Abstract
Anthrax caused by Bacillus anthracis represents a major bioterroristic threat. B. anthracis produces lethal toxin (LeTx), a combination of lethal factor (LF) and protective antigen that plays a major role in anthrax pathogenesis. We demonstrate that human neutrophil α-defensins are potent inhibitors of LF. The inhibition of LF by human neutrophil protein (HNP-1) was noncompetitive. HNP-1 inhibited cleavage of a mitogen-activated protein kinase kinase and restored impaired mitogen-activated protein kinase signaling in LeTx-treated macrophages. HNP-1 rescued murine macrophages from B. anthracis-induced cytotoxicity, and in vivo treatment with HNP-1-3 protected mice against the fatal consequences of LeTx.
Keywords: defensin, innate immunity, Bacillus anthracis
Bacillus anthracis is a Gram-positive, spore-forming, rod-shaped bacterium (1) secreting three toxin proteins: lethal factor (LF), protective antigen (PA), and edema factor (EF). LF is a metalloprotease that cleaves distinct mitogen-activated protein kinase (MAPK) kinases (MKKs) (2), EF is a calmodulin- and Ca2+-dependent adenylate cyclase (3), and PA is a transporter of the two factors into host cells by receptor-mediated endocytosis (4). Individually, none of these proteins is toxic. However, the combination of LF and PA, called lethal toxin (LeTx), and that of EF and PA, called edema toxin (EdTx), are highly toxic to mammalian hosts (5). Inactivation of the LF gene in B. anthracis reduces virulence by >1,000-fold, suggesting that anthrax pathology is largely determined by LF (6). Thus, LF represents a prime target for the rational design of therapeutic agents against anthrax.
Neutrophils are the first cells recruited to sites of infection. Once recruited to an inflammatory site, neutrophils serve as professional phagocytes, which rapidly engulf and kill microorganisms by oxygen-dependent or -independent mechanisms. Neutrophil antimicrobial peptides such as defensins and cathelicidins contribute as oxygen-independent bactericidal effectors (7).
Defensins are a small cationic peptide family characterized by their β-sheet-dominant structure and three disulfide bridges (8). Among the three subfamilies, α-, β-, and θ-defensins, human α-defensins represent small cationic peptides composed of 29-35 aa. Of the six identified human α-defensins, four subtypes, human neutrophil proteins 1-4 (HNP-1-4), are expressed primarily by granulocytes (9) and certain lymphocyte populations (10). HNP-1-3 have identical amino acid sequences except for the first N-terminal residue. They are the most abundant azurophilic granule peptides, constituting 30-50% of the granule proteins (11). Notably, mouse neutrophils lack homologs of HNP-1-3 (12). It is well known that HNP-1-3 act as natural peptide antibiotics, displaying microbicidal activity against numerous bacteria, fungi, and viruses (13). Here we describe a function of HNP-1-3, namely neutralizing activity against LF, the major toxin of B. anthracis.
Materials and Methods
Synthetic Peptides and Recombinant Proteins. Synthetic HNP-1 and HNP-2 were obtained from Bachem. For the mouse experiment, HNP-1-3 were purified from human buffy coats (Deutsches Rotes Kreuz, Berlin) (14). Synthetic LL-37 was generously provided by Hubert Kalbacher (University of Tübingen, Tü-bingen, Germany). Recombinant LF and PA were purchased from Calbiochem or purified from recombinant B. anthracis strains kindly provided by Stephen H. Leppla (National Institute of Allergy and Infectious Diseases, Bethesda) (15, 16).
Spore Experiments. B. anthracis (Sterne) spores were prepared as described (17). RAW 264.7 cells were seeded in 96-well plates at a density of 4 × 104 cells per well in RPMI medium 1640 containing serum without antibiotics. For the assay, 2 × 105 spores per well and the described amounts (see Fig. 1a) of HNP-1 were added to the cells in serum-free RPMI medium 1640. Eight hours after infection, cytotoxicity was determined by a Cyto Tox 96 cytotoxicity assay (Promega). For the in vitro killing assay, 2 × 105 spores in serum-free RPMI medium 1640 were incubated in the presence or absence of 1 μM HNP-1. At each time point, colony-forming units (CFUs) were determined.
