Abstract
Ectokinases can phosphorylate extracellular proteins and external domains of membrane proteins influencing cell adhesion, movement, and cellular interactions. An ectokinase with the properties of casein kinase 2 (CK2) has been previously described, but little is known about the structural characteristics that allow this enzyme to be exported from the cell. Transfection of human embryonic kidney-293 cells with cDNAs coding for the catalytic (CK2α or CK2α′) and regulatory (CK2β) subunits with hemaglutinin tags allowed us to study the export of ectopically synthesized enzyme. When the catalytic (CK2α or CK2α′) and the CK2β regulatory subunits are cotransfected, the tetrameric enzyme composed of both subunits (holoenzyme) is detected outside the cell. This observation has been confirmed by assaying protein kinase activity in immunoprecipitates obtained with antihemaglutinin antibody by using a CK2-specific peptide substrate and by Western blots as well as by immunofluorescence of nonpermeabilized cells. Transfection with cDNA of catalytic or regulatory subunit alone does not result in export of these subunits. A study of the kinetics of appearance of the ectopically synthesized protein at different times after transfection indicates that a 5- to 7-h delay after the synthesis of the protein before it appears in the extracellular compartment. Using mutations of CK2α that eliminate phosphorylating activity [CK2α(Asp-156-Ala)] or that make it less sensitive to heparin inhibition [CK2α(Lys-75-Glu,Lys-76-Glu)] demonstrated that these mutations do not prevent the holoenzyme to be exported from the cells.
Keywords: ectokinase, protein phosphorylation
There are a number of reports that protein kinases are present on the external side of the cellular membrane and that these ectokinases are responsible for phosphorylating proteins of the extracellular matrix and extracellular domains of proteins that are attached to cells (1). Specifically, there are several reports that an ectokinase has the characteristics of protein kinase casein kinase 2 (CK2) (2-4). A CK2-like ectokinase activity has been reported to be responsible for the phosphorylation of vitronectin, an extracellular matrix protein (5, 6), and of the external domain of the β-amyloid precursor protein (7) and T lymphocyte surface proteins (8). However, there is no information about the structural features that are responsible for the export of CK2 or other ectokinases.
Protein kinase CK2 is ubiquitous in eukaryotes and is responsible for the phosphorylation of hundreds of proteins (9-11). There is strong evidence that CK2 is involved in the control of cell proliferation, apoptosis, and circadian rhythms (12-14). In addition, CK2 may play a role in controlling the activity of a number of protein kinases through a positive feedback loop with the Cdc37 protein that acts as a chaperone that activates these kinases (15, 16).
The CK2 holoenzyme is a heterotetramer composed of catalytic subunits (α and α′) and regulatory β subunits conforming α2β2, αα′β2 and α′2β2 combinations. Neither the α, α′, or β subunits contain sequences with the characteristics of signal peptides known to tag proteins that are secreted, although the α subunit of Theileria parva may be an exception (17).
Here, we have analyzed the distribution of CK2 holoenzyme and individual CK2α, α′, and β subunits produced ectopically after transfection of human embryonic kidney (HEK)-293 T cells with cDNAs coding for these proteins fused to hemagglutinin (HA) tags. The results obtained demonstrate that ≈3-4% of the holoenzyme ectopically expressed in these cells is in the extracellular compartment. When the individual catalytic or regulatory subunits are expressed, however, these subunits are not detectable externally. Experiments with mutants indicate that the catalytic activity of the enzyme is not necessary for export and that a heparin-resistant mutant can also be exported.
Materials and Methods
Transfection of HEK-293 T Cells. HEK-293 T cells were cultured in DMEM supplemented with 10% FBS/100 units/ml penicillin/100 μg/ml streptomycin (Invitrogen) at 37°C with 5% CO2. Subconfluent HEK-293 T cells were transfected (or cotransfected) with cDNAs coding for CK2 subunits of Xenopus laevis (18) (CK2α and/or CK2β) or CK2α′ of Danio rerio (19) cloned in pCEFL-HA, which generates expressed proteins with a HA epitope tag. Before transfection, the complete medium was withdrawn and replaced with transfection medium (DMEM without FBS and antibiotics). The vector constructs (2 μg of DNA) and Lipofectamine (Invitrogen) were diluted in transfection medium, and transfection was carried out for 3 h. Transfection medium was replaced with complete medium, and the transfected cells were incubated for 20 h.
