Abstract
Background:
Self-expandable metal stent (SEMS) implantation may rapidly improve the symptoms of malignant esophageal stenosis and tracheoesophageal fistulas (TEF). However, dysphagia often returns subsequently and repeated endoscopic intervention may be necessary. The aims of the study were to identify the risk factors of complications, and the frequency and efficacy of repeated endoscopic interventions; and to provide technical recommendations on appropriate stent selection.
Methods:
We analyzed retrospectively the clinical data of 212 patients with locally advanced esophageal cancer who underwent SEMS implantation.
Results:
A total of 238 SEMS implantations were performed with 99.06% technical success and 1.26% procedure-related deaths in the enrolled 212 cases. Complications occurred in 84 patients (39.62%) and in 55 cases (25.94%) repeated endoscopic procedures were required. Early reintervention 24–48 h after the stent implantations was necessary due to stent migration (12 cases), arrhythmia (2 cases), intolerable retrosternal pain (1 case) and dyspnea (1 case). An average of 1.98 repeated gastroscopies (range 1–6; median 2), 13.58 weeks (range 1.5–48; median 11) after the stent implantation were performed during the follow-up period: 37 stent repositions, 23 restent implantations, 15 endoscopic esophageal dilations and 7 stent removals. In 48 cases (87.3%) oral feeding of patients was made possible by endoscopic interventions.
Conclusions:
In a quarter of SEMS implantations, complications occur that can be successfully managed by endoscopic interventions. Our experiences have shown that individualized stent choice may substantially reduce the complications rate and make repeated endoscopic interventions easier.
Keywords: esophageal cancer, esophageal obstruction, self-expandable metal stent
Introduction
About every second esophageal cancer is identified at an advanced, inoperable stage when the therapeutic options are largely limited to the oncological treatment and palliation of symptoms.1 Self-expandable metal stent (SEMS) placement is the most common means of palliation of dysphagia caused by esophageal cancers, and it could be effective for the palliation of malignant tracheoesophageal fistulas (TEF).2 This minimally invasive endoscopic procedure could rapidly improve the symptoms of patients, but in 30–50% of the cases minor or major complications occur with the return of dysphagia.3 The early recognition and management of complications substantially influences the efficacy of therapy. Endoscopic treatment is first recommended due to its minimal invasiveness and low burden to patients.4 In contrast to the high technical and functional success rate of the first stent implantation, the endoscopic management of SEMS complications represents a real challenge even for experienced gastroenterologists.
The aims of our retrospective study were to evaluate the complications rate of SEMS implantations and to identify their predicting factors which could help in the screening of high-risk patients. We determined the frequency and efficacy of repeated endoscopic interventions for the treatment of SEMS complications. We provide technical recommendations on appropriate stent selection based on the results of a correlation analysis between the stent features and the type of complications.
Patients, material and methods
A total of 212 patients with malignant esophageal obstruction or TEF who underwent SEMS implantation between 2007 and 2014 in one of the Hungarian tertiary level referral gastroenterology centers were retrospectively enrolled in our study. The inclusion criteria were malignant esophageal obstruction or TEF confirmed by endoscopy or barium swallow/meal examination; pathologically diagnosed esophageal malignancy; unresectable tumor with advanced stage or poor surgical candidates; onco team recommended oncological treatment or palliative esophageal SEMS implantation. The study was approved by the Regional and Institutional Human Medical Biological Research Ethics Committee of the University of Szeged (ethics approval number: 3680 SZTE). Informed consent for this study was waived by the ethics committee owing to the retrospective nature of the investigation. The study was carried out under the Declaration of Helsinki.
