ABSTRACT
The currently available drugs to treat hepatitis B virus (HBV) infection include interferons and nucleos(t)ide analogs, which can only induce disease remission and are inefficient for the functional cure of patients with chronic HBV infection (CHB). Since high titers of circulating hepatitis B surface antigen (HBsAg) may be essential to exhaust the host anti-HBV immune response and they cannot be significantly reduced by current drugs, new antiviral strategies aiming to suppress serum hepatitis B surface antigen (HBsAg) could help restore virus-specific immune responses and promote the eradication of the virus. As an alternative strategy, immunotherapy with HBsAg-specific antibodies has shown some direct HBsAg suppression effects in several preclinical and clinical trial studies. However, most described previously HBsAg-specific antibodies only had very short-term HBsAg suppression effects in CHB patients and animal models mimicking persistent HBV infection. More-potent antibodies with long-lasting HBsAg clearance effects are required for the development of the clinical application of antibody-mediated immunotherapy for CHB treatment. Our recent study described a novel mAb E6F6 that targets a unique epitope on HBsAg. It could durably suppress the levels of HBsAg and HBV DNA via Fcγ receptor-dependent phagocytosis in vivo. In this commentary, we summarize the current research progress, including the therapeutic roles and mechanisms of antibody-mediated HBV clearance as well as the epitope-determined therapeutic potency of the antibody. These insights may provide some clues and guidance to facilitate the development of therapeutic antibodies against persistent viral infection.
KEYWORDS: antibody-mediated immunotherapy, chronic hepatitis B virus infection, FcγR-mediated phagocytosis, monoclonal antibodies, persistent viral infection
Introduction
Chronic hepatitis B virus (HBV) infection is a major global public health issue. It is estimated that there are approximately 248 million individuals worldwide that are persistently infected with HBV.1 Chronic HBV infection (CHB) can cause chronic hepatitis and places patients at high risk of death from liver cirrhosis (LC) and hepatocellular carcinoma (HCC). Approximately 25 to 30% of people who acquire HBV as children will develop LC and/or HCC as adults. Worldwide, approximately 78,000 people die each year because of the acute or chronic consequences of HBV infection.1,2
The successful development of preventive hepatitis B vaccines have effectively reduced new cases of HBV infection globally.3 However, there are still millions people with CHB that need an effective anti-HBV therapy to prevent the complications of the disease.4 Interferons (IFNs) and nucleos(t)ide analogs (NAs) have been approved for treatment of CHB alone or in combination therapies. Despite the fact that these drugs have demonstrated clinical benefits for CHB patients, the virus is difficult to eliminate by current available therapeutics. A favorable clinical treatment outcome is the loss of serum hepatitis B surface antigen (HBsAg), which allows therapy to be discontinued and is associated with a significantly reduced risk of developing LC and HCC. Unfortunately, based on the long-term follow-up of patients, current treatments achieve HBsAg clearance only in a small fraction (< 10%) of patients.5-10 This issue with CHB can be attributed to 2 major reasons. First, the virological key is the persistence of the intracellular HBV replication intermediate, covalently closed circular (ccc) DNA, which resides in HBV-infected cells and cannot be suppressed by current treatments.11-13 Second, the immunological key is the exhausted host anti-HBV immune response, included the functional exhaustion of either cellular or humoral antiviral immunity.14-16 Therefore, there are 2 major approaches in the current research aiming to develop novel anti-HBV therapeutic strategies. Several efforts have been made to develop cccDNA targeting antiviral strategies, but progress is still limited because of the absence of desirable experimental models and an inadequate understanding of the mechanisms of maintenance and regulation of cccDNA.17,18 On the other hand, immune restoration is likely indispensable for off-treatment virus control, even if ways to suppress cccDNA are found. A high-titer of serum HBsAg is considered the most important factor responsible for HBV immunotolerance in CHB patients.14,15,19,20 The reduction of serum HBsAg may allow the immune system to tame viral infection and promote host immune restoration. Unfortunately, neither IFNs nor NAs can induce a HBsAg reduction efficiently. New therapeutic agents and innovative treatment strategies that can effectively remove HBsAg are needed to improve the clinical management of this disease.