Isolation of Ectopically Expressed CK2 Subunits. The method of Kübler et al. (2) was used for the release of CK2 ectokinase from transfected cells by substrate-induced “shedding” of the enzyme. Briefly, subconfluent monolayer cell cultures in p60 plates (Falcon) were washed twice with buffer P (70 mM NaCl/30 mM Tris·acetate, pH 7.2/5 mM magnesium acetate/5 mM K2HPO4/0.5 mM EDTA/75 mM glucose) prewarmed at 37°C. Cells were then incubated with 1 mg/ml phosvitin (Sigma) in 1.2 ml of buffer P per plate and with 2 mM Na3VO4 (Applichem, Darmstadt, Germany). The cells were incubated with phosvitin for 15 min at 37°C with mild shaking (shedding process). Subsequently, the extracellular liquid containing the ectoCK2 was aspirated and centrifuged at 2,000 × g at 5°C for 5 min, and the supernatant fluid was then used for immunoprecipitation.
Preparation of Cell Lysates for Immunoprecipitation. After the shedding, HEK-293 T cells were lysed to assay for ectopic CK2 levels inside the cells (20). Briefly, cells were treated with ice-cold lysis solution containing PBS with 0.1% Nonidet P-40 (Sigma) and 10 μl/ml protease inhibitor mixture (Calbiochem) for 30 min at 4°C (350 μl of lysis solution per p60 plate). The resulting lysate was centrifuged at 14,000 × g for 15 min in the cold, and the supernatant was kept on ice for immunoprecipitation. Protein concentration of lysates was determined by the Bradford method (21).
Immunoprecipitation. Ha epitope-tagged proteins in cell lysates (350 μl) or in the extracellular fluid obtained by the shedding procedure (1.2 ml) were incubated with 3 μg of monoclonal anti-Ha (F-7) antibody (Santa Cruz Biotechnology) overnight at 4°C, then with 30 μl of protein A Agarose (Sigma) for 4-5 h at 4°C. After centrifugation at 14,000 × g for 5 min, the precipitates from the lysate and extracellular fluid were washed four times in buffer P, and the wash liquids were discarded.
Assays for CK2 Activity in Immunoprecipitates. For CK2 assay, immunoprecipitates were resuspended in 60 μl of kinase buffer (50 mM Hepes, pH 7.5/10 mM MgCl2/0.5 mM DTT), of which 5 μl was used for assay. Assays contained in a total volume of 30 μl included 10 mM MgCl2, 100 mM NaCl, 200 μM of the CK2 specific substrate peptide (RRADDSDDDD) (Calbiochem), and [γ-32P]ATP. In the case of the lysates, the specific activity of the ATP was 700 cpm/pmol, and the concentration was 100 μM. In the case of the extracellular liquid, specific activity was 3,000 cpm/pmol, and the concentration 20 μM. Incubations were for 20 min (lysates) and 30 min (extracellular fluid) at 37°C. The reactions were processed according to the standard assay described in ref. 18.
Control of Cell Lysis During the Shedding Procedure. The possibility of cell lysis during the shedding procedure was controlled by (i) checking for the presence of α-actin in the extracellular fluid by Western Blotting using anti-α-actin antibody (Calbiochem) or (ii) assaying for activity of the cytosolic enzyme lactate dehydrogenase. The results for the Western Blot were negative. The results for the cytosolic enzyme assay, which indicate that incubation with phosvitin did not cause any measurable increase in the very low levels of activity of the dehydrogenase, agree with those reported by Kübler et al. (2).
Western Blots. The immunoprecipitated epitope-tagged proteins from the cell lysate and extracellular liquid were dissolved in an equal volume of Laemmli denaturing buffer containing 2-mercaptoethanol and 6 M urea and heated at 100°C for 5 min, followed by 10% SDS/PAGE. Proteins were transfered to Immobilon transfer membranes (Millipore) and probed by using rabbit polyclonal anti-HA antibody (Upstate Biotechnology, Lake Placid, NY).
Indirect Immunofluorescence Microscopy of Nonpermeabilized Cells. Before transfection, cells were grown on cover slips treated with poly-l-Lys (Sigma). Twenty-four hours after transfection, cells were washed twice with ice-cold PBS and mildly fixed for 5 min at 4°C with 0.37% p-formaldehyde in PBS. This mild fixation procedure resulted in cells that were partially or totally impermeable to macromolecules or charged ionic molecules. The cells were then incubated with PBS containing 1 μg/ml propidium iodide and 1 mg/ml ribonuclease A (Sigma) for 5 min at room temperature. Subsequently, cells were blocked in PBS with 4% FBS for 30 min at room temperature. Immunofluorescence was performed by incubating the cells with the mouse monoclonal anti-HA (F-7) antibody (1:100 in PBS) for 2-3 h at 37°C in a wet chamber. Then the cover slips were washed in PBS by passing them quickly 10 times through the interphase air-liquid. Staining for the primary antibody was done with FITC-conjugated rabbit anti-mouse IgG antibody (1:50 in PBS) (DAKO) for 2-3 h at 37°C in a wet chamber. Finally cover slips were washed again as described before. The images were taken on a Zeiss Axiovert 100 confocal laser microscope.
