Abstract
Atherosclerosis is enhanced in arterial segments exposed to disturbed flow. Perturbed shear stress increases the expression of oxidation-sensitive responsive genes (such as ELK-1 and p-JUN) in the endothelium. Evidence suggests that polyphenolic antioxidants contained in the juice derived from the pomegranate can contribute to the reduction of oxidative stress and atherogenesis. The aim of the present study was to evaluate the effects of intervention with pomegranate juice (PJ) on oxidation-sensitive genes and endothelial NO synthase (eNOS) expression induced by high shear stress in vitro and in vivo. Cultured human coronary artery endothelial cells (EC) exposed to high shear stress in vitro and hypercholesterolemic mice were used in this study. PJ concentrate reduced the activation of redox-sensitive genes (ELK-1 and p-JUN) and increased eNOS expression (which was decreased by perturbed shear stress) in cultured EC and in atherosclerosis-prone areas of hypercholesterolemic mice. Moreover, oral administration of PJ to hypercholesterolemic mice at various stages of disease reduced significantly the progression of atherosclerosis. This experimental study indicates that the proatherogenic effects induced by perturbed shear stress can be reversed by chronic administration of PJ. This approach may have implications for the prevention or treatment of atherosclerosis and its clinical manifestations.
Keywords: polyphenols, p-Jun, antioxidants
Vascular endothelial cells (EC) are constantly subjected to hemodynamic forces inducing shear stress, which stimulates physiologically the release of NO by constitutively expressed endothelial NO synthase (eNOS) (1). Perturbed shear stress alone or high shear stress associated with other classical risk factors of atherosclerosis may trigger signal transduction events that in turn may lead to endothelial dysfunction and enhanced atherogenesis (2-4). It has been demonstrated that areas highly prone to atherosclerosis have unique patterns of disturbed flow, characterized by regions of flow separation, recirculation, and temporal and spatial gradients of shear stress (4). Furthermore, vascular EC under perturbed shear stress increase their production of reactive oxygen species and other free radicals that are capable of inducing oxidative stress (5-12). NO controls vascular oxidative stress and the expression of redox-regulated genes (13, 14). Evidence exists that eNOS activity is reduced at sites of perturbed shear stress (2, 5-8, 11).
There is an abundant mythological and ancient history regarding the pomegranate fruit (15-20). In Greece, pomegranate fruit (Punica granatum L.) was known as the “fruit of the dead,” and in the Hebrew tradition, pomegranates adorned the vestments of the high priest. In Babylonia, the pomegranate was an agent of resurrection, and the Persians believed that pomegranate seeds conferred invincibility on the battlefield. In China, the pomegranate fruit symbolized longevity. The fruit of the pomegranate (≈50% of total pomegranate weight) consists of 80% juice and 20% seeds. The fresh juice contains 85% water, 10% total sugars, 1.5% pectin, ascorbic acid, and polyphenolic flavonoids (20). In pomegranate juice (PJ), fructose and glucose are present in similar quantities; calcium is 50% of its ash content; and the principal amino acids are glutamic and aspartic acids (20). The soluble polyphenol content varies within the limits of 0.2-1.0%, depending on variety, and includes mainly anthocyanins, catechins, ellagic tannins, and gallic and ellagic acids (16, 17, 20). More importantly, PJ possesses potent antioxidant activity that elicits antiatherogenic properties in mice (20-22) and can inhibit cyclooxygenases and lipoxygenases (18).
We have previously shown that intervention with antioxidants and l-arginine reverses shear-stress-related redox-gene activation and increases eNOS expression in both cultured EC and hypercholesterolemic mice (11). To date, there have been no reports indicating whether antioxidant polyphenols and flavonoids contained in PJ can elicit similar protective effects. Antioxidants are well known to enhance the biological actions of NO by virtue of their capacity to stabilize NO by protecting against the oxidative destruction of NO by reactive oxygen species and other radicals. This antioxidant effect results in much higher and more prolonged cellular concentrations of NO, leading to markedly increased biological actions of NO. To determine whether proatherogenic conditions induced by high shear stress can be attenuated by PJ, we first studied cultured human coronary artery EC subjected to high shear stress. We then analyzed high- and low-prone atherosclerotic aortic areas of hypercholesterolemic mice (11, 23) after chronic oral administration of PJ.
Materials and Methods
PJ Processing. PJ concentrate (Wonderful variety, POM Wonderful, Los Angeles) was used in this study. Pomegranates were hand-picked, washed, chilled to 4°C, and stored in tanks. The fruit was then crushed, squeezed, and treated enzymatically with pectinase to yield the juice and byproducts, which included the inner and outer peels and the seeds. Pectinase hydrolyzes α-1,4-galacturonide bonds in pectin, improving extraction and filtration, and prevents formation of pectin gels. Flavonoids constitute 40% (anthocyanins, catechins, and phenols) of total polyphenols in PJ (20-22). More complex polyphenols are also present in the juice (20-22). The PJ was filtered, pasteurized, concentrated, and stored at -20°C until use.
