Abstract
Background
To determine the efficacy of low-dose, immediate-release tacrolimus in patients with myasthenia gravis (MG) with inadequate response to glucocorticoid therapy in a randomized, double-blind, placebo-controlled study.
Methods
Eligible patients had inadequate response to glucocorticoids (GCs) after ⩾6 weeks of treatment with prednisone ⩾0.75 mg/kg/day or 60–100 mg/day. Patients were randomized to receive 3 mg tacrolimus or placebo daily (orally) for 24 weeks. Concomitant glucocorticoids and pyridostigmine were allowed. Patients continued GC therapy from weeks 1–4; from week 5, the dose was decreased at the discretion of the investigator. The primary efficacy outcome measure was a reduction, relative to baseline, in quantitative myasthenia gravis (QMG) score assessed using a generalized linear model; supportive analyses used alternative models.
Results
Of 138 patients screened, 83 [tacrolimus (n = 45); placebo (n = 38)] were enrolled and treated. The change in adjusted mean QMG score from baseline to week 24 was −4.9 for tacrolimus and −3.3 for placebo (least squares mean difference: –1.7, 95% confidence interval: −3.5, −0.1; p = 0.067). A post-hoc analysis demonstrated a statistically significant difference for QMG score reduction of ⩾4 points in the tacrolimus group (68.2%) versus the placebo group (44.7%; p = 0.044). Adverse event profiles were similar between treatment groups.
Conclusions
Tacrolimus 3 mg treatment for patients with MG and inadequate response to GCs did not demonstrate a statistically significant improvement in the primary endpoint versus placebo over 24 weeks; however, a post-hoc analysis demonstrated a statistically significant difference for QMG score reduction of ⩾4 points in the tacrolimus group versus the placebo group. This study was limited by the low number of patients, the absence of testing for acetylcholine receptor antibody and the absence of stratification by disease duration (which led to a disparity between the two groups).
ClinicalTrials.gov identifier: NCT01325571
Keywords: immunology, myasthenia gravis, tacrolimus
Introduction
Myasthenia gravis (MG) is an autoimmune disease characterized by weakness and fatigability of skeletal muscles.1–3 It is a T-cell dependent, antibody- and complement-mediated autoimmune disease, in which antibodies (mainly acetylcholine receptor antibody and, to a lesser extent, MuSK, LRP4 and Agrin) target the acetylcholine receptor (AChR) or other related proteins at the neuromuscular junction.3 Symptoms range in severity and include muscle weakness (often in the ocular muscles) and difficulty in chewing and swallowing. The most severe cases of MG require intubation.4 MG is rare, with varying incidence and prevalence rates according to the timing and geographic region of reported studies.5 There are no epidemiological data available for mainland China; however, in Taiwan a prevalence of 14/100,000 was reported.6
Treatment strategies depend on specific patient characteristics (i.e. disease severity or organ function), and have the overall aim of achieving disease remission and normal functioning while minimizing the risk and severity of adverse events (AEs).7 Conventional treatments for MG include cholinesterase inhibitors, acute immune-modulatory therapies (i.e. immunoglobulin or plasmapheresis), resection of the thymus gland and immunosuppression with glucocorticoids (GCs), azathioprine, cyclophosphamide or mycophenolate mofetil.7,8 Although long-term suppression of the immune system with GC can be an effective treatment for MG, some patients respond inadequately or have poor tolerance to GC. Furthermore, long-term GC use is associated with AEs such as weight gain, osteoporosis and Cushing’s syndrome.1,9 Therefore, treatments that enable dose reduction and elimination of GC therapy are needed.
Tacrolimus, used to prevent solid organ transplant rejection, is a macrolide compound with immunosuppressive effects that act through the inhibition of T-cell activation.10 The efficacy and safety of tacrolimus in treating patients with MG who were unresponsive or intolerant to oral GC have been explored in open-label trials.11 In these trials, tacrolimus treatment led to improvements in quantitative MG (QMG) score12–14 and to improvements in MG activities of daily living (MG-ADL) in patients who were intolerant to GCs.12,14,15 Longer-term (⩽5 years) open-label studies in GC-dependent or GC-unresponsive patients have demonstrated the ability of tacrolimus to reduce the severity of MG symptoms, with most patients reporting improved function and/or a reduction in GC dose.13,15
To date, there has been only one randomized, double-blind, placebo-controlled study of tacrolimus for MG treatment.16 It was designed to evaluate the ability of tacrolimus to reduce GC dose over a 28-week period while maintaining a stable, minimal manifestation disease state: no significant difference in the primary outcome measure was reported. Similarly, no differences were observed in secondary efficacy measures, including QMG score and MG-ADL; however, the study was not powered to detect these differences.16
Here, we report a randomized, double-blind, placebo-controlled study conducted in China to investigate the efficacy of tacrolimus in the treatment of patients with MG who have inadequate response to GC therapy.
Methods
This was a randomized, double-blind, placebo-controlled, multicentre, phase III clinical trial, conducted across 13 sites in China, of tacrolimus capsules in patients with MG who had inadequate response to GC treatment. The study was conducted in accordance with the International Conference on Harmonization guidelines for Good Clinical Practice, the current revision of the Declaration of Helsinki and the current regulatory rules of China. The study was approved by appropriate independent ethics committees and written informed consent was obtained from all patients.
