ABSTRACT
Bacillus Calmette-Guerin (BCG) is a potent agent for the prevention of tuberculosis. Current studies have regarded BCG as an immunomodulator. However, there is little information on whether it can be used to inhibit airway inflammation and airway remodeling caused by asthma. Therefore, in this study, we investigate the role of epithelial–mesenchymal transition (EMT) in airway inflammation and airway remodeling as well as the possible therapeutic mechanism of BCG for the treatment of asthma. Wistar rats were sensitized and challenged by ovalbumin for 2 weeks or 8 weeks. BCG was subcutaneously administered daily before every ovalbumin challenge to determine its therapeutic effects. The 2 weeks model group showed extensive eosinophilia, chronic inflammatory responses, bronchial wall thickening, airway epithelium damage, increased levels of transforming growth factor β 1 (TGF-β1) in both bronchoalveolar lavage fluid and sera, decreased expression of epithelial marker E-cadherin, and increased expressions of mesenchymal markers α-smooth muscle actin (α-SMA) and Fibronectin (Fn). Except for inflammatory responses, all responses were more significant in the 8 weeks model group which displayed characteristics of airway remodeling including subepithelial fibrosis, smooth muscle hypertrophy, and goblet cell hyperplasia. When compared with the model groups, BCG administration inhibited airway inflammation and airway remodeling, decreased TGF-β1 levels, upregulated expression of E-cadherin, and downregulated expression of α-SMA and Fn. The present study suggests for the first time that increased secretion of TGF- β1 induced by asthmatic chronic inflammation may result in EMT, which is one of the most important mechanisms of airway inflammation and airway remodeling seen with asthma. BCG alleviates airway inflammation and airway remodeling by preventing TGF-β1 induced EMT, therefore BCG may be a new therapy for treating asthma.
KEYWORDS: airway inflammation, airway remodeling, asthma, Bacillus Calmette-Guerin, epithelial–mesenchymal transition, transforming growth factor β 1
Introduction
Asthma is a chronic inflammatory disorder of the airways characterized by airway hyperresponsiveness, inflammation, and airway remodeling.1 The inflammatory mediators secreted by infiltrating inflammatory cells in peribronchial and perivascular areas not only induce inflammatory lesions but also contribute to the airway remodeling. Airway remodeling is characterized by subepithelial fibrosis, smooth muscle hypertrophy and hyperplasia, mucus gland hypertrophy, and increased deposition of extracellular matrix (ECM).2 This remodeling is thought to be the pathological basis for irreversible airway hyperresponsiveness and airway obstruction.3
The mechanisms responsible for the pathologic features of asthma are incompletely understood. Recent reports have shown that transforming growth factor-β1 (TGF-β1) may have an effect on asthma pathology. TGF-β1 family proteins are multifunctional cytokines that play pivotal roles in diverse biologic processes including cell growth and survival, cell and tissue differentiation, development, inflammation, immunity, and tissue remodeling and repair.4 Primarily, TGF-β1 is a master regulator of immune responses and controls the initiation and resolution of inflammatory responses. TGF-β1 does this through the regulation of chemotaxis, activation and survival of lymphocytes, natural killer cells, dendritic cells, macrophages, mast cells and granulocytes.5 TGF-β1 can inhibit the differentiation of immune cells and can also inhibit cytokine production to achieve its anti-inflammatory effect. However, TGF-β1 can also lead to the rapid accumulation of various inflammatory cells at the site of inflammation and induce differentiation of Th17 cells. These cells secrete large amounts of IL-17 and sustain acute inflammation by promoting the secretion of other inflammatory cytokines to exert its pro-inflammatory effect.6 Secondly, TGF-β1 takes a central role in airway remodeling by inducing proliferation and chemoattraction of fibroblasts. TGF-β1 also regulates fibroblast differentiation into myofibroblasts to synthesize ECM proteins fibronectin and collagen, which finally contract the ECM.6,7 Furthermore, TGF-β1 has been identified as a ‘master switch’ in the induction of epithelial–mesenchymal transition (EMT), which is considered a promoter of airway remodeling.8 EMT involves the conversion of differentiated epithelial cells into fibroblasts and myofibroblasts.6 Besides, recent studies have shown that fibroblasts play a role as initiators, modulators, and upholders of inflammation.9 These results suggest that EMT may be a possible mechanism of airway inflammation. Thus, We hypothesized that TGF-β1 induced EMT may be involved in both the process of airway inflammation and airway remodeling.
