Abstract
Dengue virus (DENV) infection in the presence of reactive, non-neutralizing IgG (RNNIg) is the greatest risk factor for dengue hemorrhagic fever (DHF) or shock syndrome (DSS). Progression to DHF/DSS is attributed to antibody-dependent enhancement (ADE); however, since only a fraction of infections occurring in the presence of RNNIg advance to DHF/DSS, the presence of RNNIg alone cannot account for disease severity. We discovered that DHF/DSS patients respond to infection by producing IgGs with enhanced affinity for the activating Fc receptor IIIA due to afucosylated Fc glycans and IgG1 subclass. RNNIg enriched for afucosylated IgG1 triggered platelet reduction in vivo and was a significant risk factor for thrombocytopenia (OR 11.00, p=0.0139). Thus, therapeutics and vaccines restricting production of afucosylated, IgG1 RNNIg during infection may prevent ADE of DENV disease.
Antibody-dependent enhancement (ADE) has been shown to occur in a variety of in vitro and in vivo dengue virus (DENV) infection models, but ADE does not explain why fewer than 15% of human DENV infections that occur in the presence of reactive, non-neutralizing IgG (RNNIg) progress to dengue hemorrhagic fever (DHF) or shock syndrome (DSS) (1–7). This gap in our knowledge of the determinants responsible for progression to severe disease impedes the rational design of flavivirus vaccines that avoid eliciting ADE-mediating antibodies, and limits proactive care to patients who are at higher risk for developing DHF/DSS. We therefore determined if a unique property of these RNNIg, seen in the small proportion of patients that progress to DHF/DSS, could account for ADE.
The currently accepted model of ADE in DENV infection posits that RNNIg opsonizes viral particles and mediates increased infectivity of Fc gamma receptor (FcR)-expressing cells(8–10). Studies have shown that immune complex (IC)/FcR-mediated internalization of DENV virus can result in more infected cells(11, 12), enhanced fusion(7, 13) and suppression of innate immune signaling(14, 15). Severe DENV disease has been associated with specific combinations of virus serotypes and preexisting serotype immunity(16), viral genetic factors(17–20), and host factors(21–24) including association of asymptomatic DENV infection with the lower binding R131 polymorphism of FcγRIIa (25, 26). How these and other factors may contribute to ADE of human dengue disease remains unknown.
Most FcRs exhibit low affinity for monomeric IgG and are only engaged upon formation of multivalent IgG ICs. FcR-mediated effector functions include positive mechanisms such as antibody-dependent cellular cytotoxicity (ADCC), phagocytosis, and pro-inflammatory cytokine production, as well as negative functions, such as inhibition of inflammatory immune responses. Whether Fc domains within an IC engage activating or inhibitory FcRs is determined by Fc structure, which, in turn, is determined by IgG subclass and the precise composition of an N-linked glycan present on the CH2 domain of each heavy chain(27–29). Humans have four IgG subclasses (IgG1–4) with IgG1 and IgG2 in highest abundance in serum, together constituting 90–95% of antigen-specific IgG (30). Each subclass is distinct in its ratio of binding to activating/inhibitory Type I FcγRs, with IgG1 having significantly higher affinities for activating Type I FcγRs than IgG2 (Table S1). The Fc glycan has a core hepatasaccharide structure that can be modified by addition of specific residues; these modifications are dynamic and regulate the biological activity of IgGs by modulating Fc structure. Glycans lacking a core fucose have enhanced affinity for the activating FcγRIIIA and can confer improved effector activity compared to fucosylated Fc glycans(31). Fucosylated, sialylated glycoforms (sFc) confer binding ability of Fc to Type II FcγRs, which mediate a variety of modulatory activities (Table S1)(30, 32). We have previously demonstrated substantial variation in determinants of Fc structure between individuals(30), suggesting that some people may be predisposed to ADE of disease by virtue of production of IgGs that engage activating FcRs with higher affinity.
To determine whether individuals with severe DENV disease have a specific IgG Fc domain structure that might contribute to ADE, we analyzed the distribution of IgG subclass and Fc-associated glycoforms from hospitalized patients who were positive for anti-DENV IgG in the early phase of disease, indicating prior infection with DENV or another flavivirus that elicited a cross-reactive IgG response(33)(34). Here, we refer to these patients who were positive for anti-DENV IgG in the early phase of disease as RNNIg+. RNNIg+ patients in this study were infected with any of the four viral serotypes and had clinical diagnoses either of dengue fever (DF), the relatively mild disease form, or DHF/DSS (Table S2). Samples were obtained at an early time point (day 4–8 of fever) and a convalescent time point (>30 days post hospital discharge). As a comparator group for early viral infection, we studied anti-hemagglutinin (HA) IgG from subjects enrolled in a controlled influenza A virus (IAV) infection study. The IAV cohort was comprised of healthy adults who had serum taken prior to, and 7 days after intranasal inoculation with a wild-type IAV strain (A/California/04/2009/H1N1)(35).