Results
HEK-293 cells in culture were transfected with the pCEFL-HA vector containing cDNAs for the CK2 subunits from X. laevis or CK2α′ from D. rerio. Twenty-four hours after transfection, the washed cells are treated with phosvitin, a CK2 substrate, to cause shedding of the ectokinase as described in Materials and Methods (3). The extracellular liquid and lysate of these cells were immunoprecipitated with an anti-HA antibody, and the phosphorylating activity present in the immunoprecipitates was measured with a specific CK2 peptide substrate. This approach limits the assay to measure only the ectopically expressed tagged enzyme, eliminating the detection of the endogeous activity.
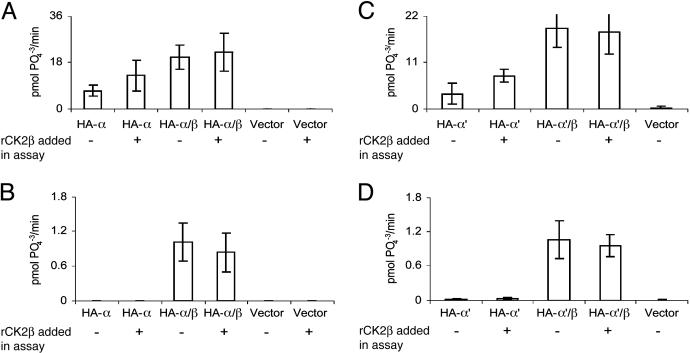
Expression of Recombinant CK2 in HEK Lysate and Extracellular Liquid. Fig. 1 shows the results obtained with cells transfected with vectors coding for CK2α or CK2α′ and of cells cotransfected with the catalytic (CK2α or CK2α′) and CK2β subunits. As a control, activity was measured in cells transfected with empty vector. The results obtained in immunoprecipitates of the lysate of cells transfected with CK2α and cotransfected with CK2α and CK2β are shown in Fig. 1 A. As expected, the transfections of CK2 subunits generate significant ectopic CK2 kinase activity in the lysates, whereas empty vector gives negligible activity. In lysates from cells transfected only with CK2α, phosphorylating activity increased significantly when recombinant CK2β was added to the assay, indicating that the lysate contained a significant percentage of free CK2α that could be stimulated by interacting with the added CK2β. As evidenced in previous studies (22), when the cells are transfected with CK2α and CK2β, the addition of free CK2β in the assay system did not significantly alter CK2 activity.
Fig. 1.
CK2 activity in immunoprecipitates of the ectopically expressed enzyme present in lysates and in the extracellular liquid. Immunoprecipitates were obtained with anti-HA monoclonal antibody after treatment of cell lysates and of the extracellular fluid as described in Materials and Methods. The assay of CK2 phosphorylating activity was also performed as described in Materials and Methods by using a CK2-specific peptide substrate. Where indicated, recombinant X. laevis CK2β (1 pmol) (rCK2β) was added to the assay mixture. (A and B) The results obtained with cells transfected with X. laevis CK2α, CK2α and CK2β, or empty vector. (A) The activity of lysates. (B) The activity of extracellular liquid. (C and D) Results are presented with cells transfected with D. rerio CK2α′, D. rerio CK2α′ and X. laevis CK2β, or empty vector. (C) The activity of lysates. (D) The activity extracellular liquid. The results presented were generated by duplicate experiments for each group of transfected cells. The bars indicate maximum and minimum values.
Fig. 1B shows the activity measured in immunoprecipitates of the extracellular liquid after phosvitin treatment from the same group of cells described for Fig. 1 A. CK2 activity can be detected only with cells cotransfected with catalytic and regulatory subunits. The activity detected amounts of 3-4% of the activity present in the lysate of cells transfected with CK2α and CK2β genes and is measured through the use of high-specific-activity [γ-32P]ATP. Exogenous CK2β subunit did not stimulate the activity detected outside the cells, indicating that there are no free CK2α subunits and that these subunits are conforming the holoenzyme tetramer or higher aggregates of CK2 (23). The empty vector again gives zero activity of CK2. The absence of activity in the external fluid of cells transfected with only CK2α, which had abundant activity in lysates, strongly supports the premise that the external activity found in the cells transfected with the holoenzyme is not due to cell lysis.
Very similar results were obtained when transfection was performed with CK2α′ from D. rerio (19) and CK2β from X. laevis (18) (Fig. 1 C and D). When transfections were carried out with CK2α′ alone, activity was seen only in the lysate, whereas the HA-α′2β2 tetramer was exported out of the cells. This level is again ≈3% of that of the lysate.
