Abstract
Obstetric haemorrhage is associated with increased risk of serious maternal morbidity and mortality. Postpartum haemorrhage is the commonest form of obstetric haemorrhage, and worldwide, a woman dies due to massive postpartum haemorrhage approximately every 4 min. In addition, many experience serious morbidity such as multi-organ failure, complications of multiple blood transfusions, peripartum hysterectomy and unintended damage to pelvic organs, loss of fertility and psychological sequelae, including posttraumatic stress disorders. Anticipation of massive postpartum haemorrhage, prompt recognition of the cause and institution of timely and appropriate measures to control bleeding and replacement of the lost blood volume and restoration of oxygen carrying capacity (i.e. haemoglobin) and correction of the ‘washout phenomenon’ leading to coagulopathy will help save lives. Obstetric shock index may help in avoidance of underestimation of blood loss and the use of tranexamic acid, oxytocics and timely peripartum hysterectomy, if appropriate, will help save lives. Triple P procedure has been recently developed as the conservative surgical alternative for women with abnormal invasion of the placenta and has been shown to significantly reduce the blood loss and to reduce inpatient stay.
Keywords: compression sutures, HAEMOSTASIS, obstetric shock index, peripartum hysterectomy, Triple P procedure
Introduction
Approximately 830 women die every day around the world as a result of pregnancy or childbirth-related complications with obstetric haemorrhage remaining a major cause of maternal morbidity and mortality.1 A World Health Organization (WHO) systematic analysis into the global causes of maternal deaths from 2003 to 2009 found haemorrhage to be the leading direct cause of maternal mortality followed by hypertensive disorders and sepsis. Overall, haemorrhage accounted for 27.1% of all maternal deaths worldwide.2
Even in the developed world, obstetric haemorrhage remains one of the major causes of maternal death. The Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK (MBRRACE-UK) Confidential Enquiries into Maternal Deaths found the mortality rate as a direct result of haemorrhage to be 0.55 per 100,000 maternities in the United Kingdom from 2011 to 2013.3
Obstetric haemorrhage encompasses both antepartum and postpartum haemorrhage (PPH). This review article discusses the risk factors, causes and management of PPH as it is the most common cause of severe maternal morbidity and mortality.
Definition
PPH refers to excessive bleeding (more than 500 mL) from the genital tract following delivery. Women delivering by Caesarean section, however, generally lose more blood; therefore, a higher cut-off value of 1000 mL is used for significant blood loss.4 Massive PPH refers to the loss of 30%–40% of the patient’s blood volume. The estimation of blood loss can be inaccurate and therefore the American College of Obstetrics and Gynaecology has defined it as a drop of more than 10% in the haematocrit value compared to that pre-delivery.
PPH is classified as Primary if bleeding occurs within the first 24 h following delivery of the foetus. Secondary PPH occurs between 24 h and 12 weeks post-delivery.
Although the above arbitrary values guide clinical management, consideration should be given to factors that may predispose women to haemodynamic decompensation despite losing less blood, for example, women with preceding anaemia or those with low body mass index (BMI).5,6 The diagnosis of PPH, therefore, remains a subjective clinical assessment, which includes any degree of blood loss that threatens the woman’s haemodynamic stability.7
Risk factors
Risk factors for PPH may present antenatally or intrapartum. These are listed in Table 1. Clinicians must be aware of these factors when counselling women on the settings and place of delivery.
Table 1.
Risk factors for PPH.
| • Age > 40 years |
| • High BMI |
| • Asian and African ethnicity |
| • Multiple pregnancy |
| • Placenta praevia |
| • Anaemia and grand multiparity |
| • Pre-eclampsia |
| • Hypertension |
| • Previous PPH |
| • Instrumental delivery |
| • Macrosomia |
| • Retained placenta |
| • Suspected or proven placental abruption |
| • Previous Caesarean sections – placenta accreta or percreta |
| • Prolonged third stage of labour |
BMI: body mass index; PPH: postpartum haemorrhage.
