Abstract
Background:
Anterior cervical discectomy with fusion is an effective treatment for patients having cervical radiculopathy and myelopathy. To reduce morbidity associated with autograft taken from the iliac crest without sacrificing high fusion rates, a novel technique that harvests bone from the vertebral body adjacent to the operative disc space has been proposed. The effects of square and round bone graft harvest techniques on the mechanical stability of the osteopenic donor vertebrae are unknown. We analyzed the biomechanical implications of the technique by subjecting osteopenic models to uniaxial compression to compare yield strengths of surgically altered and unaltered specimens.
Methods:
Biomechanical grade polyurethane foam was cut into 60 different 12 mm × 17 mm × 20 mm blocks. The foam had a density of 10 pounds per cubic foot, simulating osteoporotic bone. Rectangular prism (4 mm × 4 mm × 6 mm) and cylindrical cores (r = 2 mm, h = 8 mm) were removed from 20 blocks per group. Twenty samples were left intact as a control group. Anterior plate screws were applied to the models and a Polyether ether ketone (PEEK) interbody spacer was placed on top. Samples underwent uniaxial compression at 0.1 mm/s until mechanical failure. Points of structural failure were determined using a 0.1% offset on a force–displacement curve and compared to determine the reductions in compressive strength.
Results:
The mean force eliciting structural failure for intact samples was 450.6 N. Average failure forces for rectangular prisms and cylindrical cores removed were 383.2 and 395.4 N, respectively. Removal of a rectangular prismatic core of the necessary volume resulted in a 15.0% reduction in compressive strength, while removal of a cylindrical core of comparable volume facilitated a reduction of 12.2%.
Conclusion:
Local autograft harvested from adjacent vertebrae reduces morbidity associated with a second surgical site while minimally reducing the compressive strength of the donor vertebra in an osteopenic model, lending credence to the efficacy of this technique in elderly patient populations.
Keywords: biomechanics, osteoporosis, spine surgery, autograft, ACDF
Introduction
Anterior cervical discectomy with fusion (ACDF) is a common surgical intervention for patients having radiculopathy, myelopathy, or myeloradiculopathy when more conservative methods have proven ineffective.1 Shifts in demography toward an aging population have steadily increased the prevalence of ACDF in a geriatric population, with a median age of 53.2 years at the time of surgery.2 The increase in average age of ACDF patients results in greater prevalence of osteoporosis in potential surgical candidates. Since the procedure’s introduction by Robinson and Smith, numerous interbody materials have been investigated to promote vertebral fusion across the disc space. The current gold standard of treatment is to utilize autograft taken from the patient’s iliac crest. The procedure yields impressive fusion rates of 83% to 99%.1 Despite effective induction of vertebral fusion, this operation is associated with high levels of donor site morbidity and a decrease in quality of life secondary to donor site pain that may persist beyond normal periods of postoperative patient monitoring.3–6 In order to mitigate iliac crest morbidity, other materials have been studied to replace autologous grafting, including hydroxyapatite and cadaveric allograft. Like autograft, hydroxyapatite yields strong fusion rates (89%) but has been associated with multiple problems at the graft site, including graft collapse and the loss of sagittal alignment, though not to the degree of clinical significance.7
Cadaveric allograft has been used in hopes that its anatomical properties make it an efficacious substitute for autograft. Although some research has found allograft to be comparable to autograft with up to 91% fusion, meta-analyses have revealed a notable drop in fusion rates to as low as 74%.1,8 Allograft also presents cost concerns, as it requires harvesting, sterilization, storage, and transportation of graft prior to insertion. In order to reduce the morbidity associated with iliac harvesting while preserving the favorable properties of autograft in promoting vertebral fusion, novel harvest sites have been proposed including the manubrium and the clavicle.9,10 Unfortunately, although these investigations tended to find reduced donor site morbidity, the proposed procedures have failed to directly address the increased risk of infection and complication associated with a second surgical site.
