Abstract
Background
The Spanish Guidelines for COPD (GesEPOC) describe four clinical phenotypes: non-exacerbator (NE), asthma-COPD overlap syndrome (ACO), frequent exacerbator with emphysema (EE), and exacerbator with chronic bronchitis (ECB). The objective of this study was to determine the frequency of COPD phenotypes, their clinical characteristics, and the availability of diagnostic tools to classify COPD phenotypes in clinical practice.
Materials and methods
This study was an epidemiological, cross-sectional, and multi-centered study. Patients ≥40 years old with a post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity ratio of <0.7 and who were smokers or former smokers (with at least 10 pack-years) were included. The availability of diagnostic tools to classify COPD phenotypes was assessed by an ad hoc questionnaire.
Results
A total of 647 patients (294 primary care [PC], 353 pulmonology centers) were included. Most patients were male (80.8%), with a mean age (SD) of 68.2 (9.2) years, mean post-bronchodilator FEV1 was 53.2% (18.9%) and they suffered a mean of 2.2 (2.1) exacerbations in the last year. NE was the most frequent phenotype (47.5%) found, followed by ECB (29.1%), EE (17.0%), and ACO (6.5%). Significant differences between the four phenotypes were found regarding age; sex; body mass index; FEV1; body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE)/body mass index, airflow obstruction, dyspnea and exacerbations (BODEx) index; modified Medical Research Council dyspnea scale; respiratory symptoms; comorbidi-ties; hospitalizations; and exacerbations in the last year. Physicians considered that >80% of the diagnostic tools needed to classify COPD phenotypes were available, with the exception of computed tomography (26.9%) and carbon monoxide transfer test (13.5%) in PC, and sputum eosinophilia count in PC and pulmonology centers (40.4% and 49.4%, respectively).
Conclusion
In Spanish clinical practice, almost half of the patients with COPD presented with NE phenotype. The prevalence of ACO according to the Spanish consensus definition was very low. In general, physicians indicated that they had the necessary tools for diagnosing COPD phenotypes.
Keywords: chronic obstructive pulmonary disease, phenotype, diagnosis, emphysema, chronic bronchitis, ACO
Introduction
COPD is a complex disease with significant heterogeneity in clinical presentation, physiology, response to therapy, and prognosis. In order to approach this heterogeneity, an attempt has been made to group patients with similar characteristics that could be associated with a differential clinical outcome by using the term clinical phenotype.1,2 Clear examples of COPD phenotypes associated with different outcomes have been described, such as the frequent exacerbator3 or the overlap COPD and asthma phenotypes.4
The Spanish guidelines for the treatment of COPD (Guía Española de la EPOC, GesEPOC) propose four different COPD phenotypes: NE, ACO, EE, and ECB.5 This approach has been adopted after the consensus of the different stakeholders in the health care management of COPD patients, namely respiratory medicine specialists, primary care and internal medicine physicians, as well as specialized nurses and physiotherapists – by their respective scientific societies – and supported by the health care authorities. However, the distribution of these phenotypes is largely unknown in a clinical practice setting.
The main objectives of this study were to determine the distribution of COPD phenotypes according to the Spanish COPD Guidelines in clinical practice in PC and pulmonology centers, to explore differences in terms of their demographic and clinical characteristics, and to evaluate the availability of diagnostic tools for the classification of COPD phenotypes.
Materials and methods
Study design and organization
This was an observational, cross-sectional, and multicentered study conducted from November 2012 to December 2013.
The sample population included patients who attended PC and pulmonology centers in Spain. Recruitment was prospective, whereby the first six patients with a diagnosis of COPD, who attended the clinic for a checkup and met all the inclusion criteria, were consecutively included. The clinical data (at the study visit and from the medical history during the previous year) and available resources from the center were collected.
This study was approved by the Ethics Committee of Hospital Clínic i Provincial de Barcelona, Spain. All participants provided written informed consent.
The study was carried out with the collaboration of a Clinical Research Organization (TFS, Barcelona, Spain), which monitored the local physicians. Telephone monitoring was carried out in 10% of the participating physicians in order to guarantee the scientific and methodological rigor of the study.
Centers that participated and patient selection
A total of 193 physicians (104 from PC and 89 from pulmonology centers) from centers all over the regions of Spain (except La Rioja region, which represents 0.68% of the total population in Spain) participated in this study. They were selected based on their previous experience in COPD research and their interest in this study. The list of investigators participated in this study is provided in Supplementary material.
Patients who met the following criteria were included in this study: ≥40 years of age, smoker or ex-smoker (at least 10 pack-years), clinically stable COPD (at least 1 month following recovery from the last exacerbation), and a post-bronchodilator forced spirometry FEV1/FVC ratio <0.7 or a pre-bronchodilator FEV1/FVC ratio <0.7 and FEV1 <80% (when a post-bronchodilator test was not available). Patients with other chronic respiratory diseases (eg, cystic fibrosis, severe bronchiectasis, cancer, and restrictive lung disease), inability to complete quality of life questionnaires, or participating in a clinical trial were excluded.
Variables
Each physician completed a questionnaire on the availability of diagnostic tools necessary to correctly classify clinical COPD phenotypes in their clinical practice.
Sociodemographic characteristics and clinical COPD data from patients were collected. The degree of dyspnea was assessed according to the m-MRC6 and the COPD severity level was measured using the BODEx index7 or the BODE capacity index,8 depending on the availability of the methods. Comorbidities were assessed by a COPD comorbidity index (COTE).9 The level of physical activity was assessed by the self-reported daily walking time as previously described.10 Patients also completed the CDLM questionnaire11 and CAT.12
Clinical COPD phenotypes were classified according to the GesEPOC criteria.13 The following algorithm was used to determine phenotype: 1) patients with 0 or 1 exacerbation in the previous year were classified as NE; 2) patients who experienced at least two exacerbations in the previous year and clinical/radiological or functional evidence of emphysema were classified as EE; 3) exacerbators with cough and expectoration for 3 months of the year over two consecutive years were classified as ECB; and 4) patients who met two major criteria or one major and two minor criteria as defined later in the text were considered as ACO. Major criteria were previous history of asthma, sputum eosinophilia, and bronchodilator response to salbutamol higher than 15% and 400 mL. Minor criteria were high total IgE, history of atopy, two separated bronchodilator responses to salbutamol higher than 12% and 200 mL, and blood eosinophils >5%. Since the Spanish criteria were considered very restrictive,14–16 we also used the ACO definition proposed in the COPD History Assessment in Spain (CHAIN) cohort;16 major criteria were previous history of asthma, bronchodilator response to salbutamol higher than 15% and 400 mL, and minor criteria were IgE >100 U, history of atopy, two separated bronchodilator responses to salbutamol higher than 12% and 200 mL, and blood eosinophils >5%. To be diagnosed with ACO, a patient must fulfill one major or two minor criteria. Characteristics of patients classified as having ACO by both set of criteria were compared.