Antibody-based immunotherapy is widely used to treat cancer, autoimmune diseases and inflammation. For viral infectious diseases, polyclonal antibodies of hyperimmune human IgG preparations are used for the prevention and treatment of acute infections of rabies, vaccinia, varicella-zoster virus (VZV), influenza viruses and HBV. A humanized monoclonal antibody (mAb) against respiratory syncytial virus (RSV, Palivizumab) is used to prevent RSV infection.21 Generally, the neutralizing antibodies can block the steps viruses take to enter into cells by several different mechanisms, thus playing a preventive role in viral infection diseases, but they are mostly impotent for established viral infections, particularly for treating persistent viral infections. The therapeutic role of antibodies for persistent viral infections has lacked understanding until recently. This is especially true for HIV, which has dominated most of the reports dedicated to broadly neutralizing monoclonal antibodies (mAbs) during the past 5 y. Several studies have reported that certain unusual neutralizing antibodies could be used therapeutically to treat established simian immunodeficiency virus (SIV) infections. This virus has the same envelope proteins as the human immunodeficiency virus type 1 (HIV-1). Some potent broadly neutralizing antibodies (bnAbs) against HIV can suppress plasma virus titer over a 3-log change in SHIV-infected rhesus monkeys.22,23 The latest research in clinical trials further demonstrated that the in vivo administration of such bnAbs had potent anti-viral activity in HIV-infected human individuals, which supported the idea that antibody-mediated immunotherapy might be useful for the clinical treatment of HIV-1 infection.24-26 These findings underline the therapeutic potential of antibody-based immunotherapy in the fight against persistent viral infections. Similar to HIV, the hepatitis B virus (HBV) causes chronic, even life-long infection. The 2 viruses share several characteristics: they both replicate via reverse-transcription-dependent replication, both viral genomes can integrate into the host genome, they both cause serious public health problems and both require more effective drugs. The early explorations of monoclonal (mAb)-based treatments of chronically HBV-infected humans and animals only demonstrated transient viremia suppression effects that were very similar to the effects of treatments based on hepatitis B immune globulin (HBIG), which is prepared from the plasma of donors who have high counts of HBsAg antibodies.27,28 More potent antibodies, particularly those that have more prolonged viral suppression effects, are essentially required for the further development of antibody-based immunotherapy strategies for chronic HBV infection.
Epitope-dependent therapeutic effects of anti-HBsAg mAbs
There are several accessible epitopes on HBV large, middle and small surface proteins that have been identified, including but not limited to those shown in Fig. 1A. One famous epitope has only been presented on the HBV large surface protein surrounding the aa21-aa47 of preS1 region. MAbs specific to this epitope, such as MA18/7, 4D11 and 7H11,29,30 usually have potent neutralization activities because this epitope is located in the HBV cellular receptor (NTCP) binding site (RBD).31,32 The mAbs recognizing aa33-aa52 of the preS2 region, which is located at the translocation motif (TLM) of the middle and large surface proteins, were found to have HBV genotype-specific binding activity.33-35 For the small HBsAg, at least 3 epitope clusters on the viral particle surface were noted in previous studies (Fig. 1A and B).36,37 The majority of small HBsAg-specific antibodies raised by vaccination or natural infection recovery recognize the conformation-dependent “a” determinant located within the first loop containing aa124-aa137 and the second loop comprising aa139-aa147.38 High-affinity mAbs to “a” determinant (sB mAbs) generally exhibited potent neutralization activities similar to that of mAbs for preS1 RBD because the “a” antigenic loop is responsible for the initial interaction between the virus and cell surface heparin sulfate proteoglycans.39-42 There are 2 independent linear epitopes located in the surface-exposed antigenic loop in the major hydrophilic region (MHR), which surrounds the “a” determinant region.37 The first one contains aa119-aa125 within the N-terminus of the first loop, which includes a CXXC motif.43 It is usually found in protein-disulfide isomerase-related proteins and is evolutionarily and cross-genotype conserved.41,44 The binding activities of mAbs to this epitope (sA mAbs) are highly tolerant to common immune-escape HBV mutants, such as G145R, K141E and D144A.36 The second one contains aa139-aa147 within the second loop. The binding of mAbs to this epitope (sE mAbs) are highly sensitive to immune-escape HBV mutants, similar to those of “a” determinant mAbs.36,37 According to previous studies, it is possible that the antibodies in HBIG predominantly recognize the conformational “a” determinant and/or second loop epitope.36,40,45
Figure 1.