Women with previous Caesarean delivery as well as placenta praevia have a high incidence of placental abnormalities including placenta accreta, increta and percreta;8,9 placental location and anatomy should therefore be fully investigated in these women with a high index of suspicion of abnormal placental invasion.
Those who have a suspected diagnosis of abnormal placental invasion should have their care transferred to a multi-disciplinary setting with the appropriate level of expertise.
PPH can also occur in women with no identifiable risk factors; therefore, clinicians should be prepared to manage this complication at every delivery and anticipation is essential to save lives.
Causes
In a guideline on the prevention and management of PPH, the Society of Obstetricians and Gynaecologists of Canada summarized the causes of PPH as one or more of four simple processes:
Uterine atony – PPH will occur if the uterus does not contract well enough to arrest bleeding at the placental site.
Retained products – retained products of conception or blood clots will contribute to PPH.
Trauma – genital tract trauma may cause bleeding and lead to large volume PPH especially if not identified promptly.
Coagulation abnormalities – clotting disorders can lead to PPH alone or in combination with the other factors. These abnormalities may be congenital or acquired.
These causes can be thought of as the ‘Four T’s’ as a memory aid: Tone, Tissue, Trauma and Thrombin (Table 2).
Table 2.
The ‘Four Ts’ mnemonic for causes of PPH.
| T | Cause | Approximate incidence |
|---|---|---|
| Tone | Uterine atony | 80% |
| Tissue | Retained tissue, invasive placenta | 5% |
| Trauma | Genital tract laceration or tear, uterine rupture, uterine inversion | 13% |
| Thrombin | Coagulopathy, disseminated intravascular coagulation (DIC) | 2% |
Diagnosis and management
The diagnosis of PPH requires clinicians to recognize excessive bleeding and follow a systematic method to identify the cause. Its effective management requires a multi-disciplinary approach.
Clinical diagnosis is based on the following:
Blood loss > 500 mL following vaginal delivery.
Blood loss > 1000 mL following Caesarean section.
Signs of haemodynamic instability in the context of excessive bleeding following delivery.
Substantial drop in the haematocrit (American College of Obstetricians & Gynecologists [ACOG] 1998).
Once PPH has been diagnosed, there must be a multi-disciplinary and multi-professional team approach in managing it, as ongoing patient resuscitation should occur alongside identification and treatment of the cause of bleeding.
Tone
In the third stage of labour, myometrial contraction is responsible for separation of placental membranes as well as haemostasis, which is achieved by constriction of uterine blood vessels as the myometrium contracts. Active management of the third stage, which includes prophylactic use of uterotonic agents, controlled cord traction and usually early cord clamping has been shown to reduce the risk of primary blood loss of greater than 500 mL.7 Uterotonics which increase the efficiency of uterine contractions such as oxytocin and ergometrine have been in use since the 19th century for the treatment of atonic PPH.4
Uterine atony is the most common cause of PPH4 and is managed initially by bimanual uterine compression or massage as well as uterotonic treatments. Following delivery, careful examination of the uterus should be performed to assess uterine contraction and tone.
A management algorithm – ‘HAEMOSTASIS’ – has been described to aid systematic and stepwise management of atonic PPH (Box 1).10
Box 1. Management algorithm for atonic postpartum haemorrhage: HAEMOSTASIS.
