ABSTRACT
Treatment of multiple myeloma has undergone significant change in the last decade with the introduction of new immunomodulatory agents, proteasome inhibitors, and immunotherapeutic approaches. Elotuzumab is a humanized monoclonal antibody targeting CS1, which is a member of the SLAM (Signaling Lymphocyte Activation Molecule) family of proteins, expressed on the surface of myeloma plasma cells. Here we review the preclinical investigations that led to the development of elotuzumab and the clinical studies that resulted in its approval for the treatment of relapsed/refractory multiple myeloma. Although preclinical data looked very promising, elotuzumab monotherapy did not result in objective clinical responses in patients with relapsed/refractory multiple myeloma. However, combination treatment with immunomodulators and proteasome inhibitors resulted in substantial clinical activity in relapsed/refractory MM. Currently, there are several clinical trials ongoing investigating the role of elotuzumab in newly diagnosed myeloma patients and in patients receiving maintenance therapy.
KEYWORDS: antibodies, CS1, elotuzumab, immunotherapy, monoclonal, multiple myeloma, SLAM family of receptors
Introduction
Multiple myeloma (MM) is a clonal plasma cell disorder associated with significant morbidity and mortality. The American Cancer Society expects about 30,000 new MM cases in 2017 for the US alone with about 12,500 myeloma-related deaths.1 With the advent of new therapies, such as proteasome inhibitors and immunomodulatory agents, the last decade has seen an unprecedented improvement in outcomes with a 5-year survival approaching 50%.2 Nevertheless, patients continue to experience repeated relapses requiring salvage therapies and ultimately most patients will succumb to the disease.
In particular, the treatment of MM patients with high-risk cytogenetics has been disappointing and so far we have not been able to identify a convincing way of dealing with this more therapy-resistant and aggressive subtype of myeloma. Immunotherapies carry the potential to overcome high relapse rates and the more aggressive clinical course of high-risk MM because these novel approaches potentially target the tumor cell irrespective of its biologic characteristics.
Recently, there has been great interest in harnessing the immune system to fight cancer and major improvements have been made in solid tumors, for example with the introduction of checkpoint inhibitors. At the same time, we have also seen major advances in hematologic malignancies with 2 new monoclonal antibodies being approved in 2015, the anti-CS1 antibody elotuzumab and the anti-CD38 antibody daratumumab. Here we review in detail the novel anti-myeloma agent elotuzumab describing its target antigen, the characteristics of the monoclonal antibody itself as well as preclinical studies and clinical trials that led to its approval.
The signaling lymphocyte activation molecule (SLAM) family of receptors
SLAM family receptors are a group of type 1 transmembrane receptors broadly expressed on immune cells.3 They share a similar structure with an extracellular domain consisting of an Ig variable (V)-like domain, an Ig constant 2 (C2)-like domain, a transmembrane domain and a cytoplasmic tail.4 The cytoplasmic domain of SLAM family proteins consists of one or more copies of the immunoreceptor tyrosine-based switch motif (ITSM) that can bind to adaptor molecules SAP (SLAM associated protein) and EAT-2 (Ewing sarcoma-associated transcript-2) and Src homology 2 (SH2)-containing phosphatase.5,6 Engagement of the N-terminal Ig domains of SLAMF receptors with their cognate ligands results in the recruitment of these intracellular molecules, leading to signaling transduction events that ultimately modulate a variety of immune responses.6
Most SLAM family receptors are “self-ligands” and are involved in homotypic self-associations through their extracellular domain. SAP adaptors are essential components of the SLAM pathway and in their absence, SLAM receptors function as inhibitory signals preventing cellular activation. SAP adaptors prevent coupling of the SLAM receptor to the inhibitory pathway mediated by SH2 domain-containing containing protein tyrosine phosphatase (SHP)-1, SHP-2 and SH2 domain-containing inositol phosphatase (SHIP)-1 and also recruit Src-related tyrosine kinase Fyn that leads to downstream phosphorylation and activation of the cells. EAT-2 has a similar mechanism of preventing SLAM binding to inhibitor pathway mediators and at the same time recruiting phospholipase C which leads to granule polarization enhancing NK cell activity.7
The SLAM family of receptors has been implicated in the pathogenesis of different diseases including chronic infections, autoimmune diseases, and cancer.8,9 The SLAM family comprises a total of 9 members, however, studies in MM have been limited to CD150 (SLAMF1), CD48 (SLAMF2), CD229 (SLAMF3), CD244 (SLAMF4), CD352 (SLAMF6), and CS1 (SLAMF7). SLAM family member CS1 is the one receptor where its clinical development as a novel immunotherapy for MM is most advanced.
Surface molecule CS1 (SLAMF7, CD319)
Like other members of the SLAM family, CS1 has an extracellular domain consisting of an Ig variable like and a constant domain, a transmembrane domain and a cytoplasmic tail. But unlike most other SLAM proteins, CS1 contains only a single immunoreceptor tyrosine based switch motif (ITSM). CS1 has been shown to exist in 2 isoforms: CS1-long (CS1-L) and CS1-short (CS1-S), the difference being that the latter lacks the tyrosine residues that contribute to the ITSMs.10 Unlike other SLAM members CS1 does not bind to SAP but only to EAT-2 and the expression of the latter is limited to natural killer (NK) cells while it is absent from CD4+ T cells, B cells, and plasma cells.