Stent implantations were performed under intravenous sedation (5–10 mg midazolam) with or without intravenous analgesics (10–20 mg nalbuphine). Various types of esophageal stents were used from the following manufacturers: Leufen Medical GmbH (Berlin, Germany); Boston Scientific Corporation (Minneapolis, Minnesota, USA); Taewoong Medical Co. Ltd, (Seoul, South Korea); Changzhou Health Microport Medical Device Co. Ltd (Changzhou, Jiangsu, China); Endo-Technik (Solingen, Germany); ENDO-FLEX GmbH (Voerde, Germany); Accura Medizintechnik GmbH (Karben, Germany); BVM Medical Ltd (Trinity Lane, Leicestershire, UK); Micro-Tech Co. Ltd (Nanjing, China). The diameter of the body of the applied stents was 18 or 20 mm, and the stent material and its coating were identical in products made by the same manufacturers (polytetrafluoroethylene, silicone, polyurethane, etc.; nitinol, steel, etc.). The pattern (weave, braided, knits, etc.) and shape of stents varied widely depending on the altered anatomical situations. The selection of the optimal stent depends on the disease location and the length of obstruction. In the case of tumors of the upper third of the esophagus and the cardia, specific SEMS were inserted (cardia umbrella stent, antireflux valve, antimigration property, etc.) which could reduce the risk of foreign body sensation in the pharynx and the severity of gastroesophageal reflux. The choice between partially and fully covered stents was individualized, depending on the risk of restenosis and migration: fully covered SEMS was preferred in the case of a long and significant stenosis, in contrast to partially covered stents, which were applied in cases with higher migration risk. The stent was at least 2 cm longer than the endoscopically measured length of stenosis. The proximal and distal ends of the neoplasia were marked with external metal markers. If the stenosis was too tight to allow the passage of the stent delivery system and the endoscopic visualization of the distal part of the esophagus, endoscopic balloon or bougie dilation was performed first. After the removal of the endoscope, the stent was inserted into the right position with guidewire assistance under X-ray control, and finally the proper SEMS position was verified endoscopically (Figure 1).
Figure 1.
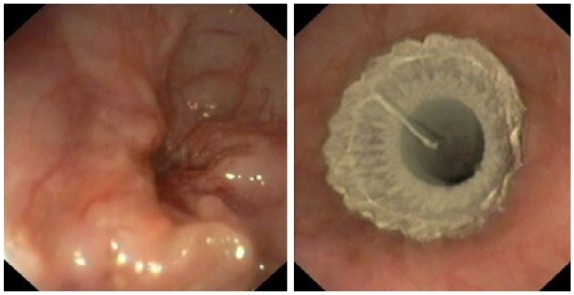
Subtotal malignant esophageal obstruction. Completely covered stent implantation after bougie dilation of stenosis.
Our study had three different aims: to evaluate the success and complication rate of esophageal stent implantation; to determine the frequency and efficacy of repeated endoscopic interventions related to SEMS complications; to propose technical recommendations for optimized stent selection based on the results of our study. The efficacy of SEMS implantation was characterized by technical and functional success rate. The intervention was considered technically successful if the stent was opened correctly in the proper position confirmed by X-ray and endoscopy, and functionally successful if the oral feeding of the patients became possible 24 h after the intervention. Minor complications were defined as mild to moderate events related to stent implantation which could be treated conservatively without the need for hospitalization (gastroesophageal reflux, emesis, retrosternal pain, mild dysphagia, etc.). All patients received opiate pain killers and in the case of distal obstruction prophylactically proton pump inhibitor therapy with or without prokinetic drugs to avoid the reflux. Major complications were defined as severe, often life-threatening complications, which required repeated hospitalization and endoscopic interventions (TEF, stent migration or obstruction, aspiration pneumonia, arrhythmia, hematemesis, etc.). We determined the rate, type, frequency and efficacy of repeated endoscopic examinations; furthermore, we analyzed the characteristics of patients, the SEMS types and the SEMS implantations to identify the risk factors and success of reinterventions.
Statistical analysis was performed using SPSS software version 22 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were expressed as mean and median with ranges. We used logistic regression analysis, Fisher’s exact test and χ2 test to identify the factors that can modify the risk of SEMS complications.