Because CK2β has no catalytic activity, this method cannot be directly used to assay the export of free CK2β. However, it is possible to test whether the presence of free CK2β can stimulate exogenous recombinant CK2α added in the assay. These experiments showed that the immunoprecipitate of the lysate of cells transfected only with CK2β did stimulate almost 3-fold the catalytic activity of added recombinant CK2α, indicating the presence of free β subunit in the lysates. The immunoprecipitate of the extracellular liquid of these same cells, however, did not contain free CK2β because it did not significantly stimulate the activity of external CK2α (not shown).
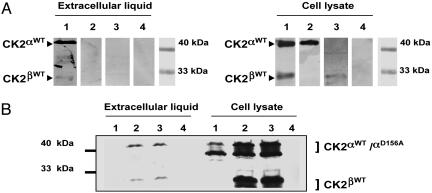
Detection of Extracellular Subunits by Western Blots. The identification of the subunits of CK2 transfected in the HEK-293 T cells in the extracellular liquid was also achieved by immunoprecipitation and Western blot analysis using monoclonal and polyclonal anti-HA antibodies (Fig. 2A). It is evident that only in the case of cells cotransfected with both subunits of CK2 it is possible to detect the presence of these subunits in the extracellular medium. As seen above, although there is strong expression of the subunits in the lysates of cells transfected with the single CK2α or CK2β subunits, these subunits were not detectable in the extracellular liquid.
Fig. 2.
Detection by Western blots of ectopically expressed CK2 subunits after transfection of HEK-293 T cells. Immunoprecipitates using anti-HA antibody were obtained from lysates and extracellular fluid as described in Materials and Methods. The immunoprecipitated proteins were resolved by SDS/PAGE and revealed with the anti-HA polyclonal antibody. (A) The results obtained in the extracellular fluid and in lysates when cells were cotransfected with X. laevis CK2α and CK2β (lane 1), only with CK2α (lane 2), only with CK2β (lane 3), or with the empty pCEFF-HA vector (lane 4). The position of molecular mass markers is also indicated. (B) The results for similar experiments in which wild-type and mutant subunits were transfected into HEK-293 T cells. Transfections were with CK2α(Asp-156-Ala) (lane 1) (24), CK2α(Asp-156-Ala) and CK2β (lane 2), CK2α wild type and CK2β (lane 3), or empty vector (lane 4). Western blots for extracellular fluid and lysates are presented. The positions of molecular mass markers and the expected migration of CK2α and CK2β and degradation products are indicated by brackets.
The question of whether a catalytically inactive CK2 could be exported out of the cells was also tested by using this technique. In Fig. 2B, cells were cotransfected with CK2β and either the CK2α wild type or an inactive catalytic subunit [HA-CK2α(Asp-156-Ala)] in which the catalytic Asp-156 has been changed to Ala (24). The Western blot shows that, indeed, this inactive catalytic subunit can be found in the extracellular liquid when it is cotransfected with CK2β. It is interesting that the Western blots of the enzyme present in the extracellular liquid do not show the extensive degradation seen in the lysates of these cells. This observation might indicate that only the intact holoenzyme subunits can be exported or that the external enzyme is less susceptible to proteolysis.
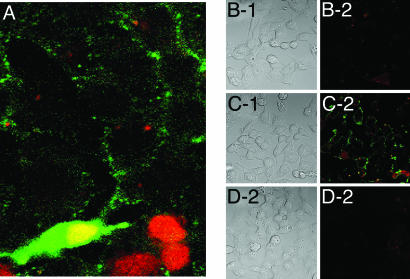
Immunofluorescence of Nonpermeabilized Cells Transfected with CK2. Immunofluorescence has been used as a third method to look at the export of CK2 holoenzyme from transfected cells. When transfected cells were fixed under mild conditions (see Materials and Methods), the cells were partially or totally impermeable to external macromolecules or charged compounds. Permeabilization or the lack thereof was tested by the capacity of cells to show red nuclear staining with propidium iodide. With cells that were not permeable, the antibodies against the HA tag should be able to detect only the external enzyme that has been exported and is bound to the transfected cells. Fig. 3A shows a patch of cells fixed under mild conditions and treated with a green fluorescent chromophore to detect the anti-HA antibody and with propidium iodide to stain red the nucleus of permeabilized cells. At the bottom of Fig. 3A, there are some cells that were permeabilized as shown by the red nuclear staining. One of these permeabilized cells was transfected with both CK2 subunits and is stained green in cytoplasm and nucleus, which appears yellow as a result of overlapping green and red colors. The upper portion of Fig. 3A features cells that are not permeabilized but that show green staining at the cell border, pointing to the presence of extracellular enzyme. Figs. 3 B-1 and B-2 show photographs of phase contrast and immunofluorescence of cells that were not permeabilized and that had been transfected with CK2α. Figs. 3 C-1 and C-2 show similar pictures of nonpermeabilized cells that had been transfected with CK2α and CK2β. Figs. 3 D-1 and D-2 show the results obtained with cells transfected with the empty vector. It can be observed that only the cells transfected with both subunits (Fig. 3C-2) have significant labeling in the cell membrane. This labeling seems to concentrate in distinct foci in the membrane. Cells transfected only with CK2α (Fig. 3B-2) are similar to cells transfected with the empty vector (Fig. 3D-2), showing no labeling by immunofluorescence. A similar negative result was obtained with cells transfected with only CK2β (data not shown). As a control, batches of these transfections were permeabilized and treated for immunofluorescence. This procedure demonstrated that ≈40% of the cells were expressing the ectopic single subunits or the holoenzyme in their interior (data not shown). This third method therefore confirms the need for the formation of the holoenzyme for export to take place.
