Abstract
Staphylococcus xylosus is a commensal bacterium found on the skin and mucosal surfaces of SPF mice. S. xylosus is rarely pathogenic, most often causing skin lesions and dermatitis in immunocompromised mice, particularly those with impaired NADPH oxidase function. Here we report spontaneous infection with S. xylosus in Rag1−/−Tpl2−/− mice. Infection was characterized by the presence of alopecia, crusts, and scaly skin. S. xylosus was detected in the feces, skin, lymph nodes, and lungs of Rag1−/−Tpl2−/− mice and led to mortality or euthanasia due to humane endpoints. C57BL/6 mice were culture-positive for S. xylosus on the skin, and Rag1−/− and Tpl2−/− mice were culture-positive on the skin and occasionally in the feces. However, S. xylosus did not cause clinical symptoms in C57BL/6, Rag1−/−, or Tpl2−/− mice. Compared with those in Rag1−/− mice, relative concentrations of circulating monocytes, but not neutrophils or lymphocytes, were increased in Rag1−/−Tpl2−/− mice, consistent with their increased incidence of clinical symptoms. Overall, this case study suggests a novel role for Tpl2 in T-cell–independent host resistance to the otherwise commensal organism S. xylosus.
Abbreviations: Tpl2, tumor progression locus 2
Staphylococcus xylosus is a nonmotile coagulase-negative, gram-positive coccoid organism that was first identified in 1975.31 Staphylococci are common bacteria in the environment and have been linked to opportunistic infections in both humans and animals. S. xylosus is generally considered a nonpathogenic bacterium and is commonly used in the production of meat and cheese products.1,13,18,33,34,39 One reason Staphylococcal spp. are useful in food production is due to their expression of the MprF gene, which leads to the production of lantibiotics and protection against spoilage.25 S. xylosus is a commensal bacterium found on the skin of SPF C57BL/6 mice.35 All strains of S. xylosus isolated from laboratory animals have been susceptible to multiple antibiotics.10 In addition, S. xylosus has been isolated from humans, dairy cows, ewes, gerbils, and poultry.5,23
Although large numbers of mice are used in research, few reports of spontaneous S. xylosus infection are available. Spontaneous infection of athymic nude mice with S. xylosus, characterized by extensive dermatitis on the neck, thorax, and shoulders has been reported;4,29 however, S. xylosus-induced scalding dermatitis in athymic nude mice arose inconsistently, with significant dermal inflammation and the presence of gram-positive cocci in the lesions.4,29 This presentation is consistent with the induction of the localized CD4 T-cell response after experimental colonization of germ-free C57BL/6 mice with S. xylosus.24 Importantly, genetically modified mouse strains deficient in phagocyte superoxide production also show increased susceptibility to spontaneous S. xylosus infection, characterized by dermatitis and abscess formation.10,14,15,26,38 The development of severe dermatitis, morbidity, and mortality in these strains suggests that both T cells and superoxide production contribute to host resistance. Superoxide is generated through activation and formation of the NADPH oxidase complex, which is composed of 5 subunits: p22phox and gp91phox (Cybb) on the cell membrane and p40phox, p47phox, and p67phox in the cytoplasm. On activation, the 5 subunits combine at the cell membrane, generating the active NADPH oxidase and enabling superoxide production. Mice lacking the gp91phox subunit (B6.129S6-Cybbtm1Din/J) or p47phox subunit (B6p47phox−/−HLL and B6.129S2-Ncf1tm1shlN14), which are commonly used as models for chronic granulomatous disease, have been reported to develop spontaneous S. xylosus infection.4,14,26 Of note, infection in superoxide-deficient mice is also characterized by dissemination into other organs, including brain, lung, lymph nodes, and bone, whereas S. xylosus remains localized to the skin in superoxide-competent mice.10,14
In the current report, we describe spontaneous infection with S. xylosus that was associated with morbidity and mortality in Rag1−/−Tpl2−/− mice. Affected mice presented with alopecia, scaly skin, crusts, and mortality or euthanasia according to humane endpoints. However, C57BL/6, Rag1−/−, and Tpl2−/− mice did not develop clinical signs of infection even though all strains were culture-positive for S. xylosus on the skin. Rag1−/−Tpl2−/− mice occasionally had disseminated bacteria in lymph nodes and lung. Dissemination into internal organs is indicative of defects in NADPH oxidase10 and implicates Tpl2 in the production of superoxide by phagocytes in vivo.