EC Culture. Human coronary artery EC were cultured as described (11, 24, 25). Cells were incubated at 37°C for 4 days in a humidified atmosphere of 95% air and 5% CO2. The incubation medium (delipidated DMEM) was supplemented with 10 ng/ml human epidermal growth factor, penicillin/streptomycin, amphotericin B, and glutamine (11, 24, 25).
Flow Apparatus for Shear Stress. EC were subjected to laminar shear stress by constant angular velocity in a cone-and-plate viscometer at a relatively low level of 1 dyne/cm3 (mean shear stress in veins) at 5 dyne/cm3 (1 dyne = 10 μN) and at a higher level of 15 dyne/cm3 (shear stress in arteries) 1 day after reaching confluence, as described in detail (11). The viscometer consists of a cone at a 0.5° angle rotating on top of a 94 × 16-mm cell culture dish. Flow conditions achieved are considered laminar because the parameter R (r2ωα2/12ν) was <4 (R1 and R15, 0.006 and 0.03 dyne/cm2, respectively) (11). On the basis of previous studies (20-22), EC were exposed to different flows for 24 h in the absence or presence of 7-14 μl of PJ (from 0.2 to 0.4 μmol total polyphenols).
Treatment of Mice. Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication no. 85-23, revised 1996) and the Guidelines of the American Heart Association. We used male hypercholesterolemic low-density-lipoprotein receptor-deficient (LDLR-/-) mice [n = 48, mean weight 42.1 ± 10.3 g (mean + SD)] receiving a cholate-free high-cholesterol diet (21% milk fat, 1.5% cholesterol, and 19.5% casein; no. 8137, Harlan/Teklad, Madison, WI), as described (11, 23, 26, 27). This diet raised the mean plasma cholesterol levels to ≈1,200 mg/dl and plasma triglyceride levels to 400 mg/dl. Both the preventive and therapeutic effects of PJ were tested. To assess whether PJ influenced stress-responsive genes, 48 mice were randomly divided into four groups (n = 12 each): in protocol 1, one group of 3-month-old mice received PJ in drinking water for 4 weeks before the disease was accelerated by superimposing a fatty diet and then continued on the juice for an additional 24 weeks. In protocol 2, another group of mice was first allowed to develop the disease for 6 months and then given PJ in the drinking water for up to an additional 24 weeks.
The administration of PJ was based on previous studies conducted in mice (20, 21). Concentrated PJ was diluted in water (6.25 ml of concentrated juice in 1 liter of water). This solution was given to the treated groups of mice in both protocols, whereas only water was given to the placebo control groups of mice in both protocols. Because the mice drank an average of 5 ml per day, they effectively drank 31 μl of PJ per day, which is equivalent to 0.875 μmol total polyphenols per day (20, 21).
Blood Determinations, Preparation of Arterial Samples, and Western Blot Analysis. At the end of the treatment period, mice were killed by CO2 asphyxia. Blood was drawn from the inferior vena cava into heparinized tubes (11, 23, 26, 27). Plasma cholesterol was determined enzymatically (23, 26, 27), and plasma isoprostane 8-epi-PGF2α was determined by using a commercially available immunoassay (Cayman Chemical, Ann Arbor, MI), as described (28, 29). The aorta was continuously immersed in PBS containing 10 μg/ml aprotinin and 0.1 mmol/liter PMSF from the time of dissection until the computerized determination of the atherosclerotic lesion area was completed (11, 23, 26-29). Immunostaining of macrophage-derived foam cells in paraffin-embedded arterial sections was made by using the F4/80 monoclonal antibody (Serotec, dilution 1:250) against mouse foam cells (23, 26-29), whereas oxidation-specific epitopes were identified by using the MDA-2 monoclonal antibody (23, 27, 28). Intercellular junctions were stained with 0.25% silver nitrate to visualize the endothelium in the high- and low-prone atherosclerotic aortic regions (located mainly in the proximal aorta), as described (11). EC in the high-prone regions have variable shapes and random orientation, whereas EC in the low-prone region are elongated and aligned in the direction of the blood flow (11, 30, 31). Arterial segments from healthy vessels served as controls. Tissue sections (5 μm) from the different arterial regions were homogenized in 250 μl of protein extraction buffer for determination of protein content by Western blot, as described (19, 21, 28). Approximately 15 μg of protein extract (from either arteries or cultured cells) were separated by 10% SDS/PAGE (11, 25-30). The gel was transblotted onto a nitro-cellulose membrane, blocked with 10% milk powder in Tris-buffered saline (pH 7.4) with 0.1% Tween 20 (TBS-T) overnight, and incubated with monoclonal antibodies against ELK-1 (I-20, goat antibody) and eNOS III (N-20, epitope corresponding to an amino acid sequence mapping at the N terminus of NOS-III; no crossreactivity with NOS-I or -II neuronal and inducible forms, respectively) purchased from Santa Cruz Biotechnology, as described (11). Antibody against the phosphorylated form of JUN (p-JUN) (420110-S) was purchased from Calbiochem. After five washes with TBS-T, the signal was detected with the aid of a chemiluminescence kit (Amersham Pharmacia enhanced chemiluminescence kit) (11, 25-30). Membranes were normalized with a polyclonal antibody against γ-tubulin protein (Sigma). Semiquantitative densitometry of Western blots was performed by using a Scan LKB (Amersham Pharmacia) (11, 25-30).