H: Ask for HELP and Hands on the uterus – uterine massage
A: Assess and resuscitate (vital signs, intravenous fluids, blood and blood products)
E: Establish aetiology, Ensure availability of blood and Ecbolics (oxytocin)
M: Massage uterus
O: Oxytocics – oxytocin infusion/prostaglandins
S: Shift to theatre – bimanual compression
T: Tissue and trauma – exclude/manage/proceed to tamponade balloon
A: Apply B-Lynch/modified compression sutures
S: Systematic pelvic devascularization (uterine/ovarian/quadruple/internal iliac)
I: Interventional radiology – uterine artery embolization if appropriate
S: Subtotal/total abdominal hysterectomy
Once uterine atony is diagnosed, the following mechanical, pharmacological or surgical measures should be instigated to ensure uterine contraction and cessation of haemorrhage:
Bimanual uterine massage
Once PPH is identified, the clinician should perform a bimanual examination of the uterus; if the uterus is soft or ‘boggy’, bimanual massage is initiated where one hand is placed in the vagina compressing the uterus and the other hand massages the fundus through the abdominal wall.
Pharmacological management
Oxytocin, ergot alkaloids and prostaglandins are commonly used uterotonic agents. Prophylactic uterotonics should be offered routinely in all women as they reduce the risk of PPH by 60%.11
Syntocinon
Oxytocin is the first choice for the prevention of PPH and has fewer side effects compared to Ergot Alkaloids.
Ergometrine
Ergometrine is an Ergot Alkaloid (alpha adrenergic, dopaminergic and serotonin receptor agonist), which causes both the upper and lower segments of the uterus to contract. Its use is contraindicated in severe cardiac disease, hypertension as well as eclampsia.
Syntometrine (a combination of oxytocin and ergometrine)
Misoprostol
Misoprostol has been shown to be not as effective in comparison with oxytocin in reducing the incidence of PPH. However, in settings where no alternatives are available or in situations where resources are limited, misoprostol (600 µg orally) may be used to manage PPH.
Carboprost (Hemabate)
Carboprost is a synthetic analogue of prostaglandin F2α (15-methyl-PGF2α), which has oxytocic properties and therefore causes uterine contractions. It, therefore, has a role in managing atonic PPH.
Tranexamic acid (1 g intravenously)
Tranexamic acid is an anti-fibrinolytic agent and should be considered in all cases of atonic and traumatic PPH to reduce the bleeding from the placental site and the site of trauma, respectively. It helps stabilize the blood clot by preventing breakdown of the formed clot (i.e. fibrin) to fibrin degradation products.
Balloon tamponade
Uterine tamponade can be attempted if bleeding continues despite conservative measures by using various balloons. These include the ‘Bakri SOS’ balloon or the Sengstaken–Blakemore oesophageal catheter. More commonly available Foley catheters can also be used.5 Approximately 300–400 mL of sterile water or saline is used to infiltrate the catheter balloon to achieve the appropriate level of counter pressure to cease bleeding. Condous et al. have described the ‘tamponade test’, which has a positive predictive value of 87.5% for satisfactory management of PPH. If the test is positive, that is, the tamponade effect ceases bleeding, then the likelihood of need for further, more radical surgical intervention is minimal. If, however, the test is negative, then there is often the need for further intervention.12
Surgical management
Surgical intervention is recommended if there is no response to uterotonics and other conservative measures.
Compression sutures
In 1997, B-Lynch et al.13 described and published his brace suturing technique (Figure 1) for the management of PPH when all conservative measures have failed, as an alternative to hysterectomy. This method is easy to apply and effective in achieving haemostasis by compressing the uterus without occluding the uterine vessels. Since then, many variations of the classic technique have been described. More recently, El-Sokkary et al.14 have published the ‘modified Lynch suture’ which has been found to be superior to the classic technique. The aim of these compression sutures is to control bleeding from the placental site by apposing the anterior and posterior uterine walls.
Figure 1.

Application of the B-Lynch brace suture.