Analysis of Fc-associated glycan composition showed that RNNIg+ patients with DENV infection exhibited a specific elevation of afucosylated Fc glycoforms (afucFc) on IgGs reactive with the dengue virus envelope protein (ENV), relative to patients infected with IAV (Figure 1A). There was no evidence for differences in other glycoforms (Figure 1B). Increased afucFc anti-ENV IgG during the early phase of infection persisted during the convalescent phase (Figure 1A). In addition to the afucFc, anti-ENV IgGs in early infection were more likely to be IgG1 than IgG2 subclass (Figure 1C), with both modifications (afucFc and IgG1) enhancing capacity of the Fc to engage the activating FcγRIIIA.
Fig. 1. Afucosylated Fc glycoforms and IgG1 subclass are enriched in dengue infection.

(A) Anti-ENV IgGs in early DENV or convalescent DENV infection (conv) show an increased abundance of afucosylated Fc glycans (AfucFc) compared with healthy adults (uninfected), IgGs on day 7 post IAV infection (IAV) or Octagam or Flebogam intravenous immunoglobulin (IVIG) preparations. (B) No difference among sialylated Fc glycoforms (sFc) was observed. (C) Anti-ENV IgGs were skewed in distribution toward the IgG1 subclass during early or convalescent DENV infection compared with anti-HA IgGs from uninfected healthy adults, patients on day 7 of IAV infection or Octagam or Flebogam IVIG preparations. (D) DENV patient IgGs that were reactive with DENV NS1 protein, cross-reactive/reactive with Zika virus ENV, or with HA protein were also elevated in afucFc relative to anti-HA IgG from IAV patients. (E) Zika ENV-reactive IgGs in early DENV infection were skewed in distribution toward the IgG1 subclass.
To determine whether elevated afucFc was specific for anti-ENV IgGs, we tested early infection IgGs that were reactive with DENV NS1 protein, cross-reactive/reactive with Zika virus ENV(36), or that were specific for IAV HA protein. These other IgG specificities, including anti-HA IgGs, were similarly elevated in afucFc compared with anti-HA IgG from IAV patients (Figure 1D), indicating that a global shift in IgG Fc structure had occurred in early DENV infection. In addition, IgGs reactive with the ENV protein of the infecting DENV serotype, or that were reactive with ENV proteins from the non-infecting DENV serotypes, were equivalent in abundance of afucFc in a subgroup analysis (Figure S1). Zika ENV-reactive antibodies were also elevated in IgG1/IgG2 ratio, while IgGs reactive with DENV NS1 or IAV HA proteins were not (Figure 1E), indicating that modulation of Fc fucosylation was a more general feature in DENV infection than subclass bias.
Stratification of patients by clinical diagnosis showed that anti-ENV and anti-HA IgGs from DHF/DSS patients were elevated in afucFc compared with IgGs from either DF or IAV patients (Figure 2A). Stratification of patients based on presence of thrombocytopenia (TH+) during disease course, which is a requisite criterion for DHF diagnosis, showed that TH+ patients had similarly elevated afucFc anti-ENV and anti-HA IgGs, with afucFc ³ 10% being a significant risk factor for TH+ (p=0.0139, OR 11.00, 95% CI 1.635–74.00, RR 1.833) (Figure 2B). TH+ also correlated with an increased IgG1/IgG2 ratio for anti-DENV ENV (Figure 2C). Total IgG from TH+ patients had measurably higher affinity for FcγRIIIA by surface plasmon resonance (Figure S2).
Fig. 2. Activating Fc phenotype in dengue infection correlates with disease severity.