Patients
All patients included in the study were aged 18–70 years with a definitive diagnosis of MG, meaning a positive result from repetitive low-frequency electric stimulation showing a decrement >10% in amplitude of compound muscle action potential (CMAP), a negative result from repetitive high-frequency electric stimulation and at least two of the following conditions: (1) fluctuating skeletal muscle weakness; (2) positive muscle fatigability test; and (3) positive neostigmine test. Notably, neostigmine test was required to be positive in all patients.
AChR antibody testing was absent because it was difficult to determine the inclusion of patients based on antibody testing in some centres owing to methodology problems. Following the criteria proposed by other trials,16,17 our diagnostic criteria not including antibody information was agreed during a meeting of investigators in China.
All patients included in the study had an inadequate response to GC treatment and a QMG score ⩾7. To facilitate the selection of such patients, a definition of ‘inadequate response’ was agreed during an investigators’ meeting in 2011 that took into consideration the common clinical practice at that time in China. Patients were required to have received prednisone at ⩾0.75 mg/kg/day or 60–100 mg/day as an active treatment for at least 6 weeks prior to study enrolment. Inadequate response was defined as meeting any two of the following four criteria: (1) QMG score improved by <25%; (2) manual muscle test (MMT) score improved by <25%; (3) MG-ADL score improved by <25%; and (4) Osserman’s classification grade failed to reduce.
Patients were excluded if they met any of the following criteria: received pyridostigmine dose >240 mg/day within 2 weeks prior to study initiation, blood purification therapy or immunoglobulin therapy within 8 weeks, or other immunosuppressive agents within 12 weeks; ocular MG; a QMG swallowing score ⩾2; a QMG respiratory score of 3; abnormal liver function; uncontrolled diabetes, concomitant pancreatitis during the 12-week period prior to the trial; hyperkalaemia; severe complications, such as infection; a history of therioma; HIV infection; active tuberculosis or hepatitis; myocardial disease, acute coronary events or severe arrhythmias; or uncontrolled hypertension.
There was a 7-week screening phase followed by a 24-week treatment phase. After the baseline visit (first day of drug administration), follow-up assessments were conducted at weeks 4, 8, 12, 16, 20 and 24 (±5 days). A follow-up telephone call was conducted at week 26.
Interventions
Patients were randomly assigned in a 1:1 ratio to 3 mg tacrolimus (PROGRAF®, Astellas Pharma US, Inc., Northbrook, USA) capsules or to a matched placebo; both were administered once daily for 24 weeks and taken orally after an evening meal.
Patients in both groups were permitted to receive concomitant GC and pyridostigmine. Patients continued to receive their dose of GC for 4 weeks after randomization; from week 5, the dose was decreased at the investigator’s discretion based on disease condition. Other immunosuppressant drugs were not permitted.
Outcomes
The primary efficacy outcome measure was a reduction, relative to baseline, in QMG score (Supplementary Table 1) by the end of the trial. Secondary efficacy endpoints were an improvement in Osserman’s classification grade (Supplementary Table 1) according to the University of Virginia’s modification of Osserman’s classification,18,19 improvement in MG-ADL and MMT scores (Supplementary Table 1) and a reduction in GC dose and dropout rate. To overcome the potential influence of cholinesterase inhibitors, each clinical evaluation was performed at least 4 h after the last dose of pyridostigmine.
Information on AEs was collected at each visit. A complete physical examination and electrocardiogram were conducted at screening and at the end of the trial. Blood and urine samples were collected at designated time points for routine tests including serum biochemistry, complete blood count, blood lipids, urinalysis and measurement of blood concentration of tacrolimus.
Withdrawal criteria included exacerbation of disease (a QMG swallowing function score ⩾2, a QMG respiratory score of 3 or a QMG score that had increased by ⩾50%) and creatinine levels >25% of the upper limit of normal.
Randomization and blinding
Randomization was performed using a stratified block randomization method (the stratification factor was site, and the block size for each site was 2) with a statistical software package (SAS® v9.1.3). Independent biostatisticians generated randomization numbers and used them to assign patients randomly to each group in a 1:1 ratio.
The treatment groups were recursive by order of randomization number, and the predetermined values of parameters such as block size and seed number were recorded in blind codes. The study drug and the placebo, which appeared identical except for the randomization number on the label, were dispensed by order of randomization number. During blind coding, both the blind codes for the first unblinding (only either group A or group B were assigned to each patient) and those for the second unblinding (both group A and group B) were indicated as either the study group or placebo group.
Statistical methods
To ensure 80% power to detect the superiority of tacrolimus, assuming a two-sided type I error of 0.05, 170 patients (85 patients per treatment group) were required. However, because of the strict inclusion/exclusion criteria, investigators anticipated that patient numbers would be limited and thus recommended a smaller sample size. Taking the dropout rate into consideration, the final sample size was determined to be 40 per group.