Exacerbation was defined as an acute increase in respiratory symptoms that require treatment with antibiotics and/or systemic corticosteroids or treatment in a hospital setting.
Statistical analysis
A descriptive analysis for the total sample and by clinical setting (PC and pulmonology centers) according to the COPD phenotype was performed.
Values were expressed as mean and standard deviation for continuous variables and absolute and relative frequencies (percentages) for categorical variables. Frequencies of COPD phenotypes were described by percentages and their 95% confidence interval (95% CIs). A χ2 test or Fisher’s exact test was used to compare categorical variables. Parametric tests (Student’s t-test or ANOVA) were used to compare values with a normal distribution and non-parametric tests (Mann–Whitney U test or Kruskal–Wallis test) for data without a normal distribution.
Statistical significance for all tests was defined as P<0.05 bilateral. The statistical software package SAS® version 9.2 (SAS Institute, Cary, NC, USA) was used for all analyses.
Results
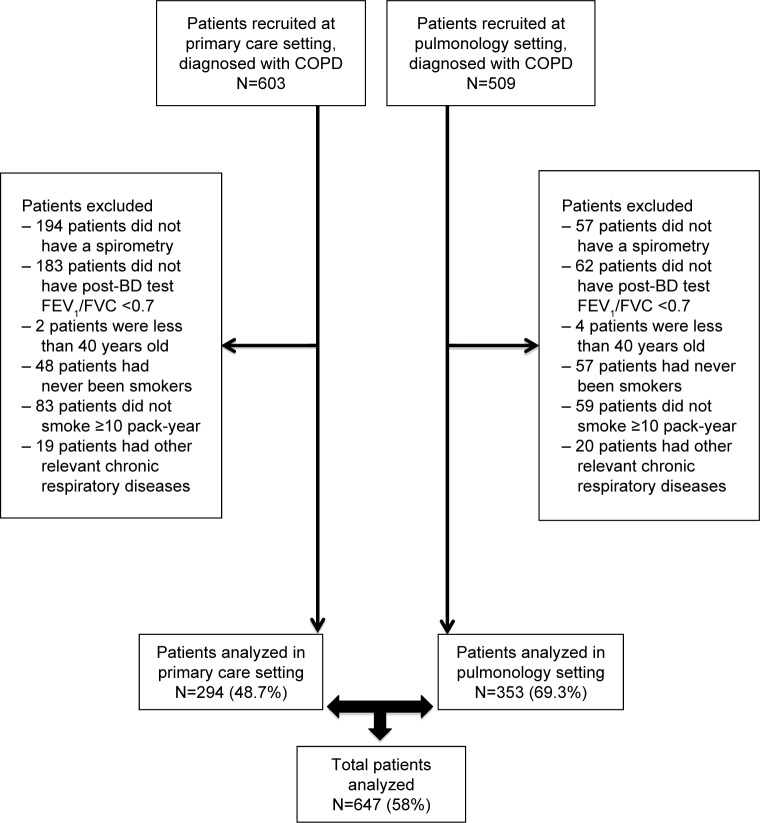
A total of 1,114 patients with COPD were selected, of whom 647 (58.1%) met all the inclusion criteria. STROBE flow diagram of patient recruitment according to PC or pulmonology settings is shown in Figure 1.
Figure 1.
STROBE flow diagram of patient recruitment according to primary care or pulmonology settings.
Abbreviations: FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; post-BD, post-bronchodilator.
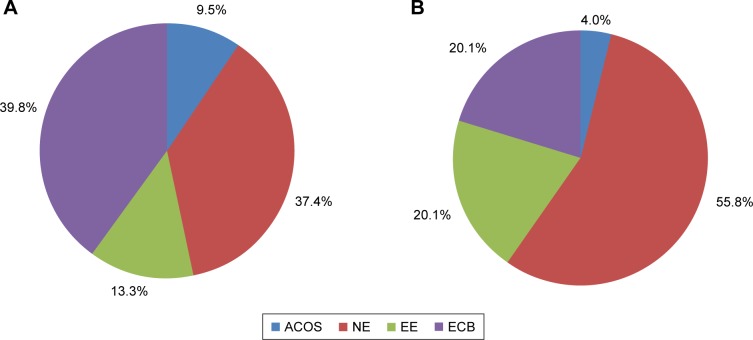
Distribution of clinical phenotypes and its demographic and clinical characteristics
In the overall population, patients were predominantly classified as NE (47.5%) or ECB (29.1%). The distribution of clinical phenotypes according to recruitment centers (PC or pulmonology centers) is shown in Figure 2. The most frequent phenotype in PC was ECB (39.8%) and in pulmonology was NE (55.8%). Demographic and clinical characteristics by clinical phenotypes are displayed in Table 1. Statistically significant differences regarding age, sex, and BMI within the four phenotypes were observed. The main differences observed were that all phenotypes showed a higher percentage of males except those with ACO, which presented a similar proportion of both sexes. Moreover, patients with the ACO phenotype were significantly younger than those with the other phenotypes. Regarding clinical characteristics, significant differences in FEV1, BODE/BODEx index, m-MRC scale, respiratory symptoms, comorbidities, hospitalizations, and exacerbations in previous year were also observed among phenotypes. In particular, patients with ACO and NE phenotypes presented more preserved lung function compared to those with ECB and EE phenotypes. Furthermore, BODE/BODEx index and m-MRC scale also showed significantly lower scores (indicating lower severity) in the ACO and NE phenotypes compared to ECB and EE phenotypes. In contrast, COTE index was higher in ACO patients (more frequent anxiety, depression, gastric ulcers). CAT scores were worse among exacerbators, particularly among ECB.
Figure 2.
Distribution of clinical COPD phenotypes by care settings. (A) Primary care and (B) pulmonology settings.