Bacillus Calmette-Guerin (BCG) has been widely used to prevent human tuberculosis and has been recognized as a potent immunomodulator for decades.10 It has also been shown to provide some protection against asthma.11 The effect of BCG on asthma has long attracted the attention of the public and scientific community. Numerous experiments have confirmed that BCG can enhance the Th1 immune response,12,13 and inhibit the production of some inflammatory mediators.14,15 However, the exact therapeutic mechanisms of BCG in asthma are largely unknown and there is little information on whether BCG can be used to inhibit asthma induced airway inflammation and airway remodeling.
Therefore, to provide new ideas and methods for asthma clinical treatment, we investigate the role of TGF-β1 induced EMT in airway inflammation and airway remodeling, and the possible therapeutic mechanism of BCG for the treatment of asthma in rats.
Results
Effect of BCG on airway inflammation
The degree of airway inflammation was evaluated by histopathological analysis of lung lesions and total cell and EOS cell counts of BALF. Compared with rats in control group, the 2 weeks model group showed significant inflammatory cell infiltration around the blood vessels and bronchi along with submucosal edema. However, the 8 weeks model group did not show any further changes in airway inflammation as compared with the 2 weeks group model. In contrast, the treatment groups (BCG or budesonide daily administered) exhibited less inflammatory cell infiltration and edema (Fig. 1A). In addition, the number of total cells and EOS cells in model groups were obviously increased compared with the control rats, but there was no significance between the 2 weeks model group and 8 weeks model group (Fig. 1B). However, after BCG or budesonide treatment, the increased cell counts were markedly decreased (Fig. 1B).
Figure 1.
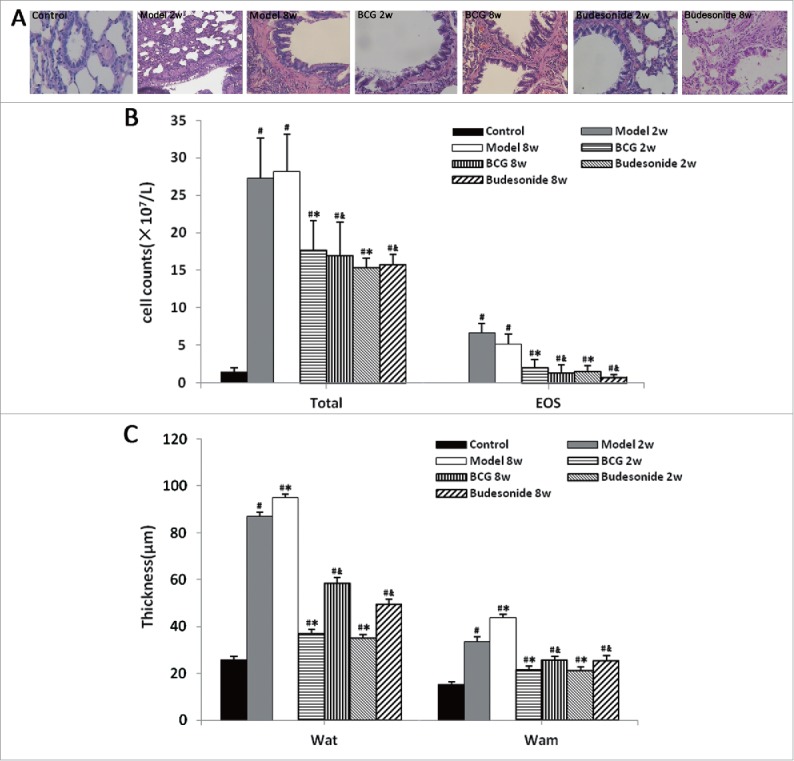
BCG attenuated airway inflammation and airway remodeling in a rat model of asthma. (A) Lung histopathologic change of the control group (Control), 2 or 8 weeks model group (model 2w or 8w), 2 or 8 weeks BCG treatment group (BCG 2w or 8w), 2 or 8 weeks budesonide treatment group (budesonide 2w or 8w), analyzed by H&E staining (magnification × 100). (B) Total and EOS cell counts in BALF of all 7 groups. (C) The thicknesses of airway wall (WAt) and airway smooth muscle (WAm) of all 7 groups. Values are expressed as mean ± SD (n = 8) Significant at: #P < 0.01 vs. control group; *P < 0.01 vs. 2 weeks model group; &P < 0.01 vs. 8 weeks model group.