Statistical Analysis. Results are expressed as mean ± SD. The difference among groups was evaluated by a one- or two-factor ANOVA by two independent investigators in a blinded fashion. Statistical significance was accepted at P < 0.05.
Results
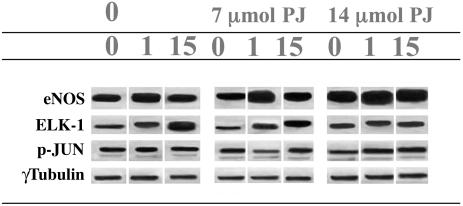
Effects of PJ in Cultured EC. Under the experimental conditions used (11), basal eNOS activity was increased by ≈50% at 1 dyne/cm2 shear stress but only 15% at 15 dyne/cm2 shear stress (Table 1 and Fig. 1). After concomitant cell exposure to 7 μl of PJ, eNOS activity showed a significant further increase at all levels of shear stress (Table 1 and Fig. 1). These effects were concentration-dependent, because cell exposure to 14 μl of PJ induced further increments of eNOS activity (Table 1 and Fig. 1).
Table 1. Effects of PJ in cultured human coronary artery EC exposed to shear stress generated in vitro (0, 1, 5, and 15 dynes per cm2) on e-NOS, ELK-1, and the p-JUN protein levels measured by Western blot.
| Control, dyne
|
7 μl of PJ, dyne
|
14 μl of PJ, dyne
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 5 | 15 | 0 | 1 | 5 | 15 | 0 | 1 | 5 | 15 | |
| eNOS | 2.1 ± 0.4 | 3.0 ± 0.5* | 2.5 ± 0.5 | 2.3 ± 0.3 | 2.2 ± 0.5 | 3.3 ± 0.5† | 3.1 ± 0.5† | 2.8 ± 0.4† | 2.3 ± 0.5 | 4.1 ± 0.6‡ | 3.0 ± 0.6‡ | 3.0 ± 0.5‡ |
| ELK-1 | 2.0 ± 0.4 | 3.2 ± 0.5 | 4.0 ± 0.6§ | 5.3 ± 0.5§ | 2.0 ± 0.3 | 2.6 ± 0.4¶ | 3.0 ± 0.6† | 3.7 ± 0.3¶ | 1.9 ± 0.4 | 2.4 ± 0.3∥ | 2.8 ± 0.4∥ | 3.3 ± 0.4∥ |
| p-JUN | 1.0 ± 0.3 | 1.3 ± 0.3 | 1.8 ± 0.3* | 2.0 ± 0.4* | 0.9 ± 0.2 | 1.1 ± 0.2 | 1.6 ± 0.4 | 2.0 ± 0.3 | 0.8 ± 0.3 | 1.0 ± 0.4 | 1.5 ± 0.4 | 1.6 ± 0.3† |
Control untreated EC or EC treated with PJ diluted in the cellular medium were exposed to laminar shear stress for 24 hours. Densitometric analysis of blots normalized with γ-tubulin are representative of the mean ± SD of five different experiments. p, significance by Bonferroni's corrected t test.
P <0.05 vs. 0 dyne.
P <0.05 vs. respective force in control untreated cells.
P <0.01 vs. respective force in control untreated cells.
P <0.001 vs. 0 dyne.
P <0.02 respective force in control untreated cells.
P <0.01 vs. respective force in control untreated cells.
Fig. 1.
Effect of shear stress (0, 1, or 15 dynes per cm2) on eNOS, ELK-1, and p-Jun protein levels measured by Western blot in cultured human coronary artery EC. Control EC (0) or EC treated with 7 or 14 μl of PJ were exposed to laminar shear stress for 24 h.
Shear stress also increases oxidation-sensitive ELK-1 expression (11). Treatment with 7 μl of PJ significantly attenuated the increase in ELK-1 protein levels compared with the untreated control at the same flow or shear stress (P < 0.02) (Table 1 and Fig. 1).
Again, treatment with 14 μl of PJ further decreased ELK-1 expression (Table 1 and Fig. 1). As already observed (11), p-JUN protein expression was increased by 100% at 15 dyne/cm2 (Table 1 and Fig. 1). PJ (14 μl) significantly attenuated the increase in p-JUN expression at 15 dyne/cm2 (Table 1 and Fig. 1).