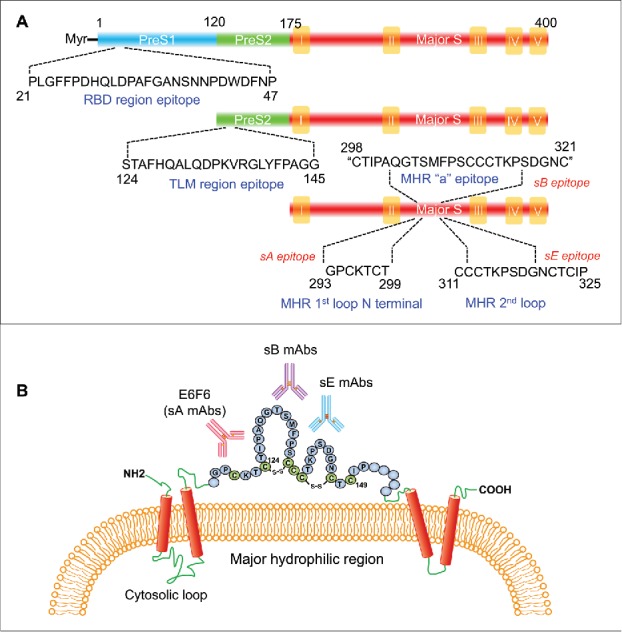
The epitopes and domain characterizations of HBV surface proteins. (A) A schematic diagram depicting the binding sequences of mAbs targeting the HBV surface proteins. (B) The epitope localization of the mAbs targeting HBV small surface protein (HBsAg). TLM = Translocation motif; RBD = receptor binding domain; MHR = major hydrophilic region.
Most mAbs against the abovementioned HBV surface-exposed epitopes could neutralize HBV infection in vitro to various degrees.41 However, their therapeutic uses still need to evaluated in vivo. Our recent study investigated the therapeutic efficacy of mAbs against various epitopes in HBV transgenic (HBV-Tg) mice.41 The HBV-Tg mice we used for the study had a terminally redundant 1.3-fold HBV genome insertion that produces viral particles HBsAg and HBeAg at high levels comparable to those of patients with chronic HBV infection.46 Although the HBV particles produced by HBV-Tg mice do not enter murine hepatocytes, HBV-Tg mice are a suitable model for evaluating anti-HBV antibody-mediated viral clearance. Our results demonstrated that the anti-HBV therapeutic efficacy of the mAb is highly dependent on its binding epitope, and the efficacy does not predominantly correlate with the mAb's binding activity or in vitro neutralizing capability. The mAbs specific to aa119-aa125 within the N-terminal of the first loop (sA mAbs) exhibited more striking therapeutic effects than those that recognize other epitopes. Interestingly, although the mAbs to the preS1 RBD region have very potent in vitro HBV-neutralizing capability, little or no viral suppression, either at the HBsAg level or with the virus titer (HBV DNA), was observed in mice that received these mAbs. The mAbs recognizing the “a” determinant and/or second loop had significant suppression effects to HBsAg and HBV DNA, but their effects were more transient than those of sA mAbs. This finding was consistent with the observations found in previous clinical trials where patients with chronic HBV infection were treated with mAbs against “a” determinant and/or second loop. One of the sA mAbs (E6F6) that completely differs from other mAbs and exhibits striking therapeutic effects in multiple HBV murine models without significant side effects. We demonstrated that a single infusion of E6F6 dramatically suppressed the levels of HBsAg and HBV DNA over 3.0 log-change for several weeks in HBV mice. These results suggested that the binding epitope differences may significantly impact the in vivo viral clearance potency of mAbs, thereby underlining the importance of mAb epitope characterization and clustering in the selection and in vivo evaluation of antiviral therapeutic antibodies.47
Therapeutic effects and mechanisms of E6F6 mAb
Theoretically, the in vivo administration of virus-specific antibodies has multiple therapeutic functions. Neutralizing antibodies that bind to and inactivate viral envelope proteins block virus entry and therefore prevent the spread of infection.21 Moreover, some antibodies might have intrinsic effector functions that facilitate the direct clearance of circulatory viruses, viral antigens or virus-producing cells via antibody-dependent cell-mediated cytotoxicity (ADCC), complement lysis (CDC) or phagocytosis (ADCP). In addition, antibody-virus complexes bind to Fc receptors that are expressed by immune effector cells that can trigger a multitude of innate and adaptive responses against viruses.48,49 In HBV-Tg mice, a neutralization effect could not be observed because mouse hepatocytes do not support HBV infection. Thus, the HBV suppression effects of E6F6 in HBV-Tg mice should be derived from antibody-dependent viral clearance. In our results, no significant ALT elevation or evidence of histological hepatitis was observed, suggesting that