Our group has recently proposed a novel ACDF technique that reduces donor site morbidity and eliminates a second surgical site by harvesting bone from the vertebral body adjacent to the operative disc space, which is then pulverized and inserted into the lumen of a polyether ether ketone (PEEK) interbody spacer to promote vertebral fusion.11 Osteotomes are used to harvest a small rectangular prism of bone from the lateral inferior portion of the inferior vertebral body. The technique as described by our group harvests the bone from the left side of the vertebra per surgeon preference; however, the right side is also acceptable. While much work has been done on the compressive resistance of vertebral bodies,12–17 the novelty of our technique renders the observation of mechanical properties of cervical vertebrae surgically altered in this unique way. A previous study examining a similar technique of harvesting bone from cervical vertebrae elected to forego the use of screws used to anchor anterior plates and carried out bone harvest along the midline of both the superior and inferior vertebral bodies.18 Our investigation examined the mechanical implications of the new technique by subjecting vertebral models with anchored anterior plate screws to uniaxial compression in order to compare points of structural failure of surgically altered specimens with unaltered specimens.
Methods
Specimen Preparation
Three groups of 20 blocks were manufactured from biomechanical grade rigid polyurethane foam (Sawbones; Pacific Research Laboratories Inc, Vashon Island, Washington). Individual blocks were cut to 12 mm × 17 mm × 20 mm in order to mimic the average dimensions of the C3–C7 vertebral bodies as determined from computed tomography measurements of 60 consecutive cervical spines. Closed form foam was chosen for its standardized properties representative of human cancellous bone. Specifically, 10 pounds per cubic foot (pcf) foam was chosen for its mechanical properties similar to that of osteoporotic bone in order to mimic an elderly patient population having varying degrees of bone density loss.19,20 The closed cell structure of rigid 10 pcf foam provided a carefully controlled model that avoided the variability associated with human cadaveric biomechanical testing. Cancellous foam was deemed an appropriate model, as repeated studies by Fields et al have demonstrated the dominance of vertical trabeculae inside the cancellous bone in providing biomechanical stability, both with and without a cortical shell.14,15
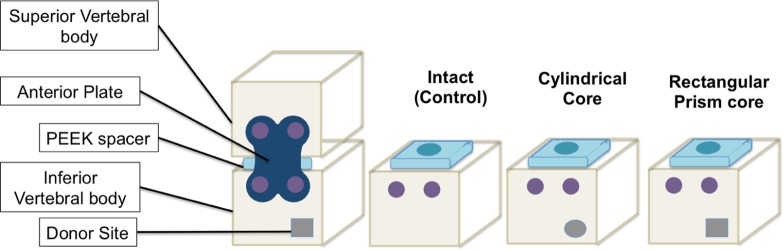
Each sample had 4 mm cancellous screws, 16 mm in length affixed 3.7 mm and 11.8 mm, respectively, from the right lateral border of the anterior face to mimic screws anchoring an anterior plate placed slightly off the midline, as described in the novel technique description,11 to maximize room for the bone harvest site without sacrificing screw purchase (Figure 1).
Figure 1.
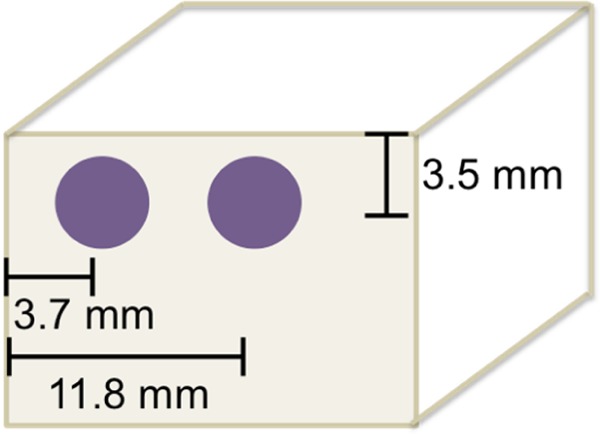
Cancellous screw placement slightly off midline allows for bone harvest site without sacrificing screw purchase.
The volume of bone material required for harvest was determined by calculating one half of the volume of the lumen of a 7-mm lordotic PEEK vertebral spacer (Depuy-Synthes, Paoli, Pennsylvania) such that a continuous column of bone may be placed in contact with both end plates adjacent to the resected disc space, with the remaining volume filled with demineralized bone matrix per the novel technique. This volume was found to be 96.2 mm3.