Statistical analyses were performed using the statistical software package SAS® v9.1.3 (or later versions). All significance testing was two-tailed at a 0.05 significance level. All randomized patients who received at least one dose of study drug were included in the safety analysis set (SAF); those who received one dose of study drug and had at least one efficacy measurement were included in the full analysis set (FAS); all patients who received 80–120% of their planned dose, who did not use prohibited drugs and who had no efficacy protocol deviations or violations were included in the per-protocol set (PPS). Demographic and baseline characteristics, summarized by between-group differences, were compared using t tests (for continuous variables) or Fisher’s exact test (for categorical variables). For the primary efficacy endpoint, generalized linear models were used to compare the change in total QMG scores between the two groups; compound symmetry and unstructured mixed-effects models were used in pre-specified supportive analyses of the primary efficacy endpoint. FAS was the primary analysis set for efficacy endpoints; supportive analyses used both the FAS and the PPS.
A post-hoc analysis using last observation carried forward was conducted to establish whether there was an improvement for total QMG score of ⩾4 points, which, according to a previous study, represents the minimal clinically relevant difference.20
Results
Patients
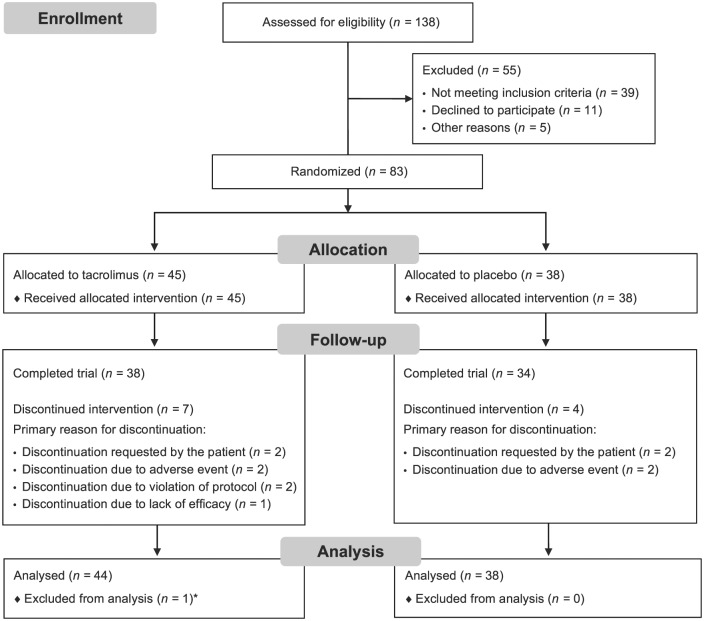
Patients were enrolled from 13 sites across China. The date of first informed consent was 30 March 2011; the last patient was evaluated 22 May 2014. In total, 138 patients were screened for inclusion; of these, 83 patients were randomized and received at least one dose of study drug [tacrolimus (n = 45); placebo (n = 38)] (Figure 1). One patient in the tacrolimus group received the study drug but had no efficacy measures assessed and thus was excluded from the FAS. One patient was excluded from the FAS and PPS owing to having no assessment of QMG scale at any post-baseline visit. Ten patients were excluded from the PPS owing to protocol violations.
Figure 1.
Patient disposition.
*The patient was excluded from both the full analysis set and the per-protocol set due to no assessment of quantitative MG scale at any post-baseline visit.
There were no significant differences in baseline demographics between treatment groups apart from the duration of MG (Table 1), which was significantly lower at baseline for the tacrolimus group (27.9 months) than the placebo group (63.5 months; p = 0.028). The most common medical histories (>5% overall) were thymectomy (17.1%), thymoma (15.9%), hypertension (13.4%), hyperlipidaemia (7.3%) and diabetes mellitus (6.1%), with some non-statistically significant differences between groups. Seven patients (15.6%) in the tacrolimus group received additional treatments for diabetes, versus one patient (2.6%) in the placebo group; two patients (4.4%) in the tacrolimus group received agents acting on the renin–angiotensin system, versus five patients (13.2%) in the placebo group. All patients in the SAF received systemic GC [prednisone (96.4%) or methylprednisolone (6.0%)], with no significant differences in therapy dose between treatment groups (median daily GC dose at baseline was 40.0 mg for both groups). Cholinesterase inhibitors were received by 85.5% of patients, with similar proportions between the two groups. The mean daily dose for cholinesterase inhibitors at baseline was 163.3 mg in the tacrolimus group versus 146.8 mg in the placebo group.
Table 1.
Baseline characteristics (FAS).