Effects of PJ in Hypercholesterolemic Mice. Plasma cholesterol levels were not significantly affected by PJ consumption in protocol 1 (781 ± 66 and 766 ± 77 mg/dl in the placebo- and PJ-treated mice, respectively) or protocol 2 (795 ± 73 and 785 ± 64 mg/dl in the placebo- and PJ-treated mice, respectively). Plasma isoprostanes in mice treated with PJ were reduced compared with placebo-treated mice in protocol 1 (108 ± 19 vs. 140 ± 31 pg/ml, P < 0.05) as well as in protocol 2 (128 ± 15 vs. 156 ± 28 pg/ml, P < 0.05).
Overall, atherosclerotic lesions in low- and high-prone areas of placebo groups of mice were larger than those observed in pomegranate-treated groups of mice in both protocols 1 and 2 and consisted of many more lipid-laden macrophage foam cells (F4/80 immunostaining) and oxidation-specific epitopes (MDA-2 immunostaining) (Table 2 and Fig. 2). There was an ≈20% decrease in both lesion area and foam cell formation in pomegranate-treated mice. These antiatherogenic properties of PJ were consistent with previous findings (20-22).
Table 2. Effects of 31 μl of PJ per day or placebo on atherosclerosis progression in hypercholesterolemic mice fed a high-fat diet.
| PJ group (n = 12)
|
Placebo group (n = 12)
|
|||||
|---|---|---|---|---|---|---|
| HV | LP | HP | HV | LP | HP | |
| Protocol 1 | ||||||
| Atherosclerosis (lesion area × 103 μm2) | 1.78 ± 0.53 | 12.98 ± 1.86† | 105.99 ± 20.58‡ | 1.95 ± 0.43 | 18.64 ± 1.55 | 134.72 ± 18.68 |
| F4/80 immunostaining (% of positive sections) | 4 ± 2 | 25 ± 8‡ | 48 ± 18‡ | 5 ± 2 | 33 ± 9 | 56 ± 24 |
| MDA-2 immunostaining (% of positive sections) | 8 ± 3‡ | 25 ± 8† | 53 ± 20‡ | 13 ± 6 | 56 ± 18 | 75 ± 25 |
| Protocol 2 | ||||||
| Atherosclerosis (lesion area × 103 μm2) | 2.09 ± 0.78 | 23.90 ± 2.55*† | 151.56 ± 20.13*† | 2.81 ± 0.86‡ | 29.96 ± 2.44† | 187.89 ± 34.11† |
| F4/80 immunostaining (% of positive sections) | 7 ± 4‡ | 32 ± 10*‡ | 59 ± 23*‡ | 9 ± 3† | 41 ± 12‡ | 74 ± 25† |
| MDA-2 immunostaining (% of positive sections) | 6 ± 3§ | 32 ± 10§ | 60 ± 21* | 18 ± 5 | 62 ± 20 | 83 ± 28 |
In protocol 1, one group of 3-month-old mice received PJ in drinking water for 4 weeks before the disease was accelerated by a fatty diet and then continued on PJ for an additional 24 weeks. In protocol 2, another group of mice was first allowed to develop the disease for 6 months and then was given PJ in drinking water for up to an additional 24 weeks. In both protocols, the PJ solution diluted in water was given to the treated mice, whereas only water was given to the placebo control groups of mice. Atherosclerotic lesion area and both the number of F4/80 and MDA-2 positive arterial section were assessed by computer-assisted imaging analysis, as described in Materials and Methods. P, significance by Bonferroni's corrected t test. HV, healthy vessel areas; LP, low-prone atherosclerotic areas; HP, high-prone atherosclerotic areas.
P < 0.05 vs. respective placebo control group of mice.
P < 0.01 vs. respective group in protocol 1.
P < 0.05 vs. respective group in protocol 1.
P < 0.01 vs. respective placebo control group of mice.
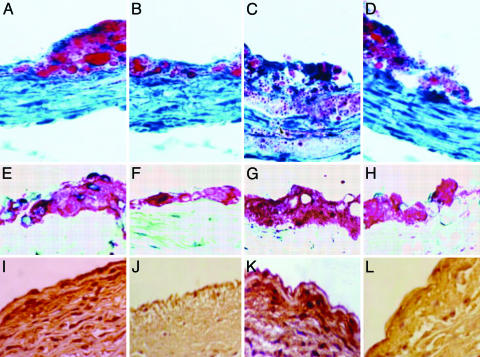
Fig. 2.
