Abstract
Background
Sarcopenia and decreased bone-mineral density (BMD) are common in elderly people, and are major comorbidities of obstructive airway disease (OAD). However, the relationship between sarcopenia and BMD in each OAD phenotype, especially asthma–COPD overlap syndrome (ACOS), is not yet clear. We aimed to evaluate differences in BMD according to the presence of sarcopenia in each OAD phenotype.
Materials and methods
Among the research subjects in KNHANES IV and V (2008–2011), 5,562 were ≥50 years old and underwent qualified spirometry and dual-energy X-ray absorptiometry. A total of 947 subjects were included in the study: 89 had asthma, 748 COPD, and 110 ACOS.
Results
In the COPD and ACOS phenotypes, T-scores were lower in the sarcopenia group than the nonsarcopenia group. Prevalence rates of osteopenia and osteoporosis were higher in the sarcopenia group than the nonsarcopenia group. (P<0.001 and P=0.017, respectively). The sarcopenia group had higher risks of developing osteopenia, osteoporosis, and low BMD than the nonsarcopenia group in the ACOS phenotype (OR 6.620, 95% CI 1.129–38.828 [P=0.036], OR 9.611, 95% CI 1.133–81.544 [P=0.038], and OR 6.935, 95% CI 1.194–40.272 [P=0.031], respectively). However, in the asthma phenotype, the sarcopenia group showed no increased risk compared with the nonsarcopenia group.
Conclusion
In the ACOS phenotype, individuals with sarcopenia had a higher prevalence rate and higher risks of osteopenia and osteoporosis than those without sarcopenia among all OAD phenotypes.
Keywords: asthma, chronic obstructive pulmonary disease, asthma–COPD overlap syndrome, sarcopenia, bone-mineral density
Introduction
As the elderly population increases rapidly worldwide, various complications and morbidities are placing an increasing burden on the public health care system, as well as individual patients.1 Obstructive airway disease (OAD) constitutes a major proportion of the burden of incommunicable diseases on the public health care system in adults. OAD is classified as asthma, COPD, and asthma–COPD overlap syndrome (ACOS), although the distinctions are not clear in practice.2 Age-related decreased bone-mineral density (BMD) is an important health problem among elderly people that contributes to disability and premature mortality.3,4 Decreased BMD may result in osteopenia and osteoporosis, of which the latter is more serious.
Sarcopenia is a syndrome characterized by progressive and generalized loss of skeletal muscle (SM) and strength, with risks of adverse outcomes, such as physical disability, poor quality of life, and death.5,6 A recent study showed that sarcopenia is closely correlated with decreased BMD and osteoporosis.7 Therefore, sarcopenia has a variety of effects on quality of life.
Sarcopenia and decreased BMD are common in elderly people, and are major comorbidities of OAD.8–10 The association between sarcopenia and decreased BMD in healthy people is known, but the relationship between sarcopenia and BMD in each OAD phenotype, especially ACOS, is not clear.11,12 We conducted a study on subjects from the Korean National Health and Nutrition Examination Survey (KNHANES) IV and V (2008–2011) to evaluate change in BMD according to the presence or absence of sarcopenia in each OAD phenotype.
Materials and methods
Study participants
KNHANES is a nationwide cross-sectional survey that has been conducted by the South Korean Centers for Disease Control and Prevention since 1998. The data used were collected in KNHANES IV and V, nationwide cross-sectional surveys of the South Korean population conducted in 2008–2011, which used stratified, multistage, clustered probability sampling to select a representative sample of the civilian, noninstitutionalized South Korean population.
KNHANES consists of three components: a health interview, a nutrition survey, and a health examination. Data were collected in household interviews, and direct standardized physical examinations were conducted at mobile examination centers. From this, data on both males and postmenopausal females aged ≥50 years with valid spirometry and dual-energy X-ray absorptiometry (DXA) results were used for analysis.
Written informed consent was provided by all participants. The study protocol for the analysis of the KNHANES 2008–2011 data was reviewed and approved by the institutional review board (approval 2008–04EXP-01-C, 2009–01CON-03-C, 2010–02CON-21-C, and 2011–02CON-06-C) of the South Korean Centers for Disease Control and Prevention. The requirement for additional institutional review-board approval was waived, because the KNHANES data set is publicly available.