Materials and Methods
Mice.
Wild-type (C57BL/6J) and Rag1−/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Tpl2−/− mice backcrossed for more than 10 generations onto the C57BL/6 genetic background were kindly provided by Dr Philip Tsichlis and Thomas Jefferson University (Philadelphia, PA). Rag1−/− mice were crossed with Tpl2−/− mice to generate Rag1−/−Tpl2−/− mice. C57BL/6 and Tpl2−/− mice were crossed to generate Tpl2+/– mice; the wild-type and Tpl2−/− mice used in this report were bred from heterozygous matings. Genotyping of mice as Tpl2+/+, Tpl2+/–, or Tpl2−/− was performed on tissue samples harvested by either tail snip or ear punch. DNA was extracted by using the E.Z.N.A Tissue DNA Kit (Omega Bio-Tek, Norcross, GA) and evaluated by PCR analysis using the following primer sequences: shared forward primer, 5′ CTT CAG TCA TCT TAA CAC TCA GGC 3′; WT reverse primer, 5′ CTG CTT GGA ACT TGC TGT TCT AGA TG 3′; and Tpl2−/− reverse primer, 5′ CTG CAC GAG ACT AGT GAG ACG TGC 3′. Mice were bred and maintained in sterile microisolation cages at the University of Georgia (Athens, GA) according to IACUC-approved guidelines for laboratory animals. The central animal facility at the University of Georgia monitors all mouse cages daily and routinely tests for the presence of pathogenic infection in female sentinel cages. Throughout this study, sentinels consistently tested negative for various endoparasites, ectoparasites, and viral infections including mouse parvovirus, mouse hepatitis virus, Sendai virus, pneumonia virus of mice, Mycoplasma pulmonis, and lymphocytic chroiomeningitis virus. Rag1−/−Tpl2−/− mice were euthanized when they met criteria for humane endpoints including ruffled fur, hunched posture, and evidence of infection. All euthanized mice in this case report were male and ranged in age from 6 wk to 4 mo old. All mice were euthanized with CO2 gas followed by cervical dislocation to confirm death.
Blood and tissue collection.
Blood was collected through terminal cardiocentesis into microvette tubes containing EDTA (Starstedt, Nümbrecht, Germany). Total WBC (reported as number of cells per microliter) were measured automatically (HemaTrue Veterinary Hematology Analyzer, Heska, Loveland, CO). Skin (including subcutaneous areas), spleen, lymph nodes (mesenteric and inguinal), lung, brain, and fecal samples were collected into sterile PBS by using aseptic technique, homogenized in BHI broth (Becton Dickenson, Franklin Lanes, NJ), and plated on blood agar (Becton Dickenson), MacConkey agar, and phenylethyl alcohol agar (Thermo Fisher Scientific, Waltham, MA) at 35 °C with 5% CO2. S. xylosus was identified by matrix-assisted laser desorption/ionization–time of flight mass spectrometry7 by the University of Georgia Veterinary Diagnostic Laboratories using the Vitek system (bioMérieux, Durham, NC). Additional skin samples were fixed in 10% neutral buffered formalin for 24 h at room temperature. Complete cross-sections of formalin-fixed tissue were placed in cassettes, embedded in paraffin, sectioned at 4 μm, mounted on glass slides, and stained with hematoxylin and eosin or Masson trichrome and periodic acid–Schiff–hematoxylin. Histologic sections were evaluated by a veterinary pathologist (TN).
