ABSTRACT
While bivalent and quadrivalent HPV vaccines have been used for about 10 years, a nonavalent vaccine against HPV types 6/11/16/18/31/33/45/52 and 58 has been recently approved by FDA and EMA and is now commercially available. The objective of our study was to evaluate the potential impact of the nonavalent vaccine on HPV infection and related low- and high-grade squamous intraepithelial lesions (LSIL, HSIL), compared to the impact of the quadrivalent vaccine, in a female population living in Sicily (Italy).
Low estimates of HPV vaccine impact were calculated as prevalence of HPV 6/11/16/18/31/33/45/52 and 58 genotypes, alone or in association, but excluding presence of other HPV types; high estimates were calculated as prevalence of HPV 6/11/16/18/31/33/45/52 and 58 genotypes alone or in association, in the presence of other HPV types.
The nonavalent HPV vaccine showed increased impact, compared to the quadrivalent vaccine. Estimates of potential impact varied from 30.9% (low estimate) to 53.3% (high estimate) for LSIL, and from 56.9% to 81,0% for HSIL. The proportion of additional cases potentially prevented by the nonavalent vaccine was 14.4%–23.8% for LSIL, and 19.0%–32.8% for HSIL.
The benefit of the nonavalent vaccine compared to the quadrivalent vaccine was more than 80% for both low and high impact estimates for LSIL and more than 50% for both low and high impact estimates for HSIL.
The present study confirms that the switch from a first generation HPV vaccines to a nonavalent vaccine would increase the prevention of cervical HSIL in up to 90% of cases.
KEYWORDS: HPV infection, impact estimate, nonavalent, quadrivalent, squamous intraepithelial lesions, vaccine
Introduction
Demonstration of the role of persistent infection with high-risk (HR) Human Papillomaviruses (HPV) as the causal agent of cervical cancer1 made the development of first and second generation prophylactic vaccines possible.2,3 In addition to cervical carcinoma, HR HPV, such as HPV 16 and 18, are considered responsible for a significant number of cervical low- and high-grade squamous intraepithelial lesions (LSIL and HSIL, respectively), as well as a subset of cancers of the vulva, vagina, penis and anus,4,5,67 and also a subset of neoplastic lesions of head and neck. On the other hand, infection with low-risk (LR) HPV types, such as HPV 16 and 11 genotypes, is associated with 90% of anogenital warts in men and women. Furthermore, a subset of neoplastic lesions of the head and neck and all genital warts in men and women8 and also causes recurrent respiratory papillomatosis, a very difficult to treat pathological entity with high recurrence rates.9
Two HPV vaccines have been licensed and are at the moment available in Europe: the bivalent (Cervarix, GSK biologicals) HPV vaccine, which prevents infections with the HR HPV 16 and 18; and the quadrivalent HPV vaccine, (Gardasil, Sanofi Pasteur MSD), which, in addition to HR 16 and 18, also targets the LR HPV 6 and 11. Both vaccines in clinical trials exhibited a high level of clinical efficacy associated with a reassuring safety profile.10,11
Recently, the FDA (2014) and EMA (2015) approved a nonavalent HPV vaccine (Merk, Sanofi Pasteur MSD, 9vHPV, trade name Gardasil9) which, in addition to the four genotypes of the quadrivalent vaccine, also targets five additional HR genotypes, namely, HPV 31/33/45/52/58, which are the most frequently detected types in invasive cervical cancer wordlwide, after HPV 16 and HPV 18.12,13
In a previous report, the nonavalent vaccine exhibited an efficacy of 96.7% in preventing cervical, vaginal and vulvar HSIL related to the newly added HPV genotypes, while confirming the clinical efficacy and safety profile of the quadrivalent vaccine.14 Moreover, Serrano et al. estimated that the addition of HPV 31/33/45/52 and 58 to the genotypes already included in the quadrivalent vaccines could expand the prevention of invasive cervical cancers worldwide from 70% to 90%.15
Also, a recent systematic review and meta-analysis,16 suggested a potential for the nonavalent vaccine to further reduce precancerous lesions and cervical cancer.17,18
The aim of our study was to evaluate the potential impact of a candidate nonavalent HPV vaccine on HPV infection and HPV-related HSIL and LSIL, compared with the presently utilized quadrivalent HPV vaccine, in a female population living in Sicily, southern Italy.