the ADCC and CDC may not be involved in E6F6-mediated HBV suppression. Further experiments in different mouse strains, including nude, SCID, Rag2−/− and NOD-SCID as well as complement-depleted HBV mice, confirmed that the direct antiviral effects of E6F6 were independent of ADCC and CDC. However, phagocyte depletion via λ-Carrageenan significantly reduced the HBV suppression effects of E6F6. Notably, compared with mice treated with isotype-control mAb, both intracellular E6F6 and HBV (immune-complex) were significantly increased in liver Kupffer cells, neutrophils and phagocytes in peripheral blood lymphocytes. Moreover, the abolishment of the interaction between the E6F6 Fc region and the mouse Fcγ receptor via mAb Fc mutations disabled the HBV suppression effects of E6F6 in HBV-Tg mice. These results demonstrate that E6F6 mediates the highly efficient viral immunoclearance via Fcγ receptor-dependent phagocytosis. Consistent with these observations, recent studies on HIV-1 therapeutic antibodies also revealed that the Fc-FcγR interactions are essentially required to achieve full therapeutic activity through the clearance of IgG-opsonized virions and the elimination of HIV-infected cells.48,50 Taken together, we propose that Fc-FcγR interactions play a key role in antibody-mediated viral clearance (Fig. 2). Therefore, the enhancement of the FcγR-mediated effector functions through the augmented activation of the FcγR-mediated pathways via mAb Fc modifications may lead to antiviral therapeutic antibodies with improved in vivo efficacy.
Figure 2.
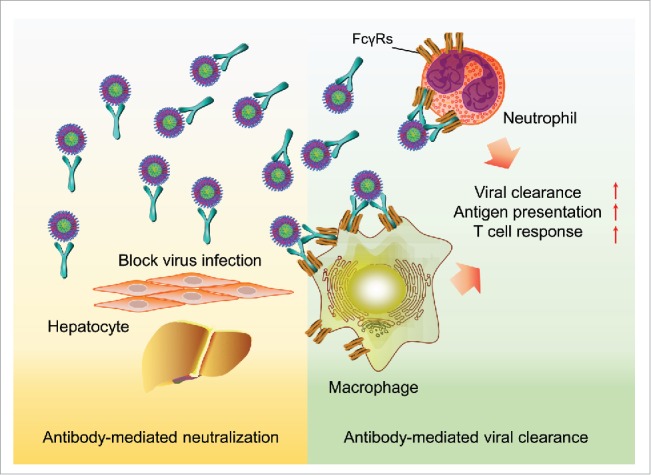
A graphic summary of the therapeutic roles and mechanisms of antibody-mediated immunotherapy for HBV infection.
In addition to the potent immune-clearance effects of E6F6 in HBV-Tg mice, we further demonstrated the immune-modulation effects of this mAb. When a hydrodynamic injection HBV mouse model was used to mimic the adaptive tolerance phase of chronic HBV infection in immmuno-competent mice, successive infusions of E6F6 lead to a sustained HBsAg reduction and to an increased number of HBcAg-specific interferon-γ-secreting T-cells and HBsAg-specific and HBcAg-specific CD8+T-cells, suggesting that E6F6 treatment facilitated the restoration of the anti-HBV T-cell response. Given that FcγR-mediated immune-clearance has multiple effector functions on several aspects of adaptive immune response, including stimulation of antigen processing and presentation, the modulation of antigen-presenting cell function, and regulation of T-and B-cell responses (Fig. 2), the passive administration of antiviral mAbs with potent viral clearance effects should also stimulate host antiviral immunity, therefore providing the opportunity for the induction of long-term humoral and cellular immune responses.
Although the neutralization activity of E6F6 does not play a predominant role in the treatment of established HBV infection, its significance may be apparent in patients whose viral load had been greatly reduced by pretreatment of E6F6 or other antiviral drugs. Using human-liver chimeric FRG mice, which support robust in vivo HBV infection,51 we demonstrated that regimens of E6F6 efficiently blocked initial HBV infection and viral spreading from infected hepatocytes. This effect of E6F6 is possibly attributed to both its potent viral clearance capacity and its inhibitory activity for viral entry, and it may play an important role in the prevention of HBV reactivation, thereby facilitating sustained HBV suppression. In summary, E6F6 can suppress HBV via 3 different modes: i) conducting highly efficient viral immune-clearance through Fcγ receptor-dependent phagocytosis, ii) stimulating the restoration of the anti-HBV T-cell response, and iii) blocking the viral entry and propagation of HBV in the liver (Fig. 2).