| Parameter | Tacrolimus (n = 44) |
Placebo (n = 38) |
p-value |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 41.0 (12.8) | 44.0 (12.1) | 0.284 |
| Median (range) | 40.0 (20–68) | 43.0 (24–68) | |
| Sex, n (%) | |||
| Male | 16 (36.4) | 20 (52.6) | 0.182 |
| Female | 28 (63.6) | 18 (47.4) | |
| Ethnicity, n (%) | |||
| Han | 42 (95.5) | 38 (100.0) | 0.497 |
| Others | 2 (4.5) | 0 (0.0) | |
| BMI (kg/m2) | |||
| Mean (SD) | 22.9 (2.9) | 23.8 (3.1) | 0.179 |
| Median (range) | 22.6 (17.8–28.3) | 24.0 (16.6–31.2) | |
| Duration of MG (months*) | |||
| Mean (SD) | 27.9 (37.8) | 63.5 (90.2) | 0.028 |
| Median (range) | 13.3 (0.0–196.7) | 33.00 (1.9–455.7) | |
| Concomitant conditions, n (%) | |||
| Diabetes mellitus | 4 (9.1) | 1 (2.6) | 0.369 |
| Hypertension | 4 (9.1) | 7 (18.4) | 0.330 |
| Hyperlipidaemia | 2 (4.5) | 4 (10.5) | 0.405 |
| Baseline daily GC dose (mg) | |||
| Mean (SD) | 42.1 (13.5) | 40.8 (10.9) | NA |
| Median (range) | 40.0 (30.0–50.0) | 40 (20.0–80.0) | NA |
| QMG score | |||
| Mean (SD) | 14.3 (4.3) | 13.6 (3.7) | 0.432 |
| Median (range) | 14.0 (7–24) | 14.0 (7–21) | |
| Osserman’s scale grading | |||
| 1 | 0 (0) | 0 (0) | 0.606* |
| 2 | 16 (36.4) | 15 (39.5) | |
| 3 | 21 (47.7) | 19 (50.0) | |
| 4 | 7 (15.9) | 4 (10.5) | |
| 5 | 0 (0) | 0 (0) | |
| Activities of daily living scale† | |||
| Mean (SD) | 6.2 (3.3) | 5.6 (3.1) | 0.383 |
| Median (range) | 5.5 (1–14) | 6.0 (0–12) | |
| Manual muscle test | |||
| Mean (SD) | 21.3 (14.0) | 21.2 (15.4) | 0.986 |
| Median (range) | 20.0 (3–62) | 17.0 (2–72) |
A significant difference in duration of MG between groups (p = 0.028). There were no other statistically significant differences in other demographic variables; †Total score.
BMI, body mass index; FAS, full analysis set; GC, glucocorticoid; MG, myasthenia gravis; SD, standard deviation; QMG, quantitative MG.
Primary efficacy endpoint
The change in adjusted mean QMG score from baseline to week 24 was larger in patients receiving tacrolimus versus placebo (–4.9 versus –3.3, respectively) (Figure 2), but the difference did not reach statistical significance [least squares (LS) mean difference: –1.7; 95% CI: –3.5 to 0.1; p = 0.067). At week 16 there was a statistically significant improvement in QMG score from baseline with tacrolimus compared with placebo (LS mean difference: –2.07; p = 0.012).
Figure 2.
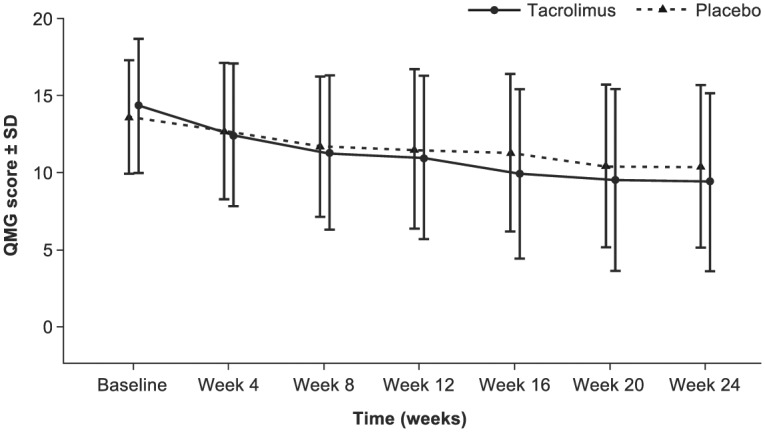
Mean QMG score with tacrolimus versus placebo over 24 weeks [FAS (LOCF)].
FAS, full analysis set; LOCF, last observation carried forward; QMG, quantitative myasthenia gravis; SD, standard deviation.
In supportive analyses, the LS mean difference in QMG score at week 24 versus placebo reached statistical significance for both the compound symmetry model and the unstructured covariance structure in the FAS population (–1.9, p = 0.012; and −1.8, p = 0.046, respectively) and the PPS population (–1.8, p = 0.022; and −1.9, p = 0.044).
Secondary endpoints
There were no statistically significant differences between tacrolimus and placebo for any of the secondary efficacy endpoints at week 24 (Table 2). In a supportive analysis using the PPS, a significant improvement in Osserman’s classification grade from baseline was observed with tacrolimus treatment (p = 0.035). In the tacrolimus group, there were more patients with an Osserman’s classification grade that decreased from baseline by ⩾1 grade versus placebo. For MG-ADL and MMT, the decreases from baseline were higher for the tacrolimus group than the placebo group. Mean change in daily GC dose at week 24 was −16.4 mg and −15.3 mg for tacrolimus and placebo, respectively (p = 0.767). There was no statistically significant difference between treatment groups in the number of patients completing the trial at week 24.
Table 2.