Results
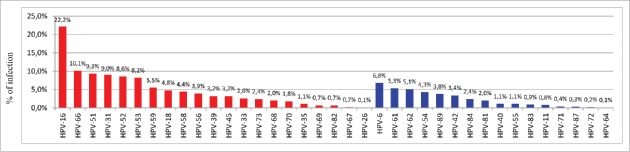
Of the 1794 samples with a positive HPV finding, 1244 (69.3%) were single infections and 550 (30.6%) multiple infections. HR HPV types, alone or in combined infection with LR types, were present in 1466 (81.7%) samples. A total of 37 HPV types were identified, of which 21 were HR HPV types and 16 LR HPV types (Fig. 1). As far as the HPV types comprised in the nonavalent vaccine were concerned, detection rates were 22.2% for HPV 16, 4.8% for HPV 18, 9.0% for HPV 31, 2.6% for HPV 33, 3.2% for HPV 45, 8.6% for HPV 52, 4.4% for HPV 58, 6.8% for HPV 6 and 0.8% for HPV 11. Altogether, 584/1794 (32.5%) samples harboured at least one of the four HPV types covered by the current quadrivalent vaccine (HPV 6/11/16 and 18), while 984 (54.8%) samples harboured at least one of the nine genotypes included in nonavalent vaccine, implying a significantly higher estimated coverage of HPV infection from the nonavalent vaccine than the current quadrivalent vaccine (54.8% vs 32.5%; p<0.001).
Figure 1.
Type-specific distribution of high-risk and low risk HPV among 1794 women HPV+.
Of the 501 samples with a known histological diagnosis of LSIL, a total of 362 LSIL were HPV positive. The most frequently detected type was HPV 16, found in 14.8% of LSIL; frequencies for types other than HPV 16 that are contained in the nonavalent vaccine were 4.4% for HPV 6, 0.8% for HPV 11, 3.8% for HPV 18, 9.2% for HPV 31, 2% for HPV 33, 2.8% for HPV 45, 5.4% for HPV 52 and 3.8.% for HPV 58. Of the 64 cases of ≥HSIL diagnoses, a total of 58 (90.6%) were HPV positive. The most frequently detected type was HPV 16, found in 42.2% of ≥HSIL; frequencies for types other than HPV 16 that are contained in the nonavalent vaccine were 3.1% for HPV 6, 1.6% for HPV 18, 12.5% for HPV 31, 7.8% for HPV 33, 4.7% for HPV 45, 6.2% for HPV 52 and 3.1% for HPV 58 (Table 1).
Table 1.
n (%) HPV-types in histological diagnosis.