Fig. 3.
Immunofluorescence of cells that are not permeabilized or are partially permeabilized. HEK-293 T cells that had been transfected with CK2 subunits were fixed under mild conditions that result in partial permeabilization and no permeabilization of the cells. Cells were treated with propidium iodide (1 μg/ml) to stain the nuclei of permeabilized cells and with anti-HA antibody to detect the expression of the tagged CK2 subunits (see Materials and Methods). (A) Cells cotransfected with CK2α and CK2β. These cells were fixed under mild conditions; thus, only some are permeabilized. (B-1 and B-2) Nonpermeabilized cells transfected with CK2α only. (B-1) Phase contrast. (B-2) Immunofluorescence. (C-1 and C-2) Cells cotransfected with CK2α and CK2β. (C-1) Phase contract. (C-2) Immunofluorescence. (D-1 and D-2) Cells transfected with the empty vector. (D-1) Phase contrast. (D-2) Immunofluorescence.
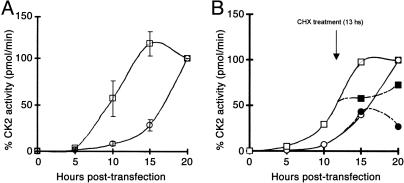
Time Course of the Export of CK2 Holoenzyme. Another question we address relates to the kinetics of the export of CK2. Fig. 4 shows the CK2 activity, measured by using specific peptide substrate, which appears in the anti-HA immunoprecipitates at specific times after transfection. In Fig. 4A, active CK2 appears in the cell lysate after a latency of ≈5 h and is maximal at 15 h. In the extracellular liquid, the activity appears after ≈12 h. The activities of both lysate and extracellular liquid after 20 h are taken arbitrarily as a 100% value. The absolute values at this time corresponded to 18 pmol of 32P incorporated per min for lysate and 0.8 pmol of 32P incorporated per min for extracellular liquid. To test whether de novo protein synthesis was required for the export process, 10 μg/ml cycloheximide was added to the culture medium of the transfected cells 13 h after transfection, and the activity was measured in the lysate and extracellular liquid at 15 and 20 h. The results obtained (Fig. 4B) indicate that cycloheximide caused a total inhibition in the increment of activity appearing in immunoprecipitates of the cell lysate at 15 and 20 h, showing that it rapidly blocked new protein synthesis. However, a similar cycloheximide treatment did not affect the increment of activity detected in the extracellular fluid at 15 h after transfection, but at 20 h after transfection, the effect of the inhibitor is evident in the ectokinase measurements.
Fig. 4.
Time course of the appearance of ectopic CK2 activity in lysates and extracellular fluid after transfection. HEK-293 T cells were cotransfected with CK2α and CK2β at time 0. The CK2 activity of immunoprecipitates obtained by using anti-HA antibody of lysates and extracellular liquid was measured as detailed in Materials and Methods by using a specific CK2 peptide substrate. Samples were collected at the times indicated. The activity value obtained at 20 h in the lysate and extracellular liquid was set arbitrarily at 100% activity. (A) The relative activity values obtained in the lysate (□) and the relative activity values obtained in the extracellular fluid (○). The bars indicate the maximum and minimum values obtained in two separate experiments. (B)An experiment similar to the one shown in A, except that at 13 h after transfection a set of cells was treated with cycloheximide (CHX) at a concentration of 10 μg/ml. □, CK2 activity measured in the lysate of untreated cells; ▪, CK2 activity in the lysate of cells treated with cycloheximide at 13 h; ○, CK2 activity in the extracellular liquid of untreated cells; •, CK2 activity in the extracellular liquid of cells treated with cycloheximide at 13 h.