Artery ligation
In cases of continued bleeding, systematic pelvic devascularization can be attempted. This involves ligation of the uterine arteries, tubal branch of the ovarian arteries and the internal iliac arteries. If bleeding persists, one can attempt a ‘quadruple ligation’. This involves ligation of uterine vessels as well as ligation of both tubal branches of ovarian arteries in the mesosalpinx.5
Interventional radiology – uterine artery embolization
Uterine artery embolization can be considered in the management of haemodynamically stable patients. It involves catheterization of uterine arteries followed by injection of embolic material. A 5-year review from two centres in the United Kingdom has reported a success rate of 89.4%.15
Hysterectomy
If all attempts at arresting bleeding have failed, subtotal or total hysterectomy is attempted as a last resort and life-saving measure. The decision to perform a hysterectomy, although devastating for patients, should not be delayed in cases of haemodynamic instability. The decision to escalate surgical management to hysterectomy should be made by the most senior obstetrician. Subtotal hysterectomy is indicated in cases where the bleeding source is the upper segment of the uterus. In cases of placenta praevia or where cervical or vaginal tears contribute to bleeding, a total hysterectomy is indicated (Figure 2).5
Figure 2.
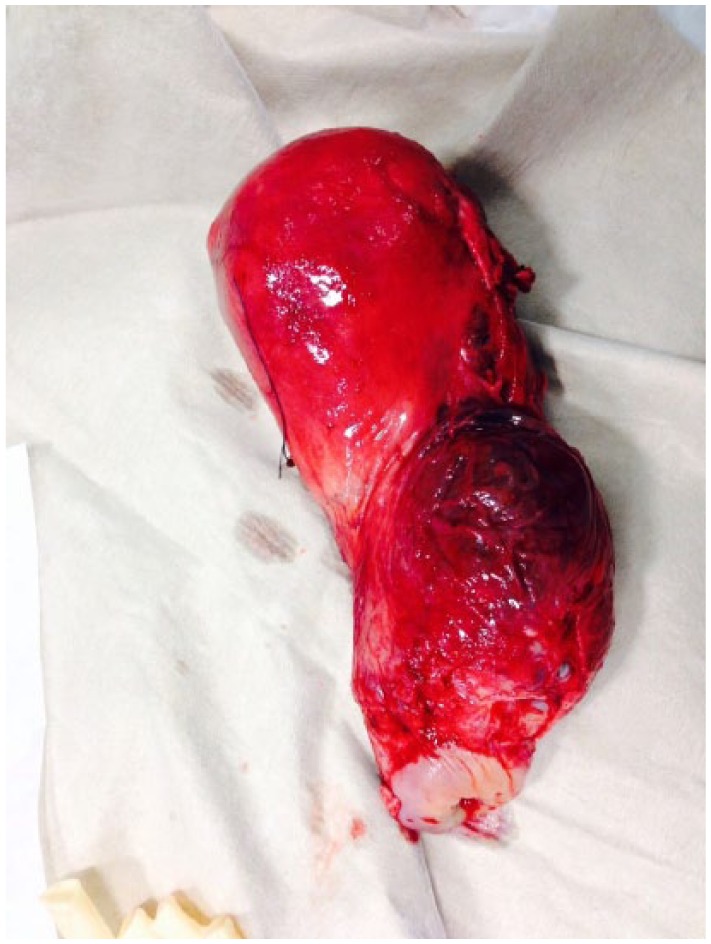
Peripartum hysterectomy for abnormal invasion of the placenta.
Tissue
Retained products of conception can cause either primary or secondary PPH. In cases of retained placenta, intravenous or intramuscular oxytocin is administered alongside controlled cord traction in an attempt to aid expulsion of placenta. In cases of retained placenta, manual removal should be attempted under anaesthesia. A blunt curette (often under ultrasound guidance) is used to prevent Asherman’s syndrome.
Cases of secondary PPH are often due to a combination of retained products alongside an element of infection (endometritis) and are therefore managed with antibiotics; surgical evacuation of products may become necessary if there is clinical suspicion or ultrasound evidence of retained products.