Abbreviations: ACO, asthma-COPD overlap syndrome; ECB, exacerbator with chronic bronchitis; EE, exacerbator with emphysema; NE, non-exacerbator.
Table 1.
Baseline characteristics according to the clinical COPD phenotype
| Characteristics | ACO (n=42) | ECB (n=188) | EE (n=110) | NE (n=307) | Total (n=647) |
|---|---|---|---|---|---|
| Sex (male)*, n (%) | 21 (50.0) | 157 (83.5) | 90 (81.8) | 255 (83.1) | 523 (80.8) |
| Age (years)*, mean (SD) | 64.2 (9.0) | 69.5 (8.6) | 70.0 (9.1) | 67.2 (9.3) | 68.2 (9.2) |
| BMI (kg/m2)*, mean (SD) | 28.0 (5.3) | 28.3 (4.5) | 26.1 (4.5) | 27.2 (4.3) | 27.4 (4.5) |
| Pack-year, mean (SD) | 39.4 (17.7) | 42.8 (21.2) | 48.5 (25.5) | 42.9 (23.6) | 43.6 (23.0) |
| Dyspnea (m-MRC scale)*, mean (SD) | 1.8 (0.8) | 2.1 (0.8) | 2.2 (1.0) | 1.5 (0.8) | 1.8 (0.9) |
| Dyspnea scale (m-MRC scale)*, n (%) | |||||
| ≤1 | 16 (38.1) | 40 (21.5) | 27 (24.8) | 150 (49.5) | 233 (36.4) |
| ≥2 | 26 (61.9) | 146 (78.5) | 82 (75.2) | 153 (50.5) | 407 (63.6) |
| Respiratory symptoms, n (%) | |||||
| Dyspnea on exertion* | 37 (88.1) | 172 (91.5) | 106 (96.4) | 255 (83.1) | 570 (88.1) |
| Daily expectorations* | 29 (69.1) | 171 (91.0) | 47 (42.7) | 148 (48.2) | 395 (61.1) |
| Wheezing* | 30 (71.4) | 95 (50.5) | 47 (42.7) | 75 (24.4) | 247 (38.2) |
| Chronic cough* | 36 (85.7) | 176 (93.6) | 82 (74.6) | 193 (62.9) | 487 (75.3) |
| Post-bronchodilator spirometry, mean (SD) | |||||
| FEV1 (mL)* | 1,748.0 (679.8) | 1,475.0 (503.6) | 1,338.4 (544.1) | 1,574.1 (599.3) | 1,516.5 (577.7) |
| FEV1 (%)* | 61.5 (28.1) | 54.8 (21.0) | 47.9 (16.4) | 53.0 (16.2) | 53.2 (18.9) |
| Exacerbations in previous year*, mean (SD) | 3.2 (2.5) | 3.6 (1.7) | 3.7 (1.9) | 0.7 (0.7) | 2.2 (2.1) |
| Hospitalizations in previous year*, mean (SD) | 0.5 (0.8) | 0.8 (0.9) | 0.8 (1.2) | 0.1 (0.3) | 0.5 (0.8) |
| BODE index*, mean (SD) | 2.6 (1.8) | 4.0 (2.3) | 4.1 (2.7) | 2.7 (1.9) | 3.3 (2.3) |
| BODEx index*, mean (SD) | 3.2 (1.7) | 4.0 (1.9) | 4.5 (2.3) | 2.4 (1.6) | 3.3 (2.0) |
| COTE index*, mean (SD) | 2.2 (3.2) | 1.6 (2.2) | 1.1 (1.7) | 1 (1.7) | 1.3 (2.0) |
| Comorbidities, n (%) | |||||
| Anxiety* | 17 (40.5) | 72 (38.3) | 37 (33.6) | 64 (20.9) | 190 (29.4) |
| Depression* | 17 (40.5) | 50 (26.6) | 28 (25.5) | 51 (16.6) | 146 (22.6) |
| Diabetes without neuropathy* | 15 (35.7) | 57 (30.3) | 27 (24.6) | 56 (18.2) | 155 (24.0) |
| Gastric ulcers* | 13 (31.0) | 25 (13.3) | 16 (14.6) | 30 (9.8) | 84 (13.0) |
| Coronary artery disease* | 2 (4.8) | 23 (12.2) | 21 (19.1) | 31 (10.1) | 77 (11.9) |
| Sleep apnea* | 13 (31.0) | 35 (18.6) | 12 (10.9) | 38 (12.4) | 98 (15.2) |
| CAT score*, mean (SD) | 16.6 (8.4) | 21.9 (7.7) | 18.8 (7.6) | 13.3 (7.1) | 16.9 (8.3) |
| CDLM score*, mean (SD) | 1.4 (0.5) | 1.3 (0.5) | 1.3 (0.5) | 1.2 (0.3) | 1.3 (0.4) |
Note:
P-values <0.05.
Abbreviations: ACO, asthma-COPD overlap syndrome; BMI, body mass index; BODE, body mass index, airflow obstruction, dyspnea, and exacerbations index; BODEx, body mass index, airflow obstruction, dyspnea, and exercise capacity index; CAT, COPD assessment test; CDLM, Capacity of Living during the Morning questionnaire; COTE, COPD specific comorbidity test; ECB, exacerbator with chronic bronchitis; EE, exacerbator with emphysema; m-MRC, modified Medical Research Council dyspnea scale; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEV1 (%), percentage of predicted FEV1; FVC (%), percentage of predicted FVC; NE, non-exacerbator; SD, standard deviation.
Characteristics of patients according to the type of centers (primary care vs pulmonology)
No significant demographic differences were observed between patients with regard to age, sex, and BMI according to the recruitment centers (PC or pulmonology centers); however, patients in PC centers were significantly more frequent active smokers. Regarding the clinical characteristics, patients in pulmonology centers had more severe impairment in FEV1 (%) and more dyspnea on exertion, but patients who attended PC had more respiratory symptoms and exacerbations in previous year and greater impact on their quality of life as evaluated by CAT and CDLM (Table 2).
Table 2.