Effects of PJ on high-prone (HP) aortic atherosclerotic lesion areas in hypercholesterolemic mice. (A and B) Comparison of the extent of lipid staining by oil red O in representative sections of the aorta of a placebo treated-control mouse (A) and in a PJ-treated mouse (B) from experimental protocol 1. Cryosections 10-μm-thick were stained with oil red O and counterstained with hematoxylin. (C and D) Comparisons of representative sections of the aortic complex lesions of a placebo-treated control mouse (C) and in a PJ-treated mouse (D) from experimental protocol 2. (E and F) Immunohistochemical staining of aortic sections for macrophages of a placebo-treated control mouse (E) and in a PJ-treated mouse (F) from experimental protocol 1. (G and H) The same immunostaining in a control mouse (G) and in a PJ-treated mouse (H) from the experimental protocol 2. (I-L) Immunohistochemical staining of aortic sections for oxidation-specific epitopes (MDA-2 monoclonal antibody). Placebo-treated control mouse (I) and PJ-treated mouse (J) from experimental protocol 1. The same immunostaining in a control mouse (K) and in a PJ-treated mouse (L) from experimental protocol 2. See Materials and Methods for more technical details. (Magnification: A-H, ×63; I-L, ×125.)
When considering shear-stress-related areas, the artery can be visualized as possessing healthy vessel areas and low- and high-prone atherosclerotic areas. In protocol 1, densitometric analysis of Western blots showed that, in comparison with healthy vessel areas, basal eNOS activity before PJ treatment was decreased by ≈25% and 45% (P < 0.01) in low- and high-prone areas, respectively (Table 3). After PJ administration, eNOS activity increased significantly both in low- and high-prone areas (Table 2). These results were also obtained in protocol 2 (Table 3). ELK-1 was increased by ≈90% in low-prone areas (P < 0.01 vs. healthy vessel areas) and 280% in high-prone areas (P < 0.001 vs. healthy vessel areas) in control mice. After PJ treatment, ELK-1 protein levels were significantly decreased by 20% in low-prone areas and 25% in high-prone areas (Table 3). Similar PJ-related beneficial effects on ELK-1 expression were observed in protocol 2 (Table 3). We observed that p-JUN protein levels were significantly increased in high-prone areas in comparison with healthy vessel areas in both protocols (P < 0.05 for both), but the increase was less striking than that of ELK-1 protein. PJ treatment was somewhat less effective and prevented the increase in p-JUN protein levels only in high-prone areas of treated mice in protocol 1 but not in protocol 2 (Table 3). Thus, therapeutic beneficial effects (e.g., increasing eNOS and decreasing redox gene expression in perturbed shear stress areas) were elicited by PJ during chronic intervention.
Table 3. Effects of 31 μl of PJ per day or placebo on shear-stress responsive genes in hypercholesterolemic mice fed a high-fat diet in both protocols 1 and 2.
| PJ group (n = 12)
|
Placebo group (n = 12)
|
|||||
|---|---|---|---|---|---|---|
| HV | LP | HP | HV | LP | HP | |
| Protocol 1 | ||||||
| eNOS | 3.4 ± 0.4 | 3.2 ± 0.4* | 2.6 ± 0.2* | 3.3 ± 0.4 | 2.5 ± 0.5 | 1.8 ± 0.4 |
| ELK-1 | 1.0 ± 0.2 | 1.6 ± 0.4† | 2.4 ± 0.4† | 1.1 ± 0.2 | 2.0 ± 0.3 | 3.1 ± 0.5 |
| p-JUN | 1.4 ± 0.4 | 1.5 ± 0.4 | 1.7 ± 0.3† | 1.5 ± 0.4 | 1.6 ± 0.3 | 2.0 ± 0.4 |
| Protocol 2 | ||||||
| eNOS | 3.1 ± 0.4 | 2.9 ± 0.4* | 2.2 ± 0.4* | 3.0 ± 0.4 | 2.3 ± 0.3 | 1.5 ± 0.4§ |
| ELK-1 | 1.2 ± 0.3 | 2.0 ± 0.3* | 2.9 ± 0.5* | 1.3 ± 0.3 | 2.4 ± 0.4§ | 3.8 ± 0.6§ |
| p-JUN | 1.6 ± 0.5 | 1.7 ± 0.4 | 1.9 ± 0.4 | 1.6 ± 0.4 | 1.8 ± 0.4 | 2.2 ± 0.5 |
Densitometric analysis of Western blots normalized with γ-tubulin are representative of the mean ± SD of each mouse in study groups. P, significance by Bonferroni's corrected t test. HV, healthy vessel areas; LP, low-prone atherosclerotic areas; HP, high-prone atherosclerotic areas.
P < 0.01 vs. respective placebo control group of mice.
P < 0.05 vs. respective placebo control group of mice.
P < 0.05 vs. respective group in protocol 1.