CS1 is highly expressed on NK cells, CD8+ cytotoxic T cells, a small proportion of CD4+ T cells and mature dendritic cells.11 CS1 is expressed on B cells at all differentiation stages but its expression is highest at the early pro-B cell, later-stage activated B cells, and differentiated plasma cells.12 Monocytes when activated show surface expression of CS1 and this upregulation of CS1 has been shown to be mediated by NF-kB pathway activation.13 Expression is limited on granulocytes, immature DCs and haematopoietic stem cells. Hsi et al. showed that CS1 expression in non-lymphoid tissue is limited as determined by gene expression profiling and immune histochemical staining with anti-CS1 antibody.14 However, other studies have indicated CS1 gene expression in other non-haematopoietic organs like kidney, heart, pituitary, skeletal muscles and certain areas of the brain.15
CS1 in multiple myeloma
Antigen CS1 is highly expressed on myeloma cells of both MM patients with standard and high-risk disease. It can be found on the malignant plasma cells at all the different stages of plasma cell dyscrasias from monoclonal gammopathy of undetermined significance (MGUS) through smoldering myeloma, multiple myeloma, and plasma cell leukemia. CS1 expression persists at relapse, even though expression levels seemed to be somewhat lower in patients with relapsed/refractory disease.14 Soluble CS1 was also detected in the blood of 90% of patients with newly diagnosed MM while it was absent in normal controls. Interestingly, there was a positive correlation of CS1 serum levels with more advanced stages of the disease.16
Unfortunately, the exact role of CS1 in the pathogenesis of MM has not yet been identified. Like normal B cells and plasma cells, MM cells do not express EAT-2, the main pathway for CS1 activation. It has been shown that CS1 is localized to uropod membranes of MM where it promotes cell-cell adhesion between individual MM cells and also between MM cells and stromal cells.16 In addition, decreased adhesion of MM cells to bone marrow stromal cells (BMSC) was seen in vitro when CS1 expression was knocked down using short interfering RNA. When MM cells were co-cultured with BMSCs in the presence of elotuzumab there was a dose dependent inhibition of cell viability suggesting that elotuzumab might overcome the stimulatory effects of BMSCs on MM growth.16
Anti-CS1 antibody elotuzumab
The broad and consistent expression of CS1 in MM made it an attractive target for immune-based therapies and as a consequence antibodies targeting CS1 were generated. Hsi et al. immunized female BALB/c mice with CS1 protein and generated monoclonal antibodies using standard hybridoma technique. They subsequently identified antibodies binding to the extracellular domain of CS1.14 Rice et al. performed comparative studies with the 2 antibodies, MuLuc63 (IgG2a) and MuLuc90 (IgG2b), in L363 MM xenograft models and found that MuLuc63 had improved tumoricidal activity compared with MuLuc90.17 This led to the humanization of MuLuc63 and the development of HuLuc63 (IgG1), which was later named elotuzumab.
Preclinical development of elotuzumab
Rice et al. developed 2 variants of the HuLuc63 antibody described above: (1) HuLuc63-Ala with a mutation in the Fc region that impairs binding to Fc receptors on NK cells and (2) HuLuc63-LF with low levels of fucosylation in the Fc region that increases binding to Fc receptors. In a MM xenograft model they showed that HuLuc63-LF had superior in vivo anti-tumor activity and HuLuc63-Ala had no anti-tumor activity compared with the non-modified HuLuc63 suggesting that antibody-dependent cell mediated cytotoxicity (ADCC) is a major mechanism of action for elotuzumab. HuLuc63 also demonstrated dose-dependent ADCC against L363 and OPM2 myeloma cell lines.17 Accordingly, depletion of NK cells from peripheral blood mononuclear cells and blocking of the Fc receptors (FcR) on NK cells also significantly decreased the anti-tumor effects of HuLuc63 antibody.14
Van Rhee et al. showed that bortezomib pretreatment of myeloma cell line OPM2 led to enhanced dose-dependent ADCC with elotuzumab and this effect was lost after pre-treatment of the effectors with FcR blocking antibodies. They also demonstrated that the enhanced elotuzumab-mediated ADCC after bortezomib treatment was not due to an increased gene or surface expression of CS1 in primary myeloma cells. In a xenograft model of MM the combination of bortezomib and elotuzumab substantially inhibited tumor growth in comparison to either bortezomib or elotuzumab alone.18 Similarly, Tai et al showed that HuLuc63 mediated ADCC against primary MM cells from patients refractory to bortezomib. Pre-treatment of myeloma cell line MM1 with subtoxic doses of dexamethasone, bortezomib, lenalidomide, AKt inhibitor perifosine or MEK inhibitor further enhanced cytotoxicity with HuLuc63. HuLuc63 also induced ADCC against MM1S and MM1R cell lines adherent to bone marrow stromal cells (BMSC). Finally, pretreatment of effector cells with lenalidomide enhanced elotuzumab-mediated lysis of primary myeloma cells and MM cell lines.16
CS1 is highly expressed on NK cells and binding of elotuzumab to NK cells may lead to NK cell activation and further amplified cytotoxicity. Collins et al. showed that when NK cells were treated with elotuzumab variants elo-F(ab’), which lacks the Fc of the antibody, or elo-G2M3, which shows reduced FcR binding, both antibodies were able to increase the expression of activation marker CD69 on NK cells. Elotuzumab-treated NK cells showed cytotoxicity toward CS1-negative cell line K562 suggesting that it can augment NK cell function even outside of ADCC. However, elotuzumab pretreatment of NK cells did not enhance autologous NK-NK cell killing, an effect that was based on an increased surface expression of MHC class I molecules on NK cells, protecting these cells from NK cell-mediated cytotoxicity.19