Clinical characteristics of patients at both settings
| Characteristics | PC N=294 |
Pulm N=353 |
P-value |
|---|---|---|---|
| Sex, % men | 77.9 | 83.3 | 0.08 |
| Age, mean (SD) | 68.5 (9.2) | 67.9 (9.1) | 0.40 |
| BMI kg/m2, mean (SD) | 27.7 (4.3) | 27.1 (4.7) | 0.09 |
| Current smoker, % | 36.0 | 20.4 | <0.0001 |
| Dyspnea on exertion, % | 84.7 | 90.9 | 0.02 |
| Sputum production, % | 72.1 | 51.8 | <0.0001 |
| Wheezing, % | 48.3 | 29.7 | <0.0001 |
| Chronic cough, % | 85.3 | 66.8 | <0.0001 |
| FEV1 postbr, mL, mean (SD) | 1,637 (588) | 1,416 (551) | <0.0001 |
| FEV1 postbr, %, mean (SD) | 56.4 (20.7) | 50.5 (16.8) | <0.0001 |
| BODEx index, mean (SD) | 3.2 (1.9) | 3.4 (2.0) | 0.19 |
| COTE index, mean (SD) | 1.4 (2.2) | 1.2 (1.9) | 0.15 |
| Mod/Sev Exac prev year, mean (SD) | 2.2 (1.8) | 1.6 (1.8) | <0.0001 |
| CAT score, mean (SD) | 19.5 (8.3) | 14.9 (7.8) | <0.0001 |
| CDLM score, mean (SD) | 1.3 (0.5) | 1.2 (0.4) | <0.001 |
| LABA + LAMA (free or fixed dose combination), % | 25.6 | 25.9 | 1 |
| LABA + LAMA + ICS (free combination), % | 42.9 | 52.4 | 0.0177 |
Note: Categorical variables: Fisher’s exact test, two-tailed P-value; continuous variables: Student’s t-test.
Abbreviations: BMI, body mass index; BODEx, body mass index, airflow obstruction, dyspnea, and exercise capacity index; CAT, COPD assessment test; CDLM, Capacity of Living during the Morning questionnaire; COTE, COPD specific comorbidity test; ICS, inhaled corticosteroids; LABA, long-acting beta-agonist; LAMA, long-acting antimuscarinic; m-MRC, modified Medical Research Council dyspnea scale; FEV1, forced expiratory volume in 1 s; FEV1 (%), percentage of predicted FEV1; PC, primary care; Pulm, pulmonology; SD, standard deviation; postbr, post-bronchodilator; Mod/Sev Exac prev, moderate/severe exacerbations previous year.
Impact of different criteria for ACS
According to the CHAIN classification of ACO,16 the proportion of patients with an ACO phenotype increased significantly from 6.5% to 15.2%, particularly in PC where it increased from 4% to 12.8% compared with an increase from 9.5% to 18% in the pulmonology center (Figure 3). No significant demographic or clinical differences between patients diagnosed with either of the two classifications were observed (Table 3).
Figure 3.
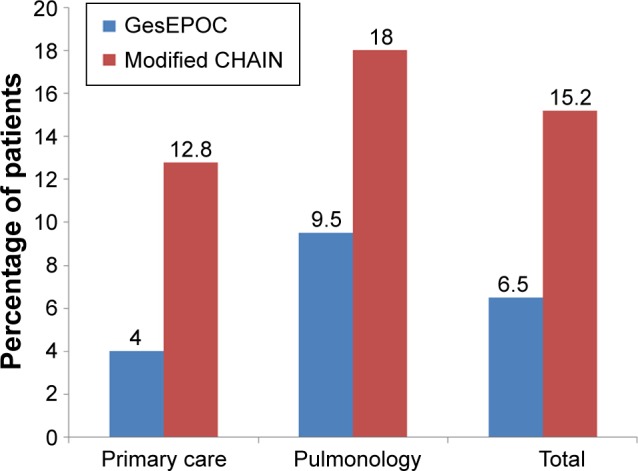
Distribution of ACO patients according to GesEPOC and modified GesEPOC (CHAIN) criteria.
Abbreviations: ACO, asthma-COPD overlap syndrome; CHAIN, COPD History Assessment in Spain; ECB, exacerbator with chronic bronchitis; EE, exacerbator with emphysema; NE, non-exacerbator.
Table 3.
Characteristics of ACO patients according to GesEPOC and modified CHAIN criteria
| Characteristics | Modified CHAIN (n=98) |
GesEPOC (n=42) |
|---|---|---|
| Sex (male), n (%) | 63 (64.3) | 21 (50.0) |
| Age (years), mean (SD) | 66.8 (9.8) | 64.2 (9.0) |
| Pack-year, mean (SD) | 39.9 (23.6) | 39.4 (17.7) |
| Dyspnea scale (m-MRC scale), mean (SD) | 1.8 (0.8) | 1.8 (0.8) |
| Post-bronchodilator spirometry, mean (SD) | ||
| FEV1 (mL) | 1,650.6 (613.0) | 1,748.0 (679.8) |
| FEV1 (%) | 58.7 (23.7) | 61.5 (28.1) |
| Hospitalizations in previous year, mean (SD) | 0.4 (0.7) | 0.5 (0.8) |
| Exacerbations in previous year, mean (SD) | 2.8 (2.5) | 3.2 (2.5) |
| BODE index, mean (SD) | 2.8 (1.9) | 2.6 (1.8) |
| BODEx index, mean (SD) | 3.1 (2.0) | 3.2 (1.7) |
| COTE index, mean (SD) | 1.8 (2.9) | 2.2 (3.2) |
| CAT score, mean (SD) | 17.9 (9.2) | 16.6 (8.5) |
| CDLM score, mean (SD) | 1.4 (0.6) | 1.4 (0.6) |
Abbreviations: BODE, body mass index, airflow obstruction, dyspnea, and exacerbations index; BODEx, body mass index, airflow obstruction, dyspnea, and exercise capacity; CAT, COPD assessment test; CHAIN, COPD History Assessment in Spain; CDLM, Capacity of daily living during the morning; COTE, COPD specific comorbidity test; m-MRC, modified Medical Research Council dyspnea scale; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; FEV1 (%), percentage of predicted FEV1; FVC (%), percentage of predicted FVC; SD, standard deviation.