Two different donor bone core shapes were examined to determine whether the geometry of the harvest site significantly altered the biomechanical response to uniaxial compression. In the first group, 2 and 4 mm osteotomes were used to resect a 96 mm3 rectangular prism of bone material (4 mm wide, 4 mm tall, and 6 mm deep) into the lower left quadrant of the anterior face of the vertebral models (Figure 2A). The lateral edge of the harvest site was located 2 mm medial to the left lateral border of the model, and the inferior edge of the harvest site was 2 mm superior to the inferior end plate. In the second group, a drill equipped with a 5/32-in (3.97 mm) bit was used to remove a 99 mm3 cylinder of bone material (D = 3.97, H = 8; Figure 2B). The core was centered 4 mm from the left lateral and inferior borders of the anterior face of the model. For the sake of consistency, all harvest sites were on the left side of the model; in the clinical setting, the technique can be applied to either side according to surgeon’s preference. The final group was left unaltered as a control group.
Figure 2.
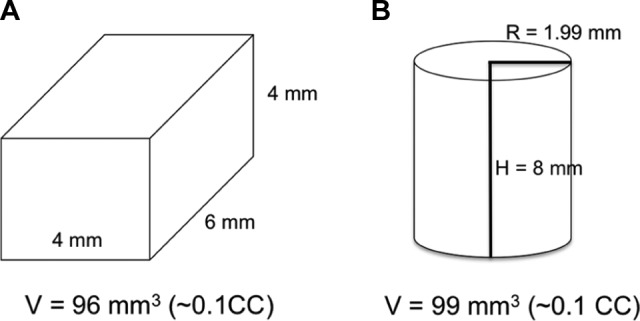
A, Rectangular core dimensions had a total volume 96 mm3. B, Cylindrical core dimensions with total volume 99 mm3.
Mechanical Testing
Prior to testing, a 7-mm PEEK lordotic vertebral interbody device was placed on the superior surface of the samples such that the anterior face of the device aligned with the anterior face of the corresponding sample (Figure 3). Each sample was subjected to continuous uniaxial craniocaudal compression using an Instron 4468 test frame at a rate of 5 mm/s with a 1 kN load cell. During compressions, the load cell was suspended from a hinge, allowing motion in the anteroposterior plane to accommodate the lordotic curvature of the spacer and ensure uniform compression, avoiding the introduction of stress risers in the anterior plane. Because the models were machine cut with high precision and the hinge accommodated stress risers associated with the geometry of the spacer, it was not deemed necessary to pot the samples in plaster as is often performed in studies examining cadaveric vertebrae.12,21 Instead, samples were allowed to rest freely on the inferior platen (Figure 4).
Figure 3.
Experimental apparatus with anterior plate anchoring screws and interbody spacer placed on the superior end plate.
Figure 4.
Square core sample prior to and after compression. At failure, the fracture propagates through the harvest site.
During each compression, force and gauge displacement were monitored via MTS Testworks 4 acquisition software, allowing for the calculation of final points of structural failure, that is, compression fracture. Points of structural failure were determined for each sample using a 0.1% offset applied to the compression–displacement curve and compared to determine the reduction in compressive strength associated with each bone core type removed.
Statistical Analysis
A 1-way analysis of variance (ANOVA) was used to determine whether there was a statistically significant difference in force displacement among the intact, circular, and square groups. Before beginning the ANOVA, the distributions of the structural failure data in each group were examined for normality and were assumed to be normally distributed due to nonsignificant Shapiro-Wilk tests for all 3 groups. However, the Levene F test revealed that the assumption of homogeneity of variance was not met (P = .0093). Therefore, the Welch F test was used. An α level of .05 was used for all analyses.
Results
Both the rectangular and cylindrical harvested bone cores represented less than 3% of the total vertebral body volume. The anterior cross-sections of the harvest sites occupied less than 5.5% for cylindrical cores and less than 7.2% for the rectangular prism of the total anterior surface area available, leaving adequate room for anterior instrumentation once the graft had been harvested.
Compared to intact controls, both the rectangular and cylindrical cores presented a decrease in point of structural failure. Intact samples exhibited an average failure at 450.6 N. Samples with a rectangular prism removed failed with an average compressive force of 383.2 N, and those with a cylindrical core removed failed on average with 395.4 N of force (Figure 5). Taking into account the measured area of the spacer in contact with the superior end plate during compressions, the yield points were 3.6, 3.1, and 3.2 MPa for intact, rectangular core, and cylindrical core samples, respectively. Thus, removal of a rectangular prismatic core of the necessary volume facilitated a 15.0% reduction in compressive strength, while removal of a cylindrical core of comparable volume facilitated a reduction of 12.2%.