Challenges and future perspective
There are some unresolved mechanisms that need to be addressed concerning the therapeutic roles of E6F6. Further investigations may focus on investigating the molecular mechanism and structural basis of how and why binding epitope differences impact the in vivo viral clearance potency. Our preliminary data revealed a unique characteristic for immune complexes (ICs) of E6F6-like mAbs and HBV viral particles that is completely different from mAbs targeting other epitopes. Examinations of the mAb-viral particle ICs using electron microscopy and low-speed centrifugation demonstrated that the E6F6-like mAbs only form smaller antibody–viral particle ICs and do not induce any viral particle aggregation, whereas mAbs to other epitopes profoundly induce viral particle aggregation. Our recent cryo-electron microscopy (cryo-EM) reconstruction analyses of the E6F6 Fab fragment in complexes with spherical HBsAg particles suggested that the 2 E6F6 arms might directly target 2 adjacent HBsAg monomers on a single HBsAg octahedron particle with limited inter-particle crosslinking, thereby preventing the formation of large antibody–viral particle ICs (unpublished data). As previous reports suggested that the size of antibody-opsonized particles strongly affects their phagocytotic efficacy, it is reasonable to speculate that macrophages phagocytose smaller ICs more efficiently than larger ones because the cell membrane takes more time to enclose larger particles than small particles.52-54 If further experimental evidence validates and supports this hypothesis, perhaps the size of antibody–viral particle ICs and the in vitro opsonophagocytotic efficacy would be considered new important parameters in addition to binding affinity and neutralization capabilities for the selection of mAbs with therapeutic potential in the future.
Although we provided a systematic in vivo evaluation of the data supporting the therapeutic potential of E6F6 for chronic HBV infection, all results were derived from murine models. Thus far, the therapeutic effects and possible safety concerns in human beings with chronic HBV infection are largely unknown. Moreover, the appearance of escape mutants during mAb treatment has been observed in clinical trials of anti-HIV therapeutic antibodies, suggesting the same possible issue for anti-HBV therapeutic antibodies.25 The E6F6 binding epitope (GPCK(/R)TCT) is considered one of the most important motifs required for the infectivity of HBV in previous in vitro studies.39 However, the emerging risk of escape mutants should be further evaluated in human-liver chimeric mice, particularly in long-term and multiple-dose treatment procedures. In addition to E6F6, other potent targets (epitopes) and mAbs are required to be explored for the development of a cocktail of anti-HBV antibodies if E6F6-resistant HBV mutants emerged. Although there are several challenges that need to be overcome before the final clinical applications of this antibody and other mAbs with similar potency, the development and use of antibody-mediated immunotherapy in patients with chronic HBV infection are certainly expected.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
National Science Fund (81672023) and the Excellent Youth Foundation of Fujian Scientific Committee (2015J06018) supported this work.