(A) Increased afucFc on anti-ENV or anti-HA IgG correlated with DHF/DSS (DHF). (B) Stronger correlations yet were observed when patients were stratified based on thrombocytopenia (TH+) (platelets <100,000/ul) during hospitalization. TH+ patients had elevated abundance of afucFc on anti-ENV or anti-HA IgG compared with TH− patients. (C) TH+ patient anti-ENV IgGs were skewed in distribution toward the IgG1 subclass, while anti-HA IgGs from TH+ patients were not. (D,E) Patients with the lowest recorded platelet count during hospitalization had the greatest abundance of afucFc and the highest IgG1/IgG2 ratio of anti-ENV IgGs. (F) Abundance of afucFc on anti-ENV IgG correlated with IgG1/IgG2 distribution; elevated afucFc and IgG1/IgG2 correlated with the severity of thrombocytopenia. The single patient with DSS in the cohort had the greatest abundance of afucFc and the highest IgG1/IgG2 ratio (marked with *). (G,H) Elevated hematocrit (HCT), a marker of plasma leak that distinguishes DHF/DSS from DF, correlated with anti-ENV IgGs having the greatest abundance of afucFc and the highest IgG1/IgG2 ratio.
Elevated afucFc and IgG1/2 ratio correlated not only with being TH+ during hospitalization, but also correlated with the lowest platelet count recorded for each patient during hospitalization (Figures 2D,E). Further, these two determinants of higher affinity binding to FcγRIIIA correlated with eachother and patients with the greatest abundance of afucFc IgG1 were most likely to have severe thrombocytopenia (Figure 2F). The single patient with DSS had the greatest abundance of afucFc IgG1 of all patients in the cohort (Figure 2F). In addition to clinical diagnosis and severity of TH+, elevated hematocrit, an indication of plasma leak that distinguishes DHF/DSS from DF, also correlated with the abundance of afucFc and elevated IgG1/IgG2 ratio of anti-ENV IgGs (Figure 2G,H).
The correlation between high afucFc IgG1 and severe disease indicated that this Fc structure may play a role in ADE during DENV infection. In particular, the correlation between the abundance of afucFc IgG1 and the degree of thrombocytopenia (Figures 2D,E,F) led us to hypothesize that anti-DENV IgGs that cross react with platelet antigens might contribute to platelet loss during dengue infection, and thus to ADE of dengue disease. As anti-DENV NS1 IgG has been shown to cross-react with platelets(37), we tested whether transfer of IgG from severely TH+ patients could mediate platelet reduction, in vivo, to a greater extent than IgG from TH− patients. IgGs from TH+ patients caused a drop in platelets in mice humanized for FcRs (hFcR), while even at high doses, IgG from TH− patients did not reduce platelet numbers (Figure S3). IgG from TH+ patients was treated to remove Fc glycans, producing an aglycosylated TH+ pool (TH+agly) that would no longer engage FcRs; this pool had less effect on platelet numbers, while mice lacking all FcRs (α−/−) were resistant to TH+ IgG-mediated thrombocytopenia (Figure 3A)(38). This loss of platelets was dependent on two low affinity human activating FcRs: FcγRIIA (CD32A) and FcγRIIIA (CD16A), as deletion of either receptor rendered the mice resistant to TH+ IgG-induced platelet reduction (Figure 3B).
Fig. 3. DHF IgG induces FcR-dependent platelet drop in vivo.
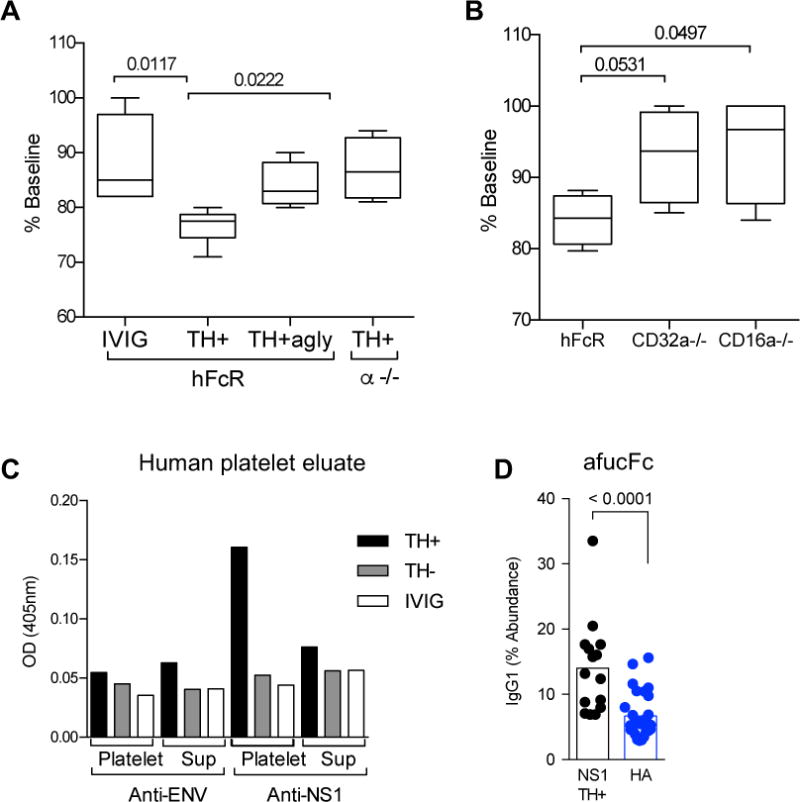
(A) IgG from TH+ patients, but not IVIG, caused a drop in platelets that was dependent on presence of the Fc glycan (TH+agly) and FcRs (α−/−). (B) Platelet drop was dependent on expression of FcgRIIA (CD32A) and FcgRIIIA (CD16A). (C) TH+ or TH− IgG or IVIG was incubated with human platelets. IgG at equivalent concentrations eluted from platelets, or the remaining supernatant (sup), reacted with DENV NS1 protein but not DENV ENV protein. Data are representative of experiment performed in duplicate. (D) NS1-specific IgG from TH+ patients had elevated afucFc glycoforms over anti-HA IgG from patients with day 7 IAV (HA).