| LSILc | HSILd | |
|---|---|---|
| LR-HPVa | n (%) | n (%) |
| 6 | 22 (4.4) | 2(3.1) |
| 11 | 4 (0.8) | — |
| 40 | 5 (1) | 2(3.1) |
| 42 | 15 (2.3) | 3(4.7) |
| 54 | 13 (2.6) | 2(3.1) |
| 55 | 2 (0.4) | — |
| 61 | 16 (3.2) | 1 (1.6) |
| 62 | 19 (3.8) | 2(3.1) |
| 71 | 2 (0.4) | — |
| 81 | 7 (1.4) | 2(3.1) |
| 83 | 4 (0.8) | — |
| 84 | 10 (2) | — |
| 89 | 11 (2.2) | — |
| HR-HPVb | ||
| 16 | 74 (14.8) | 27(42.2) |
| 18 | 19 (3.8) | 1 (1.6) |
| 31 | 46 (9.2) | 8(12.5) |
| 33 | 10 (2.0) | 5 (7.8) |
| 35 | 5 (1) | — |
| 39 | 8 (1.6) | — |
| 45 | 14 (2.8) | 3 (4.7) |
| 51 | 41 (8.2) | 3 (4.7) |
| 52 | 27(5.4) | 4 (6.2) |
| 53 | 36 (7.2) | 2 (3.1) |
| 56 | 17 (3.4) | 2 (3.1) |
| 58 | 19 (3.8) | 2 (3.1) |
| 59 | 21 (4.2) | 2 (3.1) |
| 66 | 50 (10) | 2 (3.1) |
| 67 | 1 (0.2) | — |
| 68 | 7 (1.4) | — |
| 69 | 1(0.2) | — |
| 70 | 4 (0.8) | 1(1.6) |
| 73 | 14 (2.8) | — |
| 82 | 3(0.6) | — |
= Low-risk HPV genotype;
= High-risk HPV genotype;
= Low-grade squamous intraepithelial lesions;
= High-grade squamous intraepithelial lesions
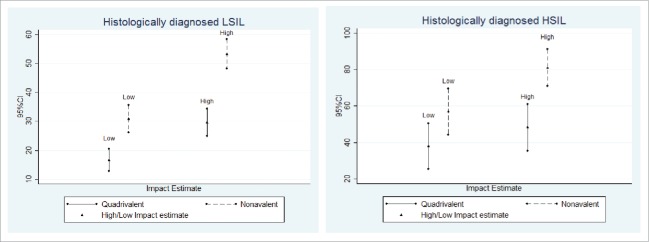
The potential impact of the quadrivalent and nonavalent HPV vaccines on LSIL and ≥HSIL histological diagnoses, as assessed by low and high impact estimates, is presented in Table 2 and Fig. 2. The nonavalent HPV vaccine showed increased impact on both categories of lesions, compared to the quadrivalent vaccine. As far as LSIL were concerned, the number of HPV infection with genotypes targeted by the quadrivalent vaccine varied between 16.6% (low estimate) and 29.6% (high estimate) while the proportion of those associated with genotypes targeted by the nonavalent vaccine varied between 30.9% (low estimate) and 53.3% (high estimate). The absolute additional impact of the nonavalent vaccine was statistically significant by low (14.4%) and high impact estimate (23.8%). The benefit of the nonavalent vaccine compared to the quadrivalent vaccine, as shown by the relative additional potential impact, was ≥80% for either low or high impact estimates. As far as histological ≥HSIL were concerned, the number of HPV genotypes targeted by the quadrivalent vaccine varied between 37.9% (low estimate) and 48.3% (high estimate); the number of viral genotypes targeted by the nonavalent vaccine varied between 56.9% (low estimate) and 81.0% (high estimate). The absolute additional impact of the nonavalent vaccine was significant by low (19.0%) and high impact estimate (32.8%). The benefit of the nonavalent vaccine compared to the quadrivalent vaccine, as shown by the relative additional potential impact, was ≥50% for low and high impact estimates.
Table 2.
Overall Proportion (Low and High Estimates) of HSIL targeted by quadrivalent and nonavalent vaccines.
| Low Estimate | High Estimate | ||
|---|---|---|---|
| Histologically diagnosed LSIL | n (%; 95% CI) | n(%; 95% CI) | |
| (N=362) | Quadrivalent vaccine | 60 (16.6; [12.7–20.4]) | 107 (29.6; [24.9–34.3]) |
| Nonavalent vaccine | 112 (30.9; [26.2–35.7]) | 193 (53.3; [48.2–58.5]) | |
| Absolute additional impact (% of additional prevented cases) | 14.4% (p<0.001) | 23.8% (p<0.001) | |
| Relative additional impact (%) | 86.7% | 80.0% | |
| Histologically diagnosed ≥HSIL | |||
| (N=58) | Quadrivalent vaccine | 22 (37.9; [25.4–50.4]) | 28 (48.3; [35.4–61.1]) |
| Nonavalent vaccine | 33 (56.9; [44.2–69.6]) | 47 (81.0; [70.9–91.1]) | |
| Absolute additional Impact (% of additional prevented cases) | 19.0% (p=0.0408) | 32.8% (p=0.0002) | |
| Relative additional impact (%) | 50.0% | 67.9% | |
Figure 2.