Various types of immunotherapies have also been studied in combination with elotuzumab and some of these are now in early phase clinical trials. Lirilumab is a fully human IgG4 anti-KIR2DL1/2/3 specific monoclonal antibody that blocks the binding of KIRs to HLA-C and thus prevents the inhibition of NK cells. Sola et al. demonstrated that combining lirilumab with elotuzumab leads to enhanced anti-myeloma activity in a dose-dependent manner especially in MM cells with low density of SLAMF7 expression. In MM xenograft models using transgenic mice that express KIR2DL3 and its ligand HLA-cw3 the combination of lirilumab and elotuzumab led to an enhanced anti-tumor effect and improved survival.20 Similarly, cytokine IL-21 increases elotuzumab-mediated ADCC in vitro but not in mouse xenograft model of MM. In contrast, agonistic anti-CD137 monoclonal antibody only had minimal effect on elotuzumab-mediated ADCC in vitro but showed significant activity in a xenograft model of MM.21
Based on these pre-clinical studies the proposed mechanisms of action of elotuzumab (Fig. 1) include:
-
1)
Antibody-dependent cell-mediated cytotoxicity (ADCC): NK cell-mediated cytotoxicity against MM cells
-
2)
Binding of elotuzumab to CS1 on NK cells leads to cellular activation and increased cytotoxicity
-
3)
Binding of elotuzumab to CS1 on MM cells inhibits cell interactions among myeloma cells and also between the myeloma cell and bone marrow stromal cells
Figure 1.
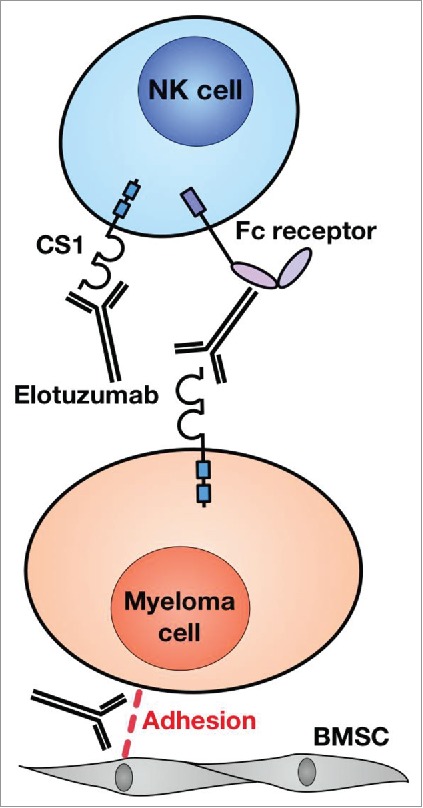
Mechanisms of action of elotuzumab in Multiple myeloma. Elotuzumab mediates antibody dependent cell mediated cytotoxicity of CS1 expressing myeloma cells by natural killer cells (NK cells) and also activates NK cells by binding to CS1. Elotuzumab also decreases the adhesion of myeloma cells to bone marrow stromal cells (BMSC) but the mechanism is not well elucidated.
Clinical trials using elotuzumab in relapsed/refractory MM
Phase I studies
The first in human phase I clinical study was conducted by Zonder et al enrolling 35 patients with relapsed/refractory MM who had received at least 2 prior lines of treatment.22 Elotuzumab was given at 6 escalating dose levels as an intravenous infusion every 14 d for 8 weeks. Those who completed 8 weeks and did not have progression of their disease were allowed to receive an additional 8-week treatment. Initially 13 of the 25 patients receiving elotuzumab developed infusion reactions like chills, fever, headache and flushing which were mostly mild. The infusion protocol was then amended to add methylprednisolone, diphenhydramine and acetaminophen before the first dose of elotuzumab and after the protocol was amended no grade 3 or 4 reactions were seen. Out of all patients, 44% developed serious adverse effects including bradycardia, chest discomfort, chills, hypersensitivity, pyrexia and acute renal failure and of these 6 were attributed to elotuzumab.22 There was a transient decrease in lymphocyte counts after the first infusion of elotuzumab including both CS1-positive NK and CD8+ T cells as well as CS1-negative CD4+ T cells and B cells and, even though there were grade 3–4 infections none of these were thought to be related to elotuzumab. Of the 34 patients that were available for evaluation, 25 completed the initial treatment and 8 patients received retreatment. Two patients experienced dose-limiting toxicity during the first cycle and both discontinued treatment. The maximum tolerated dose was not reached until the maximum planned dose of 20 mg/kg.22
Of the 31 patients tested for immunogenicity, 12 were positive for antidrug antibodies (ADA). Elotuzumab had non-linear pharmacokinetics with mean AUC increasing more than proportional for the increase in dose. Elotuzumab at doses above 10 mg /kg saturated the CS1 receptors of bone marrow-derived myeloma cells. There was no objective response observed during the study period, however, 9 patients showed stable disease.22
Jakubowiak23 et al. conducted an open label, multicenter, phase I dose escalation study of elotuzumab in combination with bortezomib in patients with MM who had previously received > 1 line of treatment. Bortezomib was administered at 1.3mg/m2 intravenously on days 1, 4, 8 and 11 of each 21-day cycle and elotuzumab was administered on days 1 and 11 of each cycle. Escalating doses of elotuzumab were administered for at least 4 cycles. Premedication with methylprednisolone, diphenhydramine and acetaminophen was given before each infusion. Of the 28 patients that were enrolled 11 had prior exposure to bortezomib and among those only 3 were considered refractory. Of all the 28 patients in the study 2 achieved a complete response (CR), 11 had a partial response (PR), and 2 progressed on the study. Interestingly, 2 of the 3 patients who were refractory to bortezomib showed a response to the combination. No dose limiting toxicity was observed in any of the patients during the first cycle. The most common grade 3 or 4 toxicities were lymphopenia (25%) and fatigue (14%). As in the previous study, a maximum tolerated dose was not attained at the maximum planned dose of 20 mg/kg. CS1 levels on bone marrow plasma cells and lymphocytes were 80% and 95% saturated at elotuzumab doses of 10 mg/kg and 20 mg/kg, respectively.
