Abstract
The ≈20S RNA ligase-containing complex (L-complex) in trypanosomatid mitochondria interacts by means of RNA linkers with at least two other multiprotein complexes to mediate the editing of mitochondrial cryptogene transcripts. The L-complex contains ≈16 proteins, including the two RNA-editing ligases (RELs), REL1 and REL2. Leishmania tarentolae REL1 and REL2 and Trypanosoma brucei REL1 were expressed as enzymatically active tandem affinity purification-tagged proteins in a Baculovirus system. When these proteins were added to mitochondrial lysates from T. brucei procyclic cells that were depleted of the cognate endogenous ligase by RNA interference down-regulation of expression, the added proteins were integrated into the L-complex, and, in the case of REL1, there was a complementation of in vitro-precleaved U-insertion and U-deletion editing activities of the 20S L-complex. Integration of the recombinant proteins did not occur or occurred at a very low level with noncognate ligase-depleted L-complex or with wild-type L-complex. A C-terminal region of the T. brucei recombinant REL1 downstream of the catalytic domain was identified as being involved in integration into the L-complex. The ability to perform functional complementation in vitro provides a powerful tool for molecular dissection of the editing reaction.
Keywords: editing, RNA-editing ligase 1, RNA-editing ligase 2, trypanosome
Several macromolecular complexes have been identified that are involved in U-insertion/deletion RNA editing in trypanosomatid mitochondria (1-3). The mitochondrial RNA-binding protein complex contains two small RNA-binding proteins and three associated proteins, and may possibly be involved in the initial annealing of the guide RNA and the mRNA (4-9). The RNA-editing terminal uridylyl transferase 1 (TUTase 1) (RET1) complex contains the RET1 tetramer and one or more unidentified components (10). RET1 has been shown to be involved in the addition of nonencoded Us to the 3′ ends of guide RNAs (11). The RNA ligase-containing L-complex was initially detected as an α-32P-ATP-labeled particle sedimenting ≈20S in glycerol gradients and migrating as a single band with an approximate size of 1,200-2,000 kDa in native gels (12, 13). This complex has been labeled the editosome but it seems appropriate to reserve this nomenclature for the entire RNA/protein supercomplex once it is more defined. The L-complex has been isolated from Trypanosoma brucei and Leishmania tarentolae mitochondria by column chromatography (13-16) and by tandem affinity purification (TAP) chromatography (17). At least 16 protein components have been identified, including the two RNA-editing ligases (RELs) (REL1 and REL2), a second 3′ TUTase (RET2), three to four RNase III-motif proteins, three related zinc finger-motif proteins, two AP-endo-exonuclease-phosphatase-motif proteins, two RNA binding-motif proteins, and several proteins lacking identifiable motifs.
The two ligases are localized in subcomplexes that have been partially defined by coimmunoprecipitation experiments and by physical fractionation (17, 18). The REL1 subcomplex also contains the MP63 (LC-4) zinc finger protein and the MP99 (LC-3) exonuclease, whereas the REL2 subcomplex contains the RET2 3′ TUTase and the MP81 (LC-1) zinc-finger protein. Other proteins such as the MP18 (LC-11) zinc-finger protein, and the MP100 (LC-2) exonuclease may also interact with these complexes. Although it is clear that RNA ligase is involved in the terminal step of the RNA-editing cascade (19-22), it is still unclear why there are two ligases in the core editing L-complex and two ligase-containing subcomplexes. Because there are basically two types of editing, one in which Us are inserted, and another in which Us are deleted, it is an attractive idea that the two ligases play specific roles in the two reactions and specific roles for REL1 in U-deletion editing and for REL2 in U-insertion editing have been proposed on the basis of in vitro titration curves for adenylation of the enzymes and deletion and insertion editing (23-25). Moreover, a TAP capture of the REL1 subcomplex that sedimented at ≈5S in a glycerol gradient appeared to catalyze precleaved U-deletion in vitro editing, whereas a TAP capture of the REL2 subcomplex appeared to catalyze U-insertion in vitro editing (18). However, in vivo down-regulation of REL1 by conditional RNA interference (RNAi) (26) or by replacement of one allele with an inactive REL1 enzyme (25) affected both U-insertion and U-deletion editing without affecting the stability or S value of the L-complex (26), whereas RNAi down-regulation of REL2 expression did not affect cell growth or editing phenotype, although both the REL2 protein and the interacting MP81 protein decreased in abundance (26, 27). REL1 RNAi also led to a decrease in the abundance of MP63 (LC-4) in the L-complex (26).
There are no reports of enzymatically active recombinant REL (rREL) rREL1 and rREL2 proteins that have been purified to homogeneity. Isolation of active, recombinant ligases would provide a powerful tool for investigating the biological roles of these enzymes in the editing reactions. In this study, we have obtained enzymatically active L. tarentolae REL1 and REL2 and T. brucei REL1 proteins from a Baculovirus expression system. Furthermore, we have discovered that the recombinant proteins can each integrate into an L-complex that has been depleted of the cognate ligase by RNAi. In the case of REL1, the integration of the recombinant enzyme functionally complemented the REL1-depleted L-complex in an in vitro editing system. Finally, we used this system to show that the C-terminal region of rREL1 is required for integration into the depleted L-complex.
Materials and Methods
Cell Culture and Plasmid Constructions. T. brucei 29-13 procyclic cells (from G. Cross, The Rockefeller University, New York), which carry integrated T7 RNA polymerase and tetracycline repressor were cultured as described (10). The construction of the T. brucei head-to-head RNAi plasmids for tetracycline-induced down-regulation of REL1 and REL2 expression has been described (26).