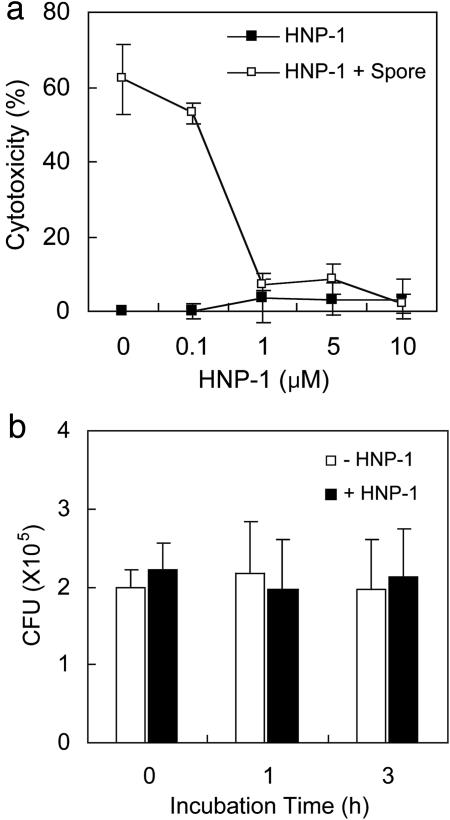
Fig. 1.
HNP-1 protects macrophages against B. anthracis-induced cell death. (a) RAW 264.7 cells were infected with B. anthracis spores and then treated with the indicated amounts of HNP-1. Cytotoxicity was determined by measuring released lactate dehydrogenase levels. (b)An in vitro killing assay was performed against spores in the presence or absence of 1 μM HNP-1. After the indicated incubation times, colony-forming units (CFU) were determined.
Cytotoxicity Assay. One day before the assay, RAW 264.7 cells were seeded in a 96-well plate at a density of 3 × 104 cells per well in RPMI medium 1640 containing serum. For the assay, 400 ng/ml LF, 1,600 ng/ml PA, and the described amounts (see Fig. 2) of HNPs were added simultaneously to cells in serum-free RPMI medium 1640 or RPMI medium 1640 supplemented with 5% FCS. Five hours after treatment, cell viability was determined by methyl thiazole tetrazolium (MTT) assay.
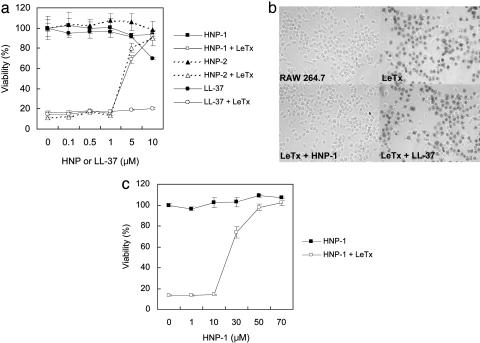
Fig. 2.
Human α-defensins protect macrophages against cytolysis by anthrax LeTx. (a) RAW 264.7 cells were treated with LeTx (400 ng/ml LF and 1,600 ng/ml PA) in the presence of the indicated amounts of HNP-1, HNP-2, or LL-37. Cell viability was determined by methyl thiazole tetrazolium (MTT) assay. (b) RAW 264.7 cells were treated with LeTx (400 ng/ml LF and 1,600 ng/ml PA) in the presence of 7 μM HNP-1 or LL-37. Five hours after treatment, cells were stained with trypan blue. (c) Viability of RAW 264.7 cells was determined by MTT assay after treatment with LeTx (400 ng/ml LF and 1,600 ng/ml PA) and various concentrations of HNP-1. This assay was performed in RPMI medium 1640 supplemented with 5% FCS.
In Vitro MKK3b Cleavage Assay. 35S-labeled MKK3b was in vitro translated from pcDNA-MKK3b [with the kind help of Jiahuai Han (The Scripps Research Institute, La Jolla, CA)] by using TNT Quick Coupled Transcription/Translation Systems (Promega). In vitro translated MKK3b was incubated at 37°C for 1 h in reaction buffer (20 mM Hepes and 1 mM CaCl2 at pH 7.2) with the indicated amounts (see Fig. 4a) of LF and either HNP-1 or magainin I.