Summary of secondary efficacy endpoints (FAS).
| Week 24 | Adjusted mean change from baseline (SE)* |
Chi-square† | LS mean difference | p-value | |
|---|---|---|---|---|---|
| Tacrolimus | Placebo | ||||
| Osserman’s classification score | 2.85 | 0.092 | |||
| MG-ADL score | −3.7 (0.4) | −2.8 (0.4) | −0.9 (–2.0–0.2) | 0.099 | |
| MMT score | |||||
| Manual muscle test | −12.4 (1.2) | −9.3 (1.3) | −3.0 (–6.7–0.6) | 0.097 | |
| Cranial nerves muscle force | −4.9 (0.5) | −3.9 (0.6) | −1.0 (–2.5–0.5) | 0.201 | |
| Human body muscle force | −7.5 (0.9) | −5.4 (1.0) | −2.1 (–4.9–0.7) | 0.137 | |
| GC dose change (mg/day) | −16.4 (2.5) | −15.3 (2.7) | −1.1 (–8.5–6.3) | 0.767 | |
| Patients who completed the trial (%) | 86.4 | 89.5 | − | − | 0.745‡ |
A generalized linear model, using the observed cases to compare the difference in the change of MG-ADL, MMT and GC dose from baseline. The response variable was the difference in indication at baseline and at the end of the trial. Baseline score was the covariate and treatment group was the fixed effect; † A non-parametric Mann–Whitney U test was used to compare the Osserman’s classification change from baseline between the treatment groups. ‡ Fisher’s exact test was used to compare the number of patients who completed the trial between treatment groups.
FAS, full analysis set; GC, glucocorticoid; LS, least squares; MG-ADL, myasthenia gravis-related activities of daily living; MMT, manual muscle test; SE, standard error.
Post-hoc analysis
The number of patients who achieved the minimal clinically relevant difference in total QMG score (reduction of ⩾4) was significantly higher in the tacrolimus group (68.2%, 30/44 patients) than the placebo group (44.7%, 17/38 patients; p = 0.044) (Figure 3).
Figure 3.
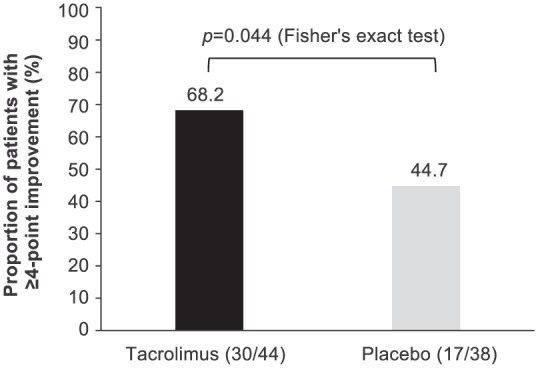
Patients with clinically important improvement in QMG score (⩾4) between baseline and week 24 [FAS (LOCF)].
FAS, full analysis set; LOCF, last observation carried forward; QMG, quantitative myasthenia gravis.
Safety
The incidence and severity of AEs were similar across the treatment groups (Table 3). There was no statistically significant difference in the proportion of patients considered by the investigator to have potentially drug-related AEs (73.3%: tacrolimus; 76.3%: placebo; p = 0.804). A total of 11 patients (13.3%) reported serious AEs (SAEs) with no statistically significant difference between the two groups (placebo: 15.8%; tacrolimus: 11.1%; p = 0.747). The SAEs reported by nine patients were considered potentially related to the trial drug, with no statistically significant difference between treatment groups (p = 0.726). No deaths occurred during the trial. Six patients discontinued treatment due to AEs (three per group), and all events, except one in the placebo group, were assessed as being serious and related to treatment [tacrolimus: worsening of MG (n = 2) and worsening of MG and lung infection (n = 1); placebo: worsening of MG (n = 1) and increased blood glucose, increased glycosylated haemoglobin and increased lipid levels (n = 1, all experienced by the same patient)].
Table 3.
Summary of serious adverse events and potentially drug-related adverse events reported in any treatment group (SAF).
| Proportion of patients, n (%) | Tacrolimus (n = 45) |
Placebo (n = 38) |
|---|---|---|
| Patients with any adverse event | 36 (80.0) | 31 (81.6) |
| Patients with any serious adverse event* | 5 (11.1) | 6 (15.8) |
| Myasthenia gravis | 3 (6.7) | 4 (10.5) |
| Lung infection | 1 (2.2) | 1 (2.6) |
| Diabetes mellitus | 1 (2.2) | (0.0) |
| Dyspnoea | 1 (2.2) | (0.0) |
| Bronchitis | 0 (0.0) | 2 (5.3) |
| Atrial fibrillation | 0 (0.0) | 1 (2.6) |
| Patients with any potentially drug-related adverse event | 33 (73.3) | 29 (76.3) |
| Reported in >5% in any treatment group* | ||
| Upper respiratory tract infection | 9 (20.0) | 6 (15.8) |
| Myasthenia gravis | 7 (15.6) | 6 (15.8) |
| Nasopharyngitis | 6 (13.3) | 5 (13.2) |
| Diarrhoea | 3 (6.7) | 6 (15.8) |
| Hypertension | 0 (0.0) | 5 (13.2) |
| Bronchitis | 0 (0.0) | 4 (10.5) |
| Urinary tract infection | 3 (6.7) | 1 (2.6) |
| Diabetes mellitus | 3 (6.7) | 0 (0.0) |
| Blood lactate dehydrogenase increased | 0 (0.0) | 2 (5.3) |
| Insomnia | 0 (0.0) | 2 (5.3) |
| Sleep disorder | 0 (0.0) | 2 (5.3) |
There was no significant difference between treatment groups in the number of patients who reported at least one adverse event (p = 1.000; Fisher’s exact test), at least one serious adverse event (p = 0.747; Fisher’s exact test) or at least one potentially drug-related adverse event (p = 0.804; Fisher’s exact test). * If a patient experienced more than one episode of an adverse event, the patient was counted only once within a preferred term.