Statistics.
Data were compared relative to Rag1−/− Tpl2−/− mice by using the Fisher exact test or one-way ANOVA with Tukey posthoc testing by using Prism software (GraphPad Software, La Jolla, CA). Differences were considered significant when the P value was 0.05 or less (0.016 or less with multiple comparisons).
Case Report
Over a 1-y period, we noted thickened scaly skin that required euthanasia or led to mortality in several male naive Rag1−/−Tpl2−/− mice in our mouse colony. The skin of affected Rag1−/−Tpl2−/− mice was characterized by ruffled fur, mild alopecia, scabs, and scattered discrete crusts (Figure 1 A). Microscopic analysis revealed a markedly hyperplastic epidermis focally covered with a serocellular crust composed of neutrophils, cellular debris, and bacteria. Regions of fibrosis beneath the epidermis indicated chronic infection, and acute hemorrhage between the epidermis and superficial dermis signified a severe inflammatory condition. Mononuclear inflammatory cell infiltrates were present also (Figure 1 B). Masson trichrome and periodic acid Schiff– hematoxylin staining revealed regions of fibrosis in Rag1−/−Tpl2−/− mice (Figure 1 C). Necropsy and diagnostic testing, consisting of differential culture followed by mass spectrometry, revealed that all mice demonstrating clinical symptoms were positive for S. xylosus. Additional bacterial species identified included Enterococcus gallinarum, Leuconostoc spp., and Corynebacterium mastitidis, but the presence of these bacterial species did not correlate with the incidence of skin lesions. Collectively, these findings strongly suggested that skin lesions were due to spontaneous S. xylosus infection, which occurred at different time points throughout the 1-y period in 4 separate cages of male mice among a total of 29 naive Rag1−/−Tpl2−/− cages, corresponding to an infection incidence rate of 13.8%. None of the 23 cages of naïve Rag1−/− mice or 152 cages of naïve Tpl2+/– × Tpl2+/– mice (comprising Tpl2+/+ [C57BL/6], Tpl2+/–, and Tpl2−/− mice) showed clinical signs of infection or required euthanasia during the same time period.
Figure 1.
Gross morphology and histopathology of Rag1−/−Tpl2−/− mice. (A) Rag1−/−Tpl2−/− mouse (right) showing ruffled fur, alopecia, crusts, and scabbing next to a Rag1−/− mouse (left). (B–D) Representative histology of skin from (B) Tpl2−/−, (C) Rag1−/− , and (D) Rag1−/−Tpl2−/− mice stained with hematoxylin and eosin. The outline indicates focal edema with fibrin in the superficial dermis immediately beneath the epidermis; the small arrows indicate mononuclear inflammatory cells. (E) Representative histology from skin of Rag1−/−Tpl2−/− mice stained with Masson trichrome and periodic acid–Schiff–hematoxylin. The arrow indicates a region of fibrosis, and the arrowhead indicates normal collagen. A, adipose; D, dermis; E, epidermis; F, fibrosis; M, cutaneous muscle; SC, serocellular crust.
Several occurrences of spontaneous S. xylosus infection in Rag1−/−Tpl2−/− mice prompted the investigative staff to euthanize a cohort of male C57BL/6, Rag1−/−, Tpl2−/−, and Rag1−/−Tpl2−/− mice and examine them for the presence of S. xylosus on the skin as well as in the feces, spleen, lymph nodes, lung, and brain. The organism was detected on the skin of all mice and in the feces of 2 Tpl2−/− mice, a single Rag1−/− mouse, and all Rag1−/−Tpl2−/− mice (Table 1). These data are consistent with reports of S. xylosus on the skin of SPF C57BL/6 mice.35 Fecal testing of Rag1−/−Tpl2−/− mice included female cages and confirmed that colonization with S. xylosus in Rag1−/−Tpl2−/− mice was not sex-specific, although dermatitis, morbidity, and mortality were restricted to male mice. In addition, aerobic culture of S. xylosus documented dissemination to lymph nodes in one Rag1−/−Tpl2−/− mouse and lung in another (Table 1). These data further implicate S. xylosus as the causative agent of dermatitis in the current outbreak and suggest that the additional absence of Tpl2 predisposes in immunocompromised Rag1−/− mice to S. xylosus infection, thus indicating the importance of Tpl2 in innate immune cells.