Fig. 4.
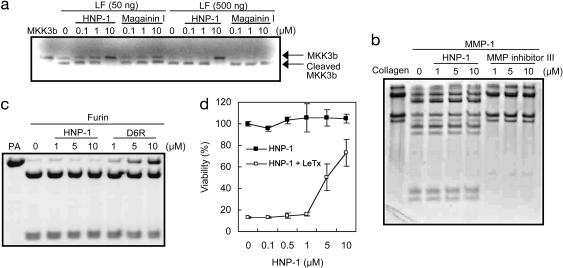
HNP-1 is an inhibitor of LF. (a) In vitro translated MKK3b was incubated for 1 h with the indicated amounts of LF and either HNP-1 or magainin I. Cleavage of MKK3b was analyzed by SDS/PAGE and autoradiography. (b) Collagen was incubated with MMP-1 in the presence of the indicated amounts of HNP-1 or MMP inhibitor. (c) PA was incubated with furin in the presence of HNP-1 or hexa-d-arginine (D6R). (d) RAW 264.7 cells were incubated with HNP-1 at 37°C. After 1 h, the medium was removed and replaced with fresh medium containing LeTx (400 ng/ml LF and 1,600 ng/ml PA). Cells were incubated further at 37°C for 5 h. Viability was determined by MTT assay.
Matrix Metalloproteinase Type 1 (MMP-1) and Furin Assay. For the MMP-1 inhibition assay, 10 μg of native collagen (Type I, Sigma) was incubated at room temperature for 1 h with 100 ng of MMP-1 (Sigma) in the presence of the described amount of HNP-1 or MMP Inhibitor III (C19H29N3O4, Calbiochem). The reaction was performed in 30 μl of reaction buffer consisting of 50 mM Hepes, 200 mM NaCl, 10 mM CaCl2, and 0.05% Brij 35 and was analyzed by 7% SDS/PAGE.
In the furin inhibition assay, PA (10 μg) was incubated in a volume of 30 μl with recombinant furin (New England Biolabs) in 50 mM Hepes, 200 mM NaCl, 10 mM CaCl2, and 0.05% Brij 35. The indicated amounts (see Fig. 4c) of HNP-1 or furin inhibitor II (Calbiochem) were added to the reaction.
Kinetic Characterization. Chromogenic substrate was synthesized (Jerini Peptide Technologies, Berlin) as described (18). To determine the IC50 value and inhibition type, we measured initial enzyme rates. To ensure initial kinetics, proteolysis was followed only 5% toward completion. For IC50 value determination, 10 nM LF was preincubated with 0-10 μM HNP-1 for 30 min at room temperature, and the reaction was started by adding substrate to reach a final concentration of 100 μM. A competition assay was performed without preincubation of LF or HNP-1. To examine the effect of DTT, HNP-1 was treated with 20 mM DTT at room temperature for 1 h and then dialyzed against 20 mM Hepes and 1 mM CaCl2 by using a 1-kDa cutoff dialysis membrane. LF (10 nM) was preincubated with DTT-treated or untreated HNP-1 at room temperature for 15 min, and the relative activity was measured by monitoring p-Nitroanilin release from the substrate. In all kinetic experiments, the reaction buffer was 20 mM Hepes/1 mM CaCl2.
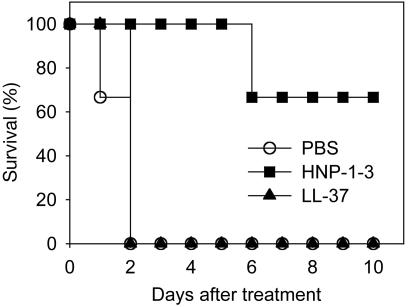
Mouse Protection Experiments. Seven- to 8-week-old female BALB/c mice were treated with i.v. injection of LeTx (50 μg of LF and 50 μg of PA in 0.2 ml of PBS) into one tail vein, immediately followed by i.v. injection with the indicated doses (see Fig. 6) of purified HNP-1-3 or synthetic LL-37 diluted in 0.2 ml of PBS into the other tail vein. The survival of mice was monitored for 10 days after toxin treatment. Experiments were conducted according to the German animal protection law.
Fig. 6.