Figure 5.
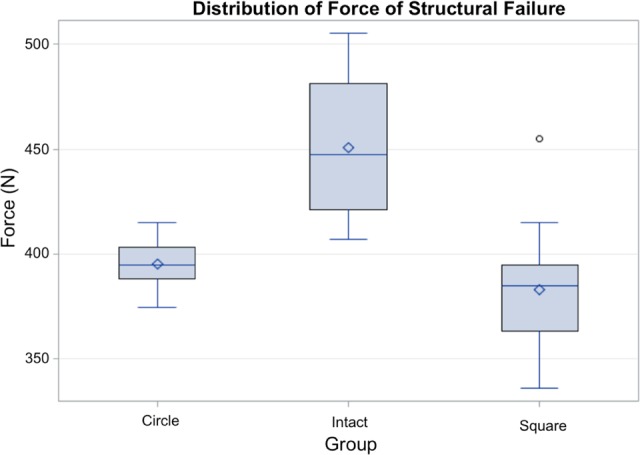
Distribution of forces at 0.1% strain. Removal of a cylindrical core (circle) demonstrated a 12.2% reduction in structural failure point when compared to intact samples. Removal of a rectangular prism (square) of bone facilitated a 15.0% reduction in structural failure point.
The 1-way ANOVA of the average structural failure force in newtons found a statistically significant main effect, Welch F2,31.59 = 28.76, P < .0001, indicating that the 3 groups differed in average force displacement. Post hoc comparisons using the Tukey honest significant difference (HSD) test revealed that the mean of the intact group (M = 450.49, standard deviation [SD] = 32.77) was significantly greater than the means of both circular (M = 395.41, SD = 11.61) and square (M = 383.17, SD = 26.28) groups. The means of the circular and square groups were not significantly different from each other.
Discussion
Anterior cervical discectomy with fusion is a standard surgical procedure for patients presenting with radiculopathy and myelopathy. The current gold standard of treatment is iliac crest autograft, favored for its osteogenerative and osteoinductive properties.1,22 Despite the advantages offered by the current gold standard autograft, significant setbacks arise in determining an effective location from which the autograft is harvested. Patients commonly experience hip pain concurrent with and sometimes long after the subsidence of their neck symptoms associated with their pathology, discectomy, and fusion.23 Additionally, the opening of a second surgical site necessitates a longer operation, increases the time under anesthesia, and the time for additional complications to arise. Iliac crest harvest requires a second surgical incision with an infection rate of up to 5.6%.24
In order to reduce morbidity associated with autograft taken from the iliac crest without sacrificing high fusion rates, our group has described a novel ACDF technique in which bone is harvested from the vertebral body adjacent to the operative disc space.11 The ability to safely use autograft from a local source makes the novel technique useful in patients in whom it has been historically more difficult to achieve fusion via allograft alone, such as smokers and diabetics, as it provides a means for supplying a continuous column of autologous cancellous bone inside a spacer that will promote rapid bone fusion via its osteoinductive and osteoconductive properties. Previous authors have described a technique in which anterior and posterior osteophyte shavings are utilized as a grafting material to promote fusion.25 Although this method avoids the opening of a second surgical site, preliminary comparison of the properties of the osteophyte dust with iliac crest chips has demonstrated a reduced osteogenic potential and increased resorption, a feature that has been hypothesized to be due to increased osteoclast load.26 Our proposed technique provides an alternative autologous graft source that preserves the use of a single surgical site while using the oseogenic properties of both cortical and cancellous bones from the vertebral body.
The present study provides preliminary data supporting the biomechanical efficacy of this technique, demonstrating a 15.0% and 12.2% reduction in compressive point of failure in vertebral models with square and cylindrical bone cores resected, respectively. While the decrease in the force of structural failure is statistically significant, a small decrease is to be expected from any reduction in vertical trabeculae.14,15 We feel that the decrease in structural failure force associated with the procedure does not pose a clinically significant risk or subject the patients to increased risk of catastrophic mechanical failure of the donor vertebra. Compressive axial forces necessary to induce fracture of the donor vertebra prior to healing are sufficiently high to cause significant trauma elsewhere in the cervical spine, notably the resected disc space and C1 and C2, which have been demonstrated to be more susceptible to fracture from axial forces than other cervical segments.21,27 Of note, in order to isolate effects on the vertebral body, the model design chosen for this study eliminated compressive strength afforded by the facets. Facet strength has been shown to play an important role in high force axial compression and so would provide extra stabilization in an in vivo setting.28,29 Interestingly, while there was a statistically significant difference in structural failure force between unaltered and surgically altered specimens, no such significant difference was found between the different geometrical shapes of the harvest site. We expected that the corners of the rectangular cores would cause sharp convergence of vertical stress lines, resulting in local stress concentrations at the point of convergence surges and increasing the likelihood of structural failure at angles of the concentrator. However, the similar structural failure forces between the 2 core models suggested that whatever concentrations did occur did not yield a clinically substantial effect.