L. tarentolae (Lt) and T. brucei (Tb) REL1 and LtREL2 Expression in Escherichia coli and Baculovirus. For antibody production, the full-length LtREL1 and LtREL2 genes were cloned into the pMAL c2x vector (New England Biolabs). The plasmids were transformed into E. coli BL21 DE3 (Stratagene), and expression was induced with 0.3 mM isopropyl-1-thio-β-d-galactopyranoside at 15°C overnight. The rREL1 and rREL2 proteins fused with the maltose-binding protein were purified by binding to amylose resin (New England Biolabs) and eluting with 10 mM maltose. The recombinant proteins were further purified by SDS/PAGE. Polyclonal antibodies against REL1 and REL2 were prepared by Covance Research Products (Denver, PA). Monoclonal antibodies against the T. brucei MP81, MP63, REL1, and MP42 proteins were kind gifts of K. Stuart (Seattle Biomedical Research Institute, Seattle). Anti-calmodulin-binding peptide (CBP) polyclonal antibody was purchased from Upstate Cell Signaling Solution. Western blot analysis was performed by using the SuperSignal West Pico and West Femto chemiluminescent system (Pierce).
For expression in the Baculovirus system, LtREL1, TbREL1, and LtREL2 with C-terminal TAP tags (17) were inserted into the BamHI-EcoRI sites of the pFastBac plasmid (Invitrogen). We did not succeed in expressing TbREL2. The fusion protein genes were transposed into the Baculovirus bacmid DNA (Invitrogen) by transformation of E. coli DH10Bac cells (Invitrogen). Sf9 insect cells (Invitrogen) at a cell density of ≈1 × 106/ml were then transfected with isolated high molecular weight viral DNA. The cells were harvested after 48 h of culture at 27°C. Lysis of the insect cells and purification of tagged recombinant protein followed the TAP-purification protocol (17). Briefly, the cells were lysed in 20 mM Tris·HCl (pH 7.5)/10 mM MgCl2/60 mM KCl/0.5% Nonidet P-40 and mild sonication. The cleared supernatant was bound to IgG Sepharose FF (Amersham Pharmacia Biotech), and the bound TAP-tagged protein was cleaved from the column with tobacco etch virus protease (Invitrogen). The CBP-tagged product was then bound to calmodulin agarose (Stratagene) and eluted with 2 mM EGTA. Lt rREL1 and Lt rREL2 were further purified by Superose 6 chromatography (AKTA Explorer, Pharmacia Biotech) in 20 mM Tris·HCl (pH 7.8)/10 mM MgCl2/150 mM NaCl/1 mM DTT/10% glycerol at a flow rate of 0.2 ml/min. A single peak was eluted at ≈15-16 ml for both rREL1 and rREL2. For the Mono Q purification of Tb rREL1, the protein was bound in 50 mM Hepes (pH 7.6)/10 mM MgCl2/1 mM DTT and eluted with a salt gradient. For protein quantitation, the samples were run on 10-20% Tris-glycine denaturing gels that were stained with Sypro ruby protein gel stain (Molecular Probes). The protein bands were visualized in the Typhoon 900 PhosphorImager, and protein quantitation was performed by using the Protein Assay Standard Set (Pierce). The purified recombinant proteins were stored in aliquots at -70°.
Extract Preparation, Glycerol Gradient Sedimentation, and Native Gel Electrophoresis. Highly purified mitochondrial fractions were obtained by hypotonic-shear cell breakage and Renografin density gradient centrifugation (28). Purified mitochondria were stored in 100-mg (wet weight) aliquots at -80°C. Each aliquot was lysed with 0.5% Nonidet P-40 in 50 mM Hepes (pH 8.1)/10 mM MgCl2/60 mM KCl. The 500-μl extract was clarified in the Optima TLX centrifuge for 30 min at 70,000 rpm and centrifuged on a 10-30% glycerol gradient in the SW41 rotor (Beckmann) for 20 h at 30,000 rpm. The fractions were diluted two-fold with Tris-glycine native sample buffer (Novex, San Diego) and electrophoresed on an 8-16% native gel (Invitrogen), or with SDS loading buffer for 10-20% denaturing gradient gel electrophoresis. The gels were blotted onto Protran nitrocellulose membrane (Schleicher & Schuell) for Western analysis. Sedimentation values were calculated by using catalase (11S), thyroglobulin (19S), and small ribosome subunits from E. coli (30S). For binding to REL1-depleted L-complex, 100 pmol of purified recombinant ligases (unless otherwise specified) was added to the lysate derived from a single aliquot of frozen mitochondria before clarification and gradient centrifugation.
RNA Substrates and in Vitro Ligation and Precleaved Editing Assay. The following RNA substrates were chemically synthesized [(Oligos Etc. (Wilsonville, OR) and Xeragon (Zurich)] and gel-purified: 5′ fragment, 5′-GCACUACACGAUAAAUAUAAAAAG-3′; 5′-UU fragment, 5′-GCACUACACGAUAAAUAUAAAAAGUU-3′; 3′ fragment, 5′-AACAUUAUGCUUCUUddC-3′; AG bridge RNA (brRNA), 5′-AAGAAGCAUAAUGUUAGCUUUUUAUAUUUAUCGUGUAGUCddG-3′; and 0 brRNA, 5′-AAGAAGCAUAAUGUUCUUUUUAUAUUUAUCGUGUAGUCddG-3′.