Results
SEMS implantation
In the 212 enrolled cases, 238 SEMS implantations were performed due to malignant esophageal obstructions caused by predominantly primer esophageal tumors (83.49%) or lung cancers (13.68%). In 33 cases, TEF was present at the time of SEMS implantation. The characteristics of patients and stent implantations are summarized in Tables 1 and 2. The technical success rate of the first SEMS implantations was 99.06%. In two cases the cardia stent spontaneously migrated into the stomach immediately after the implantation, and the reposition was not feasible. In one case intolerable retrosternal pain and severe dyspnea and in three cases development of severe complications (arrhythmia, pneumonia) hampered the oral feeding of patients, therefore the functional success rate decreased to 97.64%. Procedure-related death was 1.26%: two patients died due to malignant supraventricular arrhythmia and aspiration pneumonia less than 24 h after stent implantation.
Table 1.
Clinical and demographic data of enrolled patients.
| Clinical and demographic data of patients (n = 212) | |
|---|---|
| Women/men | 46/166 (21.7%/78.30%) |
| Mean age (years) | 63.9 (range 22–93; median 63) |
| Malignant esophageal obstruction: | |
| - lung cancer | 29 (13.68%) |
| - esophageal cancer | 177 (83.49%) |
| - breast cancer | 2 (0.94%) |
| - gastric cancer | 2 (0.94%) |
| - hypopharyngeal cancer | 1 (0.47%) |
| - mediastinal metastasis of rectal cancer | 1 (0.47%) |
| Location of obstruction: | |
| - upper third of the esophagus | 97 (45.75%) |
| - middle third of the esophagus | 95 (44.81%) |
| - lower third of the esophagus | 20 (9.43%) |
| Tracheoesophageal fistula at the time of stent placement | 33 (15.67%) |
Table 2.
Characteristics of stent implantation.
| Characteristics of SEMS placement | |
|---|---|
| Patients/SEMS | 212/238 |
| one SEMS placement | 189 (89.15%) |
| two SEMS placement | 21 (9.91%) |
| three SEMS placement | 1 (0.47%) |
| four SEMS placement | 1 (0.47%) |
| Partially/fully covered SEMS | 39/199 (16.39%/83.11%) |
| Technical success rate | 99.06% |
| Functional success rate | 97.64% |
| Procedure-related death | 1.26% |
SEMS, self-expandable metal stent.
SEMS complications
In total, major and minor complications were observed in 84 of 212 (39.6%) patients (Table 3). Retrosternal pain (13.68%) and stent migration (6.57%) were the most frequent early complications, and they appeared less than 4 weeks after SEMS implantation. Fatal complications were seen in three cases. One patient died 24 h after stent implantation due to aspiration pneumonia, respiratory insufficiency and septic shock. In two cases malignant supraventricular tachycardia occurred with cardiovascular instability immediately after stenting. These patients died despite their admission to the intensive care unit and the removal of the stent. After the 4-week follow up, stent obstruction caused by tumor overgrowth or ingrowth (15.09%), migration (10.38%) and new TEF formation (7.08%) were the most common complications. No correlation was found between clinical/procedural factors (sex, age, tumor type, location, necessity of dilation during stenting, coverage of SEMS, and presence of TEF at the time of stenting) and the development of complications (Table 4).
Table 3.
Complications of SEMS implantation. Acute complications occur less than 4 weeks after SEMS implantation.
| Complications of SEMS placement (n = 84/212) | |||
|---|---|---|---|
| Acute | Chronic | ||
| Retrosternal pain | 29 (13.68%) | Occlusion | 32 (15.09%) |
| Migration | 14 (6.57%) | Migration | 22 (10.38%) |
| Hemorrhage | 4 (1.89%) | Fistula formation | 15 (7.08%) |
| Arrhythmia | 2 (0.94%) | Perforation | 1 (0.47%) |
| Perforation | 1 (0.47%) | ||
| Pneumothorax | 1 (0.47%) | ||
| Aspiration pneumonia | 1 (0.47%) | ||
| Complication of anesthesia | 1 (0.47%) | ||
SEMS, self-expandable metal stent.
Table 4.