In the clinical setting, it is essential that surgeons not resect more bone than is necessary to create a continuous column of corticocancellous autograft between the 2 end plates adjacent to the resected disc space. The scope of this study was limited to cylindrical and rectangular prism of the dimensions noted in Table 1. With this in mind, we would not recommend resecting cores larger than have been discussed, as this may increase the likelihood for fracture. Furthermore, we used fixed screws in all of our tests. Future studies may shed light on differing dimensions for the harvest site and the use of variable versus fixed screws, potentially widening the array of harvest and screw options available.
Table 1.
Vertebral Body and Harvested Bone Dimensions.a
| Vertebra | C3 | C4 | C5 | C6 | C7 |
|---|---|---|---|---|---|
| Body anterior area (mm2) | |||||
| Total | 224.2 | 228.9 | 243.0 | 258.1 | 306.3 |
| Body volume (cm3) | |||||
| Total | 3.7 | 3.8 | 3.9 | 4.4 | 5.2 |
| Percentage anterior face area | |||||
| Cylindrical core | 5.4% | 5.2% | 4.9% | 4.6% | 3.9% |
| Square core | 7.1% | 7.0% | 6.6% | 6.2% | 5.2% |
| Percent volume | |||||
| Cylindrical core (−0.1 cm3) | 2.7% | 2.6% | 2.5% | 2.3% | 1.9% |
| Square core (0.1 cm3) | 2.6% | 2.6% | 2.5% | 2.2% | 1.8% |
aHarvested cylindrical and prismatic (square) cores represented less than 3% of total vertebral body volume. Cylindrical cores occupied less than 5.5% of the anterior face, while the square profile of the prism cores occupied less than 7.2% of the anterior face of the average cervical vertebra.
Our investigation focused on axial loading of the donor vertebral body. The application of an anterior plate anchors the 2 vertebral bodies adjacent to the resected disc space, effectively fixing the donor vertebral body to its more superior neighbor. As such, any flexion, extension, or lateral bending occurring across this space as a result of bending moments in the neck is negligible. It is standard practice at our facility to discharge patients with an Aspen collar to be worn for 6 weeks postoperatively, protecting the harvest and graft sites during the most vulnerable period of healing. Numerous studies investigating the kinematics of cervical collars have been published exhibiting the efficacy of these devices in limiting gross cervical flexion, extension, and lateral bending.30–34 Biomechanical studies by Nightingale et al examining the strength of cervical spines have demonstrated that cervical spine structural failure resulting from bending moments occurs at the extremes of cervical range of motion and most commonly occurs due to failure of ligamentous structures.35,36 Since such extremes in range of motion are not achievable while a collar is being worn, and the anterior plate eliminates range of motion across the resected disc space, we elected to forego the testing of bending moments in this preliminary investigation.
The use of osteoporotic polyurethane foam allowed for a consistency in the biomechanical properties between samples. Although we feel that the use of 10 pcf foam more closely modeled osteoporotic patients, a result of such a low-density model was a decrease in structural failure force relative to similar studies on cadaveric models.18 This decrease in failure force was likely accentuated by the reduced surface area for compression introduced by the superior placement of the interbody spacer as has been noted to occur in previous compressive studies.37 Though the superior end plate plays a relatively minor role in providing overall compressive strength relative to cancellous trabeculae,14,15 use of cadaveric models and biomechanical foam models equipped with plates modeling the cortical shell will provide additional anatomically accurate data in future studies.
