Abstract
Objectives
To evaluate the safety and efficacy of botulinum toxin type A in the treatment of bilateral primary axillary hyperhidrosis.
Design
Multicentre, randomised, parallel group, placebo controlled trial.
Setting
17 dermatology and neurology clinics in Belgium, Germany, Switzerland, and the United Kingdom.
Participants
Patients aged 18-75 years with bilateral primary axillary hyperhidrosis sufficient to interfere with daily living. 465 were screened, 320 randomised, and 307 completed the study.
Interventions
Patients received either botulinum toxin type A (Botox) 50 U per axilla or placebo by 10-15 intradermal injections evenly distributed within the hyperhidrotic area of each axilla, defined by Minor's iodine starch test.
Main outcome measures
Percentage of responders (patients with ⩾50% reduction from baseline of spontaneous axillary sweat production) at four weeks, patients' global assessment of treatment satisfaction score, and adverse events.
Results
At four weeks, 94% (227) of the botulinum toxin type A group had responded compared with 36% (28) of the placebo group. By week 16, response rates were 82% (198) and 21% (16), respectively. The results for all other measures of efficacy were significantly better in the botulinum toxin group than the placebo group. Significantly higher patient satisfaction was reported in the botulinum toxin type A group than the placebo group (3.3 v 0.8, P<0.001 at 4 weeks). Adverse events were reported by only 27 patients (11%) in the botulinum toxin group and four (5%) in the placebo group (P>0.05).
Conclusion
Botulinum toxin type A is a safe and effective treatment for primary axillary hyperhidrosis and produces high levels of patient satisfaction.
What is already known on this topic
Primary hyperhidrosis is a chronic disorder that can affect any part of the body, especially the axillas, palms, feet, and face
Current treatments are often ineffective, short acting, or poorly tolerated
What this study adds
Botulinum toxin type A was significantly better than placebo on all measures of sweating
Patient satisfaction was high and few adverse events were reported
Effects of treatment remained apparent at 16 weeks
Introduction
Primary hyperhidrosis is a chronic idiopathic disorder of excessive sweating that mainly affects the axillas, the palms, the soles of the feet, and the face. Focal hyperhidrosis causes appreciable social problems in both private and professional life.1 Profuse sweating can result in skin maceration and secondary microbial infections.2 Current treatments for axillary hyperhidrosis are often ineffective, short acting, or not well tolerated.3
Botulinum toxin type A has been used successfully in a range of medical disorders including strabismus, blepharospasm, focal dystonias, and spasticity associated with juvenile cerebral palsy and adult stroke.4 In hyperhidrosis, botulinum toxin type A acts by blocking the release of acetylcholine from overactive cholinergic sudomotor nerve fibres. These innervate eccrine sweat glands, so excessive sweating is reduced. Several small, predominantly open label studies and one placebo controlled study have shown that botulinum toxin type A is safe and relieves symptoms of hyperhidrosis for 3 to 14 months.2,5–15 We report a 16 week multicentre randomised controlled trial to evaluate the safety and efficacy of botulinum toxin type A in bilateral primary axillary hyperhidrosis.
Methods
Recruitment
From March to October 1999, 465 patients from 17 European dermatology and neurology clinics were screened for bilateral primary axillary hyperhidrosis that was sufficient to interfere with daily living. Patients were eligible for the trial if gravimetric tests showed that they produced ⩾50 mg sweat per axilla over five minutes while at rest at room temperature and were not receiving any other treatment for hyperhidrosis. Three hundred and twenty screened patients met these criteria and were randomised. We obtained ethics committee approval for the participating centres, and participants gave written informed consent.
Study design and treatment
Participants were randomised to receive treatment in a ratio of 3:1 (botulinum toxin type A to placebo) with a block size of four (stratified by centre). This ratio was chosen to maximise safety information about the active treatment.
The hyperhidrotic area for treatment was defined by Minor's iodine starch test.16 Participants received a single treatment of 50 U total botulinum toxin type A per axilla (Botox, Allergan, Irvine, CA) or placebo (Botox vehicle) as multiple (10-15) intradermal injections evenly distributed within the hyperhidrotic area. Active treatment and placebo were indistinguishable. The trial drug was reconstituted with 4 ml of 0.9% preservative free sterile saline (2 ml for each axilla). Participants were requested to shave both axillas two days before an assessment and not to use antiperspirants or deodorants for at least 24 hours before a visit. Follow up assessments were at 1, 4, 8, 12, and 16 weeks after treatment.