Potential impact of quadrivalent and nonavalent HPV vaccines on LSIL and ≥HSIL histological diagnosis.
Discussion
In a recent European study, the estimated annual number of new cervical cancers in 2013 was 34,7089; approximately 50% of cases being caused by HPV 16/18 and 25% by HPV 31/33/45/52/58.19
The International Agency for Research on Cancer (IARC) has identified twelve HPV types as carcinogenic to humans: HPV16/18/31/33/35/39/45/51/52/56/58/59.20 Therefore, assuming a 100% vaccine efficacy and coverage, estimation of the epidemiological burden of HPV-related cervical cancers and precancerous SIL suggests that the first generation, bivalent and quadrivalent vaccines targeted against HR-HPV16 and 18 could prevent 25,267 cervical cancers, accounting for 72.8% of total European cases.9
As being HSIL identified as the precursors of cervical cancer, they are considered appropriate as surrogate outcomes to evaluate HPV vaccine efficacy against cervical cancer worldwide. Nevertheless, in this study the impact of first (quadrivalent) and second (nonavalent) generation of HPV vaccines was also evaluated on cervical LSIL, notwithstanding the fact that LSIL are not considered as precancerous lesions. Indeed, taking also into consideration psychological impact on HPV infected women and economic potential burden due to medical follow-up and treatment, the assessment of HPV vaccines on LSIL development may be worthwhile.
In general, the attribution of cervical lesions to specific HPV genotypes is complicated by the occurrence of infections with multiple HPV types in the same lesion; therefore, the potential benefit of a HPV vaccine is not easily assessable, especially when viral types not prevented by vaccination are also detected in the same lesion. In this study, therefore, the impact of the vaccine was measured by low and high estimates, considering the presence of single or multiple HPV infections, following a previous approach.10 However, since high estimate considers that the HPV types targeted by vaccine are the cause of a lesion even when they are associated with other viral genotypes, the risk exists that high estimate may lead to overestimation of the degree of effectiveness of the vaccine. Therefore, it can be reasonably assumed that the real potential impact of vaccine is in the middle between high and low estimates.
In the group of LSIL tested in our study, the benefit of the nonavalent vaccine with respect to cervical lesion as tested in our study, the benefit of the vaccine with respect to quadrivalent vaccine was ≥80% for either low and high impact estimates, while in the group of HSIL was ≥50% for either low and high impact estimates. With high estimate calculations, the overall additional impact of HPV vaccine nonavalent was remarkable for lesions effectively prevented by the second generation vaccine HPV (additional absolute impact).
The Edith III study,21 showed that from 43% (low estimate) to 65% (high estimate) of cervical HSIL were associated with HPV types targeted from the quadrivalent vaccine. In a recent study on potential impact of a nonavalent vaccine on the occurrence of HPV related diseases, the authors showed that the second generation vaccine could prevent from 77% to 90% of cervical HSIL.10 This potential benefit on the prevention of all grades of cervical lesions could have a positive impact on the public health systems by reducing total costs related to the management of these lesions.
In conclusion, the present study on a female population in Sicily (southern Italy) confirms, in accordance with previous result (17), that the switch from a bivalent or quadrivalent (first generation HPV vaccines) to (second generation) a nonavalent HPV vaccine, would increase the prevention of high and low grade cervical lesions up to 90% and to 60% of cases respectively, offering a much wider protection.
It should be stressed that a significant reduction in precancerous lesions and HPV-related tumors can be reached only if vaccination coverage reaches more than 80%.22
Implementation of nonavalent vaccination programs could become, thus, a cost effective public health prevention approach, based on the potential to produce substantial incremental benefit.
Methods
Study subjects
The analysis involved samples from 4376 patients who consecutively came to the Virology laboratory of the Department of Sciences for Health Promotion and Mother and Child Care (Policlinico University of Palermo, Italy), between January 2010 and December 2015, with a request for HPV testing by their doctor. All samples were from women attending different gynecology outpatient clinics or private practice gynaecologists. Women were asked for information on their diagnosis of cervical disease, if any. All cervical samples were tested for HPV DNA presence as described below; cases of histologically confirmed diagnosis of cervical LSIL and HSIL or cancer (≥HSIL) were registered and the samples were evaluated as described below. All participants signed written, informed consent prior to entering the study. The informed consent and the study protocol were approved by the institutional review board at the Policlinico University of Palermo.