Measurements
Trained interviewers administered a standardized questionnaire to the study subjects on their medical history, smoking frequency, and physical inactivity level. Questions regarding a positive/negative asthma diagnosis by a physician and the diagnosis date, as well as questions about whether the respondents heard wheezing sounds from their chest while exercising, were included in the asthma disease-related questions in the medical history questionnaire. KNHANES contains data on vitamin D, alkaline phosphatase (ALP), parathyroid hormone, spirometry, and DXA. The procedure was performed in the same manner as in a previous study.13
Health-related quality of life was evaluated using a validated Korean version of the EuroQol five-dimensional (EQ-5D) questionnaire, which generates assessment scores on five dimensions of health: self-care, usual activities, pain/discomfort, and anxiety/depression. The responses in each dimension were divided into three categories: no problem, moderate problem, or extreme problem. In addition, respondents assessed their health status using a visual analogue scale, which ranged from 0 (worst imaginable health) to 100 (best imaginable health). Height and body weight were measured with participants in disposable examination gowns during visits to the examination center. The diagnosis of osteoporosis was made using the World Health Organization (WHO) T-score criteria, and the maximum BMD value for Japanese patients was used as a reference, due to the lack of established diagnostic criteria for South Koreans.14,15
Definition
OAD was divided into three groups: asthma, COPD, and ACOS. Patients that had been diagnosed with asthma by a physician before the age of 40 years with forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ≥0.7 were assigned to the asthma group. Patients in the COPD group were ≥50 years of age with FEV1/FVC <0.7. Patients diagnosed with asthma by a physician before the age of 40 years or with wheezing during exercise for 1 year from among patients aged ≥50 years with FEV1/FVC <0.7 were assigned to the ACOS group. Body-mass index (BMI) value was calculated as body weight/height2. SM was defined as body mass minus bone mass, and SM index (SMI) value was defined as SM/height2.16 Appendicular SM (ASM) was defined as the sum of the lean soft-tissue mass for the arms and legs.17 Appendicular SMI (ASMI) value was defined as ASM/height2.18 Fat-mass index (FMI) value was estimated as fat mass/height2, and appendicular FMI (AFMI) value defined as appendicular fat mass/height2. Sarcopenia was defined according to the criteria for the Asia Working Group for Sarcopenia.19 Osteopenia and osteoporosis correspond to conditions in which the spine or hip has a BMD T-score of −1 to −2.5 and a T-score <−2.5, as determined by the DXA evaluation according to WHO criteria. Low BMD was defined as a T-score <−1 in the femur, femoral neck, or lumbar area.
Statistical analysis
The independent-sample t-test was used to compare the demographic and hematological characteristics and differences in BMD between the nonsarcopenia and sarcopenia groups in each OAD phenotype. Results are presented as means ± standard deviation. The χ2 test was performed to assess the prevalence rates of osteopenia and osteoporosis according to sarcopenia group in each OAD phenotype. Correlation analyses were conducted to confirm the correlations among SMI, ASMI, and T-score by area in each OAD phenotype. To confirm the relationships of SMI, ASMI, and T-score, multivariate linear regression analysis was performed after adjustment for several variables. To compare the risks of osteopenia, osteoporosis, and low BMD in each OAD phenotype according to the presence of sarcopenia, multivariate logistic analyses were performed using models where several variables were corrected for. All analyses were conducted using SPSS software (version 18.0; SPSS Inc, Chicago, IL, USA). In all analyses, P<0.05 was taken to indicate statistical significance.
Results
Subject characteristics
Among the research subjects of KNHANES IV and V 2008–2011, 5,562 were ≥50 years of age and had undergone spirometry and DXA. The study population consisted of 947 subjects: 89 had asthma, 748 had COPD, and 110 had ACOS. In the asthma phenotype, subjects in the sarcopenia group had lower weight, BMI, SMI, ASMI, FMI, and AFMI compared with those in the nonsarcopenia group. In the COPD phenotype, subjects in the sarcopenia group were older, had lower weight, BMI, SMI, ASMI, FMI, AFMI, FEV1 (liters), FEV1 (percentage), and FVC compared with those in the nonsarcopenia group. In the ACOS phenotype, subjects in the sarcopenia group had lower weight, BMI, SMI, ASMI, FEV1 (liters), FEV1 (percentage), and FVC and higher ALP compared with those in the nonsarcopenia group. EQ-5D values of the sarcopenia group were lower than the nonsarcopenia group in the COPD phenotype (P=0.035); however, with regard to the EuroQol (EQ) visual analogue scale, there were no significant differences in any of the OAD phenotypes. Differences in other characteristics among the groups are shown in Table 1.
Table 1.