References
- [1].Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015; 386:1546-55; PMID:26231459; https://doi.org/ 10.1016/S0140-6736(15)61412-X [DOI] [PubMed] [Google Scholar]
- [2].Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al.. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095-128; PMID:23245604; https://doi.org/ 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis b vaccination in taiwan and the incidence of hepatocellular carcinoma in children. N Eng J Med 1997; 336:1855-9; PMID:9197213; https://doi.org/ 10.1056/NEJM199706263362602 [DOI] [PubMed] [Google Scholar]
- [4].Liaw YF, Chu CM. Hepatitis b virus infection. Lancet 2009; 373:582-92; PMID:19217993; https://doi.org/ 10.1016/S0140-6736(09)60207-5 [DOI] [PubMed] [Google Scholar]
- [5].Heathcote EJ, Marcellin P, Buti M, Gane E, De Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, et al.. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology 2011; 140:132-43; PMID:20955704; https://doi.org/ 10.1053/j.gastro.2010.10.011 [DOI] [PubMed] [Google Scholar]
- [6].Buster EH, Flink HJ, Cakaloglu Y, Simon K, Trojan J, Tabak F, So TM, Feinman SV, Mach T, Akarca US, et al.. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology 2008; 135:459-67; PMID:18585385; https://doi.org/ 10.1053/j.gastro.2008.05.031 [DOI] [PubMed] [Google Scholar]
- [7].Gish RG, Chang TT, Lai CL, de Man R, Gadano A, Poordad F, Yang J, Brett-Smith H, Tamez R. Loss of HBsAg antigen during treatment with entecavir or lamivudine in nucleoside-naive HBeAg-positive patients with chronic hepatitis B. J Viral Hepat 2010; 17:16-22; PMID:19622117; https://doi.org/ 10.1111/j.1365-2893.2009.01146.x [DOI] [PubMed] [Google Scholar]
- [8].Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow-up. Hepatology 2007; 45:1187-92; PMID:17465003; https://doi.org/ 10.1002/hep.21612 [DOI] [PubMed] [Google Scholar]
- [9].Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N, et al.. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2010; 51:422-30; PMID:20049753; https://doi.org/ 10.1002/hep.23327 [DOI] [PubMed] [Google Scholar]
- [10].Zoutendijk R, Reijnders JG, Brown A, Zoulim F, Mutimer D, Deterding K, Petersen J, Hofmann WP, Buti M, Santantonio T, et al.. Entecavir treatment for chronic hepatitis B: adaptation is not needed for the majority of naive patients with a partial virological response. Hepatology 2011; 54:443-51; PMID:21563196; https://doi.org/ 10.1002/hep.24406 [DOI] [PubMed] [Google Scholar]
- [11].Petersen J, Thompson AJ, Levrero M. Aiming for cure in HBV and HDV infection. J Hepatol 2016; 65:835-48; PMID:27270043; https://doi.org/ 10.1016/j.jhep.2016.05.043 [DOI] [PubMed] [Google Scholar]
- [12].Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015; 64:1972-84; PMID:26048673; https://doi.org/ 10.1136/gutjnl-2015-309809 [DOI] [PubMed] [Google Scholar]
- [13].Durantel D, Zoulim F. New antiviral targets for innovative treatment concepts for hepatitis B virus and hepatitis delta virus. J Hepatol 2016; 64:S117-31; PMID:27084032; https://doi.org/ 10.1016/j.jhep.2016.02.016 [DOI] [PubMed] [Google Scholar]
- [14].Chen SH, Wu HL, Kao JH, Hwang LH. Persistent hepatitis B viral replication in a FVB/N mouse model: impact of host and viral factors. PLoS One 2012; 7:e36984; PMID:22615863; https://doi.org/ 10.1371/journal.pone.0036984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Reignat S, Webster GJ, Brown D, Ogg GS, King A, Seneviratne SL, Dusheiko G, Williams R, Maini MK, Bertoletti A. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med 2002; 195:1089-101; PMID:11994415; https://doi.org/ 10.1084/jem.20011723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bertoletti A, Gehring AJ. Immune therapeutic strategies in chronic hepatitis B virus infection: virus or inflammation control? PLoS Pathog 2013; 9:e1003784; PMID:24367255; https://doi.org/ 10.1371/journal.ppat.1003784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cai D, Mills C, Yu W, Yan R, Aldrich CE, Saputelli JR, Mason WS, Xu X, Guo JT, Block TM, et al.. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob Agents Chemother 2012; 56:4277-88; PMID:22644022; https://doi.org/ 10.1128/AAC.00473-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T, et al.. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 2014; 343:1221-8; PMID:24557838; https://doi.org/ 10.1126/science.1243462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris) 2010; 58:258-66; PMID:20116937; https://doi.org/ 10.1016/j.patbio.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kennedy PT, Sandalova E, Jo J, Gill U, Ushiro-Lumb I, Tan AT, Naik S, Foster GR, Bertoletti A. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 2012; 143:637-45; PMID:22710188; https://doi.org/ 10.1053/j.gastro.2012.06.009 [DOI] [PubMed] [Google Scholar]