Clinical samples
Routine laboratory diagnosis of HPV infection was performed on all samples using routine laboratory procedures.23 DNA was extracted by the use of a QIAamp Mini Kit (Qiagen). HPV detection was carried out with the Linear ArrayHPV Genotyping Test (Roche Diagnostics), which allows the identification of 16 types considered as LR HPV (HPV 6, HPV 11, HPV 40, HPV 42, HPV 54, HPV 55, HPV 61, HPV 62, HPV 64, HPV 71, HPV 72, HPV 81, HPV 83, HPV 84, HPV 87 and HPV 89) and 21 types (HPV 16, HPV 18, HPV 26, HPV 31, HPV 33, HPV 35, HPV 39, HPV 45, HPV 51, HPV 52, HPV 53, HPV 56, HPV 58, HPV 59, HPV 66, HPV 67, HPV 68, HPV 69, HPV 70, HPV 73, and HPV 82) considered as definitive or probable HR HPV types.24,25 Since Linear Array HPV is unable to correctly determine HPV 52 status in women co-infected with either HPV 33, 35, and/or HPV 58,26 samples with ambiguous HPV 52 status were retested with the INNO-LiPA HPV assay (Innogenetics), and only those cases confirmed HPV 52 with this assay were considered as truly positive for HPV5227 and included in the assay.
After HPV testing and evaluation of histological diagnoses, three series of cervical samples were selected and examined as follows:
-
-
Group I: 1794 consecutive samples with a positive HPV result, to evaluate the impact of the two vaccines on HPV infection rate, independently of the presence of cervical SILs,;
-
-
Group II: 501 consecutive samples with histologically diagnosed LSIL;
-
-
Group III: 59 consecutive samples with HSIL and 5 carcinoma in situ, as histologically diagnosed. For ease of computation, these two categories were grouped together, and thus represented a total of 64 cases of ≥HSIL samples.
Statistical methods
Outcomes of HPV test and histological diagnosis were expressed as counts and percentages. Low estimate of the vaccine impact was calculated as the prevalence of HPV genotypes (HPV 6/11/16 and 18 for the quadrivalent, and HPV 6/11/16/18/31/33/45/52 and 58 for the nonavalent vaccine), alone or in association, by excluding the presence of any other HPV type; high estimate was calculated as the prevalence of HPV genotypes (HPV 6/11/16 and 18 for the quadrivalent, and HPV 6/11/16/18/31/33/45/52 and 58 for the nonavalent vaccine) alone or in association, also in the presence of any other HPV type. The absolute additional potential impact of the nonavalent vaccine, i.e., the proportion of additional cases potentially prevented by the nonavalent vaccine compared to the quadrivalent vaccine, was calculated as (nnonavalent-nquadrivalent)/N × 100, with n being the number of lesions potentially prevented and N the total number of lesions. The relative additional potential impact of the nonavalent vaccine compared to the quadrivalent vaccine was calculated as (nnonavalent-nquadrivalent)/nquadrivalent × 10010. The chi-square test was used to assess the association between categorical variables; z-test for proportions was used to calculate 95% Confidence Intervals (CIs) of the quadrivalent and the nonavalent vaccine coverage on HSIL, as well as of the absolute and relative additional impact of the nonavalent vaccine compared to the quadrivalent vaccine. The impact of nonavalent HPV vaccine compared to the quadrivalent vaccine was considered statistically significant if 95% CIs were not overlapping. The data were analyzed by means of Stata/SE 14.0.
Disclosure of potential conflicts of interest
Antonino Perino has been involved in scientific meetings and congress events with Sanofi-Pasteur MSD and Glaxo Smith Kline.