Discussion
The present study illustrates that prolonged supplementation with PJ can largely correct the perturbed shear-stress-induced proatherogenic disequilibrium by increasing eNOS activity and decreasing redox-sensitive transcription factors both in vitro in cultured human coronary artery EC and in vivo in hypercholesterolemic mice. Hypercholesterolemic mice spontaneously develop atherosclerosis, which can be accelerated by feeding the mice a diet rich in fats (13). Both the preventive (protocol 1) and therapeutic (protocol 2) effects of PJ were tested. Accordingly, the purpose of this study was to determine whether PJ, like other natural sources of antioxidants, reduces oxidative stress, oxidation-specific epitopes, and atherogenesis in arteries from hypercholesterolemic mice. Vascular disorders such as atherosclerosis cause disturbed blood flow in the affected regions, and this leads to perturbed shear stress that, in turn, causes endothelial damage. Moreover, endothelial dysfunction is attributed to increased oxidative stress and decreased NO production and action (14). Thus, the protective effects of NO in the vasculature are lost, and atherosclerosis develops as a result. Administration of antioxidants in experimental models has been shown to reduce the severity of atherosclerosis by reducing oxidative stress and increasing NO production and action (14). In the present study, PJ supplementation to low-density lipoprotein receptor-deficient mice, which are under oxidative stress, resulted in substantially lower plasma lipid peroxidation (assessed by plasma isoprostanes) than in control mice. Furthermore, our findings of reductions in macrophage foam cell formation, oxidation-specific epitopes, and lesion area in atherosclerotic prone lesion regions (low- and high-prone areas) in PJ-treated mice clearly confirm the correlation between antioxidative effects and antiatherogenic properties, elicited by PJ in both protocols, as was observed in other studies (20-22).
The second aim of the present study was to determine possible beneficial effects of PJ on oxidation-sensitive gene expression and eNOS at sites of disturbed shear stress. The present study demonstrates that modulation of redox-sensitive transcription factors (ELK-1 and p-JUN) and eNOS expression is associated with antiatherogenic activity in such areas. These effects are similar to those elicited by antioxidants (vitamins E and C) and l-arginine (11). Polyphenols are the most abundant antioxidants in our diets. The main classes of polyphenols are phenolic acids (mainly caffeic acid) and flavonoids [the most abundant in the diet are flavanols (catechins plus proanthocyanidins), anthocyanins, and their oxidation products], which account for one- and two-thirds of polyphenols, respectively (32). Polyphenols are reducing agents that may protect the body's tissues against oxidative stress and associated pathologies such as cancer, chronic heart disease, vascular diseases, and inflammation (32, 33). It is important to understand the nature of the main polyphenols ingested, their dietary origin, the amounts consumed in different diets, their bioavailability, and the factors controlling their bioavailability. PJ is rich in antioxidants of the polyphenolic class, which includes tannins and anthocyanins. These antioxidants are more potent, on a molar basis, than many other antioxidants, including vitamins C and E, coenzyme Q-10, and α-lipoic acid. The antioxidant level in PJ was found to be higher than in other natural juices such as blueberry, cranberry, and orange, as well as in red wine (34). Additional experiments will address the direct comparison of PJ-elicited effects to those of antioxidants and l-arginine.
Polyphenols from red wine can reduce low-density lipoprotein (LDL) aggregation in vitro and in vivo (34-36), and PJ administered to hypertensive patients causes also a significant decline in systemic blood pressure (37). Finally, a recent study shows that PJ consumption for 3 years by patients with carotid artery stenosis reduced common carotid intima-media thickness, blood pressure, and LDL oxidation (38). Accordingly, an early study showed that tea pigment (and possibly polyphenols) exerted some antiatherosclerotic effects (39). More recently, it was shown that short- and long-term black tea consumption reverses endothelial dysfunction in patients with chronic heart disease (40). Similarly, the ingestion of polyphenols contained in purple grape juice had beneficial effects on endothelial function in patients with coronary heart disease (41). Taken together, these data suggest that polyphenols can protect arteries from vascular damage via antioxidant effects and NO restoration. However, certain large clinical trials using different antioxidants have failed to show any beneficial effects in terms of prevention of major cardiovascular events (5, 12, 14). One possible explanation of this divergence is that the models used in experimental studies, although very useful to study pathophysiological mechanisms, may not precisely reflect the disease in humans (5, 12, 14). Alternatively, the doses of antioxidants used in those few studies may not have been appropriate, and/or the progression of disease may have been too severe.
The data in the present study show that elevation of the redox factors ELK-1 (a ternary complex factor of the Ets family) and p-JUN, which are associated with perturbed shear stress (11), can be reversed by intervention with PJ in cultured human coronary artery EC and in hypercholesterolemic mice. Indeed, eNOS, p-JUN, and ELK-1 are “primed” to respond to systemic activation stimuli in high-versus low-prone areas that represent areas with the most and least atherosclerosis in mice and pigs. Using PJ therapeutic intervention, we demonstrate that it is possible to attenuate this proatherogenic scenario. Antioxidant protection elicits decreased cellular production and release of oxygen radicals in the vascular wall, inhibits endothelial activation of oxidation-sensitive genes, and improves the biologic activity of NO through a cell- or tissue-specific antioxidant action (14, 42).
Conclusion
Therapeutic intervention with antioxidant polyphenols contained in PJ may promote a sustained correction of the perturbed shear-stress-induced proatherogenic profile in vitro and in vivo. These findings may have important implications for the prevention of atherosclerosis and its clinical sequelae.