Characteristics of nonsarcopenia group vs sarcopenia group in each OAD phenotypes
| Characteristics | Asthma
|
COPD
|
ACOS
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonsarcopenia (n=76) |
Sarcopenia (n=13) |
P-value | Nonsarcopenia (n=497) |
Sarcopenia (n=251) |
P-value | Nonsarcopenia (n=75) |
Sarcopenia (n=35) |
P-value | |
| Age (years) | 61.28±7.39 | 62.23±9.07 | 0.678 | 65.05±7.64 | 67.41±8.32 | <0.001 | 66±7.6 | 67.06±6.73 | 0.483 |
| Female (%) | 81.6% | 69.2% | 0.289 | 25.2% | 19.1% | 0.065 | 42.7% | 34.3% | 0.403 |
| Height (m) | 1.56±0.08 | 1.56±0.07 | 0.596 | 1.64±0.09 | 1.63±0.08 | 0.295 | 1.60±9.23 | 1.60±7.65 | 0.948 |
| Weight (kg) | 61.94±8.53 | 53.88±8.76 | 0.002 | 65.74±9.73 | 56.87±8.18 | <0.001 | 63.67±9.73 | 55.65±8.23 | <0.001 |
| BMI (kg/m2) | 25.33±2.3 | 21.65±2.22 | <0.001 | 24.48±2.51 | 21.37±2.26 | <0.001 | 24.78±2.57 | 21.64±2.37 | <0.001 |
| SMI (kg/m2) | 16.1±1.91 | 14.1±1.76 | 0.001 | 17.21±1.7 | 15.02±1.35 | <0.001 | 16.84±1.79 | 14.73±1.54 | <0.001 |
| ASMI (kg/m2) | 6.6±1.08 | 5.42±0.7 | <0.001 | 7.32±0.91 | 6.21±0.71 | <0.001 | 7.08±1 | 5.99±0.8 | <0.001 |
| FMI (kg/m2) | 8.20±1.9 | 6.46±1.62 | 0.002 | 6.19±2.11 | 5.36±1.94 | <0.001 | 6.95±2.68 | 5.98±2.06 | 0.062 |
| AFMI (kg/m2) | 3.37±0.88 | 2.62±0.73 | 0.005 | 2.38±0.92 | 2.07±0.85 | <0.001 | 2.74±1.23 | 2.39±0.95 | 0.136 |
| Smoking (pack years) | 2.62±7.95 | 3.42±7.05 | 0.735 | 21.77±22.56 | 25.26±24.04 | 0.051 | 18.46±21.08 | 26.29±22.42 | 0.078 |
| EQ-5D index | 0.86±0.171 | 0.83±0.297 | 0.584 | 0.92±0.146 | 0.9±0.152 | 0.035 | 0.85±0.17 | 0.84±0.18 | 0.818 |
| EQ-VAS | 72.20±17.569 | 68.86±21.23 | 0.598 | 74.52±18.18 | 75.28±61.31 | 0.798 | 66.85±22.87 | 60.77±17.66 | 0.167 |
| Physical inactivity n (%) | 0.348 | 0.001 | 0.036 | ||||||
| Yes | 22 (28.9%) | 2 (15.4%) | 87 (17.5%) | 69 (27.5%) | 24 (32%) | 19 (54.3%) | |||
| No | 54 (71.1%) | 11 (84.6%) | 409 (82.3%) | 181 (82.1%) | 51 (68%) | 16 (45.7%) | |||
| Unknown | 0 | 0 | 1 (0.2%) | 1 (0.4%) | 0 | 0 | |||
| FEV1 (L) | 2.4±0.52 | 2.48±0.6 | 0.617 | 2.35±0.63 | 2.17±0.62 | ,0.001 | 1.85±0.62 | 1.56±0.54 | 0.017 |
| FEV1 (%) | 77.97±4.42 | 76.64±4.73 | 0.96 | 80.09±14.31 | 76.43±16.4 | 0.002 | 69.37±17.43 | 57.79±16.13 | 0.001 |
| FVC (L) | 3.08±0.69 | 3.24±0.79 | 0.462 | 3.65±0.9 | 3.46±0.84 | 0.007 | 3.12±0.91 | 2.83±0.73 | 0.101 |
| FVC (%) | 94.35±8.78 | 96.5±9.23 | 0.419 | 91.05±14.07 | 88.9±14.85 | 0.053 | 85.1±16.07 | 77.56±13.97 | 0.019 |
| FEV1/FVC | 0.78±0.04 | 0.77±0.04 | 0.325 | 0.64±0.06 | 0.62±0.07 | <0.001 | 0.6±0.09 | 0.55±0.11 | 0.018 |
| ALP (IU/L) | 237.13±69.36 | 247.15±60.61 | 0.626 | 245.56±75.71 | 255.82±76.69 | 0.082 | 240.68±70.72 | 277.6±85.54 | 0.019 |
| PTH (pg/mL) | 65.11±24.28 | 62.02±18.94 | 0.664 | 66.05±25.48 | 67.57±29.4 | 0.463 | 67.15±23.29 | 72.5±29.16 | 0.303 |
| Vitamin D (ng/mL) | 18.41±5.81 | 20.66±6.6 | 0.211 | 21.12±7.14 | 21.29±8.38 | 0.782 | 19.68±6.76 | 20.62±8.08 | 0.525 |
| Femur T-score | −0.25±0.97 | −0.51±1.3 | 0.403 | −0.14±0.97 | −0.7±0.89 | <0.001 | −0.43±0.94 | −1±0.78 | 0.001 |
| Femur neck T-score | −1.32±1.02 | −1.4±1.46 | 0.866 | −0.93±1.04 | −1.4±0.99 | <0.001 | −1.38±1.08 | −1.76±0.87 | 0.071 |
| Lumbar T-score | −1.35±1.25 | −0.8±1.68 | 0.168 | −0.78±1.38 | −1.31±1.36 | <0.001 | −1.2±1.32 | −1.89±1.24 | 0.011 |
Note: Data presented as mean ± standard deviation unless stated otherwise.