Trauma
Over 85% of women who have a vaginal delivery will sustain some degree of perinea trauma and up to 60%–70% of these will undergo suturing.16,17 Systematic examination of the genital tract should be performed to identify any trauma to the cervix, vagina or perineum. Upper vaginal or cervical tears should be repaired by an experienced obstetrician, as there is a risk of damage to the nearby structures (bladder neck and upper urethra, ureters, rectum and loops of bowel in the Pouch of Douglas).
Cervical tears are uncommon following childbirth. Small tears (<2 cm), which do not bleed, can be managed conservatively. However, larger tears or those with profuse bleeding need to be sutured.5
Thrombin
Congenital or acquired clotting disorders (e.g. factor VIII or factor IX deficiency, Von Willebrand’s disease) can cause or contribute to PPH; therefore, identification and correction of any coagulopathy can help improve the outcome. Coagulopathy may be diagnosed by:
Clinical observation;
Laboratory-based clotting studies and platelet counts;
Point-of-care testing – with the advantage of prompt availability of results.
Local transfusion policies on the use of blood and other blood-related products should be sought alongside expert advice from haematologists in cases of ongoing bleeding caused by coagulopathy.
New developments: Triple P procedure
Described in 2010 at St George’s Hospital, London, this is a conservative surgical alternative to peripartum hysterectomy for women with morbidly adherent placenta anteriorly (Figure 3) or posteriorly to reduce severe maternal morbidity and mortality associated with peripartum hysterectomy. The procedure entails perioperative placental localization and delivery of foetus via transverse uterine incision above the upper border of placenta, pelvic devascularization and placental non-separation with myometrial excision followed by reconstruction of the uterine wall.18 A recent study has shown that the Triple P procedure is associated with a statistically significant reduction in PPH and in patient hospitalization.19 Based on the institutional data, out of 52 cases of Triple P procedures (Figure 4) that have been performed thus far, no cases of peripartum hysterectomies have been performed. This procedure requires the use of prophylactic occlusion balloon catheters within the anterior division of internal iliac arteries and this may not be universally available in low-resource settings.
Figure 3.

Placenta percreta invading the anterior uterine wall and the urinary bladder.
Figure 4.

Myometrial excision with invading placenta.
Obstetric shock index
Le Bas et al.20 recently reported that during pregnancy the normal value for the shock index (i.e. pulse rate divided by systolic blood pressure) is higher than the non-pregnant adult population secondary to increase in the pulse rate and a fall in systolic blood pressure. An obstetric shock index (OSI) > 1 has been reported to be associated with increase in the likelihood of requiring a blood transfusion following PPH. Therefore, OSI may be used as simple ‘bedside’ clinical test to assess the degree of blood loss as visual estimation of blood loss is associated with significant inter- and intra-observer errors. The latest Green Top Guideline published by the Royal College of Obstetricians and Gynaecologists also highlights the importance of OSI in identifying women at the risk of adverse outcomes.21
Conclusion
PPH remains one of the leading causes of maternal morbidity and mortality globally. It is often possible to anticipate and take steps in preventing it. The effective management of PPH requires prompt recognition, rapid response and mobilization of the multi-disciplinary team. Patient resuscitation to maintain blood volume and haemodynamic stability and the simultaneous identification and treatment of the source of bleeding remain the cornerstones of management of PPH.
Acknowledgments
E.C. designed the manuscript; M.S. performed the literature search; E.C. and M.S. co-wrote the manuscript; and E.C. took the overall responsibility revising the manuscript and for ensuring the accuracy of the content.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1. World Health Organization (WHO). Maternal mortality, 15 March 2016, http://www.who.int/mediacentre/factsheets/fs348/en/ (accessed 24 October 2016).