Acknowledgments
This work was funded by a grant from the Lynda and Stewart Resnick Revocable Trust (to L.J.I. and C.N.).
Author contributions: F.d.N., V.S., L.J.I., and C.N. designed research; F.d.N., S.W.-I., L.O.L., E.C., C.B., G.M., F.P.D., G.D.R., and V.S. performed research; E.C. contributed new reagents/analytic tools; F.d.N., S.W.-I., L.O.L., E.C., C.B., G.M., F.P.D., G.D.R., V.S., L.J.I., and C.N. analyzed data; and F.d.N., L.O.L., G.D.R., L.J.I., and C.N. wrote the paper.
Abbreviations: NOS, NO synthase; eNOS, endothelial NOS; EC, endothelial cells; PJ, pomegranate juice; p-JUN, phosphorylated form of JUN.
References
- 1.Buga, G. M., Gold, M. E. & Ignarro, L. J. (1991) Hypertension 17, 187-193. [DOI] [PubMed] [Google Scholar]
- 2.Gimbrone, M. A., Jr. (1999) Am. J. Pathol. 155, 1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman, M. H., Hutchins, G. M., Bergerson, C. B., Deters, O. J. & Mark, F. F. (1981) Atherosclerosis 39, 425-436. [DOI] [PubMed] [Google Scholar]
- 4.Glagov, S., Zarins, C., Giddens, D. P. G. & Ku, D. N. (1988) Arch. Pathol. Lab. Med. 112, 1018-1031. [PubMed] [Google Scholar]
- 5.de Nigris, F., Lerman, L. O., Condorelli, M., Lerman, A. & Napoli, C. (2001) Antioxid. Redox Signal. 3, 1119-1130. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh, H. J., Cheng, C. C., Wu, S. T., Chiu, J. J., Wung, B. S. & Wang, D. L. (1998) J. Cell. Physiol. 175, 156-162. [DOI] [PubMed] [Google Scholar]
- 7.Chiu, J. J., Wung, B. S., Shyy, J. Y., Hsieh, H. J. & Wang, D. L. (1997) Arterioscler. Thromb. Vasc. Biol. 17, 3570-3577. [DOI] [PubMed] [Google Scholar]
- 8.Silacci, P., Desgeorges, A., Mazzolai, L., Chambaz, C. & Hayoz, D. (2001) Hypertension 38, 1162-1166. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Cardena, G., Comander, J., Anderson, K. R., Blackman, B. R. & Gimbrone, M. A., Jr. (2001) Proc. Natl. Acad. Sci. USA 98, 4478-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormick, S. M., Eskin, S. G., McIntire, L. V., Teng, C. L., Lu, C. M., Russell, C. G. & Chittur, K. K. (2001) Proc. Natl. Acad. Sci. USA 98, 8955-8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Nigris, F., Lerman, L. O., Ignarro, S. W., Sica, G., Lerman, A., Palinski, W., Ignarro, L. J. & Napoli, C. (2003) Proc. Natl. Acad. Sci. USA 100, 1420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Nigris, F., Lerman, A., Ignarro, L. J., Williams-Ignarro, S., Sica, V., Baker, A. H., Lerman, L. O., Geng, Y. J. & Napoli, C. (2003) Trends Mol. Med. 9, 351-359. [DOI] [PubMed] [Google Scholar]
- 13.Ignarro, L. J., Cirino, G. & Napoli, C. (1999) J. Cardiovasc. Pharmacol. 34, 879-886. [DOI] [PubMed] [Google Scholar]
- 14.Napoli, C. & Ignarro, L. J. (2001) Nitric Oxide 5, 88-97. [DOI] [PubMed] [Google Scholar]
- 15.Cemeroglu, B., Artik, N. & Erbas, S. (1992) Fluessiges Obst. 59, 335-340. [Google Scholar]
- 16.El-Nemr, S. E., Ismail, I. A. & Ragab, M. (1990) Nahrung 34, 601-606. [Google Scholar]
- 17.Narr Ben, C., Ayed, N. & Metche, M. (1996) Z. Lebensm. Unters F. A.. 203, 374-378. [DOI] [PubMed] [Google Scholar]
- 18.Shubert, Y. S., Lansky, E. P. & Neeman, I. (1999) J. Ethnopharmacol. 66, 11-17. [DOI] [PubMed] [Google Scholar]
- 19.Gil, M. I., Tomas-Barberan, F. A., Hess-Pierce, B., Holcroft, D. M. & Kader, A. A. (2000) J. Agric. Food Chem. 48, 4581-4589. [DOI] [PubMed] [Google Scholar]
- 20.Aviram, M., Dornfeld, L., Rosenblat, M., Volkova, N., Kaplan, M., Coleman, R., Hayek, T., Presser, D. & Fuhrman, B. (2000) Am. J. Clin. Nutr. 71, 1062-1076. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan, M., Hayek, T., Raz, A., Coleman, R., Dornfeld, L., Vaya, J. & Aviram, M. (2001) J. Nutr. 131, 2082-2089. [DOI] [PubMed] [Google Scholar]
- 22.Rosenblat, M., Draganov, D., Watson, C. E., Bisgaier, C. L., La Du, B. N. & Aviram, M. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 468-474. [DOI] [PubMed] [Google Scholar]
- 23.Palinski, W., Napoli, C. & Reaven, P. D. (2000) in Contemporary Cardiology: Vascular Disease and Injury, eds. Simons, D. I. & Rogers, C. (Humana, Totowa, NJ), pp. 149-174.