Availability of diagnostic tools to classify COPD phenotypes
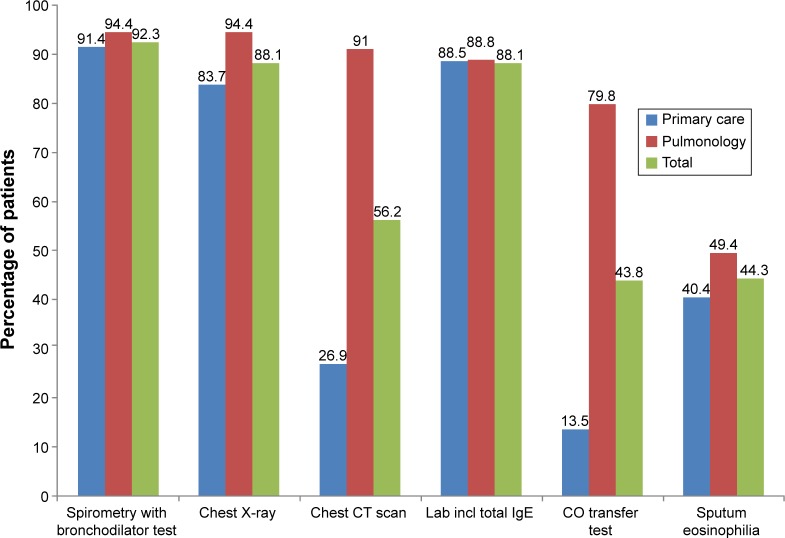
Physicians reported that over 80% of the diagnostic tools needed to classify COPD phenotypes were available. Exceptions in PC centers were computed tomography (only in 26.9%) and carbon monoxide transfer test (only in 13.5%). Sputum eosinophilia count was only available in 40.4% PC and 49.4% pulmonology centers (Figure 4).
Figure 4.
Availability of COPD diagnostic tools in clinical practice.
Abbreviations: IgE, immunoglobulin E; CT, computed tomography; CO, carbon monoxide.
Therapeutic management
Almost all patients (99.2%) received COPD maintenance treatment. The most frequent combined treatment was triple therapy: LABA/LAMA/ICS (44.2%), followed by LABA/zAMA (23.6%). Therapeutic management analyzed by clinical phenotype showed LABA/LAMA/ICS as the most common treatment for all phenotypes except for NE phenotype, in which LABA/LAMA combination was more frequently used (31.9%) (Table 4).
Table 4.
COPD treatments according to the clinical phenotype (total population)
| Treatment, n (%) | ACO (n=42) | ECB (n=188) | EE (n=110) | NE (n=307) | Total (n=647) |
|---|---|---|---|---|---|
| LABA (only monotherapy) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 11 (3.6) | 12 (1.9) |
| LAMA (only monotherapy) | 2 (4.8) | 2 (1.1) | 1 (0.9) | 26 (8.5) | 31 (4.8) |
| SABA (only monotherapy) | 0 (0.0) | 1 (0.5) | 1 (0.9) | 1 (0.3) | 3 (0.5) |
| SABA + SAMA | 0 (0.0) | 2 (1.1) | 1 (0.9) | 4 (1.3) | 7 (1.1) |
| LABA + LAMA (free or fixed dose combination) | 2 (4.8) | 33 (17.6) | 20 (18.2) | 98 (31.9) | 153 (23.6) |
| LABA + LAMA + ICS (free combination) | 25 (59.5) | 109 (58.0) | 64 (58.2) | 88 (28.7) | 286 (44.2) |
| LABA + ICS (free or fixed dose combination) | 9 (21.4) | 8 (4.3) | 10 (9.1) | 19 (6.2) | 46 (7.1) |
| LAMA + ICS (free combination) | 0 (0.0) | 13 (6.9) | 6 (5.5) | 9 (2.9) | 28 (4.3) |
| Other treatments# | 4 (9.5) | 17 (9.0) | 7 (6.4) | 48 (15.6) | 76 (11.7) |
| No treatment | 0 (0.0) | 2 (1.2) | 0 (0.0) | 3 (1.0) | 5 (0.8) |
Note:
Other treatments comprised combinations of Roflumilast, theophylline, systemic corticosteroids, antibiotics and/or mucolytics.
Abbreviations: ACO, asthma-COPD overlap syndrome; ECB, exacerbator with chronic bronchitis; EE, exacerbator with emphysema; ICS, inhaled corticosteroids; LABA, long-acting beta-2 agonists; LAMA, long-acting antimuscarinic agents; NE, no exacerbator; SABA, short-acting beta-2 agonists; SAMA, short-acting antimuscarinic agents.
Discussion
Our results have shown that the most frequent phenotype of COPD patients in Spain is the NE phenotype, with some differences related to health care settings, followed by the ECB phenotype. The ACO phenotype is the least frequent, accounting for less than 10% in PC and only 4% in pulmonology centers, although its prevalence is heavily influenced by the criteria used. Findings of this study indicate that most sites that manage patients with COPD have the tools needed to characterize COPD phenotypes according to GesEPOC;5 therefore, most patients included in this study could be appropriately classified by phenotype.
There are differences in pharmacologic treatment of COPD among the various phenotypes, which reflect the implementation of a somehow personalized treatment in clinical practice. In fact, a recent audit of patients with COPD attended in outpatient clinics in Spain found that 46.3% had their phenotype recorded in the clinical records.17
The main strength of this study lies in the fact that it was conducted on a national scale in both primary care and specialized care settings, providing us with interesting information on the available health care resources that will help characterize COPD in both settings. It also offers a good snapshot of the proportion of COPD phenotypes and their clinical characteristics. In addition, the application of a standardized protocol in data collection has helped us avoid enrolling cases whose COPD status was unconfirmed, as shown in Figure 1. In this study, 22.5% of the selected patients did not undergo a spirometry test (32.1% in PC and 11.1% in pulmonology centers) and 21% of the patients did not show airflow obstruction in the spirometry test (30.3% in PC and 12.1% in pulmonology centers). The incorrect diagnosis of COPD and the limited use of spirometry testing in the assessment of respiratory symptoms are similar to that reported by prior studies conducted in Spain, where <50% of the individuals with a COPD diagnosis in primary care had performed a spirometry test18,19 or where COPD was incorrectly diagnosed in 31% and 14% of patients seen in primary care or specialized sites, respectively.20 Nevertheless, there are some methodological considerations that should be borne in mind regarding the sites that participated in this study. Their selection was not random but based on their previous experience in COPD studies and their interest in this study; furthermore, included patients were not randomly selected. Despite these limitations, we believe that the selected sample is representative of what happens in Spain in both of the care settings, as it included a large sample of subjects from 16 of the 17 sites in the country.