- 2. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014; 2(6): e323–e333, http://www.thelancet.com/journals/langlo/article/PIIS2214-109X(14)70227-X/fulltext (accessed 24 October 2016). [DOI] [PubMed] [Google Scholar]
- 3. Saving Lives Improving Mothers’ Care: Surveillance of maternal deaths in the UK 2011-13 and lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009-13. MBRRACE-UK, December 2015, https://www.npeu.ox.ac.uk/downloads/files/mbrrace-uk/reports/MBRRACE-UK%20Maternal%20Report%202015.pdf
- 4. Mousa HA, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database Syst Rev 2007; 2: CD003249. [DOI] [PubMed] [Google Scholar]
- 5. Chandraharan E, Arulkumaran S. Surgical aspects of postpartum haemorrhage. Best Pract Res Clin Obstet Gynaecol 2008; 22(6): 1089–1102. [DOI] [PubMed] [Google Scholar]
- 6. Schuurmans N, MacKinnon C, Lane C, et al. Prevention and management of postpartum haemorrhage. J Soc Obstet Gynaecol Can 2000; 22: 271–281. [Google Scholar]
- 7. Begley CM, Gyte GML, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev 2011; 11: CD007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fitzpatrick KE, Sellers S, Spark P, et al. Incidence and risk factors for placenta accreta/increta/percreta in the UK: a national case-control study. PLoS ONE 2012; 7(12): e52893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol 2005; 192: 1458–1461. [DOI] [PubMed] [Google Scholar]
- 10. Chandraharan E, Arulkumaran S. Management algorithm for atonic postpartum haemorrhage. J Paediatrics Obstet Gynaecik 2005; 31: 106–112. [Google Scholar]
- 11. Arulkumaran S, Mavrides E, Penney GC. Prevention and management of postpartum haemorrhage (RCOG Green-top Guideline No. 52 May 2009). London: RCOG Press, 2009. [Google Scholar]
- 12. Condous GS, Arulkumaran S, Symonds I, et al. The tamponade tests for massive postpartum haemorrhage. Obstet Gynecol 2003; 101(4): 767–772. [DOI] [PubMed] [Google Scholar]
- 13. B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J ObstetGynaecol 1997; 104(3): 372–375. [DOI] [PubMed] [Google Scholar]
- 14. El-Sokkary M, Wahba K, El-Shahawy Y. Uterine salvage management for atonic postpartum hemorrhage using ‘modified lynch suture’. BMC Pregnancy and Childbirth 2016; 16(1): 251. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Ratnam LA, Gibson M, Sandhu C, et al. Transcatheter pelvic arterial embolisation for control of obstetric and gynaecological haemorrhage. J Obstet Gynaecol 2008; 28(6): 573–579. [DOI] [PubMed] [Google Scholar]
- 16. McCandlish R, Bowler U, Van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol 1998; 105: 1262–1272. [DOI] [PubMed] [Google Scholar]
- 17. Sleep J, Grant A, Garcia J, et al. West Berkshire perineal management trial. BMJ 1984; 289: 587–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chandraharan E, Rao S, Belli AM, et al. The Triple-P procedure as a conservative surgical alternative to peripartum hysterectomy for placenta percreta. Int J Gynaecol Obstet 2012; 117(2): 191–194. [DOI] [PubMed] [Google Scholar]
- 19. Teixidor Viñas M, Belli AM, Arulkumaran S, et al. Prevention of postpartum hemorrhage and hysterectomy in patients with morbidly adherent placenta: a cohort study comparing outcomes before and after introduction of the Triple-P procedure. Ultrasound Obstet Gynecol 2015; 46(3): 350–355. [DOI] [PubMed] [Google Scholar]
- 20. Le Bas A, Chandraharan E, Addei A, et al. Use of the ‘obstetric shock index’ as an adjunct in identifying significant blood loss in patients with massive postpartum hemorrhage. Int J Gynaecol Obstet 2014; 124(3): 253–255. [DOI] [PubMed] [Google Scholar]
- 21. Mavrides E, Allard S, Chandraharan E, et al. Prevention and management of postpartum haemorrhage. BJOG 2016; 124: e106–e149. [DOI] [PubMed] [Google Scholar]