- [21].Marasco WA, Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat Biotechnol 2007; 25:1421-34; PMID:18066039; https://doi.org/ 10.1038/nbt1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, et al.. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 2013; 503:224-8; PMID:24172905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Lifson JD, et al.. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 2013; 503:277-80; PMID:24172896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, et al.. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 2015; 522:487-91; PMID:25855300; https://doi.org/ 10.1038/nature14411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, Murrell B, Pfeifer N, Nogueira L, Oliveira TY, et al.. Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat Med 2017; 23:185-91; PMID:28092665; https://doi.org/ 10.1038/nm.4268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, et al.. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med 2016; 375:2037-50; PMID:27959728; https://doi.org/ 10.1056/NEJMoa1608243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Eren R, Ilan E, Nussbaum O, Lubin I, Terkieltaub D, Arazi Y, Ben-Moshe O, Kitchinzky A, Berr S, Gopher J, et al.. Preclinical evaluation of two human anti-hepatitis B virus (HBV) monoclonal antibodies in the HBV-trimera mouse model and in HBV chronic carrier chimpanzees. Hepatology 2000; 32:588-96; PMID:10960454; https://doi.org/ 10.1053/jhep.2000.9632 [DOI] [PubMed] [Google Scholar]
- [28].Galun E, Eren R, Safadi R, Ashour Y, Terrault N, Keeffe EB, Matot E, Mizrachi S, Terkieltaub D, Zohar M, et al.. Clinical evaluation (phase I) of a combination of two human monoclonal antibodies to HBV: safety and antiviral properties. Hepatology 2002; 35:673-9; PMID:11870383; https://doi.org/ 10.1053/jhep.2002.31867 [DOI] [PubMed] [Google Scholar]
- [29].Heermann KH, Goldmann U, Schwartz W, Seyffarth T, Baumgarten H, Gerlich WH. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol 1984; 52:396-402; PMID:6492255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yuan Q, Ge S, Xiong J, Yan Q, Li Z, Hao X, Tian D, Niu J, Su Z, Chen C, et al.. A novel immunoassay for PreS1 and/or core-related antigens for detection of HBsAg variants. J Virol Methods 2010; 168:108-13; PMID:20451558; https://doi.org/ 10.1016/j.jviromet.2010.04.029 [DOI] [PubMed] [Google Scholar]
- [31].Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, et al.. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012; 1:e00049; PMID:23150796; https://doi.org/ 10.7554/eLife.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schulze A, Schieck A, Ni Y, Mier W, Urban S. Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. J Virol 2010; 84:1989-2000; PMID:20007265; https://doi.org/ 10.1128/JVI.01902-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Song LW, Wang YB, Fang LL, Wu Y, Yang L, Chen JY, Ge SX, Zhang J, Xiong YZ, Deng XM, et al.. Rapid fluorescent lateral-flow immunoassay for hepatitis B virus genotyping. Anal Chem 2015; 87:5173-80; PMID:25892477; https://doi.org/ 10.1021/ac504832c [DOI] [PubMed] [Google Scholar]
- [34].Usuda S, Okamoto H, Iwanari H, Baba K, Tsuda F, Miyakawa Y, Mayumi M. Serological detection of hepatitis B virus genotypes by ELISA with monoclonal antibodies to type-specific epitopes in the preS2-region product. J Virol Methods 1999; 80:97-112; PMID:10403681; https://doi.org/ 10.1016/S0166-0934(99)00039-7 [DOI] [PubMed] [Google Scholar]
- [35].Oess S, Hildt E. Novel cell permeable motif derived from the PreS2-domain of hepatitis-B virus surface antigens. Gene Ther 2000; 7:750-8; PMID:10822301; https://doi.org/ 10.1038/sj.gt.3301154 [DOI] [PubMed] [Google Scholar]
- [36].Huang CH, Yuan Q, Chen PJ, Zhang YL, Chen CR, Zheng QB, Yeh SH, Yu H, Xue Y, Chen YX, et al.. Influence of mutations in hepatitis B virus surface protein on viral antigenicity and phenotype in occult HBV strains from blood donors. J Hepatol 2012; 57:720-9; PMID:22634131; https://doi.org/ 10.1016/j.jhep.2012.05.009 [DOI] [PubMed] [Google Scholar]