SAF, safety analysis set.
Increases in blood glucose were observed in the tacrolimus group (mean change from baseline at weeks 4 and 12 was 0.650 mmol/l and 0.568 mmol/l, respectively), but there was little change in the placebo group. Over the 24-week trial, mean creatinine levels decreased in the tacrolimus group and increased in the placebo group (week 24, mean change from baseline was −1.6 μmol/l and 0.4 μmol/l, respectively), but this difference was not statistically significant (p = 0.461). HBA1c was considered to be increased and clinically significant in 9/45 patients (20.0%) who received tacrolimus versus 4/38 patients (10.5%) who received placebo. There was a larger decrease in change from baseline alanine amino-transferase values in the tacrolimus group versus the placebo group (week 24, mean change from baseline was −7.5 U/l and −2.2 U/l, respectively). There were no notable differences between the two groups for vital signs, electrocardiogram findings or body mass index throughout the duration of the study.
Discussion
In this study, treatment with tacrolimus 3 mg did not demonstrate a statistically significant difference for QMG score reduction over 24 weeks compared with placebo. A post-hoc analysis demonstrated that there was a statistically significant difference for QMG score reduction of ⩾4 points at week 24 between the tacrolimus and placebo groups. This reduction in QMG score has been shown to be clinically significant.20
There has only been one other randomized, controlled trial for tacrolimus in MG, with similar patient numbers (n = 80) and time period (28 weeks) to the current trial.16 The primary endpoint of that trial was a reduction in mean daily GC dose given in the last 12 weeks of the study, which did not differ significantly between the treatment group and the placebo group. Furthermore, that trial did not show any statistically significant difference in QMG score.11,16 However, it should be noted that the baseline QMG score was much lower than in the current study (~5 versus ~14) and, thus, less likely to show a difference. Other studies have shown that tacrolimus led to statistically significant improvements in endpoints including QMG score12–14,21 and MG-ADLscore12,14,21 in patients with MG, including those who were GC-dependent, with inadequate response or intolerance to GCs, or those who had been unsuccessfully treated with both GCs and cyclosporine.22 Compared with our current study, open-label studies by Zhao and colleagues and Shimojima and colleagues showed a similar decrease in QMG score (~5.0) at week 24 in both patients with a similar baseline QMG score (~14) and those with different baseline QMG scores.14,21 A large, retrospective study that followed 212 patients, 110 of whom were thymectomized and immunosuppressant dependent, received tacrolimus over a mean follow-up time of 49.3 ± 18.1 months, indicating that the mean QMG score decreased from 20.5 to 0.2 at the final visit.23 Other small studies have found a small decrease in QMG score with tacrolimus. For example, Tada and colleagues demonstrated that 67% of patients achieved a three-point decrease in QMG score at 6 months, which was maintained for 1 year.13 In the long term, improvements were observed from 3 months and lasted up to 5 years, demonstrating the efficacy of tacrolimus.13,15 The dose of 3 mg tacrolimus in the current trial and the duration of 24 weeks were chosen based on previous studies of tacrolimus for the treatment of MG.14–16
A post-hoc analysis demonstrated that 68.2% of patients in the tacrolimus group achieved a ⩾4-point improvement in QMG score, compared with 44.7% of patients in the placebo group. Similarly, in the Tindall study, more patients treated with cyclosporine (40%) than placebo (11%) experienced a ⩾4-point improvement in QMG score that was sustained during the 6-month trial period. These patients had generalized MG with prominent clinical symptoms despite continuing moderate- or high-dose GC therapy.20 Recent recommendations propose the use of the MG-composite rather than QMG score in the design and implementation of clinical trials because it is weighted for clinical significance and incorporates patient-reported outcomes. In this situation, a change in value of ⩾3 points of the MG-composite score is considered the criterion for a clinically significant difference.24
In some studies, tacrolimus use in patients with MG was associated with a steroid-sparing effect.12–14 A reduction in concomitant GC dose has been observed between 4 months and 1 year after commencing tacrolimus treatment.13,14 The potential steroid-sparing effect of tacrolimus could limit the significance of the safety concerns of long-term GC use in patients with MG. However, the current trial did not show a change in concomitant GC dose with tacrolimus versus placebo at week 24. It is possible that the duration of the current trial was too short to allow for a significant change in GC dose. These results are similar to those reported in the other randomized, placebo-controlled trial of tacrolimus, in which no change was reported for the primary outcome of mean daily steroid dose during the last 12 weeks of the 28-week trial.16
Tacrolimus 3 mg administered once daily for 24 weeks was well tolerated, with AEs that were generally considered mild. A similar AE profile has been reported in other, smaller trials.12,13,15,16,21 Similar to the results of Yoshikawa and colleagues, in the current study there were no reports of AEs linked to nephrotoxicity or hypertension within the tacrolimus group. This contrasts with studies of other calcineurin inhibitors, such as cyclosporine, which have been associated with SAEs such as nephrotoxicity and hypertension.7 Notably, the occurrence of infection in the tacrolimus group was not significantly higher than in the placebo group.