Lonial5 et al. conducted an open label multicenter phase I dose escalation study of elotuzumab in combination with low dexamethasone and lenalidomide in 28 patients with relapsed/refractory MM. Escalating doses of elotuzumab were administered on days 1, 8, 15 and 22 of a 28-day cycle for the first 2 cycles and on days 1 and 15 for the remaining cycles. Lenalidomide 25 mg oral daily from day 1 to day 21 and weekly dexamethasone 40 mg were administered. Patients had active disease that had progressed on at least one previous line of treatment. 21% of the patients had received lenalidomide, 69% and 59% had received bortezomib and thalidomide, respectively, and 41% of the patients were refractory to the last line of treatment. No dose limiting toxicity was observed and the maximum tolerated dose was not reached at the maximum planned elotuzumab dose of 20 mg/kg. Out of all patients 89% experienced infusion reactions but these were mostly mild and resolved quickly. Fatigue was the most common adverse effect (AE) and the most frequent grade 3/4 AEs were neutropenia (36%) and thrombocytopenia (21%). The overall response rate was 82% with one complete response and 8 very good partial responses (VGPR) and 14 PRs and a median time to response of 50 d. Two of the 6 patients who had prior exposure to lenalidomide also achieved an objective response. Importantly of the 12 patients who were refractory to the most recent therapy 10 patients achieved an objective response with the combination. Steady state concentrations of elotuzumab above 70ug/ml were maintained at doses of 10 and 20 mg/kg and 80% of myeloma cells were observed to have fully saturated CS1 sites at this concentration.
Berdeja24 et al. studied the pharmacokinetics and safety of combining elotuzumab with lenalidomide and dexamethasone in patients with renal dysfunction in a phase Ib study. They categorized patients into normal renal function (CrCl ≥ 90mL/min, n = 8), severe renal impairment (CrCl <30 mL/min, n = 9) and end stage renal disease (dialysis-dependent, n = 9). They observed that the mean serum concentration of elotuzumab was comparable among the 3 groups and no differences were seen in the area under the concentration-time curve. No differences in the incidence of grade 3 or 4 AEs were observed and they concluded that elotuzumab can safely be used in patients with renal dysfunction without dose adjustments.
Phase II studies
Richardson25,26 et al. investigated the combination of elotuzumab, lenalidomide and dexamethasone in a phase II study in 73 patients with relapsed/refractory MM. Patients were randomized across 17 centers into dose levels of 10 mg/kg and 20 mg/kg. Of all the patients, 55% had more than 2 prior therapies, 60% of the patients had prior bortezomib, and 62% had thalidomide while patients who had previously received lenalidomide were excluded. The overall response rate was 92% at the standard 10 mg/kg dose level which included 42% VGPR and 28% PR, with a median time to first response of one month and median time to best response of 2.6 months. The median duration of responses was 34.8 months for the 10 mg/kg group. 76% of patients that received the higher dose of 20 mg/kg achieved objective response including 38% VGPR and 27% PR. Patients who had previously received thalidomide still showed an overall response of 82% with a progression free survival (PFS) of 26.9 months. Out of all patients 78% experienced at least one grade 3 AE with the most common AEs being lymphopenia (21%), neutropenia (19%), thrombocytopenia (18%), and anemia (15%). 12% of patients developed Infusion reactions and one had a grade 3 rash. Persistent neutralizing antibodies against elotuzumab were noted in 2 of the 36 patients in the 10mg/kg group.