Effect of clinical and procedural factors on the development of complications and the necessity of repeated endoscopic interventions.
| Suspected risk factors | Development of complications | Repeated endoscopic interventions |
|---|---|---|
| Sex | p = 0.216 | p = 0.272 |
| Age | p = 0.382 | p = 0.169 |
| Tumor type (esophageal or other) | p = 0.579 | p = 0.516 |
| Dilation of stenosis during stent implantation | p = 0.109 | p = 0.088 |
| Tracheoesophageal fistula at the time of stenting | p = 0.756 | p = 0.509 |
| Length of stenosis | p = 0.392 | p = 0.552 |
| Tumor location | p = 0.943 | p = 0.214 |
| Partially/fully covered stent | p = 0.539 | p = 0.339 |
Repeated endoscopic interventions
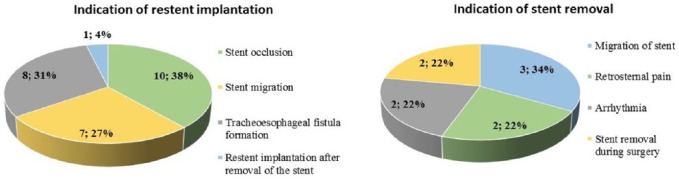
In 55 cases (25.94%) repeated endoscopic interventions were performed, the indications of which are shown in Figure 2. In 16 patients the first reintervention was performed 24–48 h after stent implantation; this was necessary due to early stent migration (12 cases), supraventricular arrhythmia (2 cases), dyspnea (1 case) and intolerable retrosternal pain (1 case). In this group, multiple endoscopies were required in every second patient during the follow up. In the case of patients with an uncomplicated early postimplantation period (24–48 h), 1.98 reinterventions (range 1–6; median 2) were performed per patient at an average of 13.58 weeks (range 1.5–48; median 11) after stenting. The type and frequency of endoscopic interventions are presented on Figure 3. Stent reimplantation occurred in 23 cases: 21 patients received two, 1 patient had three and 1 patient had four SEMSs due to stent migration (7 cases), occlusion (10 cases) or new TEF formation (8 cases) (Figure 4). Endoscopic removal of the stent due to complications (arrhythmia, retrosternal pain, migration) was unavoidable in seven cases. In 48 of 55 patients (87.27%) oral feeding was resolved by an endoscopic procedure; in six cases transient parenteral or permanent enteral feeding with a gastric tube or percutaneous endoscopic gastrostomy (PEG) was feasible.
Figure 2.
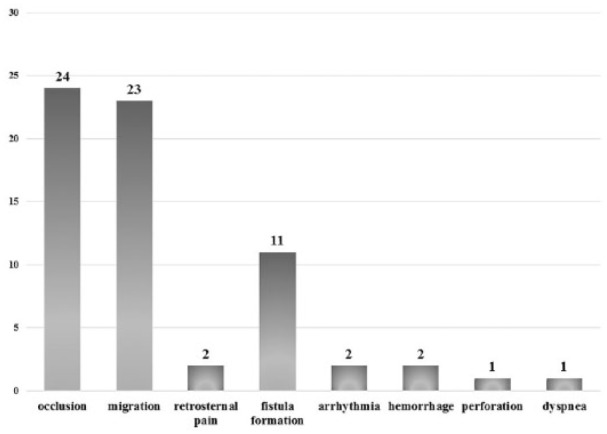
Indication of repeated endoscopic interventions.
Figure 3.
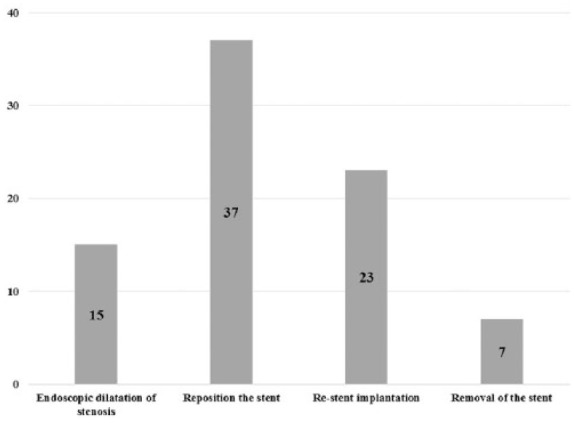
Repeated endoscopic interventions after stent implantation (55/212 patients).