At baseline, there were more patients with diabetes in the tacrolimus group (9.1%) versus the placebo group (2.6%). At week 24, there were more patients with increased blood glucose in the tacrolimus group (n = 8) versus the placebo group (n = 3). Moreover, nine patients had glycosylated haemoglobin in the tacrolimus group versus four in the placebo group. Three patients in the tacrolimus group reported an AE of diabetes, although only one of these patients shifted from normal blood glucose levels at baseline to high levels at week 12. It should be noted that all patients in the current study received a relatively high dose of concomitant steroids, which carries a risk of new-onset diabetes. In other studies of low-dose tacrolimus, treatment discontinuation due to new-onset diabetes has been reported in one patient.11
A limitation of the current trial was the small number of patients. The strict inclusion criteria, defined by consensus of Chinese clinical experts and the Centre for Drug Evaluation in China, which ensured the selection of patients with MG who had previous inadequate responses to GC therapy, coupled with the low incidence of MG, led to a relatively small number of eligible patients being treated in the study (n = 83). This small sample size compromised the statistical power to meet the primary efficacy endpoint. Another major limitation was the absence of AChR antibody testing; the diagnosis was, however, confirmed by repetitive stimulation studies and positive neostigmine test.
A potential limitation is that the trial excluded patients with ocular MG, which is normally associated with mild symptoms. Consequently, the baseline QMG score in this trial was higher than in other studies,11 indicating a population with more severe symptoms.
Furthermore, it is difficult to evaluate inadequate responses to GC therapy because the onset of remission varies greatly.7 It is possible that some of the study population were still responding to their initial GC treatment during the study period (despite a requirement of at least 6 weeks to define an inadequate response). The short duration of this study (24 weeks) made it difficult to evaluate the steroid-sparing effects of tacrolimus. Notably, the patients were only prohibited from receiving blood purification therapy 8 weeks prior to the study, which may not have been a sufficient length of time to eliminate the possibility of patients responding to these therapies during the study.
A significant limitation of this study was the disparity between the two treatment groups regarding the duration of MG (27.9 months in the tacrolimus group versus 63.5 months in the placebo group). Patients were randomly assigned in a 1:1 ratio to receive either tacrolimus or placebo and were not stratified by disease duration, so future trials should control for the duration of MG to balance the baseline characteristics of the two study groups.
Conclusion
In conclusion, low-dose (3 mg) tacrolimus for the treatment of patients with MG who had an inadequate response to GC did not demonstrate a significantly greater reduction in mean QMG score over 24 weeks versus placebo. However, a post-hoc analysis demonstrated a statistically significant difference for QMG score reduction of ⩾4 points in the tacrolimus group versus the placebo group. Unfortunately, the difference between the study groups concerning the duration of MG makes the data difficult to interpret, and this should be considered when comparing the efficacy results between the two groups.
Supplementary Material
Footnotes
Funding: Medical writing support in the development of this manuscript was provided by Helen Farrington and Michelle Utton-Mishra from SuccinctChoice Medical Communications, and funded by Astellas Pharma Global Development, Inc.
This work was supported by Astellas Pharma Global Development, Inc.
Conflict of interest statement: Huawei Shi is an employee of Astellas Pharma China, Inc. All other authors declare no conflict of interest.
Contributor Information
Lei Zhou, Department of Neurology, Huashan Hospital, Fudan University, Shanghai, China.
Weibin Liu, Department of Neurology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Wei Li, Department of Neurology, Qilu Hospital of Shandong University, Shandong, China.
Haifeng Li, Department of Neurology, Qilu Hospital of Shandong University, Shandong, China.
Xu Zhang, Department of Neurology, The Affiliated Hospital of Medical College Qingdao University, Qingdao, China.
Huifang Shang, Department of Neurology, West China Hospital, Sichuan University, Sichuan, China.
Xu Zhang, Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Zhejiang, China.
Bitao Bu, Department of Neurology, Tongji Hospital, Tongji Medical College of Huazhong University of Science & Technology, Wuhan, China.
Hui Deng, Department of Neurology, The First Hospital of Jilin University, Changchun, China.
Qi Fang, Department of Neurology, The First Affiliated Hospital of Soochow University, Jiangsu, China.
Jimei Li, Department of Neurology, Beijing Friendship Hospital Affiliated to Capital University of Medical Sciences, Beijing, China.
Hua Zhang, Department of Neurology, Beijing Hospital, Beijing, China.
Zhi Song, Department of Neurology, The Third Xiangya Hospital, Central South University, Changsha, China.
Changyi Ou, Department of Neurology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Chuanzhu Yan, Department of Neurology, Qilu Hospital of Shandong University, Shandong, China.
Tao Liu, Department of Neurology, The Affiliated Hospital of Medical College Qingdao University, Qingdao, China.
Hongyu Zhou, Department of Neurology, West China Hospital, Sichuan University, Sichuan, China.
Jianhong Bao, Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Zhejiang, China.