As purified IgG alone caused a loss of platelets, we next tested whether IgG from TH+ patients might bind to platelets directly. After incubation with human or mouse platelets, TH+ IgG could be eluted from platelets that bound the DENV NS1 protein, but not the ENV protein (Figure 3C & Figure S4). As with anti-ENV IgG from TH+ patients, anti-NS1 IgG had elevated afucFc (Figure 3D) but the IgG1/IgG2 ratio was not different (Figure S5). All together, this showed that the IgGs enhanced for FcγRIIIA binding from patients who became thrombocytopenic during DENV infection could mediate FcR-dependent platelet loss in vivo. At least three mechanisms could contribute to this platelet reduction: anti-NS1/platelet IgG may activate platelets directly through platelet FcγRIIA, and/or, cause sequestration or uptake of platelets by monocytes which express both FcγRIIIA and FcγRIIA, and/or ADCC of platelets could occur via FcγRIIIA(38).
These experiments showed that anti-DENV IgGs with enhanced affinity for FcγRIIIA could mediate ADE of disease, which is distinct from ADE of infection. Serum pools of both TH+ and TH− patients mediated ADE of DENV infection in the standard U937 cell assay (Figure S6). This result, while confirming that IgGs from RNNIg+ DENV patients can mediate ADE, is not informative in the context of our observations that enhanced dengue disease is associated with an FcRIIIA activating IgG phenotype. This is because U937 cells are FcγRIIIA−, FcγRIIA+ (39, 40)(41). Thus, we distinguish between ADE of infection and ADE of disease in our study.
That the precise Fc structure of antibodies present during DENV infection may contribute to disease severity raised the question of whether this Fc structure was present before infection or was triggered by DENV infection itself. To address this, we compared the Fc of antibodies obtained from TH+ patients during the early and convalescent phases of disease. The convalescent phase was marked by a significant drop in both afucFc and IgG1/IgG2 ratio (Figure 4A,B), indicating that, in patients with severe disease, DENV infection itself triggered an elevation in IgGs with enhanced affinity for FcγRIIIA.
Fig. 4. DENV infection contributed to activating Fc phenotype in TH+ patients.

(A) The convalescent phase of DENV infection was marked by a significant drop in both afucFc and (B) IgG1/IgG2 ratio in TH+ patients, demonstrating an aberrant IgG response during infection.
The present finding that some individuals respond to DENV infection by producing IgGs with higher affinity for FcγRIIIA indicates a host determinant of susceptibility to severe DENV disease. Further studies will determine how this patient selectivity may contribute to additional mechanisms underlying ADE of DENV disease.
Supplementary Material
One sentence summary.
Individuals who produce IgGs with higher affinity for the activating FcγRIIIA during dengue virus infection are significantly more susceptible to antibody-dependent enhancement of disease.