Regarding the resources used in the characterization of clinical phenotypes, the most significant result is probably their high availability, which indicates that characterization is feasible in clinical practice. Nevertheless, it should be noted that in primary care center, access to a lung diffusion test and computed tomography scan was limited, which could negatively affect the characterization of EE, and similarly there was little access in both health care settings to sputum eosinophilia count, which is a diagnostic criterion for the ACO phenotype. Similarly, a recent study from Turkey has indicated that obstructive airway diseases can be easily characterized in routine clinical practice based on clinical, radiological, and pulmonary function tests, and only 6% of patients were classified as having undifferentiated obstruction.21
Regarding the phenotype frequency, the most common type of phenotype was found to be NE, followed by ECB, and only 6.5% to be ACO phenotype. Phenotype distribution was similar to that found in other studies, where phenotype NE was also found to be the most common, followed by ECB.22–25 A previous study performed in Spain in a population of 3,125 patients with COPD from primary and secondary care found 60% to be NE, with 19% ECB and 16% ACO.24 More recently, a study on 831 patients all from secondary care found a similar distribution with 66% NE, 12% ECB, and 15% ACO.25 Interestingly, this distribution of phenotypes is not particular to Spain, as a large study on 3,366 COPD patients from Central and Eastern Europe found 64% to be NE, 20.4% ECB, and 7% ACO.22 However, when the distribution of clinical phenotypes by clinical setting was analyzed in our study, it was found that ECB phenotype was most commonly diagnosed in PC center (39.8%), whereas NE phenotype in pulmonology center (55.8%). Similar results were obtained in the CHAIN cohort analysis, which showed that 66% of the patients visiting pulmonology sites were of NE phenotype.25 Interestingly, there was a lower percentage of exacerbators among patients recruited at pulmonology centers, despite having a more severe airflow obstruction. This could be explained in part by a more intense therapy in patients followed in pulmonology centers.
With respect to the ACO phenotype, it is important to recall that although this is a well-known clinical phenotype, there is no agreement as to how to perform the diagnosis and, therefore, its prevalence may vary according to the diagnostic criteria used.4 The diagnosis of this phenotype in the GesEPOC guidelines was based on a set of major and minor criteria.13 According to these criteria, the prevalence of the ACO phenotype was very low in both health care settings, showing a result that was similar to that obtained in other studies, with prevalences ranging between 5% and 13%.24–27 Nevertheless, when less restrictive criteria were used in diagnosis, ie, the ones we termed modified CHAIN criteria,16 prevalence increased to 15.2% of the total sample. This prevalence is in accordance to the values reported in other studies where the diagnosis of ACO was performed using broader criteria, which included having a history of bronchial asthma before the age of 40 years, presence or a history of atopy, and positive reversibility test.14,28,29 In order to improve the diagnosis of ACO phenotype, the Spanish guidelines for asthma and COPD have published a new simplified consensus.30
Regarding the demographic and clinical characteristics, it is important to note that patients with an exacerbator phenotype were older than those with NE phenotype and showed more respiratory symptoms with a greater impact on their quality of life as evaluated by the CAT and more severe disease as determined by the BODE and BODEx indices. These data are similar to those found in the analysis of the ECLIPSE cohort31 and the COPDGene Study.32 In our study, we found very small clinical differences among exacerbator phenotypes, except for EE, which showed a lower BMI, higher smoking exposure, and greater degree of airflow obstruction. With respect to patients with the ACO phenotype, it is important to note that these patients were younger, less exposed to tobacco, and more likely to be female, compared to other phenotypes. In addition, ACO patients were less severe than other phenotypes, both in terms of BODE index and degree of obstruction. However, this group showed more respiratory symptoms (wheezing), a higher impact on their quality of life as measured by CDLM and CAT, and a greater rate of exacerbations. Moreover, they showed a greater frequency of comorbidities, such as gastric ulcers, anxiety, and depression. The demographic and clinical characteristics of the ACO phenotype are similar to those found in other studies;14,16,26,28,29 these characteristics were not modified according to the criteria used in the diagnosis, ie, either GesEPOC criteria or modified CHAIN criteria.
The majority of the patients received inhaled maintenance treatment, with the most common option being triple therapy (LABA/LAMA/ICS), followed by dual bronchodilation (LABA/LAMA). Triple therapy was more frequently used in pulmonology sites compared to PC center, while the use of LABA/LAMA combinations was similar in both settings. Interestingly, triple therapy was the first choice of treatment in all phenotypes, except for the NE phenotype patients, who received mostly dual bronchodilation (LABA/LAMA). It is important to note that a vast majority of patients with an ACO phenotype were being treated with ICS (associated with one or two bronchodilators) and only a few with single or dual bronchodilation. However, there was an excessive use of ICS in patients with NE phenotype and those with no ACO phenotype. These data concur with a recent audit of specialized clinics in Spain17 and suggest that personalized treatment according to phenotype as proposed by GesEPOC5 is becoming a reality in clinical practice.
Conclusion
To conclude, our results show that most diagnostic tools required to classify COPD phenotypes are available in clinical practice, even in PC center, allowing classification into clinical phenotypes for the majority of patients. Approximately half of the COPD patients visiting both health care settings had NE phenotype, which is the most common phenotype found among COPD patients. The prevalence of the ACO phenotype varies greatly according to the diagnostic criteria employed; however, this did not result in a change in clinical characteristics. Along with recent findings, this information should be borne in mind when updating diagnostic criteria for the ACO phenotype. The distribution of COPD treatments shows that individualized treatment related to patient phenotype is a reality in clinical practice in Spain and that its impact on results in the medium and long terms should be evaluated in future studies.