Table 1.
Detection of S. xylosusin murine organs
| Genotype | Feces | Skin | Spleen | LN | Lung | Brain |
| C57BL/6 | 0/3a | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Tpl2−/− | 2/4b | 4/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| Rag1−/− | 1/6a | 5/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Rag1–/– Tpl2–/– | 10/10 | 8/8 | 0/8 | 1/4 | 1/3 | 0/3 |
Tissue samples collected from age-matched mice were cultured for S. xylosus. Mice were sampled randomly; not all mice were evaluated for bacterial dissemination in all organs. Data are given as ‘no. mice positive/total no. of mice sampled’.
Values significantly (aP < 0.005, P < 0.06 [Fisher exact test]) different from those of Rag1−/− Tpl2−/− mice are indicated.
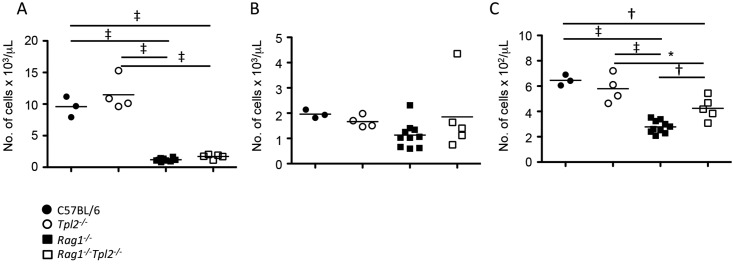
Given the presence of infection, we evaluated WBC concentrations in the circulation to evaluate immune responses generated to infection. As expected, Rag1−/− and Rag1−/−Tpl2−/− mice had fewer circulating lymphocytes, compared with C57BL/6 and Tpl2−/− mice22,32 (Figure 2). In addition, C57BL/6, Rag1−/−, Tpl2−/−, and Rag1−/−Tpl2−/− mice all had similar concentrations of circulating neutrophils (Figure 2). Both Rag1−/− and Rag1−/−Tpl2−/− mice had fewer circulating monocytes than C57BL/6 and Tpl2−/− mice, but Rag1−/−Tpl2−/− mice had more monocytes than Rag1−/− mice (Figure 2).
Figure 2.
Rag1−/−Tpl2−/− mice had increased numbers of circulating monocytes. Blood collected from age-matched C57BL/6 (n = 3), Tpl2−/− (n = 4), Rag1−/− (n = 10), and Rag1−/−Tpl2−/− (n = 5) mice was analyzed for (A) lymphocytes, (B) neutrophils, and (C) monocytes. Horizontal lines indicate mean values; data were analyzed by one-way ANOVA with Tukey posthoc testing (*, P < 0.05; †, P < 0.01; ‡, P < 0.001)
Discussion
Tumor progression locus 2 (Tpl2, also known as MAP3K8) is a serine–threonine protein kinase that is expressed in both innate and adaptive immune cells. Due to their reduced production of TNF, Tpl2−/− mice were originally described as resistant to endotoxin-induced septic shock.8 Because Tpl2 promotes TNF processing and secretion,8,28 it is being investigated as a therapeutic target for treating autoimmune diseases, especially those exacerbated by TNF, such as rheumatoid arthritis.9,11,12 The roles for Tpl2 in immune responses during autoimmune and infectious diseases are currently being investigated. Tpl2−/− mice are more susceptible to infection with Toxoplasma gondii,37 Listeria monocytogenes,19,21 Mycobacterium tuberculosis,19 and influenza,17 compared with wild-type mice. Similarly, Rag1−/−Tpl2−/− mice develop larger bacterial burdens in response to M. tuberculosis and L. monocytogenes infections than do Rag1−/− mice,19 indicating the important role of Tpl2 in regulating innate immune responses to infection.