Abbreviations: OAD, obstructive airway disease; COPD, chronic obstructive pulmonary disease; ACOS, asthma–COPD overlap syndrome; BMI, body-mass index; SMI, skeletal muscle index; ASMI, appendicular SMI; FMI, fat-mass index; AFMI, appendicular FMI; EQ-5D, EuroQol five-dimensional; EQ-VAS, EuroQol visual analogue scale; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ALP, alkaline phosphatase; PTH, parathyroid hormone.
T-scores and differences in prevalence rates of osteopenia and osteoporosis according to sarcopenia group in all OAD phenotypes
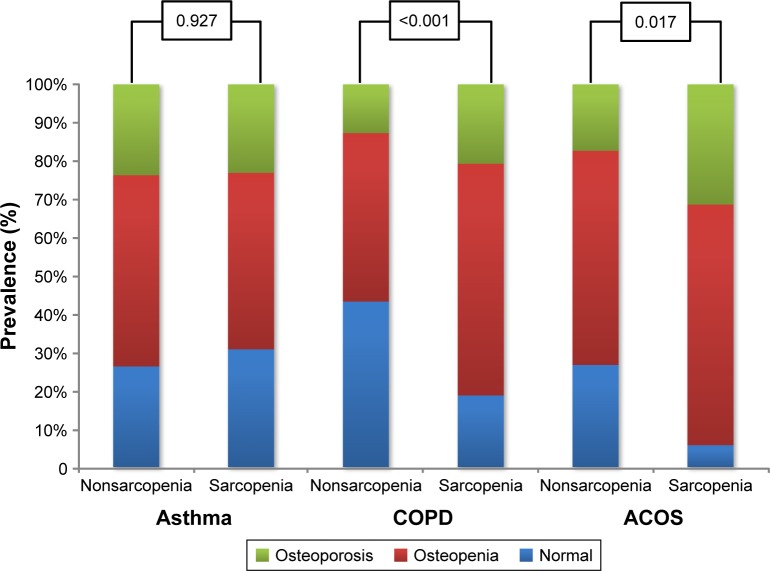
In the COPD and ACOS phenotypes, mean femur, femoral neck, and lumbar BMD T-scores were lower in the sarcopenia group than the nonsarcopenia group. However, there were no differences in these variables between the nonsarcopenia and sarcopenia groups in the asthma phenotype (Table 1). In the asthma phenotype, prevalence rates in the nonsarcopenia and sarcopenia groups for osteopenia were 50% and 46.2%, respectively, and those for osteoporosis were 23.7% and 23.1%, respectively (P=0.927). In the COPD phenotype, prevalence rates in the nonsarcopenia and sarcopenia groups for osteopenia were 44.1% and 60.6%, respectively, and those for osteoporosis were 12.7% and 20.7%, respectively (P<0.001). In the ACOS phenotype, prevalence rates in the nonsarcopenia and sarcopenia groups for osteopenia were 56% and 62.9%, respectively, and those for osteoporosis were 17.3% and 31.4%, respectively (P=0.017) (Figure 1).
Figure 1.
Prevalence rate of osteopenia and osteoporosis according to presence of sarcopenia in each obstructive airway-disease phenotype.
Abbreviation: ACOS, asthma–COPD overlap syndrome.