Effects of BCG on airway remodeling
WAt and WAm were tested to assess features of airway remodeling. Compared with control rats, the model rats exhibited obvious increases of WAt and WAm, which could be markedly inhibited by the administration of BCG or budesonide (Fig. 1C). Despite inflammatory changes, H&E-stained lung tissues of the 2 weeks model group rats showed only slight bronchial wall thickening, airway stenosis and epithelium damage as compared with control rats. However, the 8 weeks model group showed obvious enlargement of airway smooth muscle, basement membrane thickening, epithelium damage, subepithelial fibrosis, and ECM deposition as compared with control rats. The development of airway remodeling was significantly attenuated by the administration of BCG or budesonide (Fig. 1A).
Effect of BCG on the concentrations of TGF-β1 in BALF and sera
Levels of TGF-β1 in BALF and sera were detected by ELISA because TGF-β1 plays an important role in EMT. Compared with the control group, the BALF TGF-β1 levels were markedly elevated in the 2 weeks model rats and further elevated in the 8 weeks model rats (Fig. 2). After administrating BCG or budesonide, levels of BALF TGF-β1 were significantly decreased compared with model rats. The trends observed with sera TGF-β1 were similar with the trends observed for BALF TGF-β1 (Fig. 2).
Figure 2.
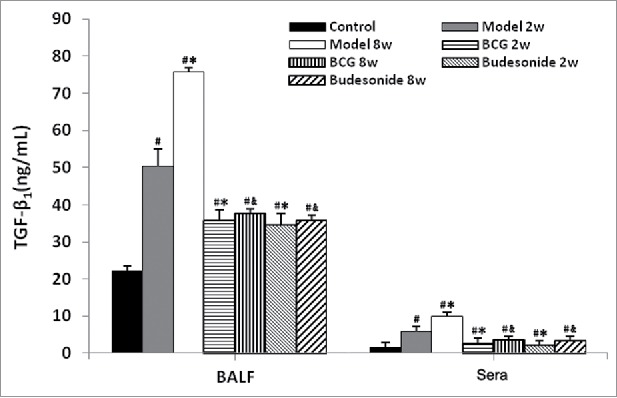
BCG decreased the levels of TGF-β1 in BALF and sera in a rat model of asthma as analyzed by ELISA. Values are expressed as mean ± SD (n = 8) Significant at: #P < 0.01 vs. control group; *P < 0.01 vs. 2 weeks model group; &P < 0.01 vs. 8 weeks model group.
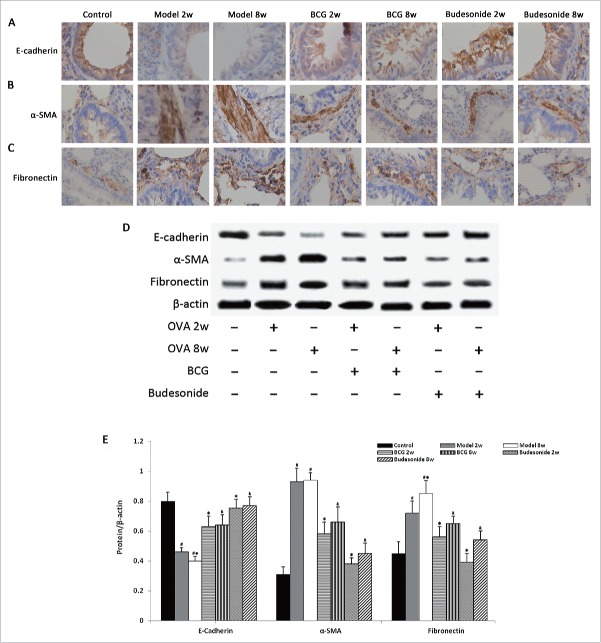
Effect of BCG on the expression of E-cadherin, α-SMA, and Fn in lung tissue
The epithelial marker E-cadherin and the mesenchymal markers Fn and α-SMA are important markers of EMT. Immunohistochemical analysis of the expression levels of E-cadherin, α-SMA, and Fn was performed. Rats challenged by OVA had decreased expression of the E-cadherin and increased expression of Fn and α-SMA. Transitions were more apparent in the 8 weeks model group than the 2 weeks model group (Fig. 3). After BCG daily administration or budesonide treatment, the transition from epithelial to mesenchymal markers was reduced significantly. Western blot data shows the same result (Fig. 3).