Acknowledgments
We thank Sheng Zhang and Robert Sherwood at the Cornell Proteomics and Mass Spectrometry Facility for helpful discussions and excellent technical support. The authors also thank the staff of the Stanford Clinical Virology Laboratory for their assistance with dengue virus serologic testing. T.T.W. thanks Barry Coller, Sarah J Schlesinger and the Rockefeller University KL2 Clinical Scholars Program for training and support. T.T.W. was supported, in part, by grant #UL1TR001866 from the National Center for Advancing Translational Sciences, NIH and the Clinical and Translational Science Award program. J.S. would like to thank Shiyu Wang and Sivaram Gunisetty for helping with preparation of sera from patient samples. K.P. was funded by HHS National Institutes of Health (NIH) (IRO1AI099385). S.B. was supported by an amfAR Mathilde Krim Fellowship in Basic Biomedical Research (109519-60-RKVA). Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the NIH under Award Numbers U19AI111825 (J.V.R.), U19AI057266 (R.A., J.W.), and U01AI115651 (R.A.). The influenza A virus challenge study was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), as well as the NIAID Extramural Clinical Research Acceleration Program. Support and infrastructure were also provided by The Rockefeller University and by Stanford University School of Medicine. Analysis of clinical samples in this work was approved by the Institutional Review Board of Rockefeller University (protocol #TWA-0804 (T.T.W.)). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The data presented in this manuscript are tabulated in the main paper and in the supplementary materials.
Footnotes
Supplementary Materials
Materials and Methods
References 42,43,44,45
References and Notes
- 1.Halstead SB, et al. Observations related to pathogenesis of dengue hemorrhagic fever. I. Experience with classification of dengue viruses. Yale J Biol Med. 1970;42:261–275. [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez D, et al. Classical dengue hemorrhagic fever resulting from two dengue infections spaced 20 years or more apart: Havana, Dengue 3 epidemic, 2001–2002. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2005;9:280–285. doi: 10.1016/j.ijid.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. The American journal of tropical medicine and hygiene. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 4.Graham RR, et al. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia I. studies in 1995–1996. The American journal of tropical medicine and hygiene. 1999;61:412–419. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- 5.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 6.Vaughn DW, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. The Journal of infectious diseases. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 7.Flipse J, Wilschut J, Smit JM. Molecular mechanisms involved in antibody-dependent enhancement of dengue virus infection in humans. Traffic. 2013;14:25–35. doi: 10.1111/tra.12012. [DOI] [PubMed] [Google Scholar]
- 8.Kliks S. Antibody-enhanced infection of monocytes as the pathogenetic mechanism for severe dengue illness. AIDS research and human retroviruses. 1990;6:993–998. doi: 10.1089/aid.1990.6.993. [DOI] [PubMed] [Google Scholar]
- 9.Endy TP, et al. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. The Journal of infectious diseases. 2004;189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 10.Libraty DH, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. The Journal of infectious diseases. 2002;185:1213–1221. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 11.Blackley S, et al. Primary human splenic macrophages, but not T or B cells, are the principal target cells for dengue virus infection in vitro. Journal of virology. 2007;81:13325–13334. doi: 10.1128/JVI.01568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kou Z, et al. Human antibodies against dengue enhance dengue viral infectivity without suppressing type I interferon secretion in primary human monocytes. Virology. 2011;410:240–247. doi: 10.1016/j.virol.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Flipse J, et al. Antibody-Dependent Enhancement of Dengue Virus Infection in Primary Human Macrophages; Balancing Higher Fusion against Antiviral Responses. Sci Rep. 2016;6:29201. doi: 10.1038/srep29201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modhiran N, Kalayanarooj S, Ubol S. Subversion of innate defenses by the interplay between DENV and pre-existing enhancing antibodies: TLRs signaling collapse. PLoS Negl Trop Dis. 2010;4:e924. doi: 10.1371/journal.pntd.0000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ubol S, Phuklia W, Kalayanarooj S, Modhiran N. Mechanisms of immune evasion induced by a complex of dengue virus and preexisting enhancing antibodies. The Journal of infectious diseases. 2010;201:923–935. doi: 10.1086/651018. [DOI] [PubMed] [Google Scholar]
- 16.OhAinle M, et al. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med. 2011;3:114ra128. doi: 10.1126/scitranslmed.3003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitmeyer KC, et al. Dengue virus structural differences that correlate with pathogenesis. Journal of virology. 1999;73:4738–4747. doi: 10.1128/jvi.73.6.4738-4747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balmaseda A, et al. Serotype-specific differences in clinical manifestations of dengue. The American journal of tropical medicine and hygiene. 2006;74:449–456. [PubMed] [Google Scholar]