Relationship of SMI and ASMI with T-score in each OAD phenotype
Relationships between SMI, ASMI, and each T-score were investigated. In all OAD phenotypes, the SMI and ASMI were significantly correlated with T-score. In the asthma phenotype, SMI and ASMI showed the highest correlation coefficients with lumbar T-score (R=0.355 [P=0.001] and R=0.321 [P=0.002], respectively). In the COPD phenotype, SMI showed the highest correlation coefficient with femur T-score (R=0.498, P<0.001), and ASMI showed the highest correlation coefficient with femur neck T-score (R=0.509, P<0.001). In the ACOS phenotype, SMI and ASMI showed the highest correlation coefficients with femur neck T-score (R=0.521 [P<0.001] and R=0.559 [P<0.001], respectively) (Table 2). In the COPD and ACOS phenotypes, we confirmed that SMI and ASMI were related to femur T-score, femur neck T-score, and lumbar T-score, even after adjusting for several variables. However, in the asthma phenotype, SMI and ASMI showed no relation to femur T-score, femur neck T-score, or lumbar T-score (Table 3).
Table 2.
Correlation analysis of SMI and ASMI, with T-score by part
| Femur T-score
|
Femur neck T-score
|
Lumbar T-score
|
||||
|---|---|---|---|---|---|---|
| Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | |
| Asthma | ||||||
| SMI | 0.312 | 0.003 | 0.290 | 0.006 | 0.355 | 0.001 |
| ASMI | 0.263 | 0.013 | 0.271 | 0.010 | 0.321 | 0.002 |
| COPD | ||||||
| SMI | 0.498 | <0.001 | 0.492 | <0.001 | 0.458 | <0.001 |
| ASMI | 0.502 | ,0.001 | 0.509 | <0.001 | 0.457 | <0.001 |
| ACOS | ||||||
| SMI | 0.506 | <0.001 | 0.521 | <0.001 | 0.455 | <0.001 |
| ASMI | 0.486 | <0.001 | 0.559 | <0.001 | 0.469 | <0.001 |
Abbreviations: SMI, skeletal muscle index; ASMI, appendicular SMI; ACOS, asthma–COPD overlap syndrome.
Table 3.
Multivariate linear analysis of SMI and ASMI, with T-score by part
| Femur T-score
|
Femur neck T-score
|
Lumbar T-score
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P-value | β | SE | P-value | β | SE | P-value | |
| Asthma | |||||||||
| SMI | 0.176 | 0.069 | 0.199 | 0.007 | 0.07 | 0.954 | 0.092 | 0.087 | 0.487 |
| ASMI | 0.069 | 0.125 | 0.582 | −0.097 | 0.124 | 0.435 | −0.037 | 0.154 | 0.811 |
| COPD | |||||||||
| SMI | 0.327 | 0.02 | ,0.001 | 0.265 | 0.021 | ,0.001 | 0.232 | 0.029 | ,0.001 |
| ASMI | 0.323 | 0.041 | ,0.001 | 0.262 | 0.042 | ,0.001 | 0.307 | 0.060 | ,0.001 |
| ACOS | |||||||||
| SMI | 0.373 | 0.054 | 0.002 | 0.206 | 0.053 | 0.044 | 0.25 | 0.08 | 0.037 |
| ASMI | 0.34 | 0.107 | 0.002 | 0.271 | 0.103 | 0.01 | 0.389 | 0.157 | 0.015 |
Notes: SMI adjusted for age, sex, height, FMI, smoking frequency, levels of blood vitamin D, PTH, ALP and FEV1 (%). ASMI adjusted for age, sex, height, AFMI, smoking frequency, levels of blood vitamin D, PTH, ALP and FEV1 (%).
Abbreviations: SMI, skeletal muscle mass index; ASMI, appendicular skeletal muscle mass index; B, standardized regression coefficient; SE, standard error; COPD, chronic obstructive pulmonary disease; ACOS, asthma–COPD overlap syndrome; FMI, fat mass index; PTH, parathyroid hormone; ALP, alkaline phosphatase; FEV1, forced expiratory volume in 1 sec; AFMI, appendicular fat mass index.
Osteopenia, osteoporosis, and low BMD in nonsarcopenia and sarcopenia in all OAD groups
A multivariate logistic regression-analysis model was used to compare the risks of developing osteopenia, osteoporosis, and low BMD between the nonsarcopenia and sarcopenia subgroups in all OAD phenotypes. In the COPD and ACOS phenotypes, the risks of developing osteopenia, osteoporosis, and low BMD were higher in the sarcopenia group than the nonsarcopenia group, even after adjusting for several variables. The sarcopenia group had higher risks of developing osteopenia, osteoporosis, and low BMD than the nonsarcopenia group in the ACOS phenotype (OR 6.62, 95% CI 1.129–38.828 [P=0.036], OR 9.611, 95% CI 1.133–81.544 [P=0.038], and OR 6.935, 95% CI 1.194–40.272 [P=0.031], respectively). However, in the asthma phenotype, the sarcopenia group showed no increased risk compared to the nonsarcopenia group (Table 4).