Figure 3.
BCG inhibits TGF-β1- induced EMT in a rat model of asthma. (A-C) Immunohistochemistry images of E-cadherin(A), α-SMA(B), and Fibronectin(C) staining in pulmonary tissue slices of all 7 groups. (D) Quantitative analysis of the western blot results of E-cadherin, α-SMA, and Fibronectin by relative densitometry intensity. Values are expressed as mean ± SD (n = 8) Significant at: #P < 0.01 vs. control group; *P < 0.01 vs. 2 weeks model group; &P < 0.01 vs. 8 weeks model group.
Discussion
Human asthma is associated with chronic inflammatory disorders accompanied by airway remodeling.1 In the present study, Wistar rats were challenged with OVA to produce airway inflammation (2 weeks model group) and airway remodeling (8 weeks model group) for imitating features of chronic asthma in humans.16 We investigated the effects of BCG on the progress of airway inflammation and airway remodeling for the first time using this model. We demonstrated that BCG treatment markedly attenuated airway inflammation and airway remodeling, reduced levels of TGF-β1 in BALF and sera, upregulated the expression of E-cadherin, and downregulated expression of α-SMA and Fn. These 3 proteins were thought to be critcal biomarkers of EMT.17
Airway inflammation is central to asthma pathophysiology. The development of airway inflammation involves a large number of inflammatory cells especially EOS cells, T lymphocytes, along with inflammatory mediators such as IL-4, IL-5, IL-9 and IL-13, that infiltrate lung tissue, gather around the bronchus, and flux into BALF.18,19 Our research found that in 2 weeks model group, rats exhibited significant inflammatory cell infiltration around the blood vessels and bronchi, submucosal edema, increased numbers of total cells and EOS cells in BALF as compared with rats in control group. However, the 8 weeks model group did not show any further changes in airway inflammation as compared with the 2 weeks group model. All these results suggested that the asthma airway inflammation model was established successfully. Daily administration of rats with BCG led to marked inhibition of inflammatory cell infiltration and inhibition of submucosal edema as compared with model groups. Elevated levels of total inflammatory cell and EOS cell counts also decreased after BCG daily administration. These findings confirm the anti-inflammatory effect of BCG on asthma.
Chronic inflammatory lesions lead to incomplete or excessive tissue repair that can result in airway remodeling,20 loss of epithelial integrity, subepithelial fibrosis, thickening of the basement membrane, increase of smooth muscle mass, and goblet cell and submucosal gland enlargement.3 Some reports using animal models have shown that prolonged allergen challenge has inhibitory effects on eosinophilic inflammation but not on airway remodeling.21,22 In the present study, inflammatory cell infiltration, bronchial wall thickening, and airway epithelium damage were observed in rats after 2 weeks of OVA exposure. With prolonged exposure, EOS cells showed a downward trend, while Wat and Wam thickening tended to increase. Meanwhile, the 8 weeks model rats showed subepithelial fibrosis, smooth muscle hypertrophy, and goblet cell hyperplasia. These results suggested that we successfully established the airway remodeling model of asthma. As expected, we found that BCG daily administration significantly reverses these airway remodeling characteristics as compared with the model rats, which suggests that BCG has an anti-remodeling effect on asthma.
TGF-β1 is one of the main mediators involved in many aspects of persistent inflammation and tissue remodeling in the asthmatic lung.6 TGF-β1 also is a powerful inducer of EMT.23 EMT has been categorized into 3 general subtypes. Type 1 EMT plays a key role in gastrulation and development, Type 2 EMT occurs during wound healing and fibrosis, and Type 3 EMT occurs in neoplastic cells that have undergone genetic and epigenetic changes, especially in genes that favor clonal outgrowth.24 TGF-β1 induced type 2 EMT involves the conversion of differentiated epithelial cells into fibroblasts and myofibroblasts,6 enables transformed epithelial cells to express α-SMA stress fibers and produces ECM through secretion of collagen and Fn, and then promotes airway remodeling.25 A recent study showed fibroblasts played a role as initiators, modulators, and upholders of inflammation.9 That indicates a pro-inflammatory role of EMT. However, there is no information of the impact of EMT on asthma airway inflammation. In our study, both the 2 weeks and 8 weeks model rats showed increased levels of TGF-β1 in BALF and sera. This was accompanied by the downregulated expression of epithelial marker E-cadherin and upregulated expression of mesenchymal markers α-SMA and Fn. These results further confirm the role of TGF-β1 induced EMT on airway remodeling and suggests that it promotes airway inflammation in asthma.