Efficacy measures
The primary efficacy variable was the incidence of responders in each treatment group at week 4. We defined responders as patients with a ⩾50% reduction from baseline in axillary sweating measured gravimetrically. A 25 percentage point difference in the number of responders between the treatment groups was considered clinically important. In addition, we analysed the percentage change from baseline and absolute sweat production. Secondary efficacy measures included the persistent responders at week 16 (patients who had not been non-responders at two consecutive visits); the size of the sweat producing area indicated by Minor's iodine starch test; and global assessment of treatment satisfaction score (table 1).
Table 1.
Patients' global assessment of treatment satisfaction scale
| Score | Description |
|---|---|
| +4 | Complete abolition of signs and symptoms (about 100% improvement) |
| +3 | Substantial improvement (some signs and symptoms remain, about 75% improvement) |
| +2 | Moderate improvement (definite improvement but a fair amount of signs and symptoms remain, about 50% improvement) |
| +1 | Slight improvement (some improvement but substantial signs and symptoms remain, about 25% improvement) |
| 0 | Unchanged |
| −1 | Slight worsening (about 25% worse) |
| −2 | Moderate worsening (about 50% worse) |
| −3 | Substantial worsening (about 75% worse) |
| −4 | Very substantial worsening (about 100% worse or greater) |
Sweat production over five minutes was measured gravimetrically in both axillas. A preweighed filter paper covered with a plastic bag was secured to the dried axilla for five minutes, after which time it was reweighed. The difference in weights represented the sweat produced. The average value from the right and left axillas was used for analysis.
Minor's iodine starch test was done both to define the area of sweating before treatment and to quantify the change in area of sweating during the trial.16 We used photographic image analysis (Canfield Scientific, Fairfield, NJ) to determine the area of sweating in cm2 for each axilla.
Safety measures
We recorded the type, incidence, severity, and cause of all spontaneously reported adverse events throughout the trial. In addition, participants had a physical examination at screening and at the end of the trial, and blood pressure, heart rate, and temperature were monitored at all visits.
Statistical analyses
We planned to enrol 300 patients (225 treated with botulinum toxin type A and 75 with placebo) to account for an expected dropout rate of less than 10%. This gave a power of 93% to detect a 25 percentage point difference between treatment groups, assuming response rates of 60% and 35% for the botulinum toxin type A and placebo groups respectively, with a two sided significance of 5%.We analysed data on an intention to treat basis, including all randomised participants. For the primary efficacy measure (incidence of treatment responders), we used the last observation carried forward method to replace missing values and evaluated differences between groups by Fisher's exact test.
For sweat production (percentage change from baseline and absolute values), area of sweating, and global assessment of treatment satisfaction, we evaluated changes within the group using a Wilcoxon signed rank test and between group differences with Wilcoxon rank sum test. Missing values for sweat production were replaced by carrying forward the last observation. We compared the number of patients classed as persistent responders (without two consecutive non-responder time points) at week 16 between treatment groups using Fisher's exact test. Baseline for all efficacy variables was defined as week 0. A P value ⩽0.05 was considered significant for all analyses.
We calculated the incidence of adverse events in each treatment group and evaluated between group differences using Fisher's exact test. We also calculated mean baseline (week 0) values and mean changes from baseline for all vital signs. Within and between group differences were assessed by using Wilcoxon signed rank and Wilcoxon rank sum tests, respectively.
Results
Participants
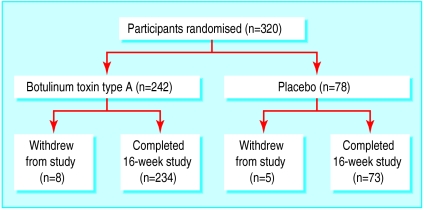
A total of 320 patients with bilateral primary axillary hyperhidrosis were randomised: 242 patients to botulinum toxin type A and 78 patients to placebo. Three hundred and seven completed the trial: 234 (97%) in the botulinum toxin group and 73 (94%) in the placebo group (figure). Of the 13 patients (eight in the botulinum toxin group and five in the placebo group) who withdrew, one withdrew because of an adverse event unrelated to trial treatment, five were lost to follow up, and seven were withdrawn because they did not comply with the protocol.