Table 4.
Osteopenia, osteoporosis, and low BMD in nonsarcopenia and sarcopenia
| Osteopenia
|
Osteoporosis
|
Low BMD
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Asthma | |||||||||
| Nonsarcopenia | 1 | 1 | 1 | ||||||
| Sarcopenia | 0.258 | 0.039–1.693 | 0.158 | 0.433 | 0.03–6.221 | 0.538 | 0.268 | 0.043–1.684 | 0.16 |
| COPD | |||||||||
| Nonsarcopenia | 1 | 1 | 1 | ||||||
| Sarcopenia | 3.124 | 2.079–4.695 | <0.001 | 5.476 | 2.866–10.464 | <0.001 | 3.131 | 2.101–4.666 | <0.001 |
| ACOS | |||||||||
| Nonsarcopenia | 1 | 1 | 1 | ||||||
| Sarcopenia | 6.62 | 1.129–38.828 | 0.036 | 9.611 | 1.133–81.544 | 0.038 | 6.935 | 1.194–40.272 | 0.031 |
Note: Adjusted for age, sex, height, AFMI, smoking frequency, levels of blood vitamin D, PTH, ALP, FEV1 (%) and physical inactivity.
Abbreviations: BMD, bone mineral density; OAD, obstructive airway disease; OR, odds ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ACOS, asthma–COPD overlap syndrome; AFMI, appendicular fat mass index; PTH, parathyroid hormon; ALP, alkaline phosphatase; FEV1, forced expiratory volume in 1 sec.
Discussion
In our study, the sarcopenia group had higher prevalence rates of osteopenia and osteoporosis than the nonsarcopenia group in COPD and ACOS phenotypes of OAD. SMI and ASMI were related to femur T-score, femur neck T-score, and lumbar T-score. The sarcopenia group had higher risks of developing osteopenia, osteoporosis, and low BMD than the nonsarcopenia group. In particular, sarcopenia showed the highest risk of decreased BMD in the ACOS phenotype. However, SMI and ASMI were not related to femur T-score, femur neck T-score, or lumbar T-score in the asthma phenotype. The sarcopenia group did not show increased risks of developing osteopenia, osteoporosis, or low BMD compared to the nonsarcopenia group.
The presence of sarcopenia was associated with decreased BMD in COPD and ACOS phenotypes. Sarcopenia was common in the COPD phenotype. Previous studies have shown that the loss of SM in the COPD phenotype is related to systemic inflammation.20,21 In addition, systemic inflammation is closely related to decreased BMD.22 Such cytokines as TNFα and IL6 are related to bone loss as well as sarcopenia, which explains the close relationship between sarcopenia and decreased BMD in the COPD phenotype.23,24 ACOS, which is a new phenotype of OAD, has not been researched in detail. However, one study showed that systemic inflammation is commonly present in patients with ACOS; ACOS resembles COPD in terms of systemic inflammation. In particular, the level of IL6, selected as a marker of systemic inflammation, is higher in ACOS than in healthy subjects and asthmatic patients.25 Therefore, systemic inflammation may contribute to the relationships between sarcopenia and decreased BMD in the ACOS phenotype.
However, sarcopenia was not related to decreased BMD in the asthma phenotype. Sarcopenia and osteoporosis share many common pathways, including sensitivity to reduced anabolic hormones, secretion, increased inflammatory cytokine activity, and anabolic or catabolic molecules released by SM or by bone cells. In particular, systemic inflammation due to inflammatory cytokine activity is a major cause of sarcopenia and bone loss.23,26 However, the rate of systemic inflammation is lower in asthma than the COPD and ACOS phenotypes.25 The major type of treatment for asthma is steroids (eg, inhaled corticosteroid or oral steroid). Steroids are representative drugs related to decreased BMD.27 Asthma patients are more likely to have steroid exposure than other phenotype groups. Therefore, the reduction in BMD due to steroids may have masked the effects of sarcopenia on BMD. Low systemic inflammation and frequent steroid exposure may be among the reasons that sarcopenia was not associated with decreased BMD in the asthma phenotype. However, as this was a retrospective study, we could not confirm the reasons for these observations, and further studies regarding this issue are warranted.