In this case study, we describe spontaneous infection of Rag1−/−Tpl2−/− mice with S. xylosus, an environmental contaminant and common commensal bacterium of barrier surfaces of mammals, including laboratory mice.23 This organism has been detected on the skin of C57BL/6 mice housed under SPF conditions.35 All of the mice in this study were culture-positive for S. xylosus on the skin, and all Rag1−/−Tpl2−/− mice, one Rag1−/− mouse, and 2 Tpl2−/− mice also cultured positive for S. xylosus in their feces. Clinical disease was never noted in Tpl2−/− or Rag1−/− mice, despite skin and fecal cultures that were positive for S. xylosus. In addition, dermatitis, morbidity, and mortality associated with S. xylosus infection were noted only in male mice, even though both male and female Rag1−/−Tpl2−/− mice were culture-positive for S. xylosus. Spontaneous S. xylosus infection in athymic nude mice reportedly occurs with increased frequency in male mice,4 although a second study noted similar frequencies in male and female mice.29 In addition, male and female mice deficient in NADPH oxidase showed similar susceptibility to opportunistic infections by S. xylosus.21 The apparent restriction of dermatitis, morbidity, and mortality due to S. xylosus to male Rag1−/−Tpl2−/− mice in the current study may reflect variability due to the small number of severe dermatitis cases or may correlate with the increased intracage aggression generally observed with male mice.20,27,36 The resultant bite wounds or skin abrasions may facilitate bacterial entry into the skin and the establishment of infection.4
Although S. xylosus is generally considered a commensal bacterium, the organism has caused opportunistic infections in immunocompromised animals. Spontaneous S. xylosus infection has previously been reported in athymic nude mice,4,29 indicating that T cells contribute to protection against spontaneous S. xylosus infection. Like athymic nude mice, Rag1−/− mice lack mature T cells,6,22,32 but they failed to develop clinical symptoms in the current study. The apparent difference in susceptibility to infection between these 2 strains is unclear. Because athymic nude mice lack a protective layer of hair, they may be more susceptible to dermal injuries that predispose to bacterial infections of the skin. S. xylosus was not detected in internal organs but was localized to the skin in nude mice.4 Dissemination into internal organs has only been reported in mice deficient in NADPH oxidase and superoxide production.10,14,26 Tpl2 is required for superoxide production in macrophages16 and peritoneal exudate cells,30 possibly through defects in formation of the NADPH oxidase complex.16 The combined defects in mature T cells and superoxide production in Rag1−/−Tpl2−/− mice is consistent with their increased susceptibility to S. xylosus infection.
The reduced numbers of circulating monocytes in Rag1−/− and Rag1−/−Tpl2−/− mice compared with C57BL/6 and Tpl2−/− mice indicate that defects in lymphocyte production affect peripheral monocyte concentrations. Interestingly, Rag1−/−Tpl2−/− mice had more circulating monocytes compared with Rag1−/− mice. The increased monocyte levels in Rag1−/−Tpl2−/− mice might indicate clinical infection with S. xylosus, given that a similar increase in monocytes occurs with S. aureus infection.2 However, experimental colonization of germ-free C57BL/6 mice with S. xylosus, among other commensals, did not influence relative numbers of blood myeloid cells.3 Perhaps clinical S. xylosus infection induces more prominent perturbations in leukocyte populations in immunocompromised hosts such as Rag1−/−Tpl2−/− mice than are induced by the same microorganism in immunocompetent hosts, where it exists as a nonpathogenic commensal.