Jakubowiak27 et al. conducted a randomized open label phase II study to compare the efficacy of a combination of elotuzumab with bortezomib and dexamethasone (EBd) to bortezomib and dexamethasone (Bd) in 153 patients with relapsed/refractory MM. Elotuzumab was administered at 10mg/kg and the median PFS was 9.7 months with EBd compared with 6.9 months for the Bd group (hazard ratio (HR) 0.72, p 0.09). The one and 2 y PFS was 39% and 18%, respectively, for the EBd group compared with 33% and 11% with the Bd group. Furthermore, in the EBd group, patients who were homozygous for the high affinity FcRIIIa V allele had a median PFS of 22.3 months compared with 9.8 months in patients with homozygous low affinity alleles. The overall response rate and median time to response was 66% and 1.4 months in the EBd group compared with 63% and 1.5 months in the Bd group. The one-year survival rate was 85% in the EBd group compared with 74% in the Bd group (HR 0.61, 95% confidence interval (CI) 0.32–1.15, 70% CI 0.43–0.85). The updated 2-year overall survival was 73% with the EBd group compared with 66% in the Bd group. There was only minimal difference regarding AEs between the 2 groups and AEs leading to drug discontinuation occurred in 32% of patients in the EBd group compared with 39% in the Bd group. No grade 3 or higher infusion reactions were seen and none of the patients discontinued elotuzumab due to infusion reactions.27
Finally, Mateos28 et al. conducted a phase II single arm study with elotuzumab at 10mg/kg in combination with thalidomide and low dose dexamethasone with or without cyclophosphamide in patients with relapsed/refractory MM. They treated 40 patients, among whom 98% had previously received bortezomib and 73% lenalidomide. They reported a median overall survival of 16.3 months, an overall response rate of 38%, and median PFS of 3.9 months.
Phase III study
ELOQUENT-2 was a phase III randomized study that compared the efficacy and safety of the combination of elotuzumab, lenalidomide, and dexamethasone (ELd) to lenalidomide and dexamethasone (Ld) alone in relapsed/refractory MM.29 The study population included 32% of patients with high-risk cytogenetics and 54% had a prior autologous stem cell transplant. Of the 646 patients enrolled in the study, 6% had previously received lenalidomide but had achieved a partial response or better and had not progressed on or within 9 months after lenalidomide treatment. 70% of the patients previously received bortezomib and 48% prior thalidomide therapy. Patients received elotuzumab at 10 mg/kg intravenously on days 1, 8, 15 and 22 of a 28-day cycle for the first 2 cycles and then on days 1 and 15 from Cycle 3 on as well as lenalidomide at 25 mg daily on days 1–21. On the days the patient receives elotuzumab, dexamethasone was given as an 8 mg intravenous and 28 mg oral dose and on the weeks off elotuzumab, 40 mg oral dexamethasone was given. The elotuzumab group showed a superior median PFS of 19.4 months (95% CI 16.6 to 22.2 months) compared with the control group 14.9 months (95% CI 12.1 to 17.2 months) with a hazard ratio of 0.70 (p < 0.001). Similarly, the overall response rate was also significantly better in the elotuzumab group, 79% compared with 66% in the control group with an odds ratio of 1.9 (95% CI 1.4 to 2.8, p < 0.001). The complete response rate was lower in the elotuzumab group (4% vs 7%) probably due to the interference of elotuzumab with immunofixation. However, VGPR rates were better in the elotuzumab group compared with the control (33% vs. 28%). The median time to best response was 2.8 months in both groups with more durable responses seen in the elotuzumab group compared with the control (21 vs. 17 months). Grade 3 or 4 neutropenia was less common (34% vs 44%) and lymphocytopenia was more common (77% vs 49%) in the elotuzumab arm compared with the control arm. The elotuzumab group had an increased rate (10%) of infusion-related reactions which were mostly low grade and only led to discontinuation of the treatment in 2 out of 321 patients. Incidence of herpes zoster infection was greater in the elotuzumab group(4.1 vs 2.2 per 100 patient-years) compared with the control group but otherwise there was no difference in overall infection rates or incidence of opportunistic infections between groups.29 This study led to FDA approval of elotuzumab in November 2015 for the treatment of relapsed/refractory MM in combination with lenalidomide and dexamethasone. A 3 y follow up of the ELOQUENT-2 trial was presented at the ASCO 2016 annual conference. The Elotuzumab group (ELd) showed significant improvement in PFS across all reported subgroups including patients with high risk cytogenetics and all age groups. Importantly, patients with del(17p) showed a significant improvement in PFS of 21.2 months compared with 14.9 months in the control group (HR 0.70, p = 0.042). Similarly patients with t(4:14) treated with ELd had improved PFS of 15.8 months compared with 5.6 months with Ld (HR 0.52, p = 0.027). This translated into an increase in the overall survival in the ELd group compared with the Ld group (29.8 vs 24.8 months, HR 0.60) in high risk patients (ISS stage 2 or 3, high risk cytogenetics t(4:14) or del 17p). This follow up analysis has shown that the benefits from the addition of elotuzumab are durable over time and continues to benefit high risk patients.30
Currently ongoing clinical trials
A number of additional trials have been initiated to evaluate elotuzumab as an anti-myeloma agent in different combinations and clinical settings and are summarized in Table 1.
Table 1.
Ongoing clinical trials with elotuzumab in multiple myeloma.