HNP-1-3 protects BALB/c mice from LeTx intoxication. Mice were injected with 50 μg of LF plus 50 μg of PA per mouse through one tail vein and then were immediately injected with PBS, 500 μg of purified HNP-1-3, or 500 μg of LL-37 through the other tail vein. Three animals per group were used for this experiment. In another set of experiments, administration of 500 μg of HNP-1-3 achieved 100% protection (data not shown).
Results
HNP-1 Rescues Macrophages from Anthrax Spore-Induced Cell Death. It has been shown that a toxin-producing B. anthracis strain Sterne kills murine macrophages (19, 20). To determine whether HNP-1 protects macrophages from toxin-producing B. anthracis, we examined the cytotoxicity of anthrax spores in HNP-1-treated RAW 264.7 cells. As shown in Fig. 1a, as little as 1 μM HNP-1 inhibited the anthrax spore-induced cytotoxicity almost completely. Surprisingly, this protective effect was independent of HNP-1's well known microbicidal activity, because HNP-1 at this low concentration (1 μM) did not show any significant sporicidal effect (Fig. 1b).
HNP-1-3 Protect Macrophages from LeTx-Mediated Cytolysis. Because B. anthracis Sterne-mediated macrophage cytotoxicity is largely determined by LeTx, we examined whether HNP-1-HNP-3 have any effect on this toxin. When RAW 264.7 cells were treated with LeTx, they succumbed to the toxin within a few hours. In marked contrast, the addition of HNP-1 completely abolished cytotoxicity (Fig. 2 a and b). This protection was observed even 24 h after LeTx and HNP-1 cotreatment (data not shown). HNP-2 (Fig. 2a) and a purified HNP-1-3 mixture (data not shown) from human leukocytes (14) showed similar protection, whereas LL-37, another neutrophil cationic peptide with a size and a net charge similar to that of HNP-1-3, did not display any significant effect (Fig. 2 a and b).
To examine whether this phenomenon is physiologically relevant and to assess potential effects of serum components, we performed the same assay under serum-supplemented conditions. In the presence of 5% FCS, HNP-1 still protected cells from LeTx-induced cytotoxicity, although a higher amount of HNP-1 was needed (Fig. 2c).
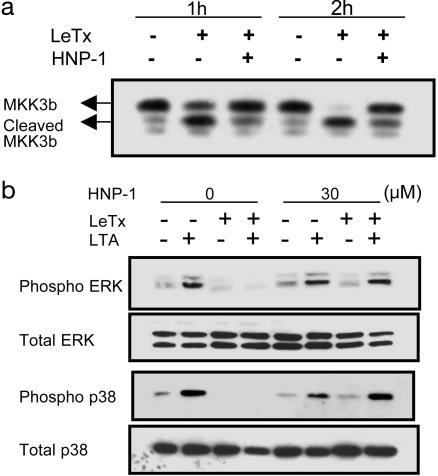
HNP-1 Inhibits Cleavage of MKK3b and Restores Impaired MAPK Signaling in LeTx-Treated Macrophages. Because LF is a protease cleaving the N terminus of MKKs, we investigated whether HNP-1 inhibited cleavage of MKK3b in LeTx-treated cells. RAW 264.7 macrophages were treated with LeTx and HNP-1 for 1 or 2 h, and the cell lysates were analyzed by using an antibody directed against the C terminus of MKK3. Within 2 h of LeTx treatment, MKK3b was almost completely converted to its cleaved form, but this cleavage was efficiently inhibited by HNP-1 (Fig. 3a).
Fig. 3.
HNP-1 inhibits proteolysis of MKK in LeTx-treated macrophages. (a) LeTx was added to RAW 264.7 macrophages with (+) or without (-) HNP-1. At the indicated time points, cell lysates were prepared and assessed by Western blotting with an anti-MKK3 antibody. (b) RAW 264.7 cells were treated (+) with LeTx (200 ng/ml LF and 1,600 ng/ml PA) and HNP-1 (30 μM). Two hours after treatment, cells were stimulated with 10 μg/ml B. subtilis lipoteichoic acid (LTA) for 30 min, and the lysates were assessed by immunoblotting with antibodies against MAPKs (Total) and their phosphorylated forms (Phospho). ERK, extracellular signal-regulated kinase.