In conclusion, we describe spontaneous S. xylosus infection in a genetically modified murine model. S. xylosus infection in Rag1−/−Tpl2−/− mice correlated with disseminated bacteria and elevated numbers of circulating monocytes compared with those in Rag1−/− mice. Overall, these data may represent an important consideration for future research using Rag−/−Tpl2−/− mice, in which underlying S. xylosus infection might alter immune status and obscure experimental interpretations.
Acknowledgments
We thank the University of Georgia (UGA)’s Veterinary Medicine Central Animal Facility for animal care; our laboratory animal veterinarian, Dr Stephen Harvey; UGA's Veterinary Diagnostic Laboratories; and UGA's Veterinary Pathology Department. We thank Dr Deborah Keys for guidance on statistical analysis. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the NIH under award no. R01AI099058 (to WTW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Addis E, Fleet GH, Cox JM, Kolak D, Leung T. 2001. The growth, properties, and interactions of yeasts and bacteria associated with the maturation of Camembert and blue-veined cheeses. Int J Food Microbiol 69:25–36. [DOI] [PubMed] [Google Scholar]
- 2.Ardura MI, Banchereau R, Mejias A, Di Pucchio T, Glaser C, Allantaz F, Pascual V, Banchereau J, Chaussabel D, Ramilo O. 2009. Enhanced monocyte response and decreased central memory T cells in children with invasive Staphylococcus aureus infections. PLoS One 4:e5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balmer ML, Schurch CM, Saito Y, Geuking MB, Li H, Cuenca M, Kovtonyuk LV, McCoy KD, Hapfelmeier S, Ochsenbein AF, Manz MG, Slack E, Macpherson AJ. 2014. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88–TICAM signaling. J Immunol 193:5273–5283. [DOI] [PubMed] [Google Scholar]
- 4.Bradfield JF, Wagner JE, Boivin GP, Steffen EK, Russell RJ. 1993. Epizootic fatal dermatitis in athymic nude mice due to Staphylococcus xylosus. Lab Anim Sci 43:111–113. [PubMed] [Google Scholar]
- 5.Byrum BR, Slemons RD. 1995. Detection of proteolytic bacteria in the upper respiratory tract flora of poultry. Avian Dis 39:622–626. [PubMed] [Google Scholar]
- 6.De Sousa MAB, Parrott DMV, Pantelouris EM. 1969. The lymphoid tissues in mice with congenital aplasia of the thymus. Clin Exp Immunol 4:637–644. [PMC free article] [PubMed] [Google Scholar]
- 7.Dubois D, Leyssene D, Chacornac JP, Kostrzewa M, Schmit PO, Talon R, Bonnet R, Delmas J. 2009. Identification of a variety of Staphylococcus species by matrix-assisted laser desorption–ionization time-of-flight mass spectrometry. J Clin Microbiol 48:941–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. 2000. TNFα induction by LPS is regulated posttranscriptionally via a Tpl2–ERK-dependent pathway. Cell 103:1071–1083. [DOI] [PubMed] [Google Scholar]
- 9.George D, Salmeron A. 2009. Cot/Tpl2 protein kinase as a target for the treatment of inflammatory disease. Curr Top Med Chem 9:611–622. [DOI] [PubMed] [Google Scholar]
- 10.Gozalo AS, Hoffmann VJ, Brinster LR, Elkins WR, Ding L, Holland SM. 2010. Spontaneous Staphylococcus xylosus infection in mice deficient in NADPH oxidase and comparison with other laboratory mouse strains. J Am Assoc Lab Anim Sci 49:480–486. [PMC free article] [PubMed] [Google Scholar]