References
- [1].Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer 2003; 88(1):63-73; PMID:12556961; https://doi.org/10.1038%2Fsj.bjc.6600688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, et al.. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369(9580):2161-70; PMID:17602732; https://doi.org/10.1016%2FS0140-6736(07)60946-5 [DOI] [PubMed] [Google Scholar]
- [3].FUTURE II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356(19):1915-27; PMID:17494925; https://doi.org/10.1056%2FNEJMoa061741 [DOI] [PubMed] [Google Scholar]
- [4].Scurry J, Wilkinson EJ. Review of terminology of precursors of vulvar squamous cell carcinoma. J Low Genit Tract Dis 2006; 10(3):161-69; PMID:16829756 [DOI] [PubMed] [Google Scholar]
- [5].Partridge JM, Hughes JP, Feng Q, Winer RL, Weaver BA, Xi LF, Stern ME, Lee SK, O'Reilly SF, Hawes SE, et al.. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis 2007; 196(8):1128-36; PMID:17955430; https://doi.org/10.1086%2F521192 [DOI] [PubMed] [Google Scholar]
- [6].Giuliano AR, Nyitray AG, Kreimer AR, Pierce Campbell CM, Goodman MT, Sudenga SL, Monsonego J, Franceschi S. EUROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer 2015; 136(12):2752-60; https://doi.org/10.1002%2Fijc.29082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Capra G, Nyitray AG, Lu B, Perino A, Marci R, Schillaci R, Matranga D, Firenze A, Caleca M, Bellavia C, Guarneri F, Giuliano A, Giovannelli L. Analysis of persistence of human papillomavirus infection in men evaluated by sampling multiple genital sites. Eur Rev Med Pharmacol Sci 2015; 19(21):4153-63; PMID:26592842 [PubMed] [Google Scholar]
- [8].Clifford GM, Gallus S, Herrero R, Muñoz N, Snijders PJF, Vaccarella S, Anh PT, Ferreccio C, Hieu NT, Matos E, et al.. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet 2005; 366(9490):991-98; PMID:16168781; https://doi.org/10.1016%2FS0140-6736(05)67069-9 [DOI] [PubMed] [Google Scholar]
- [9].Hartwig S, Syrjänen S, Dominiak-Felden G, Brotons M, Castellsagué X. Estimation of the epidemiological burden of human papillomavirus-related cancers and non-malignant diseases in men in Europe: a review. BMC Cancer 2012; 12:30; PMID:22260541; https://doi.org/10.1186%2F1471-2407-12-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Riethmuller D, Jacquard AC, Lacau St Guily J, Aubin F, Carcopino X, Pradat P, Dahlab A, Prétet JL. Potential impact of a nonavalent HPV vaccine on the occurrence of HPV-related diseases in France. BMC Public Health [Internet] 2015. [cited 2016 Jan 5]; 15:453; PMID:25934423; https://doi.org/10.1186%2Fs12889-015-1779-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Muñoz N, Kjaer SK, Sigurdsson K, Iversen O-E, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, et al.. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 2010; 102(5):325-39; PMID:20139221; https://doi.org/10.1093%2Fjnci%2Fdjp534 [DOI] [PubMed] [Google Scholar]
- [12].de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al.. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11(11):1048-56; https://doi.org/10.1016%2FS1470-2045(10)70230-8 [DOI] [PubMed] [Google Scholar]
- [13].Li N, Franceschi S, Howell-Jones R, Snijders PJF, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 2011; 128(4):927-35; https://doi.org/10.1002%2Fijc.25396 [DOI] [PubMed] [Google Scholar]
- [14].Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, Jr Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E, et al.. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372(8):711-23; PMID:25693011; https://doi.org/10.1056%2FNEJMoa1405044 [DOI] [PubMed] [Google Scholar]