- 24.de Nigris, F., Youssef, T., Ciafre, S., Anania, V., Condorelli, G., Palinski, W. & Napoli, C. (2000) Circulation 102, 2111-2117. [DOI] [PubMed] [Google Scholar]
- 25.Napoli, C., Quehenberger, O., de Nigris, F., Abete, P., Glass, C. K. & Palinski, W. (2000) FASEB J. 14, 1996-2007. [DOI] [PubMed] [Google Scholar]
- 26.Napoli, C., de Nigris, F., Welch, J. S., Calara, F. B., Stuart, R. O., Glass, C. K. & Palinski, W. (2002) Circulation 105, 1360-1367. [DOI] [PubMed] [Google Scholar]
- 27.Napoli, C., Cirino, G., Del Soldato, P., Sorrentino, R., Sica, V., Condorelli, M., Pinto, A. & Ignarro, L. J. (2001) Proc. Natl. Acad. Sci. USA 98, 2860-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Napoli, C., Ackah, E., de Nigris, F., Del Soldato, P., D'Armiento, F. P., Crimi, E., Condorelli, M. & Sessa, W. C. (2002) Proc. Natl. Acad. Sci. USA 99, 12467-12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Napoli, C., Martin-Padura, I., de Nigris, F., Giorgio, M., Mansueto, G., Somma, P., Condorelli, M., Sica, G., De Rosa, G. & Pelicci, P. (2003) Proc. Natl. Acad. Sci. USA 100, 2112-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Nigris, F., Lerman, L. O., Rodriguez-Porcel, M., De Montis, M. P., Lerman, A. & Napoli, C. (2001) Biochem. Biophys. Res. Commun. 281, 945-950. [DOI] [PubMed] [Google Scholar]
- 31.Hajra, L., Evans, A. I., Chen, M., Hyduk, S. J., Collins, T. & Cybulsky, M. I. (2000) Proc. Natl. Acad. Sci. USA 97, 9052-9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tapiero, H., Tew, K. D., Ba, G. N. & Mathe, G. (2002) Biomed. Pharmacother. 56, 200-207. [DOI] [PubMed] [Google Scholar]
- 33.Folts, J. D. (2002) Adv. Exp. Med. Biol. 505, 95-111. [DOI] [PubMed] [Google Scholar]
- 34.Aviram, M., Dornfeld, L., Kaplan, M., Coleman, R., Gaitini, D., Nitecki, S., Hofman, A., Rosenblat, M., Volkova, N., Presser, D., et al. (2002) Drugs Exp. Clin. Res. 28, 49-62. [PubMed] [Google Scholar]
- 35.Hayek, T., Fuhrman, B., Vaya, J., Rosenblat, M., Belinky, P., Coleman, R., Elis, A. & Aviram, M. (1997) Arterioscler. Thromb. Vasc. Biol. 17, 2744-2752. [DOI] [PubMed] [Google Scholar]
- 36.Aviram, M. & Fuhrman, B. (1998) Atherosclerosis 137, S45-S50. [DOI] [PubMed] [Google Scholar]
- 37.Aviram, M. & Dornfeld, L. (2001) Atherosclerosis 158, 195-198. [DOI] [PubMed] [Google Scholar]
- 38.Aviram, M., Rosenblat, M., Gaitini, D., Nitecki, S., Hoffman, A., Dornfeld, L., Volkova, N., Presser, D., Attias, J., Liker, H., et al. (2004) Clin. Nutr. 23, 423-433. [DOI] [PubMed] [Google Scholar]
- 39.Lou, F. Q., Zhang, M. F., Zhang, X. G., Liu, J. M. & Yuan, W. L. (1989) Chin. Med. J. (Engl.) 102, 579-583. [PubMed] [Google Scholar]
- 40.Duffy, S. J., Keaney, J. F., Jr., Holbrook, M., Gokce, N., Swerdloff, P. L., Frei, B. & Vita, J. A. (2001) Circulation 104, 151-156. [DOI] [PubMed] [Google Scholar]
- 41.Chou, E. J., Keevil, J. G., Aeschlimann, S., Wiebe, D. A., Folts, J. D. & Stein, J. H. (2001) Am. J. Cardiol. 88, 553-555. [DOI] [PubMed] [Google Scholar]
- 42.Ignarro, L. J. & Napoli, C. (2004) Curr. Atheroscler. Rep. 6, 278-287. [DOI] [PubMed] [Google Scholar]