BCG as a immunomodulator, leads to a reverse of Th1/Th2 imbalance which is considered to be the classical pathogenesis model of asthma, possibly through the function of regulatory T cells (Tregs) and the cytokines it secretes such as IL-10 and TGF-β.26 Tregs and IL-10 may be related to preventing airway inflammation and lung remodeling in asthmatic mice.27,28 However, another study showed BCG vaccinations reduced metrics characteristic of allergen-induced airway remodeling, but this decrease was not associated with pulmonary Tregs.29 It is suggested that other than immune regulation, there will be another mechanism for the anti-asthma effect of BCG. Our results showed daily administration with BCG could significantly decrease levels of TGF-β1 in BALF and sera and attenuate EMT. These outcomes indicate that one of the mechanisms of BCG in prevention and treatment of asthma airway inflammation and airway remodeling may be the inhibition of TGF-β1 induced EMT.
In this study, we present novel evidence that EMT lead by increased TGF-β1 is one of the most important mechanisms of airway inflammation and airway remodeling. BCG was shown to have a therapeutic effect on asthma induced airway inflammation and airway remodeling. The therapeutic mechanism may be achieved through regulation of Th1 immunity, decreasing TGF-β1 expression, and reducing EMT. The results of this study provide an experimental basis for BCG therapy in clinical asthma treatment.
Materials and methods
Asthma model set-up
Fifty-six male Wistar rats with the age of 2–3 months and the weight of 160–180 g were provided by Shanxi Medical University Experimental Animal Center (Shanxi, China). They were housed in ventilated cages, with free access to water and food. Experiments were performed under the review and approval of the Animal Ethics Committee of Shanxi Medical University.
The rats were randomly divided into 7 groups: control, 2 weeks ovalbumin (OVA) (model, BCG, budesonide), 8 weeks OVA (model, BCG, budesonide). Except for the control group, the rats were sensitized by intraperitoneal (i.p.) injection of 10% OVA suspension containing l00 mg OVA (Sigma Corporation, USA) and 100 mg aluminum hydroxide powder (Chemical Reagent Factory, Tianjin, China) on days 1 and 8. Starting on day 15, the rats were challenged by inhaling 1% OVA daily for 2 (2 weeks group) or 8 (8 weeks group) successive weeks (20 min each time). Half an hour before each OVA exposure, the model group rats were given distilled water (0.16 ml) by i.p. injection, the budesonide group rats were given budesonide (0.5mg, AstraZeneca Company, Australia) aerosol inhalation twice a day (10 min each time), and the rats in the BCG group were subcutaneously administered with BCG (0.025mg Chengdu Biological Products Institute of Limited Liability Company, China). Control animals received normal saline instead of OVA on the same schedule with model groups.
Sera, BALF, lung tissue specimen preparation
All rats were anesthetized by i.p. injection of 25% urethane (4 ml/kg, i.p.) and killed within 24 h of the last challenge. After sacrifice, rats were ligated on one side of the bronchus and the airway of other side was lavaged thrice with 1 ml normal saline and then 80% of the input volume was recovered. The lavaged samples were then centrifuged (2000 r/min for 10min at 20℃). The supernatants were collected and stored at །80℃ for enzyme-linked immuno sorbent assay (ELISA) testing. Cell pellets were collected for total cell and eosinophil (EOS) cell counting. The non-lavaged side of the lungs was fixed in 0.1 mmol/L paraformaldehyde and then made into 4-μm-thick paraffin-embedded tissue sections for histopathological analysis, immunohistochemistry, and western blot. Moreover, approximately 5 ml of whole blood was collected by cardiac puncture. The blood samples were then centrifuged (2000r/min for 10min at 4℃). Sera were taken and stored at །80℃ for ELISA testing.
BALF cell counts
Wright stain was used to count the total cells and EOS cells in bronchoalveolar lavage fluid (BALF) with a light microscope at 400 × magnification using a hemocytometer.