Conclusion
Using bone autograft from an adjacent vertebral body reduces the morbidity and infection risk associated with a second surgical site while minimally reducing the craniocaudal compressive strength of the donor vertebra. In addition, use of local autograft should result in healing rates similar to other autograft sources. The present study helps validate the biomechanical efficacy of the proposed ACDF technique, making it an important alternative to iliac crest autograft in an osteopenic patient population.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Chau AM, Mobbs RJ. Bone graft substitutes in anterior cervical discectomy and fusion. Eur Spine J. 2009;18(4):449–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walid MS, Sanoufa M, Robinson JS. The effect of age and body mass index on cost of spinal surgery. J Clin Neurosci. 2011;18(4):489–493. [DOI] [PubMed] [Google Scholar]
- 3. Heneghan HM, McCabe JP. Use of autologous bone graft in anterior cervical decompression: morbidity & quality of life analysis. BMC Musculoskelet Disord. 2009;10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pollock R, Alcelik I, Bhatia C, et al. Donor site morbidity following iliac crest bone harvesting for cervical fusion: a comparison between minimally invasive and open techniques. Eur Spine J. 2008;17(6):845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;(329):300–309. [DOI] [PubMed] [Google Scholar]
- 6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3–S15. [DOI] [PubMed] [Google Scholar]
- 7. McConnell JR, Freeman BJ, Debnath UK, Grevitt MP, Prince HG, Webb JK. A prospective randomized comparison of coralline hydroxyapatite with autograft in cervical interbody fusion. Spine. 2003;28(4):317–323. [DOI] [PubMed] [Google Scholar]
- 8. Miller LE, Block JE. Safety and effectiveness of bone allografts in anterior cervical discectomy and fusion surgery. Spine (Phila Pa 1976). 2011;36(24):2045–2050. [DOI] [PubMed] [Google Scholar]
- 9. Iwasaki K, Ikedo T, Hashikata H, Toda H. Autologous clavicle bone graft for anterior cervical discectomy and fusion with titanium interbody cage. J Neurosurg Spine. 2014;21(5):761–768. [DOI] [PubMed] [Google Scholar]
- 10. Peelle MW, Rawlins BA, Frelinghuysen P. A novel source of cancellous autograft for ACDF surgery: the manubrium. J Spinal Disord Tech. 2007;20(1):36–41. [DOI] [PubMed] [Google Scholar]
- 11. O’Neill C, Walterscheid Z, Behrend CJ, Carmouche JJ. Novel autograft site in anterior cervical discectomy/fusion: a multifaceted description and analysis. Presented at: American Academy of Orthopedic Surgeons Annual Meeting; Orlando, Florida; Mar 1–5 2016. [Google Scholar]
- 12. Ochia RS, Tencer AF, Ching RP. Effect of loading rate on endplate and vertebral body strength in human lumbar vertebrae. J Biomech. 2003;36(12):1875–1881. [DOI] [PubMed] [Google Scholar]
- 13. Kopperdahl DL, Pearlman JL, Keaveny TM. Biomechanical consequences of an isolated overload on the human vertebral body. J Orthopaedic Res. 2000;18(5):685–690. [DOI] [PubMed] [Google Scholar]
- 14. Fields AJ, Eswaran SK, Jekir MG, Keaveny TM. Role of trabecular microarchitecture in whole-vertebral body biomechanical behavior. J Bone Miner Res. 2009;24(9):1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fields AJ, Lee GL, Liu XS, Jekir MG, Guo XE, Keaveny TM. Influence of vertical trabeculae on the compressive strength of the human vertebra. J Bone Miner Res. 2011;26(2):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singer K, Edmondston S, Day R, Breidahl P, Price R. Prediction of thoracic and lumbar vertebral body compressive strength—correlations with bone-mineral density and vertebral region. Bone. 1995;17(2):167–174. [DOI] [PubMed] [Google Scholar]
- 17. Mirzaei M, Zeinali A, Razmjoo A, Nazemi M. On prediction of the strength levels and failure patterns of human vertebrae using quantitative computed tomography (QCT)-based finite element method. J Biomech. 2009;42(11):1584–1591. [DOI] [PubMed] [Google Scholar]