The 5′ fragment RNAs were 5′-phosphorylated with T4 polynucleotide kinase (Invitrogen) and [γ-32P]ATP. Complementary RNAs were annealed by heating and slow cooling. For in vitro editing assays, the ≈20S L-complex fractions were pooled and concentrated to 400 μl by using Millipore 5K NMWL centrifugal filters. The concentrated gradient fractions were stored at -20°C in 50% glycerol/20 mM Tris·HCl (pH 7.5)/10 mM MgCl2/20 mM KCl/1 mg/ml BSA/1 mM DTT. The fractions were electrophoresed on 10-20% denaturing gradient gels, and the gels were stained with Sypro ruby (Molecular Probes) to monitor the protein concentrations. The in vitro ligation and editing reactions were performed at 27°C for 1 h in 20 μl of 50 mM Tris·HCl (pH 7.5)/10 mM MgCl2/20 mM KCl/60 μg/ml BSA/1 mM DTT/1 mM ATP. UTP (2 mM) was added for the U-insertion assay. For enzymatic analysis, 75 fmol of rREL1 or 25 fmol of rREL2 were added to a 20-μl reaction with the indicated concentration of [γ-32P]ATP 5′ end-labeled RNA. The reactions were incubated at 27°C for 120 min and stopped by addition of three volumes of ethanol at -20°C. The pellets were resusupended in 80% formamide/1 mM EDTA/50 mM Tris borate (pH 8.3), and electrophoresed in a 15% polyacrylamide-urea sequencing gel. The gel was dried and exposed to a PhosphorImager cassette. The signals were quantitated in the PhosphorImager by using imagequant software (Molecular Dynamics) and the data were analyzed for Vmax and Km values by using the prism nonlinear curve-fitting program (GraphPad, San Diego).
Transfer of AMP from Charged Ligases to RNAs. Three picomoles of rREL1 was incubated with 10 μCi [α-32P]ATP at 27°C for 15 min in 50 mM Tris·HCl (pH 7.5)/10 mM MgCl2/20 mM KCl. This reaction mixture was divided into two equal aliquots. Into one aliquot, 10 pmol of 5′-phosphate RNA (AG brRNA, with ddG at the 3′ terminus) was added. The other aliquot was used as a control. The reaction was incubated for another 15 min at 27°C and stopped by phenol/chloroform extraction. The samples were electrophoresed on a 10-20% SDS/PAGE gel and blotted onto a nitrocellulose membrane (Protran, Schleicher & Schuell, Keene, NH).
Results
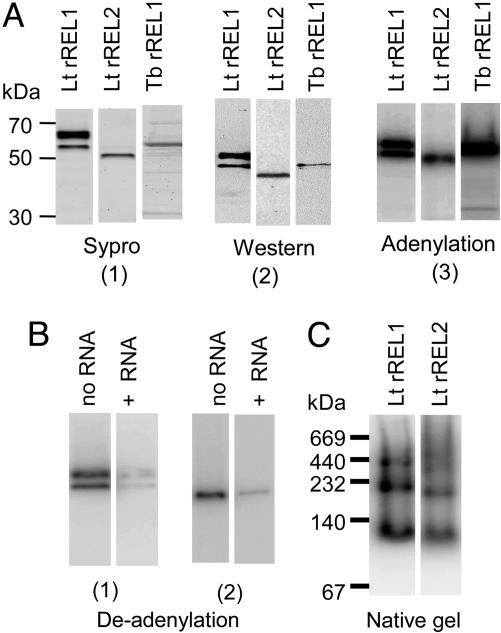
Expression of Enzymatically Active Lt REL1 and REL2 and Tb REL1 Proteins in the Baculovirus System. The Lt and Tb REL1 and REL2 genes fused in-frame with the CBP and Protein A peptides (29) were expressed in Sf9 insect cells by using the Baculovirus system. The Lt rREL1 and Lt rREL2-CBP fusion proteins were purified by binding to calmodulin agarose after release from the IgG column, whereas the Tb rREL1-CBP fusion proteins were purified by MonoQ chromatography after release from IgG without calmodulin agarose binding because of the low yield of this step. Syprostained SDS gels of the purified proteins are shown in Fig. 1A1, a Western blot in A2, and an autoradiograph of the autoadenylated proteins in A3. Lt rREL1 migrated as two bands in an SDS gel, but both bands could be labeled with [α-32P]ATP, and the bound AMP was removed by incubation with a ligatable RNA substrate, indicating that both these bands represent functional REL1 proteins (Fig. 1B1) with approximately the same specific activity. The Lt rREL2 protein migrated as a single autoadenylatable band in an SDS gel, and this band could also be deadenylated by incubation with ligatable RNA (Fig. 1B2). Purified Tb rREL1 migrated as a single band of the expected size in an SDS gel (Fig. 1A1-3).
Fig. 1.
Purification of enzymatically active Lt rREL1, rREL2, and Tb rREL1. (A) Purification and autoadenylation. (A1) SDS gel of purified Lt REL1, Lt REL2, and Tb REL1 stained with Sypro ruby. (A2) Western blot of gel in A1 with specific antisera against each protein. (A3) SDS gel of autoadenylated purified proteins. The lower Lt rREL1 band is the expected molecular weight of the CBP-fusion protein. The upper Lt rREL1 band, which has ≈2.2-fold more protein than the lower band, separates into several closely spaced bands at higher resolution. From the ratio of the adenylated bands to the Sypro-stained bands, we estimate that the enzyme in the upper band has≈70% of the specific activity of the lower band. (B1) Deadenylation of charged Lt rREL1 bands 1 and 2 by incubation with an RNA with a 5′ phosphate for 15 min. (B2) Deadenylation of charged Lt rREL2 by incubation with RNA. (C) Native gel of Lt rREL1 and Lt rREL2. The proteins were autoadenylated with [α-32P]ATP before electrophoresis. The molecular weights of the bands are approximately two, four, and eight times the molecular weights of the monomeric proteins.