Figure 4.
Indications of restent implantations (n = 23) and stent removal (n = 9).
No statistically significant correlation was found between clinical/procedural factors (sex, age, tumor type, location, necessity of dilation during stenting, coverage and manufacturer of SEMS, and presence of TEF at the time of stenting) and the necessity of repeated endoscopic interventions.
Discussion
This retrospective observational study of 212 patients has confirmed that stent implantation is easy to perform, and a safe and effective treatment in neoplastic esophageal obstruction and malignant TEF. Additionally, our results revealed that the majority of SEMS complications could be successfully managed by endoscopic interventions (stent reimplantation, dilation of stenosis, stent reposition).
The role of SEMS placement in the palliative treatment of malignant esophageal stenosis is unquestionable because it provides immediate and potentially long-lasting relief of obstructive symptoms.5,6 SEMS placement is superior to the remaining endoscopic procedures such as self-expandable plastic stent (SEPS) placement, dilation alone or argon plasma coagulation (APC), because it provides a more durable asymptomatic period and is associated with decreased risk of complications.7–9 However, previous studies have shown that the rate of SEMS-related adverse events is high, and varies between 22% and 50% depending on the location of the tumor, the presence of a fistula or tumor shelf, concomitant chemoirradiation, tumor vascularity and the stent design.7 Stenting of upper esophageal tumors represents a real therapeutic challenge due to pain and globus sensation, an elevated risk of TEF and aspiration pneumonia.10 In this location the use of a specially designed stent is recommended, the proximal end of which keeps a 2 cm distance from the upper esophageal sphincter after stenting. A retrospective study analyzed the clinical data of 104 patients with malignant proximal esophageal stenosis and concluded that SEMS placement is safe and effective, and the complication rate is not elevated compared with stenting in the distal esophagus.11 The use of newly designed esophageal stents could reduce certain types of complications such as SEMS with antireflux valve or antimigration property, and cardia umbrella stents.12 The results of previous studies have shown better long-term efficacy in the case of partially or fully covered stent placement compared with uncovered stent placement.7,13,14 The appropriate use of specially designed stents could help to prevent the development of short- and long-term adverse events. In our study, fully covered SEMS were applied most frequently (partially covered 16.39% versus fully covered 83.11%), and in every cardia or proximal esophageal tumor specially designed stents were inserted. We have noted complications in 84 cases (39.62%) which showed no correlation with tumor type, location, necessity of dilation during stenting, coverage of SEMS and the presence of TEF at the time of stenting. The difference between the risk factors of complications in the published data and our study might be caused by the retrospective study design or the relatively small number of adverse events, although it could also suggest that our stent choice was adequate. We considered that the stent design substantially influenced the effectiveness and complications of SEMS placement, therefore we always strived for individualized stent choice. We experienced that:
- There is no difference between the migration rates of partially and fully covered stents, but fully covered stents reduce the risk of tumor ingrowth. In the case of repeated endoscopic interventions, the reposition of these stents is often easy even months after stenting.
- There are differences between the coatings of SEMS. Only chemically and mechanically resistant coating could help to prevent tumor ingrowth. The time for decomposition of Teflon (polytetrafluoroethylene) coating is longer compared with silicone and polyurethane coating, therefore the stent occlusion by tumor ingrowth may occur later. In addition, the longer durability of SEMS coverage allows the stent to be repositioned an extended period after implantation, for example in cases of stent occlusion due to tumor overgrowth of the stent ends.
- Stents with a larger fitting surface at the ends compress the esophagus less, thus the risk of fistula formation at the stent ends is lower.