- 18. Pitzen T, Tan JS, Dvorak MF, Fisher C, Oxland T. Local autograft retrieval from a cervical vertebral body: biomechanical consequences. J Neurosurg Spine. 2012;16(4):340–344. [DOI] [PubMed] [Google Scholar]
- 19. ASTM International. ASTM International Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopedic Devices and Instruments. Conshohocken, Pennsylvania; ASTM International; 2012. [Google Scholar]
- 20. Poukalova M, Yakacki CM, Guldberg RE, et al. Pullout strength of suture anchors: effect of mechanical properties of trabecular bone. J Biomech. 2010;43(6):1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Panjabi MM, Crisco JJ, Vasavada A, et al. Mechanical properties of the human cervical spine as shown by three-dimensional load-displacement curves. Spine. 2001;26(24):2692–2700. [DOI] [PubMed] [Google Scholar]
- 22. Beaman FD, Bancroft LW, Peterson JJ, Kransdorf MJ. Bone graft materials and synthetic substitutes. Radiol Clin North Am. 2006;44(3):451–461. [DOI] [PubMed] [Google Scholar]
- 23. Wigfield CC, Nelson RJ. Nonautologous fusion materials in cervical spine surgery: how strong is the evidence to justify their use? Spine. 2001;26(6):687–694. [DOI] [PubMed] [Google Scholar]
- 24. Schnee CL, Freese A, Weil RJ, Marcotte PJ. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine. 1997;22(19):2222–2227. [DOI] [PubMed] [Google Scholar]
- 25. Ekanayake J, Shad A. Use of the novel ANSPACH bone collector for bone autograft in anterior cervical discectomy and cage fusion. Acta Neurochir. 2010;152(4):651–653. [DOI] [PubMed] [Google Scholar]
- 26. Ye S, Seo KB, Park BH, et al. Comparison of the osteogenic potential of bone dust and iliac bone chip. Spine J. 2013;13(11):1659–1666. [DOI] [PubMed] [Google Scholar]
- 27. Nuckley DJ, Linders DR, Ching RP. Developmental biomechanics of the human cervical spine. J Biomech. 2013;46(6):1147–1154. [DOI] [PubMed] [Google Scholar]
- 28. Teo EC, Ng HW. Evaluation of the role of ligaments, facets and disc nucleus in lower cervical spine under compression and sagittal moments using finite element method. Med Eng Phys. 2001;23(3):155–164. [DOI] [PubMed] [Google Scholar]
- 29. Moroney SP, Schultz AB, Miller JA, Andersson GB. Load-displacement properties of lower cervical spine motion segments. J Biomech. 1988;21(9):769–779. [DOI] [PubMed] [Google Scholar]
- 30. Evans NR, Hooper G, Edwards R, et al. A 3D motion analysis study comparing the effectiveness of cervical spine orthoses at restricting spinal motion through physiological ranges. Eur Spine J. 2013;22(suppl 1):10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gavin TM, Carandang G, Havey R, Flanagan P, Ghanayem A, Patwardhan AG. Biomechanical analysis of cervical orthoses in flexion and extension: a comparison of cervical collars and cervical thoracic orthoses. J Rehabil Res Dev. 2003;40(6):527. [DOI] [PubMed] [Google Scholar]
- 32. IvancicPC. Effects of orthoses on three-dimensional load–displacement properties of the cervical spine. Eur Spine J. 2013;22(1):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ivancic PC. Do cervical collars and cervicothoracic orthoses effectively stabilize the injured cervical spine? A biomechanical investigation. Spine. 2013;38(13):E767–E774. [DOI] [PubMed] [Google Scholar]
- 34. Schneider AM, Hipp JA, Nguyen L, Reitman CA. Reduction in head and intervertebral motion provided by 7 contemporary cervical orthoses in 45 individuals. Spine. 2007;32(1):E1–E6. [DOI] [PubMed] [Google Scholar]
- 35. Nightingale RW, Carol CV, Ottaviano D, et al. Flexion and extension structural properties and strengths for male cervical spine segments. J Biomech. 2007;40(3):535–542. [DOI] [PubMed] [Google Scholar]
- 36. Nightingale RW, Winkelstein BA, Knaub KE, Richardson WJ, Luck JF, Myers BS. Comparative strengths and structural properties of the upper and lower cervical spine in flexion and extension. J Biomech. 2002;35(6):725–732. [DOI] [PubMed] [Google Scholar]
- 37. Lee CK. Osteopenia and total disc prosthesis subsidence: inclusion/exclusion criteria for total disc replacement. SAS J. 2007;1(2):82–84. [DOI] [PMC free article] [PubMed] [Google Scholar]