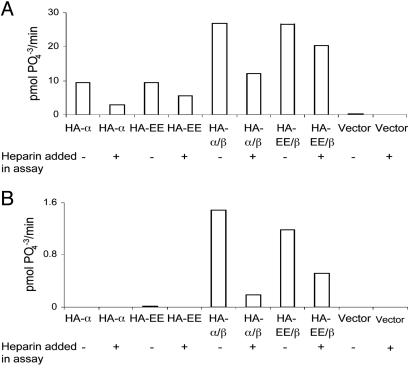
Export of Holoenzyme Integrated by a CK2α Mutant Resistant to Heparin Inhibition. Because heparin and its derivatives are potent inhibitors of CK2 phosphorylating activity (9), it has been claimed that extracellular heparin and heparan sulfate residues attached to extracellular proteins might regulate the ectokinase activity of CK2 (4). We tested therefore whether mutants of CK2α that are less sensitive to heparin could also be exported as part of the holoenzyme-ectokinase. Such a mutant with Lys-75 and Lys-76 changed to Glu [CK2α(Lys-75-Glu,Lys-76-Glu)] (25) was used for this purpose. Fig. 5 shows the result of an experiment similar to that shown in Fig. 1, in which the CK2 activity is measured in immunoprecipitates of the extracellular liquid and lysates of cells transfected with wild-type CK2α and CK2α(Lys-75-Glu,Lys-76-Glu), with or without cotransfection with CK2β.
Fig. 5.
A CK2 ectokinase resistant to heparin as a result of transfection of a mutant of CK2α. This experiment was performed as shown in Fig. 1. The CK2 activity of ectopically expressed CK2 subunits is assayed in the lysates (A) and extracellular liquid (B) of cells transfected with HA-tagged subunits. In this experiment, transfection of the mutant CK2α(Lys-75-Glu,Lys-76-Glu) (shown as HA-EE), which is resistant in vitro to heparin inhibition (25), was included either alone or in combination with CK2β. The activity was measured in the presence of heparin (1 μg/ml) or in its absence as indicated.
As observed previously, cells transfected with only the CK2α subunit, either wild-type or mutant, express the kinase activity in lysates but not in the extracellular liquid. Assays of the mutant show that CK2α(Lys-75-Glu,Lys-76-Glu) is clearly more resistant to heparin inhibition. When cotransfected with CK2β, the mutant CK2α(Lys-75-Glu,Lys-76-Glu) subunit generates a holoenzyme with activity in the lysate and in the extracellular fluid, both of which are less sensitive to heparin than the wild type.
Discussion
Abundant previous evidence demonstrated the presence of ectokinase activity in the external side of cells. There are also numerous indications that this activity has a physiological role in cell-cell interactions, cell movement, and in cell attachment to the extracellular matrix (1).
There have been a number of carefully conducted studies that indicate that one of the ectokinases has the characteristics of protein kinase CK2 (2-4). Furthermore, it has been demonstrated that this CK2-like ectokinase enzyme phosphorylates a number of external proteins or the extracellular domains of membrane proteins (5-8). However, there is a dearth of evidence concerning the mechanisms through which the CK2-like enzyme or other ectokinases are exported and about the structural features that tag these proteins for export. Another intriguing question is how CK2 is attached to the external membrane of cells. Relevant to this question is the finding that exposure of cells to a CK2 protein substrate, such as casein or phosvitin, causes the release of the CK2-like ectokinase (3). This observation suggests that the attachment of CK2-like enzyme to the external surface of the membrane might involve the protein substrate recognition domain of the enzyme. Competition for this site by the presence of an external substrate would cause the shedding of the enzyme. It cannot be discarded that substrate induces a conformational change in the membrane-bound CK2 and thus triggers release. Shedding of CK2 activity bound to the extracellular membrane is not a specific effect of phosvitin and casein, which are not physiological substrates. Shedding has also been observed with the physiological substrate vitronectin, whose external phosphorylation is increased when cells are transfected with CK2α and CK2β subunits (F.R., unpublished results). An alternative explanation for the attachment of CK2 to membrane might be that CK2 is bound to heparin or to heparan sulfate residues present in external membrane proteins. CK2 is known to have a high affinity for heparin (9).
The work presented above constitutes an approach to learn more about the mechanism and the structural requirements for the export of protein kinase CK2 by studying the localization of this enzyme after its ectopic expression in human cells that have been transfected with cDNAs coding for the X. laevis catalytic and regulatory subunits of CK2 (18) and with the D. rerio CK2α′ subunit (19).