- Flexible stents (based on our observations, especially in the case of braided stent design) do not stay straight, but also adapt to the altered anatomy of the tumorous esophagus. Therefore they increase the internal stent patency and result in a reduced and steady radial force throughout the entire length of the stent. Therefore, the use of SEMS with enhanced flexibility may prevent damage to the esophagus wall by the end of the stent (ulceration, TEF formation) and decrease the intensity of SEMS-related retrosternal pain.
- Bougie dilatation of esophageal stenosis could be necessary before stent implantation to allow the passage of the endoscope through the malignant obstruction. Therefore, stents should strive to achieve about 11–12 mm diameter considering also the ‘rule of three’.
- In the case of significant esophageal stenosis requiring bougie or balloon dilatation, smaller diameter stents could substantially result in lower retrosternal pain without elevated risk of stent migration.
- In the case of tumors of the upper third of the esophagus and in the cardia, the use of specially designed stents is essential.
- The chemically and mechanically resistant stent retrieval loop may allow the movement of the stent for a long time after implantation.
These recommendations would decrease the burden to patients and could also make the treatment of stent complications more cost effective because they may reduce the frequency of stent-related complications, the number of repeated endoscopic interventions and the necessity of stent reimplantation.
Endoscopic reinterventions can successfully treat SEMS-related complications in most of cases.15 Homann et al. analyzed the clinical data of 133 patients with unresectable esophageal cancer. They found that delayed complications occurred in 53.4% (71 of 133 patients); these patients were successfully treated by dilatation (24%), placement of a second or third stent (27%), laser therapy (16%), or placement of a feeding tube (19%). Patients with repeated endoscopic interventions had a significantly longer life expectancy (222 ± 26 days versus 86 ± 14 days, p < 0.001).16 Recurrent dysphagia occurred in one-third of patients due to tumor over- or ingrowth via the stent, noncancerous granulomatous tissue overgrowth or food impaction. In the case of stent obstruction, either endoscopic reposition, APC, exchange for a new stent or a second SEMS implantation could be effective in restoring esophageal patency.17 Incidence of stent migration ranges from 4% to 36%. This could be asymptomatic or manifest, presenting as chest pain, recurrent dysphagia or dyspnea. Stent reposition or removal of the stent with a new stent implantation are the effective endoscopic therapeutic options in these cases.18 In our study, in 48 of 55 patients (87.27%) with SEMS-related complications oral feeding was solved by endoscopic interventions (dilation, reposition, restent implantation, stent removal). We confirm that the second SEMS placement was effective in 91.31% of cases; one of the 23 stents migrated distally and one was removed due to retrosternal pain. Stent reposition might be a good alternative to SEMS reimplantation due to its effectiveness, low cost and relative simplicity. We experienced that:
- In the case of fully covered or double-covered SEMSs the reposition or removal is easy and well tolerated a few months after stenting, despite partial tumor ingrowth.
- Stents can be displaced safely for centimeters and can also be positioned in spite of a significant migration.
- Fixation of the proximal end of SEMS by hemoclips may prevent repeated stent migration.
- If repeated stent implantation is required due to a fistula or tumor overgrowth at the stent ends, we recommend distal dragging of the first stent, above which a second stent can be implanted.
- In the case of multiplex TEF with a thinned out esophageal wall, simultaneous tracheal and esophageal SEMS placement may be effective.
In most cases, TEF develops next to the proximal or distal end of the stent due to the radial force and resulting pressure necrosis.17 The study performed by Shin et al. highlighted that SEMS placement is clinically successful in 80% of patients with TEF, but during the follow up, recurrence of fistula was experienced in one third of the cases.19 We have found that the risk of fistula formation is high in patients with TEF at the time of stenting (Figure 5). In 8 of 11 cases (72.73%) of new TEF formation endoscopic reposition or a second SEMS placement solved the oral feeding of patients.
Figure 5.