Histology study of lung tissue
Paraffin-embedded tissue sections were performed hematoxylin-eosin (H&E) staining to observe the changes of airway inflammation and airway remodeling under the light microscopy (100 × magnification). On each slide, at least 10 bronchioles with 150–200 μm inner diameter were chosen. The thicknesses of airway wall (WAt) and airway smooth muscle (WAm) were determined by morphometric analysis on transverse sections using BI2000 medical image analysis system to evaluate airway remodeling.
ELISA for TGF-β1 in sera and BALF
Levels of TGF-β1 in sera and BALF were measured by ELISA-kit (Boster Biological Company, Wuhan, China), following the manufacturer's instructions. Optical density (OD) was detected by microplate reader for ELISA (Bio-Rad Laboratories, USA). TGF-β1 levels were determined by OD values.
Immunohistochemical analysis of the expression of E-cadherin, α-SMA, and Fn in lung tissues
Immunohistochemistry was performed using strept avidin-biotin complex (SABC) anti-mouse reagent (Boster Biological Company, Wuhan, China). The primary antibodies were mouse anti-rat E-cadherin, fibronectin (Fn), and α-smooth muscle actin (α-SMA) antibody (SantaCruz Company, USA). The secondary antibody was biotinylated goat anti-mouse IgG antibody (Boster Biological Company, Wuhan, China). All sections were added SABC to amplify the reaction and then stained with diaminobenzidine at room temperature. Slices were observed under a light microscope (400 ×). The positive areas were brown colors. The mean OD was then detected by BI2000 medical image analysis system in all slices.
Western blot analysis the expression of E-cadherin, α-SMA, and Fn in lung tissues
The lung tissues were homogenized, incubated in lysis buffer, added with a protease inhibitor cocktail and then centrifugally broken nucleus to obtain extracts of lung proteins. The samples were loaded for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore Company, USA), and then blocked with 5% skim milk for 2 h. The membranes were treated with the appropriate antibodies to assess the protein levels of E -cadherin, Fn, and α –SMA (SantaCruz Biotechnologies, USA) and then exposed to corresponding FITC-conjugated goat anti-mouse IgG (Boster Biological Company, Wuhan, China) for 2.5 h at room temperature. The labeled band was detected using an enhanced chemiluminescence detection kit (invitrogen company, USA). The loading control was β-actin.
Statistical methods
Data are expressed as mean ± SD The SPSS19.0 software was used for statistical analysis. Statistical comparisons between groups were evaluated by analysis of variance (ANOVA), followed by least significant difference (LSD), or Kruskal-Wallis testing, followed by Tamhane's T2 test. P values < 0.05 were considered statistically significant. #P < 0.01 vs. control group; *P < 0.01 vs. 2 weeks model group; &P < 0.01 vs. 8 weeks model group.
Abbreviations
- ANOVA
analysis of variance
- BALF
bronchoalveolar lavage fluid
- BCG
Bacillus Calmette-Guerin
- ECM
extracellular matrix
- ELISA
enzyme-linked immuno sorbent assay
- EMT
epithelial–mesenchymal transition
- EOS
eosinophil
- Fn
Fibronectin
- H&E
hematoxylin-eosin
- LSD
least significant difference
- OD
optical density
- OVA
ovalbumin
- PVDF
polyvinylidene fluoride
- SABC
strept avidin-biotin complex
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- TGF-β1
transforming growth factor β 1
- WAm
the thicknesses of airway smooth muscle
- WAt
the thicknesses of airway wall
- α-SMA
α-smooth muscle actin
Disclosure of potential conflicts of interest
No potential conflict of interest was disclosed.
Funding
This work was supported by The Natural Science Foundation of Shanxi Province of China [2013011055–1] and Foundation for Returnees of Shanxi Province of China [2011–104].