The Lt rREL1 and rREL2 proteins appear to be in an oligomeric conformation in the native state, as shown by the native gel electrophoresis in Fig. 1C. The molecular weights of the bands are approximately two, four, and eight times the molecular weights of the monomers.
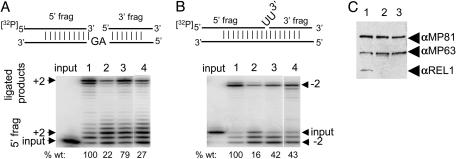
All of the recombinant ligases showed ligation activity by using a bridged nicked substrate consisting of two short RNAs hybridized to a brRNA (Fig. 2 A and C). A kinetic analysis of the L. tarentolae recombinant enzymes yielded similar Km values in the 50-100 nM range for both rREL1 and rREL2 (Fig. 2 B and D). A complete kinetic analysis of the T. brucei recombinant enzymes was not performed.
Fig. 2.
Kinetics of ligation of Lt rREL1 and Lt rREL2 with a bridged nicked RNA substrate. (A) A representative experiment is shown. Ligation was performed in 50 mM Tris·HCl (pH 7.5)/10 mM MgCl2/20 mM KCl/1 mM ATP/60 μg/ml BSA/1 mM DTT at 27°C for 120 min. Each reaction contained 75 fmol of Lt rREL1. (B) Kinetics of Lt rREL1 ligation fitted to a single site-binding Michaelis-Menton model by nonlinear regression (GraphPad prism program). The dotted lines show the 95% confidence limits for a representative reaction. The Vmax and Km values shown were from three independent experiments (± SD). (C) A representative ligation experiment with Lt rREL2 and the same substrate as in A. Each reaction contained 25 fmol Lt rREL2. (D) Data from C plotted as in B.
Binding of Homologous and Heterologous Recombinant RNA Ligases to Ligase-Depleted L-Complex in Vitro. To determine whether the rREL1 ligase could complement the loss of the endogenous ligases from the L-complex after RNAi down-regulation of REL1 expression in T. brucei procyclic cells, the purified Tb rREL1 protein was incubated with mitochondrial lysate from REL1-depleted cells. The lysates were then fractionated in a glycerol gradient, and the localization of the recombinant ligases was assayed by Western blot analysis and by autoadenylation of each gradient fraction. This experiment is shown in Fig. 3 for increasing amounts of Tb rREL1 added back to REL1-depleted T. brucei mitochondrial lysates. The relative amount of Tb rREL1 cosedimenting in the 20S L-complex region (circled), which was detected by both adenylation and Western analysis increased with the amount of protein added to the lysate and appeared to saturate at ≈250-500 pmol, giving rise to nonbound Tb rREL1 sedimenting more slowly in the gradient in fractions 3 and 4.
Fig. 3.
Binding of Tb rREL1 to REL1-depleted T. brucei L-complex. Addition of 10 pmol (A and B), 250 pmol (C and D), and 500 pmol (E and F) Tb rREL1 to 500 μl of mitochondrial lysate derived from 100 mg wet weight of purified mitochondria from day 5 tetracycline-induced REL1 RNAi-down regulated T. brucei procyclics. Half of the lysate (250 μl) was fractionated on a 12-ml 10-30% glycerol gradient at 30,000 rpm for 20 h in the SW41 rotor. Fractionation was from the top with 750 μl in each fraction. From this mixture, 10 μl of each fraction were incubated with 3 μCi of [α-32]ATP and analyzed on an 10-20% Tris-glycine polyacrylamide gel, which was blotted onto nitrocellulose membrane to detect the autoadenylated ligases, and the blot was reacted with antisera to detect the MP81, MP63, and REL1 proteins. The autoradiographs are shown in A, C, and E, and the Western blots in B, D, and F. The L-complex fractions are circled. The Con lane in each gel contains the autoadenylated purified protein alone as a marker.
It was not possible to use Western analysis to analyze the add back of the heterologous Lt rREL1 to REL1-depleted T. brucei L-complex because of the low affinity of the available specific antiserum. However, the fact that the recombinant L. tarentolae protein migrated in an SDS gel as two distinctive autoadenylatable bands above the endogenous REL1 band was used to establish that this enzyme also bound to the T. brucei REL1-depleted L-complex. As shown in Fig. 4A, the characteristic doublet of Lt rREL1 can be seen in the 20S fractions (circled), in addition to the minor endogenous REL1 and REL2 bands that were probably labeled by trace amounts of [α-32P]ATP present in the labeled rREL1 preparation. Approximately 70% of the added Lt rREL1 sedimented in the 20S region.
Fig. 4.