LeTx inhibits extracellular signal-regulated kinase (ERK) and p38 MAPK signaling through cleavage of MKK family members in activated macrophages (21). To characterize the effects of HNP-1 on LeTx-mediated impairment of MAPK signaling, macrophages were incubated with LeTx and HNP-1 for 2 h, followed by stimulation with Bacillus subtilis lipoteichoic acid (LTA). This experiment was performed under 5% FCS-supplemented conditions to achieve the efficient stimulation of Toll-like receptors by LTA. LeTx strongly inhibited LTA-induced ERK and p38 activation in macrophages, and phosphorylation of these two MAPKs was restored by HNP-1 (Fig. 3b).
HNP-1 Is an Inhibitor of LF. To verify whether HNP-1 directly inhibits the endoprotease activity of LF, we performed an in vitro cleavage assay with 35S-labeled LF substrate (Fig. 4a). In vitro translated MKK3b was almost completely cleaved within 1 h by 500 ng of LF. In contrast, in the presence of 10 μM HNP-1, proteolysis was efficiently inhibited, suggesting that HNP-1 inactivates the catalytic activity of LF. Other cationic antimicrobial peptides, magainin I (Fig. 4a) and LL-37 (data not shown), did not prevent cleavage of MKK3b by LF.
To determine the specificity of HNP-1 against LF, we examined its effects on other enzymes. Because LF is a metalloprotease, first we assessed whether HNP-1 showed inhibitory effects on host MMP-1. As shown in Fig. 4b, in the range between 1 and 10 μM, HNP-1 did not affect MMP-1 activity. It has been shown that polyarginine-containing peptides represent potent inhibitors of furin (22), which is involved in activation of anthrax toxin (23). Because cationic characteristics of HNP-1 are mainly determined by arginine residues in its amino acid sequence, we asked whether HNP-1 also behaves as a furin inhibitor. Consistent with the previous study, hexa-d-arginine (D6R) showed dose-dependent inhibition against PA cleavage by furin. In contrast, HNP-1 failed to inhibit furin activity at the same range of concentration (Fig. 4c).
Potential LF inhibitors would be expected to enter cells to exert their activity against LF, and HNP-1-3 can, indeed, be internalized into host cells (24). Given the described effects of HNP-1 on LeTx, we investigated whether HNP-1 can inhibit LF inside cells. RAW 264.7 cells were incubated with HNP-1 at 37°C for 1 h, washed extensively to remove free HNP-1, and subsequently treated with LeTx at 37°C for 5 h. As shown in Fig. 4d, treatment of macrophages with HNP-1 prevented LeTx toxicity in an HNP-1 dose-dependent manner, indicating that HNP-1 acts on LF inside cells.
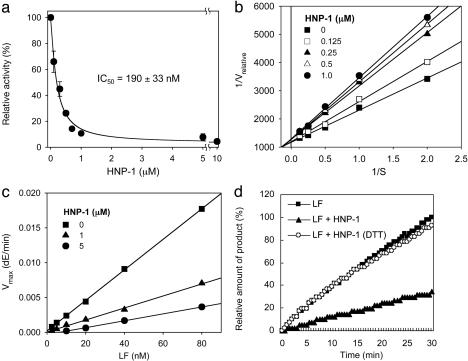
HNP-1 Behaves as a Reversible Noncompetitive Inhibitor of LF. To elucidate the mode of inhibition, we characterized the kinetics of inhibition by using a chromogenic peptide substrate (18). The initial rates of enzyme reaction in the presence of various concentrations of HNP-1 provided an IC50 value of 190 ± 33 nM (Fig. 5a). In the competition assay (Fig. 5b), HNP-1 acted noncompetitively. When Vmax versus [E] (where [E] represents concentration of enzyme) was plotted, HNP-1 was identified as a reversible noncompetitive inhibitor, showing smaller slopes than the control curve lacking HNP-1 (Fig. 5c). Reversibility testing that used ultrafiltration confirmed that the inhibition was reversible (data now shown).
Fig. 5.