References
- [1].Killeen K, Skora E. Pathophysiology, diagnosis, and clinical assessment of asthma in the adult. Nurs Clin North Am 2013; 48(1):11–23; PMID:23465443; https://doi.org/ 10.1016/j.cnur.2012.11.001 [DOI] [PubMed] [Google Scholar]
- [2].Cho JY. Recent advances in mechanisms and treatments of airway remodeling in asthma: a message from the bench side to the clinic. Korean J Intern Med 2011; 26(4):367–83; PMID:22205837; https://doi.org/ 10.3904/kjim.2011.26.4.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gras D, Bourdin A, Chanez P, Vachier I. Airway remodeling in asthma: clinical and functional correlates. Medecine Sci 2011; 27(11):959–65; PMID:22130022; https://doi.org/ 10.1051/medsci/20112711011 [DOI] [PubMed] [Google Scholar]
- [4].Lee CM, Park JW, Cho WK, Zhou Y, Han B, Yoon PO, Chae J, Elias JA, Lee CG. Modifiers of TGF-beta1 effector function as novel therapeutic targets of pulmonary fibrosis. Korean J Intern Med 2014; 29(3):281–90; PMID:24851060; https://doi.org/ 10.3904/kjim.2014.29.3.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res 2007; 13(18 Pt 1):5262–70; PMID:17875754; https://doi.org/ 10.1158/1078-0432.CCR-07-1157 [DOI] [PubMed] [Google Scholar]
- [6].Yang YC, Zhang N, Van Crombruggen K, Hu GH, Hong SL, Bachert C. Transforming growth factor-beta1 in inflammatory airway disease: a key for understanding inflammation and remodeling. Allergy 2012; 67(10):1193–202; PMID:22913656; https://doi.org/ 10.1111/j.1398-9995.2012.02880.x [DOI] [PubMed] [Google Scholar]
- [7].Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 2010; 298(6):L715–31; PMID:20363851; https://doi.org/ 10.1152/ajplung.00361.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang ZC, Yi MJ, Ran N, Wang C, Fu P, Feng XY, Xu L, Qu ZH. Transforming growth factor-beta1 induces bronchial epithelial cells to mesenchymal transition by activating the Snail pathway and promotes airway remodeling in asthma. Mol Med Reports 2013; 8(6):1663–8; PMID:24126595; https://doi.org/ 10.3892/mmr.2013.1728 [DOI] [PubMed] [Google Scholar]
- [9].Enzerink A, Vaheri A. Fibroblast activation in vascular inflammation. J Thrombosis Haemostasis 2011; 9(4):619–26; PMID:21255248; https://doi.org/ 10.1111/j.1538-7836.2011.04209.x [DOI] [PubMed] [Google Scholar]
- [10].Freyne B, Marchant A, Curtis N. BCG-associated heterologous immunity, a historical perspective: experimental models and immunological mechanisms. Trans R Soc Trop Med Hyg 2015; 109(1):46–51; PMID:25573108; https://doi.org/ 10.1093/trstmh/tru196 [DOI] [PubMed] [Google Scholar]
- [11].Souza-Machado A, Cruz AA. BCG vaccination and reduced risk of asthma. J Bras Pneumol 2010; 36(3):275–7; PMID:20625661 [DOI] [PubMed] [Google Scholar]
- [12].Cui Y, Choi IS, Koh YA, Lin XH, Cho YB, Won YH. Effects of combined BCG and DHEA treatment in preventing the development of asthma. Immunological Invest 2008; 37(3):191–202; PMID:18389439; https://doi.org/ 10.1080/08820130801967833 [DOI] [PubMed] [Google Scholar]
- [13].Ke X, Huang J, Chen Q, Hong S, Zhu D. Protective effects of combined Mycobacterium bovis BCG and interleukin-12 vaccination on airway inflammation in a murine model of allergic asthma. Clin Invest Med 2010; 33(3):E196–202; PMID:20519099 [DOI] [PubMed] [Google Scholar]
- [14].Chen WC, Liu EM, Deng Y, He Y, Chen JH, Li X, Liu W. Impact of neonatal bacillus Calmette-Guerin vaccination on lung Th17 cells and IL-17 in murine asthma model. Zhongguo Dang Dai Er Ke Za Zhi 2010; 12(8):650–3; PMID:20704801 [PubMed] [Google Scholar]
- [15].Deng Y, Li W, Luo Y, Wang LJ, Xie XH, Luo J, Luo ZX, Zhao XD, Fu Z, Liu EM. Inhibition of IFN-gamma promotes anti-asthma effect of Mycobacterium bovis Bacillus Calmette-Guerin neonatal vaccination: a murine asthma model. Vaccine 2014; 32(18):2070–8; PMID:24560675; https://doi.org/ 10.1016/j.vaccine.2014.02.007 [DOI] [PubMed] [Google Scholar]