- [15].Serrano B, Alemany L, de Ruiz PA, Tous S, Lima MA, Bruni L, Jain A, Clifford GM, Qiao YL, Weiss T, et al.. Potential impact of a 9-valent HPV vaccine in HPV-related cervical disease in 4 emerging countries (Brazil, Mexico, India and China). Cancer Epidemiol 2014; 38(6):748-56; PMID:25305098; https://doi.org/10.1016%2Fj.canep.2014.09.003 [DOI] [PubMed] [Google Scholar]
- [16].Drolet M, Bénard É, Boily MC, Ali H, Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JM, Cummings T, et al.. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15(5):565-80; https://doi.org/10.1016%2FS1473-3099(14)71073-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Van de Velde N, Boily MC, Drolet M, Franco EL, Mayrand MH, Kliewer EV, Coutlée F, Laprise JF, Malagón T, Brisson M et al.. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst 2012; 104(22):1712-23; PMID:23104323 https://doi.org/10.1093%2Fjnci%2Fdjs395 [DOI] [PubMed] [Google Scholar]
- [18].Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, Steinau M, Watson M, Wilkinson EJ, Hopenhayn C, et al.. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015; 107(6):djv086; PMID:25925419; https://doi.org/10.1093%2Fjnci%2Fdjv086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burd EM. Human Papillomavirus and Cervical Cancer. Clin Microbiol Rev 2003; 16(1):1-17; https://doi.org/ 10.1128/CMR.16.1.1-17.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Joura EA, Ault KA, Bosch FX, Brown D, Cuzick J, Ferris D, Garland SM, Giuliano AR, Hernandez-Avila M, Huh W, et al.. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev 2014; 23(10):1997-2008; PMID:25274978; https://doi.org/10.1158%2F1055-9965.EPI-14-0410 [DOI] [PubMed] [Google Scholar]
- [21].Prétet JL, Jacquard AC, Saunier M, Clavel C, Dachez R, Gondry J, Pradat P, Soubeyrand B, Leocmach Y, Mougin C, et al.. Human papillomavirus genotype distribution in low-grade squamous intraepithelial lesions in France and comparison with CIN2/3 and invasive cervical cancer: The EDiTH III study. Gynecol Oncol 2008; 110(2):179-84; https://doi.org/10.1016%2Fj.ygyno.2008.04.012 [DOI] [PubMed] [Google Scholar]
- [22].Riethmuller D, Pretet JL, Denis F, Aubin F, Pradat P, Clavel C, Dachez R, Gondry J, Carcopino X, Mougin C. Expected impact of a quadrivalent HPV vaccine in France. J Gynecol Obstet Biol Reprod 2009; 38(5):389-95; PMID:19481365; https://doi.org/ 10.1186/s12889-015-1779-1 [DOI] [PubMed] [Google Scholar]
- [23].Giovannelli L, Lama A, Capra G, Giordano V, Aricò P, Ammatuna P. Detection of human papillomavirus DNA in cervical samples: analysis of the new PGMY-PCR compared to the hybrid capture II and MY-PCR assays and a two-step nested PCR assay. J Clin Microbiol 2004; 42(8):3861-64; PMID:15297550; https://doi.org/10.1128%2FJCM.42.8.3861-3864.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24 Suppl 3:S3/1-10; PMID:16949995; https://doi.org/10.1016%2Fj.vaccine.2006.05.115 [DOI] [PubMed] [Google Scholar]
- [25].de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004; 324(1):17-27; PMID:15183049; https://doi.org/10.1016%2Fj.virol.2004.03.033 [DOI] [PubMed] [Google Scholar]
- [26].Poljak M, Cuzick J, Kocjan BJ, Iftner T, Dillner J, Arbyn M. Nucleic acid tests for the detection of alpha human papillomaviruses. Vaccine 2012; 30 Supplement 5:F100-6; https://doi.org/10.1016%2Fj.vaccine.2012.04.105 [DOI] [PubMed] [Google Scholar]
- [27].Marks M, Gupta SB, Liaw KL, Kim E, Tadesse A, Coutlee F, Sriplienchan S, Celentano DD, Gravitt PE. Confirmation and quantitation of human papillomavirus type 52 by Roche Linear Array© using HPV52-specific TaqMan© E6/E7 quantitative real-time PCR. J Virological Methods 2009; 156(1-2):152-6; https://doi.org/10.1016%2Fj.jviromet.2008.10.013 [DOI] [PubMed] [Google Scholar]