Characterization of LF inhibition by HNP-1. (a) HNP-1 inhibited 50% of LF activity at a concentration of 190 ± 33 nM. (b) Lineweaver-Burk plot indicates noncompetitive inhibition mode. (c) A plotting of Vmax versus concentrations of LF confirms that HNP-1 is a reversible noncompetitive inhibitor. dE, release of p-Nitroaniline. (d) DTT-treated HNP-1 did not show any significant effect on LF.
Because HNP-1-3 have three intramolecular disulfide bridges, we examined whether these disulfide bonds are critical for inhibition. As shown in Fig. 5d, DTT-treated HNP-1 did not show any significant inhibition, suggesting that intact disulfide linkages are essential for its inhibitor activity against LF.
HNP-1-3 Protect Mice Against LeTx Intoxication. Having identified LeTx neutralization as a biological function of HNP-1-3, we decided to exploit this activity for therapeutic intervention against anthrax attack. To this end, LeTx-sensitive BALB/cmice received LeTx i.v., immediately followed by the indicated amounts of purified HNP-1-3 i.v. (Fig. 6). Within 2 days, mice succumbed to the toxin. In striking contrast, 500 μg of HNP-1-3 protected mice from the intoxication of LeTx. In contrast, LL-37, a control antimicrobial peptide, had no effect on LeTx toxicity (Fig. 6).
Discussion
Our data reveal a biological function of HNP-1-3, namely neutralization of a secreted bacterial enzyme. Although it is undoubted that HNP-1-3 express antimicrobial activities in identified locations, such as within phagosomes of human neutrophils, where bacteria are exposed to high concentrations (mg/ml) of HNP-1-3 (25), their direct microbicidal effect in other extracellular locations is not clear because of their poor microbicidal activity in the presence of serum or physiological salt concentration (8, 26). We show that HNP-1-3 act as inhibitors of anthrax LF under physiological conditions. Supporting our finding, we recently identified several other secreted bacterial enzymes that are also inhibited by HNP-1-3 (C.K. and S.H.E.K., unpublished data). Many bacterial pathogens secrete enzymes into the host environment to avoid destruction by the immune system. Because recruited human neutrophils release abundant HNP-1-3 locally in the infection regions, we assume that HNP-1-3 achieve efficient neutralization of bacterial enzymes in vivo.
HNP-1 contains three intramolecular disulfide linkages to maintain its stable and compact conformation. It has been shown that the cyclic analogs of HNP-1 with different S—S pairings exhibit antimicrobial activity, whereas linear HNP-1 is inactive (27). Thus, it is of interest to address whether these S—S bonds are essential for the function of HNP-1 as a LF inhibitor. Our data suggest that the unique structure of HNP-1 determined by disulfide bonds is a critical requirement for inhibition, because reduced HNP-1 did not show anti-LF activity. In addition, kinetic analysis revealed that HNP-1 binds to a region remote from the active site of LF and causes a conformational change in the active center, preventing the enzyme from converting the bound substrate to its product.
The concentration of HNP-1-3 in plasma under normal physiological conditions ranges around 40 ng/ml but can rise to 0.9-170 μg/ml during severe infections (28). The local concentrations of HNP-1-3 at infection sites are expected to be much higher. In the view of our findings that HNP-1-3 are potent inhibitors of LF, it remains unclear why inhalation anthrax often causes fatal consequences in humans, even in the presence of profound natural inhibitors of LF. It is possible that the endogenous concentrations of HNP-1-3 remain below the threshold required for neutralization of the lethal effect of LeTx during infection, particularly after inhalations of high doses of B. anthracis. It is also possible that B. anthracis suppresses secretion of HNP-1-3 by human leukocytes via unknown mechanisms.
In either case, our data not only reveal that the human immune system produces potent inhibitors for LF, but they also demonstrate the potential of HNP-1-3 for immunotherapy of anthrax. Although B. anthracis itself can be treated by antibiotics, this treatment frequently fails when not initiated promptly after infection, because, even after bacterial eradication, secreted toxins remain active. These obstacles underscore the need for novel intervention strategies against anthrax. Indeed, new strategies have been exploited, based on recent progress in understanding the mechanisms of anthrax toxin such as recombinant antibodies against the toxin (29), peptide (30, 31), or small chemical inhibitors (18, 32) of LF, and polyvalent inhibitors of PA-LF interactions (33). HNP-1-3 could have several therapeutic advantages over other candidates. HNP-1-3 are multifunctional peptides. Besides their well established capacity to kill a variety of microbial pathogens, immunomodulating activities have also been described for HNP-1-3. Human neutrophil α-defensins show chemotactic activities for T cells and dendritic cells (34), and they enhance the production of antigen-specific antibodies and certain cytokines by immune cells facilitating the initiation of adaptive immune responses (35). Hence, LeTx neutralization by HNP-1-3, in combination with antibiotic eradication of B. anthracis, should be exploited for efficient prevention of fatal anthrax incidences.