Binding of Lt rREL1 and Lt rREL2 to the cognate ligase-depleted T. brucei L-complex. (A) Purified Lt rREL1 was autoadenylated with [α-32P]ATP and then mixed with REL1-depleted mitochondrial lysate. The lysate was fractionated in a glycerol gradient, and the fractions were separated in an SDS gel that was autoradiographed to identify the two labeled rREL1 bands (arrows). The ≈20S L-complex fractions are circled. (B) Purified Lt rREL2 was autoadenylated with [α-32P]ATP and mixed with REL1-depleted mitochondrial lysate. The lysate was fractionated in a glycerol gradient, and the fractions were separated in an SDS gel that was autoradiographed to detect the labeled Lt rREL2 band (arrow). (C) Binding of unlabeled purified Lt rREL1 to REL1-depleted L-complex. (C1) Sedimentation of mitochondrial lysate from wild-type cells in glycerol gradient and localization of endogenous REL1 and REL2 by autoadenylation of each fraction and SDS gel electrophoresis. (C2) Sedimentation of REL1-depleted mitochondrial lysate in glycerol gradient and localization of endogenous REL1 and REL2 by autoadenylation of each fraction and SDS gel electrophoresis. Note the decrease in relative intensity of the REL1 band in the L-complex fractions (circled). (C3) Addition of Lt rREL1 to REL1-depleted mitochondrial lysate and sedimentation in glycerol gradient with localization of endogenous and exogenous REL1 bands by autoadenylation of each fraction. Note the presence of the characteristic two bands of Lt rREL1 in the L-complex fractions (circled). (D) Lt rREL1 was incubated with REL1-depleted mitochondrial lysate and the CBP-tagged protein, and any associated proteins were captured on calmodulin agarose. The captured material was eluted with EGTA, autoadenylated, and separated in an SDS gel that was subjected to autoradiography to identify the endogenous REL1 and REL2 and the exogenous rREL1 (arrows). Lane 1, control to show the location of the endogenous REL1 and REL2 bands. Lane 2, calmodulin-eluted material. (E) The calmodulin eluted material from D was fractionated in a glycerol gradient, and each fraction was autoadenylated. The Lt rREL1 bands are associated with the L-complex (circled) in addition to being at the top of the gradient.
We also used autoadenylation of each gradient fraction to assay the add-back of unlabeled Lt rREL1. In the experiment in Fig. 4C, unlabeled Lt rREL1 was incubated with the T. brucei REL1-depleted mitochondrial lysate before centrifugation, and the localization was monitored by labeling each gradient fraction with [α-32P]ATP and separating the proteins in an SDS gel. The controls in Fig. 4 C1 and C2 show the typical patterns obtained with wild-type T. brucei mitochondrial lysate and the decrease in labeled endogenous REL1 after 5 days of RNAi down-regulation. The add-back experiment in Fig. 4C3 shows the characteristic doublet of Lt rREL1 cosedimenting with the REL1-depleted L-complex. This finding indicates that the heterologous REL1 protein also binds to the T. brucei REL1-depleted L-complex.
Similar binding experiments were performed with α-32P-ATP-prelabeled Lt rREL2 with mitochondrial lysate from REL2-depleted T. brucei. As shown in Fig. 4B, the labeled Lt rREL2 cosedimented with the REL2-depleted L-complex (circled). A similar result was obtained by using incubation of the lysate with unlabeled rREL2 followed by detection of the CBP-tagged protein by Western analysis with anti-calmodulin-binding peptide anti-serum (data not shown).
To establish that the observed cosedimentation in glycerol gradients is due to interaction of the recombinant ligases with the ligase-depleted L-complex, mitochondrial lysate from REL1-depleted T. brucei was incubated with Lt rREL1, and then calmodulin-agarose beads were added to capture the CBP-tagged Lt rREL1 together with any associated proteins. An aliquot of the captured material was autoadenylated with [α-32P]ATP and separated in an SDS gel. As shown in Fig. 4 D2, both the two CBP-tagged Lt rREL1 ligase bands and the endogenous REL1 and REL2 ligases are present in the pull-down material. Lane 1 is a control to show the location of the endogenous adenylated ligases. The material eluted from the CBP agarose was then subjected to glycerol gradient sedimentation, and each fraction was autoadenylated with [α-32P]ATP to localize the ligases (Fig. 4E). Lanes wt and con are controls to show the location of the endogenous and recombinant ligases. The presence of Lt rREL1 in the L-complex region indicates that the Lt rREL1 protein was interacting with the L-complex and not just cosedimenting.
To address the question whether the binding of the recombinant ligases was specific for the cognate ligase-depleted L-complex, α-32PAT-P-prelabeled Lt rREL1 and α-32PAT-P-prelabeled Lt rREL2 were incubated with mitochondrial lysates from wild-type T. brucei, or from T. brucei, which were down-regulated for the noncognate ligase. The lysates were sedimented in glycerol gradients, and the fractions were assayed for the presence of labeled recombinant proteins by SDS gel electrophoresis. As shown in Fig. 7A, which is published as supporting information on the PNAS web site, (Supplement figure), no detectable Lt rREL1 was bound to the L-complex from wild-type cells (Fig. 7 A1) or from T. brucei depleted of REL2 (Fig. 7 A2). In the case of the prelabeled Lt rREL2 add-back experiment shown in Fig. 7B, a very low level of binding of prelabeled Lt rREL2 to both wild-type T. brucei L-complex (Fig. 7 B1) and REL1-depleted L-complex (Fig. 7 B2) was observed, but this result was many fold lower than the level of binding to REL2-depleted L-complex (see Fig. 4B above).