The mean reported time since the onset of hyperhidrosis was 13.14 (SD 10.36) years in the botulinum toxin group and 13.62 (9.99) years in the placebo group (13.26 (10.26) years for the whole population). Other baseline variables were similar between treatment groups (table 2).
Table 2.
Characteristics of hyperhidrosis at baseline. Values are numbers (percentages) of participants
| Botulinum toxin type A (n=242) | Placebo (n=78) | Total (n=320) | |
|---|---|---|---|
| Other areas with hyperhidrosis: | |||
| Palms | 113 (47) | 35 (45) | 148 (46) |
| Soles | 98 (41) | 28 (36) | 126 (39) |
| Face | 63 (26) | 19 (24) | 82 (26) |
| Triggers for hyperhidrosis: | |||
| Emotional | 188 (78) | 65 (83) | 253 (79) |
| Physical exertion | 174 (72) | 61 (78) | 235 (73) |
| Heat | 168 (69) | 58 (74) | 226 (71) |
| No seasonal variation in hyperhidrosis | 164 (68) | 47 (60) | 211 (66) |
| Family member with hyperhidrosis | 91 (38) | 29 (37) | 120 (38) |
| Previous drugs for hyperhidrosis | 238 (98) | 77 (99) | 315 (98) |
| Other procedures for hyperhidrosis | 86 (36) | 27 (35) | 113 (35) |
Efficacy
Botulinum toxin type A effectively reduced sweating at all time points after treatment compared with placebo (table 3). The proportion of responders in the botulinum toxin type A treated group was significantly higher than that in the placebo group at all time points (95% (230) v 32% (25) at week 1, 94% (227) v 36% (28) at week 4, and 82% (198) v 21% (16) at week 16, P<0.001). In addition, the difference in responder rate between the treatment groups at all time points was much greater than the 25 percentage points that we had predefined as being clinically important (63% at week 1, 95% confidence interval 52% to 74%; 58% at week 4, 47% to 69%; and 61% at week 16, 51% to 72%). A significantly higher percentage of patients in the botulinum toxin type A treated group were persistent treatment responders at the end of the study (77%; 182/235) compared with the placebo group (18%; 13/74) (P<0.001). The results for all other measures of efficacy were also significantly better in the botulinum toxin group than the placebo group at all follow up visits (table 3).
Table 3.
Effect of botulinum toxin type A and placebo on sweat production and patient satisfaction
| No of weeks after treatment | Botulinum toxin type A
|
Placebo
|
|||
|---|---|---|---|---|---|
| No of participants | Mean (SD) | No of participants | Mean (SD) | ||
| Sweat production (% change from baseline): | |||||
| 1 | 242 | −83.0* (24.1) | 78 | −21.8 (58.7) | |
| 4 | 242 | −83.5* (21.6) | 78 | −20.8 (54.4) | |
| 16 | 242 | −69.3* (39.4) | 78 | −3.8 (93.5) | |
| Absolute sweat production (mg): | |||||
| 0 | 242 | 215.8 (178.7) | 78 | 235.7 (213.8) | |
| 1 | 242 | 28.6* (37.5) | 78 | 166.2 (178.8) | |
| 4 | 242 | 28.1* (40.5) | 78 | 153.0 (143.3) | |
| 16 | 242 | 53.7* (67.7) | 78 | 190.5 (195.6) | |
| Area of sweat production (cm2): | |||||
| 0 | 216 | 5.3 (7) | 66 | 6.0 (7) | |
| 1 | 224 | 0.1 (1.1)* | 70 | 4.1 (9.2) | |
| 4 | 219 | 0.2 (0.7)* | 65 | 4.5 (7.8) | |
| 16 | 218 | 0.2 (0.9)* | 71 | 2.3 (5.5) | |
| Satisfaction score: | |||||
| 1 | 67 | 3.1* (1.1) | 24 | 0.8 (1.4) | |
| 4 | 85 | 3.3* (0.9) | 29 | 0.8 (1.4) | |
| 16 | 204 | 2.6* (1.6) | 61 | 0.3 (1.2) | |
P<0.001 compared with placebo.