Sarcopenia is associated with many harmful clinical components, such as decreased quality of life and physical functioning and increased mortality.28,29 In addition, OAD is associated with many harmful clinical components and socioeconomic burdens,2 among which the ACOS phenotype comprises all the features of asthma and COPD. The systemic consequences of ACOS are not yet well known. Our results showed that the relationship between sarcopenia and decreased BMD in ACOS was similar to that in COPD. In particular, the risk of decreased BMD according to the presence of sarcopenia was higher in the ACOS phenotype than the COPD phenotype. These results were similar to those of recent studies indicating that ACOS has more severe systemic consequences than COPD.30,31 Our results suggest that the presence of sarcopenia in the ACOS phenotype may be related to many clinical adverse systemic effects.
Limitations
Our study had several limitations. First, recall bias and misdiagnosis could not be excluded, as self-reported asthma history was used in the analysis. However, misdiagnoses of COPD and asthma were minimized by including only patients that had asthma before the age of 40 years. Second, as the results of pre- and postbronchodilator tests could not be compared to assess pulmonary function, the presence or absence of bronchial hyperresponsiveness, which is an important characteristic of ACOS, could not be confirmed. Nevertheless, we made efforts to minimize the number of patients with ACOS in the COPD group by including questionnaire items about the presence or absence of wheezing during exercise, which suggests variations in airflow as a diagnostic criterion of ACOS. Third, we were unable to confirm the total dose of steroid used between the nonsarcopenia group and sarcopenia group. Previous studies have shown that the ACOS phenotype is associated with more frequent exacerbation compared with the COPD phenotype.31 Therefore, subjects with the ACOS phenotype may have received larger amounts of steroid than those with the COPD phenotype, which may have resulted in greater changes in OR in the ACOS phenotype. However, as this was a retrospective study, we were unable to evaluate total steroid dose used by the subjects. Fourth, we could not confirm differential markers of systemic inflammation between the nonsarcopenia group and sarcopenia group. It is likely that systemic inflammation mediated by cytokines, such as TNFα and IL6, plays a role in the relationship between sarcopenia and decreased BMD in the COPD and ACOS phenotypes. As this was a retrospective study, we were unable to evaluate cytokine levels as markers of systemic inflammation in blood samples. Fifth, this study was conducted using a cohort database of Asian (South Korean) people, and thus the results cannot be extended to Western populations. Further studies are required to address these limitations.
Sarcopenia was closely related to decreased BMD in OAD phenotypes, such as COPD and ACOS, with systemic inflammation. In the ACOS phenotype, patients with sarcopenia had higher prevalence rates and risks of osteopenia, osteoporosis, and low BMD than those without sarcopenia. The degree of decreased BMD risk according to the presence of sarcopenia was more severe compared with the COPD phenotype, indicative of the relevance to COPD phenotype but not asthma phenotype. In future studies, clinicians should consider the presence of sarcopenia and systemic consequences in patients with ACOS, as well as those with COPD.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Prince MJ, Wu F, Guo Y, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385(9967):549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 2.Beran D, Zar HJ, Perrin C, Menezes AM, Burney P. Burden of asthma and chronic obstructive pulmonary disease and access to essential medicines in low-income and middle-income countries. Lancet Respir Med. 2015;3(2):159–170. doi: 10.1016/S2213-2600(15)00004-1. [DOI] [PubMed] [Google Scholar]
- 3.Rogucka E, Bielicki T, Welon Z, Medras M, Susanne C. Variation in bone mineral density in adults in Poland: age and sex differences. Ann Hum Biol. 2000;27(2):139–148. doi: 10.1080/030144600282253. [DOI] [PubMed] [Google Scholar]