| Study | Description |
|---|---|
|
Phase 1 studies | |
| SWOG S1211 | The study evaluates combination of lenalidomide, bortezomib, dexamethasone and elotuzumab as induction treatment of newly diagnosed MM. After induction maintenance regimen with the same combination. |
| NCT02252263 | The study evaluates combination of elotuzumab with antibodies like urelumab (CD137 agonistic monoclonal antibody) and lirilumab (fully human monoclonal anti-KIR antibody that blocks the interaction between KIR2DL-1,2,3 inhibitory receptors and their ligands) in relapsed/refractory MM who have achieved a VGPR or a CR with minimal residual disease after autologous transplant. |
| NCT03023527 | The study evaluates combination of nivolumab, pomalidomide and dexamethasone with and without elotuzumab in patients who are in the first or second relapse and refractory to lenalidomide. |
| NCT02655458 | Studies elotuzumab in combination with autologous stem cell transplant followed by lenalidomide maintenance in patients who have attained at least a partial response with induction therapy. |
| Phase II studies | |
| NCT02420860 | Study evaluates the combination of elotuzumab and lenalidomide as maintenance therapy after autologous stem cell transplant in patients who undergo transplant within 18 months of induction therapy in newly diagnosed MM |
| NCT01441973 | This is a biomarker study to determine if treatment with elotuzumab in patients with high risk smoldering myeloma who have more CD56dim NK cells will improve response |
| NCT02718833 | This study combines elotuzumab with pomalidomide, bortezomib and dexamethasone to treat patients with relapsed/refractory MM who have received at least 2 previous therapies have relapsed or refractory to both lenalidomide and bortezomib |
| NCT02654132 (ELOQUENT-3) | This is an open labeled randomized trial of pomalidomide and low dose dexamethasone treated with or without elotuzumab in patients with relapsed and refractory MM. Patients included in the study are refractory to at least 2 previous lines of treatment and that should include lenalidomide and a proteasome inhibitor |
| NCT03003728 | This is a pilot study in which high risk myeloma patients, refractory to both proteasome inhibitors and immunomodulatory drugs are treated with elotuzumab combined with expanded NK cells after autologous stem cell transplantation. |
| Phase III studies | |
| NCT02726581 (CheckMate 602) | An exploratory arm of the study evaluates the clinical benefit and safety of combining elotuzumab, nivolumab, pomalidomide and dexamethasone (NE-Pd) in patients with relapsed/refractory MM. |
| NCT01891643 (ELO 1 Substudy) | patients with previously untreated MM who are not eligible for high dose therapy and autologous stem cell transplant are treated with lenalidomide and dexamethasone with or without addition of elotuzumab |
| NCT02495922 | This randomized trial evaluates the effect of elotuzumab on VRD induction/consolidation in patients with new diagnosis of multiple myeloma It also assess the effect of addition of elotuzumab to lenalidomide as maintenance therapy after autologous transplant and VRD consolidation |
| NCT01335399 (ELOQUENT-1) | This study determines whether the addition of elotuzumab to lenalidomide and dexamethasone will improve the progression free survival in previously untreated MM who are not eligible for high dose chemotherapy and autologous stem transplant due to their age or co-morbidities. |
Comparing elotuzumab to other approved anti-myeloma antibodies on the market
Darartumumab is a human IgGκ monoclonal antibody targeting CD38 and has been studied as a single agent and in combination with lenalidomide/dexamethasone and bortezomib/dexamethasone in relapsed/refractory MM. Elotuzumab and daratumumab have not been compared head to head either alone or in combination therapy in MM but as both are approved therapies for relapsed/refractory MM, treatment decisions would be based on the relative efficacy seen in the respective trials. Daratumumab monotherapy in a highly treatment refractory MM population showed an overall response rate of 36% and no dose-limiting toxicity was found in the phase I/II study. This in contrast to elotuzumab monotherapy, which did not show any significant single agent activity in the phase I study.31 In the landmark phase III POLLUX trial 569 patients with relapsed refractory MM were treated with lenalidomide/dexamethasone with or without daratumumab. The treatment schedule for lenalidomide/dexamethasone was similar to that in the ELOQUENT-2 trial mentioned above. In the daratumumab group the overall response rate was 92.9% with 43.1% of the patients achieving a complete response and another 32.7% achieving a VGPR. At a median follow up time of 13.5 months, disease had progressed in 18.5% in the daratumumab group compared with 41% in the control (HR 0.37, p<0.001) and the median PFS could not be evaluated in the daratumumab group compared with 18.4 months in the controls. There was a higher incidence of grade 3/4 neutropenia in patients treated with daratumumab 51.9% compared with 37% in the control group. Similarly, febrile neutropenia occurred in 5.7% patients in the daratumumab arm compared with 2.5% in the control arm. Rates of thrombocytopenia were similar and grade 3/4 anemia was less common in the daratumumab arm (12.4% vs 19.6%). The incidence of infusion reactions was 47.7% but were mostly grade 1 or 2 with only 5.3% with grade 3 and none with grade 4 infusion reactions. The infusion reaction most commonly manifested as cough, dyspnea or vomiting and are different from infusion reactions seen with elotuzumab which included fever, chills and hypertension. The incidence of infusion reactions with daratumumab is also higher compared with elotuzumab (10% in the ELOQUENT-2 trial). Overall, there were higher rates of grade 3/4 AEs in the daratumumab group (48.8% vs. 42%) but the discontinuation rates were similarly low in both arms of the study.32
If these results are compared with the ELOQUENT-2 trial with similar patient population, daratumumab significantly improves the response to treatment with a much higher percentage of patients achieving CR or VGPR compared with elotuzumab both as monotherapy and in combination with standard treatment regimens. The caveat though is that patients on the POLLUX trial in general had a better response to treatment with percentage of patients in the control group achieving a higher CR or VGPR compared with the ELOQUENT-2 trial. However, the significant improvement in response rates and the progression free survival with good tolerability makes us believe that in this patient population of relapsed / refractory MM, daratumumab would be a better option compared with elotuzumab in combination with lenalidomide/dexamethasone (or even bortezomib/dexamethasone) and can even be used as monotherapy in patients refractory to both bortezomib and lenalidomide.