Safety
Adverse events were similar between treatment groups in type, incidence, and severity. Most adverse events in both treatment groups were mild or moderate. The only significant difference between the two treatment groups was in the incidence of infection, predominantly common cold, which was more common in the placebo group than in the botulinum toxin group (10/78 (13%) v 14/242 (6%), P=0.049).
Twenty seven (11%) patients in the botulinum toxin group reported treatment related adverse events compared with four (5%) in the placebo group; this difference was not significant. Eleven (5%) patients in the botulinum toxin group perceived an increase in non-axillary sweating after treatment compared with none of the control group. All eleven were responders, and increases were reported at various body sites (four forehead, three palmar, two facial, one hands, one feet, one back, one chest, one trunk, one groin, and one unspecified), with five patients reporting sweating at two sites. No clinically important changes in vital signs or findings on physical examination were observed.
Discussion
Primary findings
Our results show a highly significant reduction in the amount of sweating in patients with primary axillary hyperhidrosis after intradermal injections of 50 U botulinum toxin type A. The results are based on the Botox formulation of botulinum toxin type A and cannot be generalised to other formulations or to other serotypes.
The onset of action was rapid and the effect was sustained for at least 16 weeks. Reduction in sweating was accompanied by a high level of treatment satisfaction. Botulinum toxin type A was both safe and well tolerated, with few adverse events reported. These results agree with the preliminary findings of open label studies and one recent double blind study.5
Strengths and weaknesses of study
This is one of the first double blind, placebo controlled studies of botulinum toxin type A in axillary hyperhidrosis. The follow up period was limited, but longer term follow up is in progress. Other studies have shown that the treatment remains effective for up to 14 months.5,6,9,17
Another limitation of the study is that it evaluated only a single treatment. Previous studies of botulinum toxin type A in the treatment of axillary hyperhidrosis have evaluated doses ranging from 30 U to 100 U in each axilla.6,9,11,12 Clearly, the optimal dose of botulinum toxin type A is one that effectively reduces sweating to physiologically normal levels for as long as possible while minimising side effects. Although 50 U botulinum toxin type A per axilla was safe and effective in this trial population, some patients may need the dose adjusted to achieve optimal clinical results.
Only one other study of axillary sweating has used a similar controlled design.5 However, that study had no pure control group as one axilla was treated with botulinum toxin type A and the other with placebo. Since a different botulinum toxin type A formulation was used, we cannot compare effectiveness and dose with those in our study. Both studies show that botulinum toxin type A is an effective treatment for axillary hyperhidrosis.
Implications
A highly effective treatment with few side effects has the potential to change current treatment strategies for this distressing disorder. Topical and systemic anticholinergic treatments for axillary hyperhidrosis are often ineffective, short acting, or poorly tolerated.3 Surgical intervention, such as endoscopic transthoracic sympathectomy, is effective but carries appreciable risks, including Horner's syndrome, gustatory sweating, neuralgia, and pneumothorax.18–20 In addition, up to 100% of patients having endoscopic transthoracic sympathectomy develop compensatory hyperhidrosis,19–21 resulting in dissatisfaction with the procedure in up to a third of patients.22 Another surgical treatment, excision and curettage of sweat glands in the axillas, can cause scar formation.3
A perceived increase in non-axillary sweating was reported by 11 patients (5%) in the botulinum toxin group of our study. However, these patients did not show reduced satisfaction with treatment on the global assessment of treatment satisfaction scale. This additional sweating was not quantified. Subjective reports of increased non-axillary sweating could have been the result of heightened awareness of sweat production at other sites after the axillas had been successfully treated. Alternatively, the increased non-axillary sweating could reflect a central up-regulation of the autonomic nervous system or may represent minimal compensatory sweating. Reported rates of non-axillary sweating after botulinum toxin type A were much lower than those reported after surgical treatment.19–21
In conclusion, botulinum toxin type A is an effective, safe, and well tolerated treatment for patients with primary axillary hyperhidrosis. The treatment is easily administered, patients are easily identified, and repeat treatment has been shown to be effective.10 Botulinum toxin type A is a valuable alternative to previous treatment options for this disorder.