- 11.Green N, Hu Y, Janz K, Li HQ, Kaila N, Guler S, Thomason J, Joseph-McCarthy D, Tam SY, Hotchandani R, Wu J, Huang A, Wang Q, Leung L, Pelker J, Marusic S, Hsu S, Telliez J-B, Hall JP, Cuozzo JW, Lin L-L. 2007. Inhibitors of tumor progression loci 2 (Tpl2) kinase and tumor necrosis factor α (TNFα) production: selectivity and in vivo antiinflammatory activity of novel 8-substituted-4-anilino-6-aminoquinoline-3-carbonitriles. J Med Chem 50:4728–4745. [DOI] [PubMed] [Google Scholar]
- 12.Hall JP, Kurdi Y, Hsu S, Cuozzo J, Liu J, Telliez JB, Seidl KJ, Winkler A, Hu Y, Green N, Askew GR, Tam S, Clark JD, Lin LL. 2007. Pharmacologic inhibition of Tpl2 blocks inflammatory responses in primary human monocytes, synoviocytes, and blood. J Biol Chem 282:33295–33304. [DOI] [PubMed] [Google Scholar]
- 13.Irlinger F. 2008. Safety assessment of dairy microorganisms: coagulase-negative Staphylococci. Int J Food Microbiol 126:302–310. [DOI] [PubMed] [Google Scholar]
- 14.Jackson SH, Gallin JI, Holland SM. 1995. The p47phox mouse knockout model of chronic granulomatous disease. J Exp Med 182:751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krugner-Higby L, Brown R, Rassette M, Behr M, Okwumabua O, Cook M, Bell C, Flowers MT, Ntambi J, Gendron A. 2012. Ulcerative dermatitis in C57BL/6 mice lacking stearoyl CoA desaturase 1. Comp Med 62:257–263. [PMC free article] [PubMed] [Google Scholar]
- 16.Kuriakose T, Rada B, Watford WT. 2014. Tumor progression locus 2-dependent oxidative burst drives phosphorylation of extracellular signal-regulated kinase during TLR3 and 9 signaling. J Biol Chem 289:36089–360100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuriakose T, Tripp RA, Watford WT. 2015. Tumor progression locus 2 promotes induction of IFNλ, interferon-stimulated genes, and antigen-specific CD8+ T-cell responses and protects against influenza virus. PLoS Pathog 11:e1005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macedo AC, Malcata FX, Hogg TA. 1995. Microbiological profile in Serra ewes’ cheese during ripening. J Appl Bacteriol 79:1–11. [Google Scholar]
- 19.McNab FW, Ewbank J, Rajsbaum R, Stavropoulos E, Martirosyan A, Redford PS, Wu X, Graham CM, Saraiva M, Tsichlis P, Chaussabel D, Ley SC, O'Garra A. 2013. TPL2–ERK1/2 signaling promotes host resistance against intracellular bacterial infection by negative regulation of type I interferon production. J Immunol 191:1732–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miczek KA, Maxson SC, Fish EW, Faccidomo S. 2001. Aggressive behavioral phenotypes in mice. Behav Brain Res 125:167–181. [DOI] [PubMed] [Google Scholar]
- 21.Mielke LA, Elkins KL, Wei L, Starr R, Tsichlis PN, O'Shea JJ, Watford WT. 2009. Tumor progression locus 2 (Map3k8) is critical for host defense against Listeria monocytogenes and IL1β production. J Immunol 183:7984–7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. 1992. RAG1-deficient mice have no mature B and T lymphocytes. Cell 68:869–877. [DOI] [PubMed] [Google Scholar]
- 23.Nagase N, Sasaki A, Yamashita K, Shimizu A, Wakita Y, Kitai S, Kawano J. 2002. Isolation and species distribution of Staphylococci from animal and human skin. J Vet Med Sci 64:245–250. [DOI] [PubMed] [Google Scholar]