By using three different methods that rely on the measurement of enzyme activity, immunodetection by Western blots, and immunofluorescence of nonpermeabilized cells, it has been possible to establish that ectopically expressed holoenzyme composed of catalytic (α or α′) and regulatory (β) subunits can be exported from the HEK-293 T cells. The free catalytic or regulatory subunits are not exported to a detectable degree. From activity measurements, it has been possible to determine that ≈3-4% of the ectopically expressed enzyme present in the cell lysates is exported to the extracellular compartment. These experiments allow us to state that bona fide CK2 is an ectokinase. There is no need any more to refer to it as a CK2-like ectoenzyme.
These experiments required careful controls to ascertain that the small fraction of the enzyme activity detected externally is not due to cell lysis. There are a number of facts that make the lysis explanation highly unlikely. For instance, under the same conditions used to detect CK2 subunits by Western blots, actin, a highly abundant cellular protein, could not be detected in the extracellular fluid by an actin-specific antibody. Also measurements of lactate dehydrogenase activity in the extracellular liquid to detect cell lysis did not increase when the cells were incubated with phosvitin, a treatment that leads to an important increase in CK2 activity through the shedding phenomenon (2). The results presented here that demonstrated very significant activity inside the cells transfected with the catalytic subunits HA-CK2α, HA-CK2α′, or HA-CK2α(Lys-75-Glu,Lys-76-Glu) but the absence of detectable activity in the external liquid of the same cells constitutes an additional confirmation that ectokinase activity cannot be explained by cell lysis.
When the appearance of ectopically expressed tagged enzyme was studied as a function of time after transfection, an interesting result was obtained. The detection of the newly synthesized enzyme inside the cell followed the expected kinetics, appearing 5 h after transfection and peaking at ≈15 h. However, there was a considerable delay in detection of the ectokinase activity, which only appeared in the external liquid after 10-12 h after transfection, reaching a maximum at 20 h. This finding would suggest that the ectokinase undergoes an export process that takes 5-7 h. This transit time and the intracellular location of the ectokinase undergoing the export process need to be studied further.
The effect observed with cycloheximide on the kinetics of appearance of the ectokinase demonstrated that the drug rapidly and completely stopped protein synthesis and further expression of CK2 inside the cell but did not stop the export process of the ectokinase until several hours after the treatment. This observation indicates that the export process does not require de novo protein synthesis and that it involves some sort of compartmentation of the CK2 that will emerge later as an ectokinase.
Some mutants of the CK2α subunit have been tested for their capacity to be exported. CK2α(Asp-156-Ala) is a catalytically inactive mutant that has a key Asp residue changed to Ala (24). By using Western blots, it was observed that the inactive holoenzyme containing this mutant subunit was exported into the extracellular medium as efficiently as the wild-type holoenzyme. Enzyme activity, therefore, is not required for the export process.
A second mutant, CK2α(Lys-75-Glu,Lys-76-Glu), which was described by our laboratory several years ago (25), has Lys-75 and Lys-76 changed to Glu. This mutation affects a basic region of CK2α that is important for binding heparin and other polyanion inhibitors, making it more resistant to heparin inhibition. This feature is interesting because it has been demonstrated that extracellular heparin and heparan sulfates present on external domains of proteins can inhibit the phosphorylation of the β-amyloid peptide by the CK2 ectokinase activity (4). It has been possible to demonstrate that the CK2α(Lys-75-Glu,Lys-76-Glu) mutant can be exported as part of a holoenzyme and that the resulting ectokinase activity is less sensitive to heparin inhibition. The heparin-resistant mutant protein was recovered after shedding the transfected cells with phosvitin to a similar degree as the wild-type enzyme (data not shown). This finding argues against the idea that ectoCK2 might be bound to the extracellular membrane through interaction with heparin or heparan sulfate residues because it may be surmised that the mutant would have a weaker binding of these moieties.
Understanding the physiological importance of ectokinases may be enhanced by further studies using recombinant ectopically expressed proteins modified to define the specific pathway for export and the structural requirements of this process.
Acknowledgments
We thank Dr. Jorge Sans for advice in the immunofluorescence studies and Dr. Enrique Brandan for helpful discussions. This work is part of the doctoral thesis of F.R., who is the holder of a doctoral fellowship from the Comisión Nacional de Investigación Científica y Tecnológica. This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico Grant 1030462 (to J.E.A.) and by the Wellcome Trust.
Author contributions: J.E.A. designed research; F.R. performed research; C.C.A. analyzed data; and C.C.A. and J.E.A. wrote the paper.
Abbreviations: CK2, casein kinase 2; HA, hemagglutinin; HEK, human embryonic kidney.