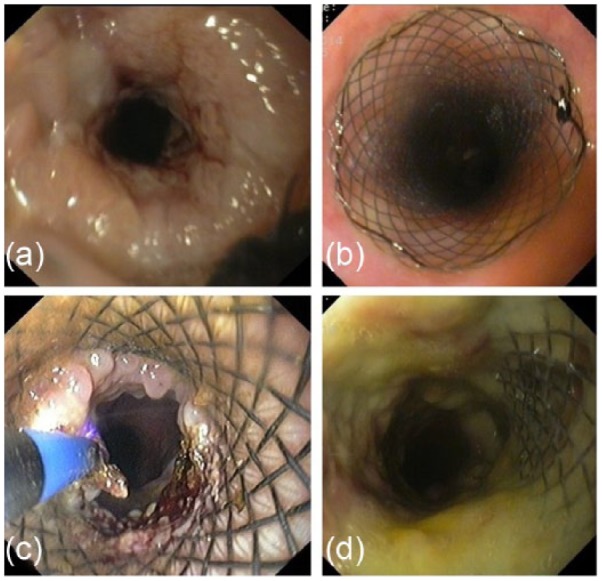
Endoscopic management of stent complications. (a, b) Restent implantation due to tumor overgrowth; (c) endoscopic argon plasma coagulation of noncancerous granulomatous tissue overgrowth at the distal end of self-expandable metal stent; (d) tracheoesophageal fistula next to the proximal end of the stent.
Retrosternal pain often occurs after stent implantation, but it is mild to moderate in most cases, and could be managed with opiate analgesics. The frequency of this minor complication varies widely among different studies from 13% to 60%.17,20 Our results correlate with the results of other studies: 29 patients (13.68%) experienced retrosternal pain, but only two of them required endoscopic intervention, stent removal (6.89%).
In one quarter of patients we should expect the development of complications. Despite the simplicity and high success rate of SEMS implantation, the treatment of SEMS-related complications represents the real clinical challenge. Our study has not found any clinical factors which could help the selection of high-risk patients. Nonetheless, we consider that the individualized stent choice could help to reduce the frequency of adverse events and make repeated endoscopic interventions easier. We recommend endoscopic interventions as the first-line treatment for SEMS-related complications because in most cases they make oral feeding possible. Our recommendations for stent selection may decrease the burden to patients and could also make the treatment of stent complications more cost effective because they may reduce the frequency of stent-related complications, the number of repeated endoscopic interventions and the necessity of stent reimplantation.
Acknowledgments
Conception and design: RB, AF, ZS. Analysis and interpretation of the data: RB, MS, ÁM, MR, AB, KF. Drafting of the article: RB, ZS, AF. Critical revision of the article for important intellectual content: TM, ZS, LC.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Renáta Bor, First Department of Medicine, University of Szeged, Szeged, Hungary.
Anna Fábián, First Department of Medicine, University of Szeged, Szeged, Hungary.
Anita Bálint, First Department of Medicine, University of Szeged, Szeged, Hungary.
Klaudia Farkas, First Department of Medicine, University of Szeged, Szeged, Hungary.
Mónika Szűcs, Department of Medical Physics and Informatics, University of Szeged, Szeged, Hungary.
Ágnes Milassin, First Department of Medicine, University of Szeged, Szeged, Hungary.
László Czakó, First Department of Medicine, University of Szeged, Szeged, Hungary.
Mariann Rutka, First Department of Medicine, University of Szeged, Szeged, Hungary.
Tamás Molnár, First Department of Medicine, University of Szeged, Szeged, Hungary.
Zoltán Szepes, First Department of Medicine, University of Szeged, Korányi Fasor 8–10, 6720 Szeged, Hungary.