- 4.Svejme O, Ahlborg HG, Nilsson JA, Karlsson MK. Low BMD is an independent predictor of fracture and early menopause of mortality in post-menopausal women: a 34-year prospective study. Maturitas. 2013;74(4):341–345. doi: 10.1016/j.maturitas.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55(5):769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 6.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Won CW, Kim BS, Choi HR, Moon MY. The association between the low muscle mass and osteoporosis in elderly Korean people. J Korean Med Sci. 2014;29(7):995–1000. doi: 10.3346/jkms.2014.29.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues AM, Roncada C, Santos G, et al. Clinical characteristics of children and adolescents with severe therapy-resistant asthma in Brazil. J Bras Pneumol. 2015;41(4):343–350. doi: 10.1590/S1806-37132015000004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Looker AC. Relationship between femur neck bone mineral density and prevalent chronic obstructive pulmonary disease (COPD) or COPD mortality in older non-Hispanic white adults from NHANES III. Osteoporos Int. 2014;25(3):1043–1052. doi: 10.1007/s00198-013-2601-5. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe R, Tanaka T, Aita K, et al. Osteoporosis is highly prevalent in Japanese males with chronic obstructive pulmonary disease and is associated with deteriorated pulmonary function. J Bone Miner Metab. 2015;33(4):392–400. doi: 10.1007/s00774-014-0605-7. [DOI] [PubMed] [Google Scholar]
- 11.Pereira FB, Leite AF, de Paula AP. Relationship between pre-sarcopenia, sarcopenia and bone mineral density in elderly men. Arch Endocrinol Metab. 2015;59(1):59–65. doi: 10.1590/2359-3997000000011. [DOI] [PubMed] [Google Scholar]
- 12.Verschueren S, Gielen E, O’Neill TW, et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos Int. 2013;24(1):87–98. doi: 10.1007/s00198-012-2057-z. [DOI] [PubMed] [Google Scholar]
- 13.Lee DW, Choi EY. Sarcopenia as an independent risk factor for decreased BMD in COPD patients: Korean National Health and Nutrition Examination Surveys IV and V (2008–2011) PloS One. 2016;11(10):e0164303. doi: 10.1371/journal.pone.0164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orimo H, Hayashi Y, Fukunaga M, et al. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19(6):331–337. doi: 10.1007/s007740170001. [DOI] [PubMed] [Google Scholar]
- 15.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4(6):368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 16.Bijlsma AY, Meskers CG, Ling CH, et al. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr) 2013;35(3):871–881. doi: 10.1007/s11357-012-9384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heymsfield SB, Smith R, Aulet M, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52(2):214–218. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 19.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Gea J, Agustí A, Roca J. Pathophysiology of muscle dysfunction in COPD. J Appl Physiol (1985) 2013;114(9):1222–1234. doi: 10.1152/japplphysiol.00981.2012. [DOI] [PubMed] [Google Scholar]
- 21.Agustí A. Systemic effects of chronic obstructive pulmonary disease: what we know and what we don’t know (but should) Proc Am Thorac Soc. 2007;4(7):522–525. doi: 10.1513/pats.200701-004FM. [DOI] [PubMed] [Google Scholar]
- 22.Liang B, Feng Y. The association of low bone mineral density with systemic inflammation in clinically stable COPD. Endocrine. 2012;42(1):190–195. doi: 10.1007/s12020-011-9583-x. [DOI] [PubMed] [Google Scholar]
- 23.Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab. 2013;20(1):1–10. doi: 10.11005/jbm.2013.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietschmann P, Mechtcheriakova D, Meshcheryakova A, Föger-Samwald U, Ellinger I. Immunology of osteoporosis: a mini-review. Gerontology. 2016;62(2):128–137. doi: 10.1159/000431091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu JJ, McDonald VM, Gibson PG, Simpson JL. Systemic inflammation in older adults with asthma-COPD overlap syndrome. Allergy Asthma Immunol Res. 2014;6(4):316–324. doi: 10.4168/aair.2014.6.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reginster JY, Beaudart C, Buckinx F, Bruyere O. Osteoporosis and sarcopenia: two diseases or one? Curr Opin Clin Nutr Metab Care. 2016;19(1):31–36. doi: 10.1097/MCO.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soen S. Glucocorticoid and bone. Clin Calcium. 2014;24(6):829–836. Japanese. [PubMed] [Google Scholar]
- 28.Hirani V, Blyth F, Naganathan V, et al. Sarcopenia is associated with incident disability, institutionalization, and mortality in community-dwelling older men: the Concord Health and Ageing in Men project. J Am Med Dir Assoc. 2015;16(7):607–613. doi: 10.1016/j.jamda.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Beaudart C, Reginster JY, Petermans J, et al. Quality of life and physical components linked to sarcopenia: the SarcoPhAge study. Exp Gerontol. 2015;69:103–110. doi: 10.1016/j.exger.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Andersen H, Lampela P, Nevanlinna A, Saynajakangas O, Keistinen T. High hospital burden in overlap syndrome of asthma and COPD. Clin Respir J. 2013;7(4):342–346. doi: 10.1111/crj.12013. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen M, Bårnes CB, Ulrik CS. Clinical characteristics of the asthma-COPD overlap syndrome: a systematic review. Int J Chron Obstruct Pulmon Dis. 2015;10:1443–1454. doi: 10.2147/COPD.S85363. [DOI] [PMC free article] [PubMed] [Google Scholar]