Functional Complementation of in Vitro-Precleaved Editing Activities of REL1-Depleted L-Complex with Heterologous rREL1. As shown previously (26), down-regulation of REL1 expression in T. brucei by RNAi produces a decrease in the levels of both in vitro-precleaved U-insertion editing and precleaved U-deletion editing by the 20S L-complex fraction. To functionally test the effect of integration of recombinant ligase into the REL1-depleted T. brucei L-complex, Lt rREL1 was added to the lysate before gradient fractionation, and the 20S L-complex fraction was then used for in vitro-precleaved editing assays with the U-insertion and U-deletion RNA substrates shown in Fig. 5 A and B. The level of guide RNA-guided plus 2U-insertion editing of the 20S L-complex increased from 22% in lane 2 of the wild-type level of editing in lane 1, to 79% in lane 3 after add-back of the recombinant protein. Addition of 100-fold excess of rREL1 directly to the 20S L-complex fraction in lane 4 instead of to the lysate only increased the extent of U-insertion editing to 27% of the wild-type level. Similar results were observed in the U-deletion editing assay (Fig. 5B). It is possible that the apparent greater efficiency of reconstitution if the enzyme is added to the lysate is due to an inhibitory effect of an excess of enzyme, but more likely is due to a requirement for binding factors present in the lysate before integration into the L-complex. However, this finding remains to be investigated in detail.
Fig. 5.
Functional complementation of in vitro editing activities. (A) The +2U-insertion precleaved RNA substrate is shown above the gel. Mitochondrial lysates were fractionated in glycerol gradients and the ≈20S L-complex region used for in vitro editing assays. Input lane, no enzyme control with input RNA substrate only. Lane 1, lysate was from 29-13 wild-type cells. Lane 2, lysate was from 5-day RNAi REL1-down-regulated cells. Lane 3, add-back of Lt rREL1 to mitochondrial lysate of 5-day RNAi REL1-down regulated cells. Lane 4, addition of 10 pmol of Lt rREL1 directly to the same sample as used for lane 2. This result represents ≈100 times that added to the mitochondrial lysate in lane 3. The percent editing is shown below each lane as compared with the editing with wild-type lysate in lane 1 as 100%. (B) The -2U-deletion RNA substrate is shown above the gel. See A for details of enzymes used for each reaction. The percent editing is shown below each lane as compared with the editing with wild-type lysate in lane 1 as 100%. (C) Western blot of same filter as in A with anti-MP81, anti-MP63, and anti-REL1 antibodies. The decrease in Tb REL1 in lanes 2 and 3 is because of the RNAi down-regulation.
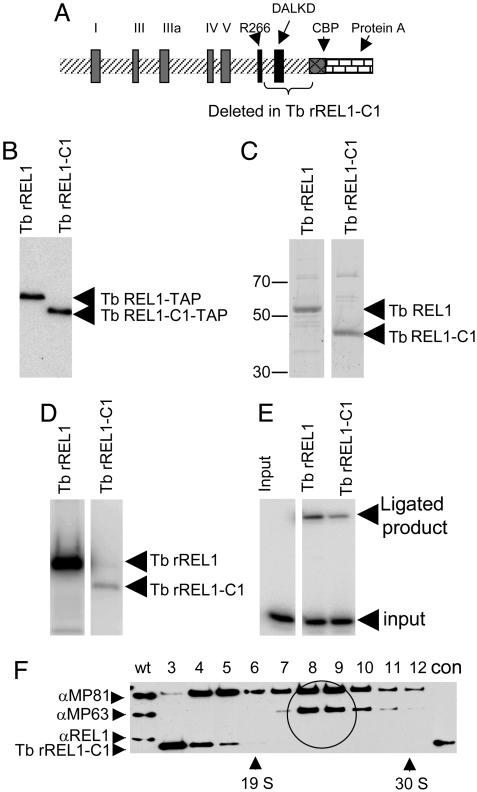
Localization of a C-Terminal Region of the Tb rREL1 Enzyme Required for Binding to the REL1-Depleted L-Complex. There is a recent body of work on defining the motifs involved in catalysis in RNA ligases (30, 31), and there are partial structures of the T4 RNA ligase (32) and the Tb REL1 enzyme (33). We decided to attempt to use the reconstitution system to localize the region of REL1 involved in binding to the REL1-depleted L-complex. Toward this end, a C-terminal deletion of 90 aa from Tb rREL1 was generated (Fig. 6A). This Tb rREL1-C1 mutant lacked the DALKD motif, but still had arginine 266 and the five motifs (labeled I-V), known to be critical for catalysis (30, 32, 34, 35). It is comparable to the Rnl2 mutant of T4 RNA ligase which Ho et al. (32) showed had a higher pH optimum for adenylation. The TAP-tagged mutant protein was purified by binding to calmodulin agarose and Mono Q chromatography (Fig. 6C). The purified mutant protein was enzymatically active in autoadenylation (Fig. 6D), although at a ≈100-fold lower level than that of wild-type rREL1 but was only 5-fold less active than wild-type rREL1 in ligation of a bridged nicked substrate (Fig. 6E), suggesting that the protein was basically folded properly. Further deletion including Arg-266 had no further effect on adenylation or ligation but did abolish the reactivity with the monoclonal antibody, whereas deletion of motif V completely abolished ligation activity (data not shown). We decided to investigate whether the Tb rREL1-C1 mutant protein was capable of in vitro reconstitution.
Fig. 6.
Isolation and add-back of the Tb rREL1-C1 deletion mutant protein. (A) Diagram of Tb rREL1 showing the five ligase motifs (I-V) and the two sites, R266 and DALKD, involved in catalysis. Note the absence of the putative mitochondrial signal sequence. The fused CBP and Protein A tags are also shown. The 90 aa C-terminal region deleted is indicated by a bracket. (B) PAP reagent (Sigma) detection of TAP-tagged Tb rREL1 and Tb rREL1-C1 from transfected insect cells. (C) The Tb rREL1 and Tb rREL1-C1 were purified by binding to IgG followed by Mono Q anion exchange chromatography. Aliquots (4 pmol) of each ligase were loaded onto an SDS gel which was stained with Sypro. (D) Autoadenylation with [α-32P]ATP of equal amounts of Tb rREL1 and Tb rREL1-C1. (E) Ligation of bridged nicked RNA substrate. (F) Add-back of 1 nmol of Tb rREL1-C1 to mitochondrial lysate from REL1-depleted T. brucei. The lysate was fractionated in a glycerol gradient, and each fraction was separated in an SDS gel that was reacted with antibodies against MP81, MP63, and REL1. The ≈20S L-complex region is circled. Note the absence of Tb rREL1-C1 in the L-complex region and the presence at the top of the gradient in fractions 3 and 4.
