that individual income is a better variable.24,25 Finally, our statistical method might not have been able to fully account for the effect of differential treatment rates, although we also note that we used rich and comprehensive data sources to adjust and control for many measurable confounders and real-life practices to assess the health outcomes.
CONCLUSIONS
The results of our study have shown that in men with insurance and equal access to care, race per se does not affect the survival from prostate cancer. Although we found differences in income, insurance status, baseline PSA level, comorbidities, and treatment by race, these did not translate into racial differences in prostate-cancer mortality or overall survival. Future work should explore methods to reduce the effect of nonclinical factors on socioeconomic disparities in healthcare outcomes. Targets might include educational initiatives and community-based programs to aid those who need additional support during and after treatment.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. Am Cancer Soc. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Moul JW, Sesterhenn IA, Connelly RR, et al. Prostate-specific antigen values at the time of prostate cancer diagnosis in African-American men. JAMA. 1995;274:1277–1281. [PubMed] [Google Scholar]
- 3.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 4.Godley PA, Schenck AP, Araamoo MA, et al. Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. J Natl Cancer Inst. 2003;95:1702–1710. doi: 10.1093/jnci/djg094. [DOI] [PubMed] [Google Scholar]
- 5.Steenland K, Rodriguez C, Mondul A, et al. Prostate cancer incidence and survival in relation to education (United States) Cancer Causes Control. 2004;15:939–945. doi: 10.1007/s10552-004-2231-5. [DOI] [PubMed] [Google Scholar]
- 6.Richardson JT, Webster JD, Fields NJ. Uncovering myths and transforming realities among low-SES African-American men: implications for reducing prostate cancer disparities. J Natl Med Assoc. 2004;96:1295–1302. [PMC free article] [PubMed] [Google Scholar]
- 7.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Nad Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 8.Polednak AP. Black-white differences in tumor grade (aggressiveness) at diagnosis of prostate cancer, 1992–1998. Elfin Dts. 2002;12:536–540. [PubMed] [Google Scholar]
- 9.Cher ML, Lewis PE, Banerjee M, et al. A similar pattern of chromosomal alterations in prostate cancers from African-Americans and Caucasian Americans. Clin Cancer Res. 1998;4:1273–1278. [PubMed] [Google Scholar]
- 10.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 11.SAS, version 8. Cary, NC: SAS Institute, Inc; 1999. [Google Scholar]
- 12.Grossfeld GD, Latini DM, Downs T, et al. Is ethnicity an independent predictor of prostate cancer recurrence after radical prostatectomy? J Urol. 2002;168:2510–2515. doi: 10.1016/S0022-5347(05)64179-1. [DOI] [PubMed] [Google Scholar]
- 13.Freedland SJ, Amling CL, Dorey F, et al. Race as an outcome predictor after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Urology. 2002;60:670–674. doi: 10.1016/s0090-4295(02)01847-2. [DOI] [PubMed] [Google Scholar]
- 14.Moul JW, Connelly RR, Lubeck DP, et al. Predicting risk of prostate specific antigen recmrence after radical prostatectomy with the Center for Prostate Disease Research and Cancer of the prostate strategic Urologic research endeavor databases. J Urol. 2001;166:1322–1327. [PubMed] [Google Scholar]
- 15.Cross CK, Shultz D, Malkowicz SB, et al. Impact of race on prostate-specific antigen outcome after radical prostatectomy for clinically localized adenocarcinoma of the prostate. J Clin Oncol. 2002;20:2863–2868. doi: 10.1200/JCO.2002.11.054. [DOI] [PubMed] [Google Scholar]
- 16.Freedland SJ, Jalkut M, Dorey F, et al. Race is not an independent predictor of biochemical recurrence after radical prostatectomy in an equal access medical center. Urology. 2000;56:87–91. doi: 10.1016/s0090-4295(00)00587-2. [DOI] [PubMed] [Google Scholar]
- 17.Kang JS, Maygarden SJ, Mohler JL, et al. Comparison of clinical and pathological features in African-American and Caucasian patients with localized prostate cancer. BJU Int. 2004;93:1207–1210. doi: 10.1111/j.1464-410X.2004.04846.x. [DOI] [PubMed] [Google Scholar]
- 18.Optenberg SA, Thompson IM, Friedrichs P, et al. Race, treatment, and long-term survival from prostate cancer in an equal access medical cate delivery system. JAMA. 1995;274:1599–1605. [PubMed] [Google Scholar]
- 19.Schapira MM, McAuliffe TL, Nattinger AB. Treatment of localized prostate cancer in African-American compared with Caucasian men: less use of aggressive therapy for comparable disease. Med Care. 1995;33:1079–1088. doi: 10.1097/00005650-199511000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman RM, Harlan LC, Klabunde CN, et al. Racial differences in initial treatment for clinically localized prostate cancer: results from the Prostate Cancer Outcomes Study. J Gen Intern Med. 2003;18:845–853. doi: 10.1046/j.1525-1497.2003.21105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shavers VL, Brown M, Klabunde CN, et al. Race/ethnicity and the intensity of medical monitoring under “watchful waiting” for prostate cancer. Med Care. 2004;42:239–250. doi: 10.1097/01.mlr.0000117361.61444.71. [DOI] [PubMed] [Google Scholar]
- 22.Bianco FJ, Jr, Wood DP, Jr, Grignon DJ, et al. Prostate cancer stage shift has eliminated die gap in disease-free survival in black and white American men after radical prostatectomy. J Urol. 2002;168:479–482. [PubMed] [Google Scholar]
- 23.Wu H, Sun L, Moul JW, et al. Watchful waiting and factors predictive of secondary treatment of localized prostate cancer. J Urol. 2004;171:1111–1116. doi: 10.1097/01.ju.0000113300.74132.8b. [DOI] [PubMed] [Google Scholar]
- 24.Dale W, Vijayakumar S, Lawlor EF, et al. Prostate cancer, race, and socioeconomic status: inadequate adjustment for social factors in assessing racial differences. Prostate. 1996;29:271–281. doi: 10.1002/(SICI)1097-0045(199611)29:5<271::AID-PROS1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 25.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Pub Health. 1997;18:341–348. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]