Figure.
Trial profile
Acknowledgments
The Hyperhidrosis Clinical Study Group includes the authors and the following investigators: R Brehler, Universitäts-Hautklinik, Munster, Germany; J Britton, General Infirmary, Leeds; W J Cunliffe, General Infirmary, Leeds; H Eimer, Universitäts-Hautklinik, Mainz, Germany; N van Geel, Universitair Ziekenhuis, Ghent, Belgium; S George, Amersham General Hospital, Amersham; S Gramvussakis, Amersham General Hospital, Amersham; C E M Griffiths, Hope Hospital, Manchester; H Hamm, Universitäts-Hautklinik, Würzburg, Germany; E Haneke, Klinikum Wuppertal, Germany; D Hohl, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland; M Hund, Universitäts-Hautklinik, Würzburg, Germany; G Itschert, Universitäts-Hautklinik, Hamburg, Germany; A Jäckel, Universitäts-Hautklinik, Heidelberg, Germany; I Kinkelin, Universitäts-Hautklinik, Würzburg, Germany; J Knop, Universitäts-Hautklinik, Mainz, Germany; J M Lachapelle, UCL Hospital, Brussels, Belgium; P Lowe, Cranley Clinic, London; O M Mainsuch, Klinikum Wuppertal, Germany; K Maslen, Charité University Hopsital, Berlin, Germany; M Meyer, Klinik Hirslanden, Zurich, Switzerland; R Motley, University Hospital of Wales, Cardiff; J M Naeyaert, Universitair Ziekenhuis, Ghent, Belgium; K Ongenae, Universitair Ziekenhuis, Ghent, Belgium; W Perkins, Queen's Medical Centre, Nottingham; D Petzoldt, Universitäts-Hautklinik, Heidelberg, Germany; M Richter, Universitäts-Hautklinik, Heidelberg, Germany; U Schlese, Charité University Hospital, Berlin, Germany; W Sterry, Charité University Hospital, Berlin, Germany; and G Street, Hope Hospital, Manchester.
Footnotes
Funding: This study was supported by Allergan.
Competing interests: MN and NJL have received fees for speaking, research grants, and consultant fees from Allergan, and NJL owns shares in the company.
References
- 1.Naver H, Aquilonius S-M. The treatment of focal hyperhidrosis with botulinum toxin. Eur J Neurol. 1997;4(suppl 2):S75–S79. [Google Scholar]
- 2.Naumann M, Hamm H, Kinkelin I, Reiners K. Botulinum toxin type A in the treatment of focal, axillary and palmar hyperhidrosis and other hyperhidrotic conditions. Eur J Neurol. 1999;6(suppl.4):S111–S115. [Google Scholar]
- 3.Stolman LP. Treatment of hyperhidrosis. Dermatol Clin. 1998;16:863–869. doi: 10.1016/s0733-8635(05)70062-0. [DOI] [PubMed] [Google Scholar]
- 4.Hallett M. One man's poison: clinical applications of botulinum toxin. N Engl J Med. 1999;341:118–120. doi: 10.1056/NEJM199907083410209. [DOI] [PubMed] [Google Scholar]
- 5.Heckman M, Ceballos-Baumann AO, Plewig G. Botulinum toxin type A for axillary hyperhidrosis (excessive sweating) N Engl J Med. 2001;344:488–493. doi: 10.1056/NEJM200102153440704. [DOI] [PubMed] [Google Scholar]
- 6.Glogau RG. Botulinum A neurotoxin for axillary hyperhidrosis. Dermatol Surg. 1998;24:817–819. doi: 10.1111/j.1524-4725.1998.tb04257.x. [DOI] [PubMed] [Google Scholar]
- 7.Heckmann M, Breit S, Ceballos-Baumann A, Schaller M, Plewig G. Side-controlled intradermal injection of botulinum toxin type A in recalcitrant axillary hyperhidrosis. J Am Acad Dermatol. 1999;41:987–990. doi: 10.1016/s0190-9622(99)70258-6. [DOI] [PubMed] [Google Scholar]