Incubation of a large excess (1 nmol) of the Tb rREL1-C1 protein with REL1-depleted mitochondrial lysate and subsequent gradient fractionation led to a localization of the protein at the top of the gradient in fractions 3 and 4 with no detectable binding of the mutant enzyme to the 20S REL1-depleted L-complex (Fig. 6F). This evidence indicates that the C-terminal 90 aa of Tb REL1 contain a region required for binding to ligase-depleted L-complex and illustrates the power of this system for a genetic dissection of the RELs.
Discussion
We presented evidence that CBP-tagged enzymatically active rREL1 and rREL2 RNA ligases can bind to the L-complex from T. brucei procyclic cells that were depleted of the cognate mitochondrial ligase by RNAi. The recombinant ligases were incubated with the mitochondrial lysate, which was then fractionated in a glycerol gradient to separate the 20S L-complex (12, 13, 17). Both homologous T. brucei recombinant ligases and heterologous recombinant ligases from L. tarentolae were equally effective in this reconstitution reaction. Evidence that the binding represents a functional complementation, at least in the case of REL1, was provided by the restoration of both U-deletion and U-insertion precleaved in vitro-editing activities to the 20S L-complex fraction by incubation of the lysate with L. tarentolae recombinant ligase. There is some preliminary indication that the recombinant ligase may interact with additional proteins before binding to the depleted L-complex, with the most likely candidates being the other components of the cognate subcomplex that are known to be present in the mitochondrial lysate of REL1 down-regulated cells (18, 26). For example, addition of 10 pmol of rREL1 directly to the 20S gradient fraction of REL1-depleted cells was less effective in restoring precleaved editing than addition of 1/100 of this amount to the lysate before fractionation. Also, there was reproducible 30-50% increase in the MP63/MP81 ratio in the reconstituted L-complex (data not shown).
Deletion of the C-terminal 90 aa from the Tb rREL1 completely abolished integration into the REL1-depleted L-complex. This evidence suggests the presence of a C-terminal region of REL1 involved in the protein/protein interactions required for integration into the REL1-depleted L-complex. Although this region contains the DALKD site-modulating catalysis, it does not contain any of the motifs required for catalysis and presumably proper folding, suggesting that catalysis and integration into the L-complex can be separated. It is not known whether proteins, other than those belonging to the REL1 and REL2 subcomplexes, can be reintegrated in vitro. Preliminary evidence suggests that recombinant Lt RNA-editing exonuclease 1 (REX1) (= LC-2 or MP100) does not integrate into REX1-depleted L-complex (X.K. and L.S., unpublished results), but this finding may be due to the generalized breakdown of the L-complex engendered by down-regulation of REX1 (36). Nevertheless, the ability to perform functional complementation of several L-complex components in vitro should allow detailed mutational analysis of conserved motifs, and may allow for an understanding of the specific biological roles of these proteins in the editing reaction.
Supplementary Material
Acknowledgments
We thank Ruslan Aphasizhev, Dmitri Maslov, and Daren Osato for helpful discussions; Hee-Jeong Choi for assistance in construction of plasmids, Ken Stuart for the monoclonal antibodies against MP81, MP63, MP42, and REL1; and George Cross for the 29-13 T. brucei cells. This work was supported in part by National Institutes of Health Grant AI09102 (to L.S.).
Author contributions: G.G. and L.S. designed research; G.G., A.M.S., X.K., K.R., M.N., and F.L. performed research; A.M.S., and M.N. contributed new reagents/analytic tools; G.G., A.M.S., and L.S. analyzed data; and G.G. and L.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TUTase, terminal uridylyl transferase; RET, RNA-editing TUTase; REL, RNA-editing ligase; rREL, recombinant REL; TAP, tandem affinity purification; L-complex, ligase-containing complex; Lt, Leishmania tarentolae; Tb, Trypanosoma brucei; RNAi, RNA interference; CBP, calmodulin-binding peptide; brRNA, bridge RNA.