Acknowledgments
We thank A. Zychlinsky for critical review of the manuscript, Dr. S. H. Leppla for the generous gift of recombinant B. anthracis strains, Dr. H. Kalbacher for LL-37, and Dr. J. Han for the MKK3b plasmid. This work was supported in part by the Anthrax-Euronet coordination program of the European Commission Sixth Framework Programme.
Author contributions: C.K., N.G., H.-W.M., M. Weiwad, Y.-H.S., and S.H.E.K. designed research; C.K., H.-W.M., Y.-H.S., and R.H. performed research; M. Wilmanns contributed new reagents/analytic tools; C.K., M. Weiwad, G.F., and S.H.E.K. analyzed data; and C.K. and S.H.E.K. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: LF, lethal factor; PA, protective antigen; MAPK, mitogen-activated protein kinase; MKK, MAPK kinase; LeTx, lethal toxin; HNP, human neutrophil protein; MMP-1, matrix metalloproteinase type 1.
References
- 1.Turnbull, P. C. (1999) J. Appl. Microbiol. 87, 237-240. [DOI] [PubMed] [Google Scholar]
- 2.Duesbery, N. S., Webb, C. P., Leppla, S. H., Gordon, V. M., Klimpel, K. R., Copeland, T. D., Ahn, N. G., Oskarsson, M. K., Fukasawa, K., Paull, K. D. & Vande Woude, G. F. (1998) Science 280, 734-737. [DOI] [PubMed] [Google Scholar]
- 3.Leppla, S. H. (1982) Proc. Natl. Acad. Sci. USA 79, 3162-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley, K. A., Mogridge, J., Mourez, M., Collier, R. J. & Young, J. A. (2001) Nature 414, 225-229. [DOI] [PubMed] [Google Scholar]
- 5.Collier, R. J. & Young, J. A. (2003) Annu. Rev. Cell Dev. Biol. 19, 45-70. [DOI] [PubMed] [Google Scholar]
- 6.Pezard, C., Berche, P. & Mock, M. (1991) Infect. Immun. 59, 3472-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gudmundsson, G. H. & Agerberth, B. (1999) J. Immunol. Methods 232, 45-54. [DOI] [PubMed] [Google Scholar]
- 8.Ganz, T. (2003) Nat. Rev. Immunol. 3, 710-720. [DOI] [PubMed] [Google Scholar]
- 9.Ganz, T., Selsted, M. E., Szklarek, D., Harwig, S. S., Daher, K., Bainton, D. F. & Lehrer, R. I. (1985) J. Clin. Invest. 76, 1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agerberth, B., Charo, J., Werr, J., Olsson, B., Idali, F., Lindbom, L., Kiessling, R., Jornvall, H., Wigzell, H. & Gudmundsson, G. H. (2000) Blood 96, 3086-3093. [PubMed] [Google Scholar]
- 11.Ganz, T., Selsted, M. E. & Lehrer, R. I. (1990) Eur. J. Haematol. 44, 1-8. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer, P. B. & Lehrer, R. I. (1992) Infect. Immun. 60, 3446-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehrer, R. I., Lichtenstein, A. K. & Ganz, T. (1993) Annu. Rev. Immunol. 11, 105-128. [DOI] [PubMed] [Google Scholar]
- 14.Harwig, S. S., Ganz, T. & Lehrer, R. I. (1994) Methods Enzymol. 236, 160-172. [DOI] [PubMed] [Google Scholar]
- 15.Park, S. & Leppla, S. H. (2000) Protein Expression Purif. 18, 293-302. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez, D. M., Leppla, S. H., Schneerson, R. & Shiloach, J. (2002) J. Ind. Microbiol. Biotechnol. 28, 232-238. [DOI] [PubMed] [Google Scholar]