- 19.Rico-Hesse R, et al. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230:244–251. doi: 10.1006/viro.1997.8504. [DOI] [PubMed] [Google Scholar]
- 20.Morrison J, et al. Dengue virus co-opts UBR4 to degrade STAT2 and antagonize type I interferon signaling. PLoS pathogens. 2013;9:e1003265. doi: 10.1371/journal.ppat.1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens HA, et al. HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens. 2002;60:309–318. doi: 10.1034/j.1399-0039.2002.600405.x. [DOI] [PubMed] [Google Scholar]
- 22.Ryan EJ, et al. Variant in CD209 promoter is associated with severity of liver disease in chronic hepatitis C virus infection. Hum Immunol. 2010;71:829–832. doi: 10.1016/j.humimm.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Loke H, et al. Susceptibility to dengue hemorrhagic fever in vietnam: evidence of an association with variation in the vitamin d receptor and Fc gamma receptor IIa genes. The American journal of tropical medicine and hygiene. 2002;67:102–106. doi: 10.4269/ajtmh.2002.67.102. [DOI] [PubMed] [Google Scholar]
- 24.Kwissa M, et al. Dengue virus infection induces expansion of a CD14(+)CD16(+) monocyte population that stimulates plasmablast differentiation. Cell host & microbe. 2014;16:115–127. doi: 10.1016/j.chom.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohsin SN, et al. Association of FcgammaRIIa Polymorphism with Clinical Outcome of Dengue Infection: First Insight from Pakistan. The American journal of tropical medicine and hygiene. 2015;93:691–696. doi: 10.4269/ajtmh.15-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia G, et al. Asymptomatic dengue infection in a Cuban population confirms the protective role of the RR variant of the FcgammaRIIa polymorphism. The American journal of tropical medicine and hygiene. 2010;82:1153–1156. doi: 10.4269/ajtmh.2010.09-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anthony RM, et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Annals of the New York Academy of Sciences. 2012;1253:170–180. doi: 10.1111/j.1749-6632.2011.06305.x. [DOI] [PubMed] [Google Scholar]
- 29.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Advances in immunology. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang TT, et al. Anti-HA Glycoforms Drive B Cell Affinity Selection and Determine Influenza Vaccine Efficacy. Cell. 2015;162:160–169. doi: 10.1016/j.cell.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herter S, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Molecular cancer therapeutics. 2013;12:2031–2042. doi: 10.1158/1535-7163.MCT-12-1182. [DOI] [PubMed] [Google Scholar]
- 32.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CDC. (Centers for Disease Control and Prevention, 2016), vol. 2016, chap. January 20, 2016.
- 34.Materials and methods are available as supplementary materials on Science Online.
- 35.Memoli MJ, et al. Validation of the wild-type influenza A human challenge model H1N1pdMIST: an A(H1N1)pdm09 dose-finding investigational new drug study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60:693–702. doi: 10.1093/cid/ciu924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Priyamvada L, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:7852–7857. doi: 10.1073/pnas.1607931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun DS, et al. Antiplatelet autoantibodies elicited by dengue virus non-structural protein 1 cause thrombocytopenia and mortality in mice. J Thromb Haemost. 2007;5:2291–2299. doi: 10.1111/j.1538-7836.2007.02754.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6181–6186. doi: 10.1073/pnas.1203954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleit HB, Wright SD, Unkeless JC. Human neutrophil Fc gamma receptor distribution and structure. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones DH, Looney RJ, Anderson CL. Two distinct classes of IgG Fc receptors on a human monocyte line (U937) defined by differences in binding of murine IgG subclasses at low ionic strength. Journal of immunology. 1985;135:3348–3353. [PubMed] [Google Scholar]
- 41.See Methods, available as supplementary materials on Science Online.
- 42.Zhang S, et al. Comparative characterization of the glycosylation profiles of an influenza hemagglutinin produced in plant and insect hosts. Proteomics. 2012;12:1269–1288. doi: 10.1002/pmic.201100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S, Williamson BL. Characterization of protein glycosylation using chip-based nanoelectrospray with precursor ion scanning quadrupole linear ion trap mass spectrometry. Journal of biomolecular techniques : JBT. 2005;16:209–219. [PMC free article] [PubMed] [Google Scholar]
- 44.Changal KH, et al. Differentiating secondary from primary dengue using IgG to IgM ratio in early dengue: an observational hospital based clinico-serological study from North India. BMC infectious diseases. 2016;16:715. doi: 10.1186/s12879-016-2053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cucunawangsih NP, Lugito A. Kurniawan, Immunoglobulin G (IgG) to IgM ratio in secondary adult dengue infection using samples from early days of symptoms onset. BMC infectious diseases. 2015;15:276. doi: 10.1186/s12879-015-1022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