- 8.Naumann M, Flachenecker P, Bröcker E-B, Toyka KV, Reiners K. Botulinum toxin for palmar hyperhidrosis. Lancet. 1997;349:252. doi: 10.1016/S0140-6736(05)64861-1. [DOI] [PubMed] [Google Scholar]
- 9.Naumann M, Hofmann U, Bergmann I, Hamm H, Toyka KV, Reiners K. Focal hyperhidrosis: effective treatment with intracutaneous botulinum toxin. Arch Dermatol. 1998;134:301–304. doi: 10.1001/archderm.134.3.301. [DOI] [PubMed] [Google Scholar]
- 10.Naver H, Swartling C, Aquilonius S-M. Treatment of focal hyperhidrosis with botulinum toxin type A. Brief overview of methodology and 2 years' experience. Eur J Neurol. 1999;6(suppl 4):S117–S120. [Google Scholar]
- 11.Odderson IR. Hyperhidrosis treated by botulinum A exotoxin. Dermatol Surg. 1998;24:1237–1241. doi: 10.1111/j.1524-4725.1998.tb04104.x. [DOI] [PubMed] [Google Scholar]
- 12.Odderson IR. Axillary hyperhidrosis: treatment with botulinum toxin type A. Arch Phys Med Rehabil. 1998;79:350–352. doi: 10.1016/s0003-9993(98)90020-x. [DOI] [PubMed] [Google Scholar]
- 13.Schnider P, Binder M, Berger T, Auff E. Botulinum A toxin injection in focal hyperhidrosis. Br J Dermatol. 1996;134:1160–1161. doi: 10.1111/j.1365-2133.1996.tb07974.x. [DOI] [PubMed] [Google Scholar]
- 14.Schnider P, Binder M, Kittler H, Birner P, Starkel D, Wolff K, et al. A randomized, double-blind, placebo-controlled study trial of botulinum toxin type A for severe hyperhidrosis. Br J Dermatol. 1999;140:677–680. doi: 10.1046/j.1365-2133.1999.02769.x. [DOI] [PubMed] [Google Scholar]
- 15.Shelley WB, Talanin TY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol. 1998;38:227–229. doi: 10.1016/s0190-9622(98)70242-7. [DOI] [PubMed] [Google Scholar]
- 16.Minor V. Ein neues Verfahren zu der klinischen Untersuchung der Schweissabsonderung. Dtsch Z Nervenheilkd. 1927;101:301–306. [Google Scholar]
- 17.Bushara KO, Park DM, Jones JC, Schutta HS. Botulinum toxin—a possible new treatment for axillary hyperhidrosis. Clin Exp Dermatol. 1996;21:276–278. doi: 10.1111/j.1365-2230.1996.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 18.Drott C, Gothberg G, Claes G. Endoscopic transthoracic sympathectomy: an efficient and safe method for the treatment of hyperhidrosis. J Am Acad Dermatol. 1995;33:78–81. doi: 10.1016/0190-9622(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 19.Drott C, Claes G. Hyperhidrosis treated by thoracic sympathectomy. Cardiovasc Surg. 1996;4:790–791. doi: 10.1016/s0967-2109(96)00048-8. [DOI] [PubMed] [Google Scholar]
- 20.Lai YT, Yang LH, Chio CC, Chen HH. Complications in patients with palmar hyperhidrosis treated with transthoracic endoscopic sympathectomy. Neurosurgery. 1997;41:110–113. doi: 10.1097/00006123-199707000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Kao MC, Chan YL, Lin YJ, Hsieh CA, Tsai JC. Endoscopic sympathectomy treatment of craniofacial hyperhidrosis. Arch Surg. 1996;131:1091–1094. doi: 10.1001/archsurg.1996.01430220085019. [DOI] [PubMed] [Google Scholar]
- 22.Herbst F, Plas EG, Fugger R, Fritsch A. Endoscopic thoracic sympathectomy for primary hyperhidrosis of the upper limbs: a critical analysis and long-term results of 480 operations. Ann Surg. 1994;220:86–90. doi: 10.1097/00000658-199407000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]