- 17.Lyons, C. R., Lovchik, J., Hutt, J., Lipscomb, M. F., Wang, E., Heninger, S., Berliba, L. & Garrison, K. (2004) Infect. Immun. 72, 4801-4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Min, D. H., Tang, W. J. & Mrksich, M. (2004) Nat. Biotechnol. 22, 717-723. [DOI] [PubMed] [Google Scholar]
- 19.Dixon, T. C., Fadl, A. A., Koehler, T. M., Swanson, J. A. & Hanna, P. C. (2000) Cell. Microbiol. 2, 453-463. [DOI] [PubMed] [Google Scholar]
- 20.Guidi-Rontani, C., Levy, M., Ohayon, H. & Mock, M. (2001) Mol. Microbiol. 42, 931-938. [DOI] [PubMed] [Google Scholar]
- 21.Park, J. M., Greten, F. R., Li, Z. W. & Karin, M. (2002) Science 297, 2048-2051. [DOI] [PubMed] [Google Scholar]
- 22.Cameron, A., Appel, J., Houghten, R. A. & Lindberg, I. (2000) J. Biol. Chem. 275, 36741-36749. [DOI] [PubMed] [Google Scholar]
- 23.Klimpel, K. R., Molloy, S. S., Thomas, G. & Leppla, S. H. (1992) Proc. Natl. Acad. Sci. USA 89, 10277-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nassar, T., Akkawi, S., Bar-Shavit, R., Haj-Yehia, A., Bdeir, K., Al-Mehdi, A. B., Tarshis, M. & Higazi, A. A. (2002) Blood 100, 4026-4032. [DOI] [PubMed] [Google Scholar]
- 25.Ganz, T. (1987) Infect. Immun. 55, 568-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang, D., Biragyn, A., Kwak, L. W. & Oppenheim, J. J. (2002) Trends Immunol. 23, 291-296. [DOI] [PubMed] [Google Scholar]
- 27.Mandal, M. & Nagaraj, R. (2002) J. Pept. Res. 59, 95-104. [DOI] [PubMed] [Google Scholar]
- 28.Panyutich, A. V., Panyutich, E. A., Krapivin, V. A., Baturevich, E. A. & Ganz, T. (1993) J. Lab. Clin. Med. 122, 202-207. [PubMed] [Google Scholar]
- 29.Maynard, J. A., Maassen, C. B., Leppla, S. H., Brasky, K., Patterson, J. L., Iverson, B. L. & Georgiou, G. (2002) Nat. Biotechnol. 20, 597-601. [DOI] [PubMed] [Google Scholar]
- 30.Tonello, F., Seveso, M., Marin, O., Mock, M. & Montecucco, C. (2002) Nature 418, 386. [DOI] [PubMed] [Google Scholar]
- 31.Turk, B. E., Wong, T. Y., Schwarzenbacher, R., Jarrell, E. T., Leppla, S. H., Collier, R. J., Liddington, R. C. & Cantley, L. C. (2004) Nat. Struct. Mol. Biol. 11, 60-66. [DOI] [PubMed] [Google Scholar]
- 32.Panchal, R. G., Hermone, A. R., Nguyen, T. L., Wong, T. Y., Schwarzenbacher, R., Schmidt, J., Lane, D., McGrath, C., Turk, B. E., Burnett, J., et al. (2004) Nat. Struct. Mol. Biol. 11, 67-72. [DOI] [PubMed] [Google Scholar]
- 33.Mourez, M., Kane, R. S., Mogridge, J., Metallo, S., Deschatelets, P., Sellman, B. R., Whitesides, G. M. & Collier, R. J. (2001) Nat. Biotechnol. 19, 958-961. [DOI] [PubMed] [Google Scholar]
- 34.Yang, D., Chen, Q., Chertov, O. & Oppenheim, J. J. (2000) J. Leukocyte Biol. 68, 9-14. [PubMed] [Google Scholar]
- 35.Lillard, J. W., Jr., Boyaka, P. N., Chertov, O., Oppenheim, J. J. & McGhee, J. R. (1999) Proc. Natl. Acad. Sci. USA 96, 651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]