- [16].Palmans E, Kips JC, Pauwels RA. Prolonged allergen exposure induces structural airway changes in sensitized rats. Am J Respir Critical Care Med 2000; 161(2 Pt 1):627–35; PMID:10673209; https://doi.org/ 10.1164/ajrccm.161.2.9902094 [DOI] [PubMed] [Google Scholar]
- [17].Scanlon CS, Van Tubergen EA, Inglehart RC, D'Silva NJ. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dental Res 2013; 92(2):114–21; PMID:23128109; https://doi.org/ 10.1177/0022034512467352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chong L, Zhang W, Nie Y, Yu G, Liu L, Lin L, Wen S, Zhu L, Li C. Protective effect of curcumin on acute airway inflammation of allergic asthma in mice through Notch1-GATA3 signaling pathway. Inflammation 2014; 37(5):1476–85; PMID:24706026; https://doi.org/ 10.1007/s10753-014-9873-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pelaia G, Vatrella A, Busceti MT, Gallelli L, Calabrese C, Terracciano R, Maselli R. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. 2015; 2015:879783; PMID:25878402; https://doi.org/ 10.1155/2015/879783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol 2008; 121(3):560–70; quiz 71-2; PMID:18328887; https://doi.org/ 10.1016/j.jaci.2008.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fraga-Iriso R, Nunez-Naveira L, Brienza NS, Centeno-Cortes A, Lopez-Pelaez E, Verea H, Ramos-Barbon D. Development of a murine model of airway inflammation and remodeling in experimental asthma. Archivos De Bronconeumologia 2009; 45(9):422–8; PMID:19464098; https://doi.org/ 10.1016/j.arbres.2009.01.014 [DOI] [PubMed] [Google Scholar]
- [22].Sakai K, Yokoyama A, Kohno N, Hamada H, Hiwada K. Prolonged antigen exposure ameliorates airway inflammation but not remodeling in a mouse model of bronchial asthma. Int Archives Allergy Immunol 2001; 126(2):126–34; PMID:11729350 [DOI] [PubMed] [Google Scholar]
- [23].Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax 2014; 69(8):760–5; PMID:24334519; https://doi.org/ 10.1136/thoraxjnl-2013-204608 [DOI] [PubMed] [Google Scholar]
- [24].Natarajan J, Chandrashekar C, Radhakrishnan R. Critical biomarkers of epithelial-mesenchymal transition in the head and neck cancers. J Cancer Res Therapeutics 2014; 10(3):512–8; PMID:25313730; https://doi.org/ 10.4103/0973-1482.137926 [DOI] [PubMed] [Google Scholar]
- [25].Ijaz T, Pazdrak K, Kalita M, Konig R, Choudhary S, Tian B, Boldogh I, Brasier AR. Systems biology approaches to understanding Epithelial Mesenchymal Transition (EMT) in mucosal remodeling and signaling in asthma. World Allergy Organization J 2014; 7(1):13; PMID:24982697; https://doi.org/ 10.1186/1939-4551-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang GS, Wang PL, Huang HQ, Shen HH. New insights into the effects of Mycobacterium bovis Bacillus Calmette-Guerin on asthma. Chinese Medical J 2009; 122(5):577–83; PMID:19323911 [PubMed] [Google Scholar]
- [27].Samary Cdos S, Antunes MA, Silva JD, Silva AL, Araujo CC, Bakker-Abreu I, Diaz BL, Fernezlian S, Parra ER, Capelozzi VL, et al. Impact of Bacillus Calmette-Guerin Moreau vaccine on lung remodeling in experimental asthma. Respir Physiol Neurobiol 2013; 189(3):614–23; PMID:23928268; https://doi.org/ 10.1016/j.resp.2013.07.025 [DOI] [PubMed] [Google Scholar]
- [28].Xia Y, Zhang JH, Ji ZH, Li XD, Yu ZW, Liu HY. Effect of bacillus calmette-guerin treatment on airway inflammation and T regulatory cells in mice with asthma. Zhongguo Dang Dai Er Ke Za Zhi 2006; 8(5):413–6; PMID:17052404 [PubMed] [Google Scholar]
- [29].Wang P, Zhang G, Qin X, Qiu Z, Li N, Chen Z, Li W, Shen H. Inhibition of allergen-induced airway remodeling by neonatal bacillus Calmette-Guerin vaccination is associated with interferon-gamma-producing T cells but not regulatory T cells in mice. Ann Allergy Asthma Immunol 2011; 107(2):163–70; PMID:17052404 [DOI] [PubMed] [Google Scholar]