Conclusions
Over the last decade there has been a paradigm shift in cancer treatment with the advent of immunotherapies such as checkpoint inhibitors directed against CTLA-4 and PD1/PDL1. Elotuzumab is the first immune-based therapy approved for multiple myeloma for relapsed/refractory disease. Even though pre-clinical data were very promising, phase I clinical trials failed to show significant single agent activity. However, in combination with bortezomib/dexamethasone or lenalidomide/dexamethasone, elotuzumab showed substantial treatment benefits. Furthermore, even though initial studies indicated significant infusion reactions, in subsequent trials elotuzumab was well tolerated if premedications were given.
Ongoing clinical trials are assessing the role of elotuzumab in newly diagnosed MM in combination with standard treatment as well as in the maintenance setting after autologous transplant. Combination therapy with IMiDs other than lenalidomide, such as pomalidomide, newer proteasome inhibitors like carfilzomib, or antibodies like nivolumab, urelumab and lirilumab are also undergoing clinical investigation. The response to elotuzumab/lenalidomide/dexamethasone appears to be durable over time even in patients with high risk disease and across all age groups and so combination therapies including elotuzumab will continue to be an important treatment option for patients with relapsed/refractory multiple myeloma.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67(1):7-30; https://doi.org/ 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66(1):7-30; https://doi.org/ 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- [3].Stefanova I, Horejsí V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science 1991; 254(5034):1016-9; https://doi.org/ 10.1126/science.1719635 [DOI] [PubMed] [Google Scholar]
- [4].Wu N, Veillette A. SLAM family receptors in normal immunity and immune pathologies. Curr Opin Immunol 2016; 38:45-51; PMID:26682762; https://doi.org/ 10.1016/j.coi.2015.11.003 [DOI] [PubMed] [Google Scholar]
- [5].Lonial S, Vij R, Harousseau JL, Facon T, Moreau P, Mazumder A, Kaufman JL, Leleu X, Tsao LC, Westland C, et al.. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol 2012; 30(16):1953-9; https://doi.org/ 10.1200/JCO.2011.37.2649 [DOI] [PubMed] [Google Scholar]
- [6].Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol 2011; 29:665-705; PMID:21219180; https://doi.org/ 10.1146/annurev-immunol-030409-101302 [DOI] [PubMed] [Google Scholar]
- [7].Dong Z, Davidson D, Pérez-Quintero LA, Kurosaki T, Swat W, Veillette A. The adaptor SAP controls NK cell activation by regulating the enzymes Vav-1 and SHIP-1 and by enhancing conjugates with target cells. Immunity 2012; 36(6):974-85; https://doi.org/ 10.1016/j.immuni.2012.03.023 [DOI] [PubMed] [Google Scholar]
- [8].Abadia-Molina AC, Ji H, Faubion WA, Julien A, Latchman Y, Yagita H, Sharpe A, Bhan AK, Terhorst C. CD48 controls T-cell and antigen-presenting cell functions in experimental colitis. Gastroenterology 2006; 130(2):424-34; https://doi.org/ 10.1053/j.gastro.2005.12.009 [DOI] [PubMed] [Google Scholar]
- [9].Raziorrouh B, Schraut W, Gerlach T, Nowack D, Grüner NH, Ulsenheimer A, Zachoval R, Wächtler M, Spannagl M, Haas J, et al.. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology 2010; 52(6):1934-47; https://doi.org/ 10.1002/hep.23936 [DOI] [PubMed] [Google Scholar]
- [10].Lee JK, Boles KS, Mathew PA. Molecular and functional characterization of a CS1 (CRACC) splice variant expressed in human NK cells that does not contain immunoreceptor tyrosine-based switch motifs. Eur J Immunol 2004; 34(10):2791-9; PMID:15368295 [DOI] [PubMed] [Google Scholar]
- [11].Bouchon A, Cella M, Grierson HL, Cohen JI, Colonna M. Activation of NK cell-mediated cytotoxicity by a SAP-independent receptor of the CD2 family. J Immunol 2001; 167(10):5517-21. [DOI] [PubMed] [Google Scholar]
- [12].De Salort J, Sintes J, Llinàs L, Matesanz-Isabel J, Engel P. Expression of SLAM (CD150) cell-surface receptors on human B-cell subsets: from pro-B to plasma cells. Immunol Lett 2011; 134(2):129-36. [DOI] [PubMed] [Google Scholar]
- [13].Kim JR, Horton NC, Mathew SO, Mathew PA. CS1 (SLAMF7) inhibits production of proinflammatory cytokines by activated monocytes. Inflamm Res 2013; 62(8):765-72. [DOI] [PubMed] [Google Scholar]
- [14].Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y, et al.. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res 2008; 14(9):2775-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, et al.. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A 2004; 101(16):6062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, et al.. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008; 112(4):1329-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Audie Rice MD, Anne van Abbema, Lynne Jesaitis, Melanie Wong, Stacey Lawson, Gao Liu, Yin Zhang, David Powers, Susan Rhodes, Ingrid Caras, Debbie Law, Daniel Afar. Eradication of Tumors in Pre-Clinical Models of Multiple Myeloma by Anti-CS1 Monoclonal Antibody HuLuc63: Mechanism of Action Studies. Blood 2006; 108:3503. [Google Scholar]