References
- 1.Simpson, L., Sbicego, S. & Aphasizhev, R. (2003) RNA 9, 265-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson, L., Aphasizhev, R., Gao, G. & Kang, X. (2004) RNA 10, 159-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panigrahi, A. K., Allen, T. E., Stuart, K., Haynes, P. A. & Gygi, S. P. (2003) J. Am. Soc. Mass Spectrom. 14, 728-735. [DOI] [PubMed] [Google Scholar]
- 4.Koller, J., Muller, U. F., Schmid, B., Missel, A., Kruft, V., Stuart, K. & Goringer, H. U. (1997) J. Biol. Chem. 272, 3749-3757. [DOI] [PubMed] [Google Scholar]
- 5.Lambert, L., Muller, U. F., Souza, A. E. & Goringer, H. U. (1999) Nucleic Acids Res. 27, 1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller, U. F., Lambert, L. & Goringer, H. U. (2001) EMBO J. 20, 1394-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leegwater, P., Speijer, D. & Benne, R. (1995) Eur. J. Biochem. 227, 780-786. [DOI] [PubMed] [Google Scholar]
- 8.Aphasizhev, R., Aphasizheva, I., Nelson, R. E. & Simpson, L. (2003) RNA 9, 62-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blom, D., Burg, J., Breek, C. K., Speijer, D., Muijsers, A. O. & Benne, R. (2001) Nucleic Acids Res. 29, 2950-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aphasizhev, R., Sbicego, S., Peris, M., Jang, S. H., Aphasizheva, I., Simpson, A. M., Rivlin, A. & Simpson, L. (2002) Cell 108, 637-648. [DOI] [PubMed] [Google Scholar]
- 11.Aphasizhev, R., Aphasizheva, I. & Simpson, L. (2003) Proc. Natl. Acad. Sci. USA 100, 10617-10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peris, M., Simpson, A. M., Grunstein, J., Liliental, J. E., Frech, G. C. & Simpson, L. (1997) Mol. Biochem. Parasitol. 85, 9-24. [DOI] [PubMed] [Google Scholar]
- 13.Rusche, L. N., Cruz-Reyes, J., Piller, K. J. & Sollner-Webb, B. (1997) EMBO J. 16, 4069-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panigrahi, A. K., Gygi, S. P., Ernst, N. L., Igo, R. P., Palazzo, S. S., Schnaufer, A., Weston, D. S., Carmean, N., Salavati, R., Aebersold, R., et al. (2001) Mol. Cell. Biol. 21, 380-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panigrahi, A. K., Schnaufer, A., Carmean, N., Igo, R. P., Jr., Gygi, S. P., Ernst, N. L., Palazzo, S. S., Weston, D. S., Aebersold, R., Salavati, R., et al. (2001) Mol. Cell. Biol. 21, 6833-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panigrahi, A. K., Schnaufer, A., Ernst, N. L., Wang, B., Carmean, N., Salavati, R. & Stuart, K. (2003) RNA 9, 484-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aphasizhev, R., Aphasizheva, I., Nelson, R. E., Gao, G., Simpson, A. M., Kang, X., Falick, A. M., Sbicego, S. & Simpson, L. (2003) EMBO J. 22, 913-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnaufer, A., Ernst, N. L., Palazzo, S. S., O'Rear, J., Salavati, R. & Stuart, K. (2003) Mol. Cell 12, 307-319. [DOI] [PubMed] [Google Scholar]
- 19.Blum, B., Bakalara, N. & Simpson, L. (1990) Cell 60, 189-198. [DOI] [PubMed] [Google Scholar]
- 20.Kable, M. L., Seiwert, S. D., Heidmann, S. & Stuart, K. (1996) Science 273, 1189-1195. [DOI] [PubMed] [Google Scholar]
- 21.Seiwert, S. D., Heidmann, S. & Stuart, K. (1996) Cell 84, 831-841. [DOI] [PubMed] [Google Scholar]
- 22.Cruz-Reyes, J. & Sollner-Webb, B. (1996) Proc. Natl. Acad. Sci. USA 93, 8901-8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz-Reyes, J., Rusche, L., Piller, K. J. & Sollner-Webb, B. (1998) Mol. Cell 1, 401-409. [DOI] [PubMed] [Google Scholar]
- 24.Cruz-Reyes, J., Zhelonkina, A. G., Huang, C. E. & Sollner-Webb, B. (2002) Mol. Cell. Biol. 22, 4652-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang, C. E., Cruz-Reyes, J., Zhelonkina, A. G., O'Hearn, S., Wirtz, E. & Sollner-Webb, B. (2001) EMBO J. 20, 4694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao, G. & Simpson, L. (2003) J. Biol. Chem. 278, 27570-27574. [DOI] [PubMed] [Google Scholar]
- 27.O'Hearn, S. F., Huang, C. E., Hemann, M., Zhelonkina, A. & Sollner-Webb, B. (2003) Mol. Cell. Biol. 23, 7909-7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braly, P., Simpson, L. & Kretzer, F. (1974) J. Protozool. 21, 782-790. [DOI] [PubMed] [Google Scholar]
- 29.Puig, O., Caspary, F., Rigaut, G., Rutz, B., Bouveret, E., Bragado-Nilsson, E., Wilm, M. & Seraphin, B. (2001) Methods 24, 218-229. [DOI] [PubMed] [Google Scholar]
- 30.Ho, C. K. & Shuman, S. (2002) Proc. Natl. Acad. Sci. USA 99, 12709-12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odell, M., Sriskanda, V., Shuman, S. & Nikolov, D. B. (2000) Mol. Cell 6, 1183-1193. [DOI] [PubMed] [Google Scholar]
- 32.Ho, C. K., Wang, L. K., Lima, C. D. & Shuman, S. (2004) Structure (London) 12, 327-339. [DOI] [PubMed] [Google Scholar]
- 33.Deng, J., Schnaufer, A., Salavati, R., Stuart, K. D. & Hol, W. G. (2004) J. Mol. Biol. 343, 601-613. [DOI] [PubMed] [Google Scholar]
- 34.Nandakumar, J. & Shuman, S. (2004) Mol. Cell 16, 211-221. [DOI] [PubMed] [Google Scholar]
- 35.Nandakumar, J., Ho, C. K., Lima, C. D. & Shuman, S. (2004) J. Biol. Chem. 279, 31337-31347. [DOI] [PubMed] [Google Scholar]
- 36.Kang, X., Rogers, K., Gao, G., Falick, A. M., Zhou, S.-L. & Simpson, L. (2005) Proc. Natl. Acad. Sci. USA. 102, 1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.