Supplementary material
Investigators of the FENEPOC study group (in alphabetical order)
Aganzo López FJ, Agüero Balbín R, Aguilo Rovira A, Alonso Mendieta V, Álvarez Martínez CJ, Álvarez Sala Walther JL, Amaro Cendon J, Amato Sotos T, Apilánez Tomás J, Aragón Fierro AJ, Arcalá Campillo E, Argemí Coletas TM, Arnedillo Muñoz A, Arnes Acevedo FJ, Arroyo Masa M, Artero Canals F, Barrueco Ferrero M, Bautista Ojeda C, Belda Díaz S, Bengoa Dolón M, Berchid S, BereciartuIbargutxi F, Beristain Urquiza AM, Bernado Bou P, Blanco Díaz MJ, Borrás Martínez P, Bravo Olalla AT, Bueno Cortés AL, Bujalance Zafra J, Calle Rubio M, Campos Pascual J, Carasol Ferrer M, Carboneros de la Fuente F, Cardeñosa López R, Carracedo Sevillano M, Carrero Reyes F, Carrizo Sierra S, Castellanos Narvaez I, Celdrán Gil J, Cervera del Pino M, Chacón Patiño ML, Chávez Plasencia JA, Codina Trenzano S, Colomer Escuder MT, Cordero Rodríguez PJ, Cordovilla Pérez R, Crespo García JR, Culebras Amigo M, Cumplido Pérez A, de Pablo Cillero F, Delgado Bregel JL, Diago Palacios J, Domenech Irles A, Donado Uña JR, Durán Dotras FJ, Egea Santaolalla C, Espíldora Hernández F, Esteban C, Esteban Calvo RM, Ferrando García, D Ferreiro Álvarez MJ, Ferrer Taylor G, Flores Segovia J, Fuster Gomila A, Galache de Dios J, Galera Martínez R, García Ibarra HD, García Martín I, García Molne A, García Rivero JL, García-Cosio FB, Gavela García JE, Gil Carbonell J, Gómez de Terreros FJ, Gómez Punter RM, Gómez Villa A, González Barcala FJ, González Fernández JM, González Fernández M, González García MI, González Orodea JI, Guallar Ballester J, Guardiola Parellada J, Guardiola Pérez JC, Guerrero Toledo T, Hernández Díaz JA, Hernández Méndez AI, Herrero Hernández JL, Hidalgo Campos I, Hidalgo Requena A, Huerta García A, Iglesias Río F, Iriberri Pascual M, Jiménez Díaz JJ, Jiménez López J, Laborda González A, López Torres JA, Lorenzo Rodríguez JL, Lores MV, Malo de Molina Ruíz R, Marcos Rodríguez PJ, Marín Arroyo M, Martín-Delgado Rodríguez JL, Martín Pérez MA, Martínez Bretones R, Martínez Carbonell JA, Martínez Jalvo JL, Martínez A, Martínez Palacios J, Martínez Pardo R, Martínez Rivera C, Martínez Uceda R, Masa Sánchez J, Mata Calderón P, Mateos Caballero L, Mayoralas Alises S, Medina Cruz MV, Mena Rodríguez MJ, Menéndez Rodríguez JJ, Merino Muñoz M, Monge García JM, MoscardóOrenes MA, Mosquera Viéitez JC, Muñoz Cabrera L, Muñoz Rino F, Navarro Fernández F, Navarro Iváñez R, Navarro Vázquez L, Nistal Rodríguez AJ, Oliván Roldán F, Pascual Pape T, Pastor Antón J, Pastor Polo A, Pastor Rull M, Pavón Freire J, Pedrosa Freire VM, Pellicer Ciscar C, Pérez Izquierdo J, Pérez Pallares J, Pérez Pérez P, Pinto González P, Ponce Lorenzo F, Prados Gonzalo H, Puente Maestu L, Quiles Catalá MD, Quintano Jiménez JA, Rabell Santacana V, Ramírez Felipe L, Rebollo Hernández E, Rey Suárez S, Riesco Miranda JA, Robles Fernández MA, Rodríguez Alarios MA, Rodríguez Anta I, Rodríguez Glez-Moro JM, Rodríguez González J, Rodríguez Hermosa JL, Rodríguez Jiménez E, Rodríguez Salgueiro J, Román Rodríguez M, Romero Caballero G, Romero Requena JM, Romero Sanz VF, Rosselló Ramonell B, RozadillaSacanell JR, Rubio Villasol L, Ruíz Ortega F, Saez Alonso Muñumer, M Saez Pérez JM, Salamanca Sánchez-Escalonilla MT, Sampol Company J, Sánchez López F, Sánchez Mera JA, Santonja Vilaplana J, Saneleuterio Brines R, Serra Batlles J, Serrano Martín de Eugenio R, Sesma Aisa F, Sibila Vidal O, Solano Reina S, Soler David V, Tallón Toledano P, Teruel Alto J, Tirado Moliner JM, Toledano Galdeano JJ, Torrecillas FJ, Torregrosa Suau O, Valero López IJ, Valverde Forcada E, Vázquez Cacheiro J, Verdaguer Miralles JM, Vicente Hernández MA, Vidal de Mesa C, Vilá Giralte X, Zorita Viota Sánchez JI.
Acknowledgments
This study was sponsored by the laboratory NOVARTIS Farmacéutica, S.A. The authors would like to thank the investigators and the centers that participated in the FENEPOC study.