Jiahong Lu, Department of Neurology, Huashan Hospital, Fudan University, Shanghai, China.
Huawei Shi, Astellas Pharma China, Inc., Beijing, China.
Chongbo Zhao, Department of Neurology, Huashan Hospital, Fudan University, 12 Wulumuqi Middle Rd, Shanghai, 200040, China.
References
- 1. Gold R, Schneider-Gold C. Current and future standards in treatment of myasthenia gravis. Neurotherapeutics 2008; 5: 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilhus NE. Myasthenia gravis. New Engl J Med 2016; 375: 2570–2581. [DOI] [PubMed] [Google Scholar]
- 3. Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015; 14: 1023–1036. [DOI] [PubMed] [Google Scholar]
- 4. Jaretzki A, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 2000; 55: 16–23. [DOI] [PubMed] [Google Scholar]
- 5. Guo J, Dang D, Li H-Z, et al. Current overview of myasthenia gravis and experience in China. Neuroimmunol Neuroinflammation 2014; 1: 127–130. [Google Scholar]
- 6. Lai CH, Tseng HF. Nationwide population-based epidemiological study of myasthenia gravis in Taiwan. Neuroepidemiology 2010; 35: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skeie GO, Apostolski S, Evoli A, et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol 2010; 17: 893–902. [DOI] [PubMed] [Google Scholar]
- 8. Drachman DB. Myasthenia gravis. N Engl J Med 1994; 330: 1797–1810. [DOI] [PubMed] [Google Scholar]
- 9. Pascuzzi RM, Coslett HB, Johns TR. Long-term corticosteroid treatment of myasthenia gravis: report of 116 patients. Ann Neurol 1984; 15: 291–298. [DOI] [PubMed] [Google Scholar]
- 10. Astellas. PROGRAF prescribing information, www.us.astellas.com/docs/prograf.pdf (accessed 11 July 2017).
- 11. Cruz JL, Wolff ML, Vanderman AJ, et al. The emerging role of tacrolimus in myasthenia gravis. Ther Adv Neurol Disord 2015; 8: 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Konishi T, Yoshiyama Y, Takamori M, et al. Clinical study of FK506 in patients with myasthenia gravis. Muscle Nerve 2003; 28: 570–574. [DOI] [PubMed] [Google Scholar]
- 13. Tada M, Shimohata T, Tada M, et al. Long-term therapeutic efficacy and safety of low-dose tacrolimus (FK506) for myasthenia gravis. J Neurol Sci 2006; 247: 17–20. [DOI] [PubMed] [Google Scholar]
- 14. Zhao CB, Zhang X, Zhang H, et al. Clinical efficacy and immunological impact of tacrolimus in Chinese patients with generalized myasthenia gravis. Int Immunopharmacol 2011; 11: 519–524. [DOI] [PubMed] [Google Scholar]
- 15. Nagaishi A, Yukitake M, Kuroda Y. Long-term treatment of steroid-dependent myasthenia gravis patients with low-dose tacrolimus. Intern Med 2008; 47: 731–736. [DOI] [PubMed] [Google Scholar]
- 16. Yoshikawa H, Kiuchi T, Saida T, et al. Randomised, double-blind, placebo-controlled study of tacrolimus in myasthenia gravis. J Neurol Neurosurg Psychiatry 2011; 82: 970–977. [DOI] [PubMed] [Google Scholar]
- 17. Soliven B, Rezania K, Gundogdu B, et al. Terbutaline in myasthenia gravis: a pilot study. J Neurol Sci 2009; 277: 150–154. [DOI] [PubMed] [Google Scholar]
- 18. Osserman K. Myasthenia gravis. New York: Grune and Stratton, 1958. [Google Scholar]
- 19. Selcen D, Dabrowski ER, Michon AM, et al. High-dose intravenous immunoglobulin therapy in juvenile myasthenia gravis. Pediatric Neurol 2000; 22: 40–43. [DOI] [PubMed] [Google Scholar]
- 20. Tindall RS, Phillips JT, Rollins JA, et al. A clinical therapeutic trial of cyclosporine in myasthenia gravis. Ann N Y Acad Sci 1993; 681: 539–551. [DOI] [PubMed] [Google Scholar]
- 21. Shimojima Y, Matsuda M, Gono T, et al. Tacrolimus in refractory patients with myasthenia gravis: coadministration and tapering of oral prednisolone. J Clin Neurosci 2006; 13: 39–44. [DOI] [PubMed] [Google Scholar]
- 22. Ponseti JM, Azem J, Fort JM, et al. Benefits of FK506 (tacrolimus) for residual, cyclosporin- and prednisone-resistant myasthenia gravis: one-year follow-up of an open-label study. Clin Neurol Neurosurg 2005; 107: 187–190. [DOI] [PubMed] [Google Scholar]
- 23. Ponseti JM, Gamez J, Azem J, et al. Tacrolimus for myasthenia gravis: a clinical study of 212 patients. Ann N Y Acad Sci 2008; 1132: 254–263. [DOI] [PubMed] [Google Scholar]
- 24. Benatar M, Sanders DB, Burns TM, et al. Recommendations for myasthenia gravis clinical trials. Muscle Nerve 2012; 45: 909–917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.