References
- 1.Redegeld, F. A., Caldwell, C. C. & Sitkovsky, M. V. (1999) Trends Pharmacol. Sci. 20, 453-459. [DOI] [PubMed] [Google Scholar]
- 2.Kübler, D., Pyerin, W., Burow, E. & Kinzel, V. (1983) Proc. Natl. Acad. Sci. USA 80, 4021-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter, J., Schnölzer, M., Pyerin, W., Kinzel, V. & Kübler, D. (1996) J. Biol. Chem. 271, 111-119. [DOI] [PubMed] [Google Scholar]
- 4.Walter, J., Schindzielorz, A., Hartung, B. & Haass, C. (2000) J. Biol. Chem. 275, 23523-23529. [DOI] [PubMed] [Google Scholar]
- 5.Seger, D., Gechtman, Z. & Shaltiel, S. (1998) J. Biol. Chem. 273, 24805-24813. [DOI] [PubMed] [Google Scholar]
- 6.Stepanova, V., Jerke, U., Sagach, V., Lindschau, C., Dietz, R., Haller, H. & Dumler, I. (2002) J. Biol. Chem. 277, 10265-10272. [DOI] [PubMed] [Google Scholar]
- 7.Walter, J., Capell, A., Hung, A. Y., Langen, H., Schnölzer, M., Thinakaran, G., Sisodia, S. S., Selkoe, D. J. & Haass, C. (1997) J. Biol. Chem. 272, 1896-1903. [DOI] [PubMed] [Google Scholar]
- 8.Apasov, S. G., Smith, P. T., Jelonek, M. T., Margulies, D. H. & Sitkovsky, M. V. (1996) J. Biol. Chem. 271, 25677-25683. [DOI] [PubMed] [Google Scholar]
- 9.Allende, J. E. & Allende, C. C. (1995) FASEB J. 9, 313-323. [DOI] [PubMed] [Google Scholar]
- 10.Meggio, F. & Pinna, L. A. (2003) FASEB J. 17, 349-368. [DOI] [PubMed] [Google Scholar]
- 11.Lichtfield, D. W. (2003) Biochem. J. 369, 1-15.12396231 [Google Scholar]
- 12.Pinna, L. A. & Meggio, F. (1997) Prog. Cell Cycle Res. 3, 77-97. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed, K., Gerber, D. A. & Cochet, C. (2002) Trends Cell Biol. 12, 226-230. [DOI] [PubMed] [Google Scholar]
- 14.Lin, J. M., Kilman, V. L., Keegan, K., Paddok, B., Rosbash, M. & Allada, R. (2002) Nature 420, 816-820. [DOI] [PubMed] [Google Scholar]
- 15.Bandhakavi, S., Mc Cann, R. O., Hanna, D. E. & Glover, C. V. (2003) FEBS Lett. 554, 295-300. [DOI] [PubMed] [Google Scholar]
- 16.Miyata, Y. & Nishida, E. (2004) Mol. Cell. Biol. 24, 4065-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bierman, R., Schnittger, L., Beyer, D. & Ahmed, J. (2003) J. Cell. Physiol. 196, 444-453. [DOI] [PubMed] [Google Scholar]
- 18.Hinrichs, M. V., Jedlicki, A., Tellez, R., Pongor, S., Gatica, M., Allende, C. C. & Allende, J. E. (1993) Biochemistry 32, 7310-7316. [DOI] [PubMed] [Google Scholar]
- 19.Antonelli, M., Daniotti, J. L., Rojo, D., Allende, C. C. & Allende, J. E. (1996) Eur. J. Biochem. 241, 272-279. [DOI] [PubMed] [Google Scholar]
- 20.Kübler, D., Pyerin, W., Burow, E. & Kinzel, V. (1989) J. Biol. Chem. 264, 14549-14555. [PubMed] [Google Scholar]
- 21.Bradford, M. M. (1976) Anal. Biochem. 72, 248-254. [DOI] [PubMed] [Google Scholar]
- 22.Korn, I., Jacob, G., Allende, C. C. & Allende, J. E. (2001) Mol. Cell. Biochem. 227, 37-44. [PubMed] [Google Scholar]
- 23.Valero, E., De Bonis, S., Filhol, O., Wade, R. H., Langowski, J., Chambaz, E. M. & Cochet, C. (1995) J. Biol. Chem. 270, 8345-8352. [DOI] [PubMed] [Google Scholar]
- 24.Cosmelli, D., Antonelli, M., Allende, C. C. & Allende, J. E. (1997) FEBS Lett. 410, 391-396. [DOI] [PubMed] [Google Scholar]
- 25.Gatica, M., Jedlicki, A., Allende, C. C. & Allende, J. E. (1994) FEBS Lett. 339, 93-96. [DOI] [PubMed] [Google Scholar]