References
- 1. Stahl M, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24: vi51– vi56. [DOI] [PubMed] [Google Scholar]
- 2. Sharma P, Kozarek R. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol 2010; 105: 258–273. [DOI] [PubMed] [Google Scholar]
- 3. Pavlidis TE, Pavlidis ET. Role of stenting in the palliation of gastroesophageal junction cancer: a brief review. World J Gastrointest Surg 2014; 6: 38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evans JA, Early DS, Chandraskhara V, et al. ; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013; 77: 328–334. [DOI] [PubMed] [Google Scholar]
- 5. Madhusudhan C, Saluja SS, Pal S, et al. Palliative stenting for relief of dysphagia in patients with inoperable esophageal cancer: impact on quality of life. Dis Esophagus 2009; 22: 331–336. [DOI] [PubMed] [Google Scholar]
- 6. Sreedharan A, Harris K, Crellin A, et al. Interventions for dysphagia in oesophageal cancer. Cochrane database Syst Rev 2009; 4: CD005048. [DOI] [PubMed] [Google Scholar]
- 7. Jee SR, Cho JY, Kim KH, et al. Evidence-based recommendations on upper gastrointestinal tract stenting: a report from the stent study group of the Korean society of gastrointestinal endoscopy. Clin Endosc 2013; 46: 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shenfine J, McNamee P, Steen N, et al. A randomized controlled clinical trial of palliative therapies for patients with inoperable esophageal cancer. Am J Gastroenterol 2009; 104: 1674–1685. [DOI] [PubMed] [Google Scholar]
- 9. Conio M, Repici A, Battaglia G, et al. A randomized prospective comparison of self-expandable plastic stents and partially covered self-expandable metal stents in the palliation of malignant esophageal dysphagia. Am J Gastroenterol 2007; 102: 2667–2677. [DOI] [PubMed] [Google Scholar]
- 10. Shim CS, Jung IS, Bhandari S, et al. Management of malignant strictures of the cervical esophagus with a newly-designed self-expanding metal stent. Endoscopy 2004; 36: 554–557. [DOI] [PubMed] [Google Scholar]
- 11. Verschuur EML, Kuipers EJ, Siersema PD. Esophageal stents for malignant strictures close to the upper esophageal sphincter. Gastrointest Endosc 2007; 66: 1082–1090. [DOI] [PubMed] [Google Scholar]
- 12. Uitdehaag MJ, Siersema PD, Spaander MCW, et al. A new fully covered stent with antimigration properties for the palliation of malignant dysphagia: a prospective cohort study. Gastrointest Endosc 2010; 71: 600–605. [DOI] [PubMed] [Google Scholar]
- 13. Hirdes MMC, Siersema PD, Vleggaar FP. A new fully covered metal stent for the treatment of benign and malignant dysphagia: a prospective follow-up study. Gastrointest Endosc 2012; 75: 712–718. [DOI] [PubMed] [Google Scholar]
- 14. Repici A, Jovani M, Hassan C, et al. Management of inoperable malignant oesophageal strictures with fully covered WallFlex(®) stent: a multicentre prospective study. Dig Liver Dis 2014; 46: 1093–1098. [DOI] [PubMed] [Google Scholar]
- 15. Homs MY, Steyerberg EW, Kuipers EJ, et al. Causes and treatment of recurrent dysphagia after self-expanding metal stent placement for palliation of esophageal carcinoma. Endoscopy 2004; 36: 880–886. [DOI] [PubMed] [Google Scholar]
- 16. Homann N, Noftz MR, Klingenberg-Noftz RD, et al. Delayed complications after placement of self-expanding stents in malignant esophageal obstruction: treatment strategies and survival rate. Dig Dis Sci 2008; 53: 334–340. [DOI] [PubMed] [Google Scholar]
- 17. Didden P, Spaander MCW, Bruno MJ, et al. Esophageal stents in malignant and benign disorders. Curr Gastroenterol Rep 2013; 15: 319. [DOI] [PubMed] [Google Scholar]
- 18. da C, Martins B, Retes FA, Medrado BF, et al. Endoscopic management and prevention of migrated esophageal stents. World J Gastrointest Endosc 2014; 6: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shin JH, Song HY, Ko GY, et al. Esophagorespiratory fistula: long-term results of palliative treatment with covered expandable metallic stents in 61 patients. Radiology 2004; 232: 252–259. [DOI] [PubMed] [Google Scholar]
- 20. Verschuur EML, Steyerberg EW, Kuipers EJ, et al. Effect of stent size on complications and recurrent dysphagia in patients with esophageal or gastric cardia cancer. Gastrointest Endosc 2007; 65: 592–601. [DOI] [PubMed] [Google Scholar]