- [37].Ijaz S, Ferns RB, Tedder RS. A 'first loop' linear epitope accessible on native hepatitis B surface antigen that persists in the face of 'second loop' immune escape. J Gen Virol 2003; 84:269-75; PMID:12560557; https://doi.org/ 10.1099/vir.0.18667-0 [DOI] [PubMed] [Google Scholar]
- [38].Tajiri K, Ozawa T, Jin A, Tokimitsu Y, Minemura M, Kishi H, Sugiyama T, Muraguchi A. Analysis of the epitope and neutralizing capacity of human monoclonal antibodies induced by hepatitis B vaccine. Antiviral Res 2010; 87:40-9; PMID:20412816; https://doi.org/ 10.1016/j.antiviral.2010.04.006 [DOI] [PubMed] [Google Scholar]
- [39].Salisse J, Sureau C. A function essential to viral entry underlies the hepatitis B virus “a” determinant. J Virol 2009; 83:9321-8; PMID:19570861; https://doi.org/ 10.1128/JVI.00678-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhao JH, Zhang YL, Zhang TY, Yuan LZ, Cheng T, Chen PJ, Yuan Q, Xia NS. A novel toolbox for the in vitro assay of hepatitis D virus infection. Sci Rep 2017; 7:40199; PMID:28079152; https://doi.org/ 10.1038/srep40199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang TY, Yuan Q, Zhao JH, Zhang YL, Yuan LZ, Lan Y, Lo YC, Sun CP, Wu CR, Zhang JF, et al.. Prolonged suppression of hbv in mice by a novel antibody that targets a unique epitope on hepatitis b surface antigen. Gut 2015; 65:658; PMID:26423112; https://doi.org/ 10.1136/gutjnl-2014-308964 [DOI] [PubMed] [Google Scholar]
- [42].Sureau C, Salisse J. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus a-determinant. Hepatology 2013; 57:985-94; PMID:23161433; https://doi.org/ 10.1002/hep.26125 [DOI] [PubMed] [Google Scholar]
- [43].Chen YC, Delbrook K, Dealwis C, Mimms L, Mushahwar IK, Mandecki W. Discontinuous epitopes of hepatitis B surface antigen derived from a filamentous phage peptide library. Proc Natl Acad Sci U S A 1996; 93:1997-2001; PMID:8700874; https://doi.org/ 10.1073/pnas.93.5.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vuori K, Pihlajaniemi T, Myllyla R, Kivirikko KI. Site-directed mutagenesis of human protein disulphide isomerase: effect on the assembly, activity and endoplasmic reticulum retention of human prolyl 4-hydroxylase in Spodoptera frugiperda insect cells. EMBO J 1992; 11:4213-7; PMID:1327760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cooreman MP, van Roosmalen MH, te Morsche R, Sunnen CM, de Ven EM, Jansen JB, Tytgat GN, de Wit PL, Paulij WP. Characterization of the reactivity pattern of murine monoclonal antibodies against wild-type hepatitis B surface antigen to G145R and other naturally occurring “a” loop escape mutations. Hepatology 1999; 30:1287-92; PMID:10534351; https://doi.org/ 10.1002/hep.510300508 [DOI] [PubMed] [Google Scholar]
- [46].Wang SH, Yeh SH, Lin WH, Wang HY, Chen DS, Chen PJ. Identification of androgen response elements in the enhancer I of hepatitis B virus: a mechanism for sex disparity in chronic hepatitis B. Hepatology 2009; 50:1392-402; PMID:19670412; https://doi.org/ 10.1002/hep.23163 [DOI] [PubMed] [Google Scholar]
- [47].Sureau C. A unique monoclonal antibody for therapeutic use against chronic hepatitis B: not all antibodies are created equal. Gut 2016; 65:546-7; PMID:26611231; https://doi.org/ 10.1136/gutjnl-2015-310978 [DOI] [PubMed] [Google Scholar]
- [48].Bournazos S, Ravetch JV. Anti-retroviral antibody FcgammaR-mediated effector functions. Immunol Rev 2017; 275:285-95; PMID:28133801; https://doi.org/ 10.1111/imr.12482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Forthal DN, Moog C. Fc receptor-mediated antiviral antibodies. Curr Opin HIV AIDS 2009; 4:388-93; PMID:20048702; https://doi.org/ 10.1097/COH.0b013e32832f0a89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 2014; 158:1243-53; PMID:25215485; https://doi.org/ 10.1016/j.cell.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol 2007; 25:903-10; PMID:17664939; https://doi.org/ 10.1038/nbt1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A 2006; 103:4930-4; PMID:16549762; https://doi.org/ 10.1073/pnas.0600997103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pacheco P, White D, Sulchek T. Effects of microparticle size and Fc density on macrophage phagocytosis. PLoS One 2013; 8:e60989; PMID:23630577; https://doi.org/ 10.1371/journal.pone.0060989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Flannagan RS, Jaumouille V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol 2012; 7:61-98; PMID:21910624; https://doi.org/ 10.1146/annurev-pathol-011811-132445 [DOI] [PubMed] [Google Scholar]