Abbreviations
- PC
primary care
- ACO
asthma-COPD overlap syndrome
- ECB
exacerbator with chronic bronchitis
- EE
exacerbator with emphysema
- NE
non-exacerbator
- BMI
body mass index
- LAMA
long-acting antimuscarinic agents
- LABA
long-acting beta-2 agonists
- ICS
inhaled corticosteroids
- m-MRC
modified Medical Research Council dyspnea scale
- BODEx
body mass index, airflow obstruction, dyspnea, and exacerbations index
- BODE
body mass index, airflow obstruction, dyspnea, and exercise capacity
- COTE
COPD specific comorbidity test
- CDLM questionnaire
Capacity of Daily Living during the Morning questionnaire
- CAT
COPD assessment test
- IgE
immunoglobulin E
Footnotes
Disclosure
All authors meet the International Committee for Medical Journal Editors criteria for authorship. Myriam Calle has received speaker fees from Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, Menarini, and Novartis. Marc Miravitlles has received speaker fees from Boehringer Ingelheim, AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, Teva, Grifols, and Novartis, and consulting fees from Bayer Schering, Boehringer Ingelheim, GlaxoSmithKline, Gebro Pharma, CLS Behring, Cipla, MediImmune, Mereo Biopharma, Teva, Novartis, and Grifols. Ricard Casamor is a full-time employee of Novartis Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
- 1.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miravitlles M, Calle M, Soler-Cataluña JJ. Clinical phenotypes of COPD: identification, definition and implications for guidelines. Arch Bronconeumol. 2012;48(3):86–98. doi: 10.1016/j.arbres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Hurst JR, Vestbo J, Anzueto A, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 4.Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21(1):74–79. doi: 10.1097/MCP.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 5.Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish COPD guidelines (GesEPOC) 2017. Pharmacological treatment of stable chronic obstructive pulmonary disease. Arch Bronconeumol. 2017;53(6):324–335. doi: 10.1016/j.arbres.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soler-Cataluña JJ, Martínez-García MA, Sánchez LS, Tordera MP, Sánchez PR. Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med. 2009;103(5):692–699. doi: 10.1016/j.rmed.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 9.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]
- 10.Miravitlles M, Cantoni J, Naberan K. Factors associated with a low level of physical activity in patients with chronic obstructive pulmonary disease. Lung. 2014;192(2):259–265. doi: 10.1007/s00408-014-9557-x. [DOI] [PubMed] [Google Scholar]
- 11.Partridge MR, Miravitlles M, Ståhl E, Karlsson N, Svensson K, Welte T. Development and validation of the Capacity of Daily Living during the Morning questionnaire and the Global Chest Symptoms Questionnaire in COPD. Eur Respir J. 2010;36(1):96–104. doi: 10.1183/09031936.00123709. [DOI] [PubMed] [Google Scholar]
- 12.Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 13.Soler-Cataluña JJ, Cosío B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012;48(9):331–337. doi: 10.1016/j.arbres.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Barrecheguren M, Román-Rodríguez M, Miravitlles M. Is a previous diagnosis of asthma a reliable criterion for asthma-COPD overlap syndrome in a patient with COPD? Int J Chron Obstruct Pulmon Dis. 2015;10:1745–1752. doi: 10.2147/COPD.S87025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golpe R, Sanjuán López P, Cano Jiménez E, Castro Añón O, Pérez de Llano LA. Distribution of clinical phenotypes in patients with chronic obstructive pulmonary disease caused by biomass and tobacco smoke. Arch Bronconeumol. 2014;50(8):318–324. doi: 10.1016/j.arbres.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Cosio BG, Soriano JB, López-Campos JL, et al. CHAIN Study Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest. 2016;149(1):45–52. doi: 10.1378/chest.15-1055. [DOI] [PubMed] [Google Scholar]
- 17.Calle Rubio M, Alcázar Navarrete B, Soriano JB, On behalf of the EPOCONSUL Study Clinical audit of COPD in outpatient respiratory clinics in Spain: the EPOCONSUL study. Int J Chron Obstruct Pulmon Dis. 2017;12:417–426. doi: 10.2147/COPD.S124482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Miguel Díez J, Izquierdo Alonso JL, Molina París J, Rodríguez González-Moro JM, de Lucas Ramos P, Alonso-Vega G. Fiabilidad del diagnóstico de la EPOC en atención primaria y neumología en España [Drug treatment of chronic obstructive pulmonary disease on two levels of patient care: degree of compliance with recommended protocols]. Factores predictivos. Arch Bronconeumol. 2003;39:203–208. doi: 10.1016/s0300-2896(03)75362-9. Spanish. [DOI] [PubMed] [Google Scholar]
- 19.Miravitlles M, de la Roza C, Naberan K, Lamban M, Gobartt E, Martín A. Use of spirometry and patterns of prescribing in COPD in primary care. Respir Med. 2007;101(8):1753–1760. doi: 10.1016/j.rmed.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Izquierdo Alonso JL, Rodríguez González-Moro JM, de Lucas Ramos P, Martín Centeno A, Gobartt Vázquez E. ¿Ha cambiado el manejo de la EPOC en España? Resultados de un estudio multicéntrico comu-nitario (VICE) [Has the treatment of COPD changed in Spain? Results of a community multicenter study (VICE)] Rev Clin Esp. 2008;208(1):18–25. doi: 10.1157/13115003. Spanish. [DOI] [PubMed] [Google Scholar]
- 21.Ozkaya S, Diricam A, Tuna T. The objective evaluation of obstructive pulmonary diseases with spirometry. Int J Chron Obstruct Pulmon Dis. 2016;11:2009–2015. doi: 10.2147/COPD.S113774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koblizek V, Milenkovic B, Barczyk A, et al. Phenotypes of COPD patients with a smoking history in Central and Eastern Europe: the POPE Study. Eur Respir J. 2017;49(5) doi: 10.1183/13993003.01446-2016. pii:1601446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miravitlles M, Huerta A, Fernández-Villar JA, et al. Generic utilities in chronic obstructive pulmonary disease patients stratified according to different staging systems. Health Qual Life Outcomes. 2014;12:120. doi: 10.1186/s12955-014-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miravitlles M, Barrecheguren M, Román-Rodríguez M. Frequency and characteristics of different clinical phenotypes of chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2015;19(8):992–998. doi: 10.5588/ijtld.15.0021. [DOI] [PubMed] [Google Scholar]
- 25.Cosio BG, Soriano JB, López-Campos JL, et al. Distribution and outcomes of a phenotype-based approach to guide COPD management: results from the CHAIN cohort. PLos One. 2016;11(9):e01607702016. doi: 10.1371/journal.pone.0160770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izquierdo-Alonso JL, Rodríguez-Gonzálezmoro JM, de Lucas-Ramos P, et al. Prevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD) Respir Med. 2013;107(5):724–731. doi: 10.1016/j.rmed.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Miravitlles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107(7):1053–1060. doi: 10.1016/j.rmed.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Menezes AM, de OcaM Montes, Pérez-Padilla R, PLATINO Team Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145:297–304. doi: 10.1378/chest.13-0622. [DOI] [PubMed] [Google Scholar]
- 30.Miravitlles M, Alvarez-Gutierrez F, Calle M, et al. Algorithm for identification of ACO: consensus between the Spanish COPD and asthma guidelines. Eur Respir J. 2017;49(5) doi: 10.1183/13993003.00068-2017. pii:1700068. [DOI] [PubMed] [Google Scholar]
- 31.Rennard SI, Locantore N, Delafont B, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints. Identification of five chronic obstructive pulmonary disease subgroups with different prognoses in the ECLIPSE cohort using cluster analysis. Ann Am Thorac Soc. 2015;12(3):303–312. doi: 10.1513/AnnalsATS.201403-125OC. [DOI] [PubMed] [Google Scholar]
- 32.Kim V, Han MK, Vance GB, et al. COPDGene Investigators The chronic bronchitic phenotype of COPD: analysis of the COPDGene Study. Chest. 2011;140(3):626–633. doi: 10.1378/chest.10-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]