- 24.Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, Quinones M, Brenchley JM, Kong HH, Tussiwand R, Murphy KM, Merad M, Segre JA, Belkaid Y. 2015. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, Kalbacher H, Nieuwenhuizen WF, Jung G, Tarkowski A, van Kessel KPM, van Strijp JAG. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor Mprf is based on modification of membrane lipids with l-lysine. J Exp Med 193:1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizzolla A, Hultqvist M, Nilson B, Grimm MJ, Eneljung T, Jonsson IM, Verdrengh M, Kelkka T, Gjertsson I, Segal BH, Holmdahl R. 2012. Reactive oxygen species produced by the NADPH oxidase 2 complex in monocytes protect mice from bacterial infections. J Immunol 188:5003–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poole TB, Morgan HDR. 1973. Differences in aggressive behaviour between male mice (Mus musculus L.) in colonies of different sizes. Anim Behav 21:788–795. [DOI] [PubMed] [Google Scholar]
- 28.Rousseau S, Papoutsopoulou M, Symons A, Cook D, Lucocq JM, Prescott AR, O'Garra A, Ley SC, Cohen P. 2008. TPL2-mediated activation of ERK1 and ERK2 regulates the processing of preTNFα in LPS-stimulated macrophages. J Cell Sci 121:149–154. [DOI] [PubMed] [Google Scholar]
- 29.Russo M, Invernizzi A, Gobbi A, Radaelli E. 2012. Diffuse scaling dermatitis in an athymic nude mouse. Vet Pathol 50:722–726. [DOI] [PubMed] [Google Scholar]
- 30.Sanz-Garcia C, Ferrer-Mayorga G, González-Rodríguez Á, Valverde ÁM, Martín-Duce A, Velasco-Martín JP, Regadera J, Fernández M, Alemany S. 2013. Sterile inflammation in acetaminophen-induced liver injury is mediated by Cot/Tpl2. J Biol Chem 288:15342–15351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schleifer KH, Kloos WE. 1975. Isolation and characterization of Staphylococci from human skin. Int J Syst Evol Biol 25:50–61. [Google Scholar]
- 32.Shinkai Y, Rathbun G, Lam K-P, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. 1992. RAG2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855–867. [DOI] [PubMed] [Google Scholar]
- 33.Stahnke LH. 1994. Aroma components from dried sausages fermented with Staphylococcus xylosus. Meat Sci 38:39–53. [DOI] [PubMed] [Google Scholar]
- 34.Stahnke LH. 1995. Dried sausages fermented with Staphylococcus xylosus at different temperatures and with different ingredient levels. Part II. Volatile components. Meat Sci 41:193–209. [DOI] [PubMed] [Google Scholar]
- 35.Tavakkol Z, Samuelson D, deLancey Pulcini E, Underwood RA, Usui ML, Costerton JW, James GA, Olerud JE, Fleckman P. 2010. Resident bacterial flora in the skin of C57BL/6 mice housed under SPF conditions. J Am Assoc Lab Anim Sci 49:588–591. [PMC free article] [PubMed] [Google Scholar]
- 36.Van Loo PLP, Van Zutphen LFM, Baumans V. 2003. Male management: coping with aggression problems in male laboratory mice. Lab Anim 37:300–313. [DOI] [PubMed] [Google Scholar]
- 37.Watford WT, Hissong BD, Durant LR, Yamane H, Muul LM, Kanno Y, Tato CM, Ramos HL, Berger AE, Mielke L, Pesu M, Solomon B, Frucht DM, Paul WE, Sher A, Jankovic D, Tsichlis PN, O'Shea JJ. 2008. Tpl2 kinase regulates T-cell interferon γ production and host resistance to Toxoplasma gondii. J Exp Med 205:2803–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Won YS, Kwon HJ, Oh GT, Kim BH, Lee CH, Park YH, Hyun BH, Choi YK. 2002. Identification of Staphylococcus xylosus isolated from C57BL/6J-Nos2tm1Lau mice with dermatitis. Microbiol Immunol 46:629–632. [DOI] [PubMed] [Google Scholar]
- 39.Zell C, Resch M, Rosenstein R, Albrecht T, Hertel C, Gotz F. 2008. Characterization of toxin production of coagulase-negative Staphylococci isolated from food and starter cultures. Int J Food Microbiol 127:246–251. [DOI] [PubMed] [Google Scholar]