- [18].van Rhee F, Szmania SM, Dillon M, van Abbema AM, Li X, Stone MK, Garg TK, Shi J, Moreno-Bost AM, Yun R, et al.. Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma. Mol Cancer Ther 2009; 8(9):2616-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Collins SM, Bakan CE, Swartzel GD, Hofmeister CC, Efebera YA, Kwon H, Starling GC, Ciarlariello D, Bhaskar S, Briercheck EL, et al.. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunol Immunother 2013; 62(12):1841-9; https://doi.org/ 10.1007/s00262-013-1493-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Caroline Sola MB, Cécile Bonnafous, Elodie Bonnet Nicolas Fuseri, Graziano Robert F, Yannis Morel, Pascale André. Lirilumab Enhances Anti-Tumor Efficacy of Elotuzumab. Blood 2014; 124:4711. [Google Scholar]
- [21].Michael Robbins MJ-K, Gennaro Dito, Pascale Andre, Hui-fen Zhang, Natalie Bezman, Robert F Graziano . Effects of IL-21, KIR Blockade, and CD137 Agonism on the Non-Clinical Activity of Elotuzumab. Blood 2014; 124:4717. [Google Scholar]
- [22].Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, Fry J, Afar DE, Singhal AK. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 2012; 120(3):552-9; https://doi.org/ 10.1182/blood-2011-06-360552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jakubowiak AJ, Benson DM, Bensinger W, Siegel DS, Zimmerman TM, Mohrbacher A, Richardson PG, Afar DE, Singhal AK, Anderson KC. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol 2012; 30(16):1960-5; https://doi.org/ 10.1200/JCO.2011.37.7069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Berdeja J, Jagannath S, Zonder J, Badros A, Kaufman JL, Manges R, Gupta M, Tendolkar A, Lynch M, Bleickardt E, et al.. Pharmacokinetics and Safety of Elotuzumab Combined With Lenalidomide and Dexamethasone in Patients With Multiple Myeloma and Various Levels of Renal Impairment: Results of a Phase Ib Study. Clin Lymphoma Myeloma Leuk 2016; 16(3):129-38; https://doi.org/ 10.1016/j.clml.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Richardson Paul G Moreau Philippe SJ, Andrzej Jakubowiak, Raab Marc S Thierry Facon, Ravi Vij, White Darrell J., Donna Reece, Lotfi Benboubker, Zonder Jeffrey A, Wei Deng, Glenn Kroog, Singhal Anil K and Sagar Lonial. A Phase 2 Study of Elotuzumab (Elo) in Combination with Lenalidomide and Low-Dose Dexamethasone (Ld) in Patients (pts) with Relapsed/Refractory Multiple Myeloma (R/R MM): Updated Results. Blood 2012; 120:202. [Google Scholar]
- [26].Richardson PG, Jagannath S, Moreau P, Jakubowiak AJ, Raab MS, Facon T, Vij R, White D, Reece DE, Benboubker L, et al.. Elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed multiple myeloma: final phase 2 results from the randomised, open-label, phase 1b-2 dose-escalation study. Lancet Haematol 2015; 2(12):e516-27; https://doi.org/ 10.1016/S2352-3026(15)00197-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jakubowiak A, Offidani M, Pégourie B, De La Rubia J, Garderet L, Laribi K, Bosi A, Marasca R, Laubach J, Mohrbacher A, et al.. Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood 2016; 127(23):2833-40; https://doi.org/ 10.1182/blood-2016-01-694604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mateos MV, Granell M, Oriol A, Martinez-Lopez J, Blade J, Hernandez MT, Martín J, Gironella M, Lynch M, Bleickardt E, et al.. Elotuzumab in combination with thalidomide and low-dose dexamethasone: a phase 2 single-arm safety study in patients with relapsed/refractory multiple myeloma. Br J Haematol 2016; 175(3):448-56. [DOI] [PubMed] [Google Scholar]
- [29].Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, Walter-Croneck A, Moreau P, Mateos MV, Magen H, et al.. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med 2015; 373(7):621-31. [DOI] [PubMed] [Google Scholar]
- [30].Sagar Lonial PGR, María-Victoria Mateos, Katja Weisel, Dimopoulos Meletios A., Philippe Moreau, Oumar Sy, Jessica Katz, Manish Gupta, Antonio Palumbo. Winship Cancer Institute, Emory University, Atlanta, GA; Dana-Farber Cancer Institute, Boston, MA; Complejo Asistencial Universitario de Salamanca-IBSAL, Salamanca, Spain; University of Tubingen, Tubingen, Germany; National and Kapodistrian University of Athens, Athens, Greece; University Hospital, Nantes, France; Bristol-Myers Squibb, Princeton, NJ; A.O.U. San Giovanni Battista di Torino - Ospedale Molinette, Torino, Italy, ELOQUENT-2 update: Phase III study of elotuzumab plus lenalidomide/dexamethasone (ELd) vs Ld in relapsed/refractory multiple myeloma (RRMM)—Identifying responders by subset analysis. J Clin Oncol 2016; 34(suppl; abstr 8037), 2016 ASCO Annual Meeting. [Google Scholar]
- [31].Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A, et al.. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med 2015; 373(13):1207-19. [DOI] [PubMed] [Google Scholar]
- [32].Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki K, et al.. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med 2016; 375(14):1319-31. [DOI] [PubMed] [Google Scholar]


