Abstract
Context
Many patients with potentially curable cancer do not complete their prescribed treatment regimens because of the toxicity. There is evidence that the common endpoints of many of these toxicities are amenable to quality of life (QOL)-directed interventions.
Objectives
This study was conducted to determine the effect of a multidisciplinary QOL-directed intervention on patients’ adherence to planned chemoradiation (CR) regimens.
Methods
The results of two randomized controlled trials that utilized the same QOL intervention were pooled to form a cohort of 61 patients with advanced localized gastrointestinal (GI) cancer. Of these 61 subjects, 29 participated in six to eight bi- to triweekly sessions that included exercise, education and relaxation, and 32 received usual medical care. The primary endpoint was completion of their prescribed CR regimens. Secondary outcomes included hospitalization during CR, rates of adverse postoperative events, and complete pathological response in those undergoing neoadjuvant therapy.
Results
Significantly more members of the intervention than the control group completed their planned CR regimens (77.8 vs. 38.2%, P = 0.003). More participants in the control (n=14) than the intervention (n=5) group (P = 0.063) required hospitalization. Among those undergoing neoadjuvant CR, those in the intervention group were significantly more likely to complete CR as planned (81.0% vs. 37.5%, P = 0.005) and less likely to be hospitalized (14.3% vs. 50.0%, P = 0.011).
Conclusion
A structured multidisciplinary QOL-directed intervention delivered to patients undergoing CR may increase the proportion of patients who complete CR as planned and reduce unplanned hospitalizations. Utilization is an important outcome in QOL-directed intervention trials.
Keywords: chemoradiation, treatment tolerance, hospitalization, prehabilitation, neoadjuvant
Introduction
Many patients with advanced cancer choose to undergo multi-modal treatment involving chemotherapy, radiation and surgery.1 This decision is potentially curative; however, the agents used may be toxic and poorly tolerated despite our best efforts to provide supportive measures and tailor dosages. The result can be catastrophic as many patients, particularly the elderly, do not complete their planned regimens as scheduled,2–4 and this lack of adherence has been posited to contribute to decreased survival rates.5,6
Whereas some side effects of cancer treatment may be more apparent to the physician (e.g., myelosuppression), others such as diarrhea, nausea, vomiting, and pain confront patients on a daily basis. It is common for such adverse symptoms to co-occur among patients receiving combined chemoradiation (CR), and to undermine important clinical outcomes, e.g., peri-operative morbidity and survival.7–9 In concert, these symptoms may ultimately produce what the patient perceives as intolerable levels of fatigue, functional decline, and dysphoria, which influence clinicians’ decisions to reduce, terminate, or delay treatments.10–14 In effect, potentially life-saving therapy may be abandoned because of its overwhelming degradation of a patient’s quality of life (QOL). Fortunately, research suggests that psychosocial support and exercise during treatment may reduce adverse symptoms and enhance patients’ adherence to planned anticancer treatment.15–18 Additionally, exercise as “prehabilitation” prior to or concurrent with cancer treatment reduces morbidity for some cancer populations.19,20
Thus, physical activity and QOL are not second-order considerations in a patient’s multimodal treatment plan. At times they may factor in their survival. Given this, it is surprising that a literature review reveals little emphasis directed towards enhancing physical activity or preserving QOL during CR, or prior to surgery for patients receiving neoadjuvant treatment. The marked dearth of research that characterizes gastrointestinal (GI) cancers, in particular, is troubling as the CR-associated side effects for patients undergoing treatment for these cancers may be particularly severe and increase the morbidity of subsequent surgeries.9,21
It seems important to gain additional knowledge about the potential impact of exercise- and QOL-directed interventions on patient tolerance and adherence to their CR regimens, as well as on surgical outcomes following neoadjuvant CR. We, therefore, studied this question with a posthoc analysis of the data of the participants with locally advanced GI cancers who participated in two QOL-directed intervention trials that featured exercise as a key component.17,18
Methods
Our research team has conducted two Institutional Review Board-approved randomized clinical trials to assess the effectiveness of a multidisciplinary QOL-focused intervention among patients receiving radiation for late-stage cancer.22,23 The two trials utilized the same structured interventions and differed only in that the second trial included six rather than the eight sessions of the first, and involved the inclusion of caregivers. Participants with GI cancers in the two trials were pooled in order to reduce heterogeneity by balancing chemotherapeutic agents, radiation doses, and their potential toxicities between the study groups, and to more accurately estimate the effect of the intervention on: 1) CR completion rates in light of reported associations between QOL and adherence to planned therapy for locally advanced GI cancers,4,14 2) unplanned hospitalizations during CR given evidence linking psychosocial support during CR to reduced health care utilization,18 and 3) surgical outcomes post neoadjuvant CR given the exercise emphasis of the intervention and recent reports of reduced peri-operative morbidity with exercise-based “prehabilitation” for GI cancers. 19,20 Only participants with GI cancers were receiving neoadjuvant CR in the previous trials. Focusing on GI cancer enabled us to examine the utility of the intervention as “prehabilitation” given its emphasis on exercise and fitness. The data analyzed in this study differ from those previously reported for Studies 1 and 2 in that only findings related to patient reported outcomes (PROs) were reported in prior publications. Data regarding completion of CR, hospitalizations during CR, and perioperative morbidity (for participants receiving neoadjuvant treatment) were retrospectively abstracted exclusively for this study after the completion of both Studies 1 and 2. Vital status was also ascertained solely for this study. PROs were not included in the analyses for this study either as dependent variables or covariates.
Participants
The inclusion and exclusion criteria of the two trials were identical and have been described previously.22,23 Participants were required to be at least 18 years of age with late-stage cancer diagnosed within the past 12 months and requiring external beam radiation, a six-month or greater life expectancy, and an expected five-year survival of 50% or less.
Design
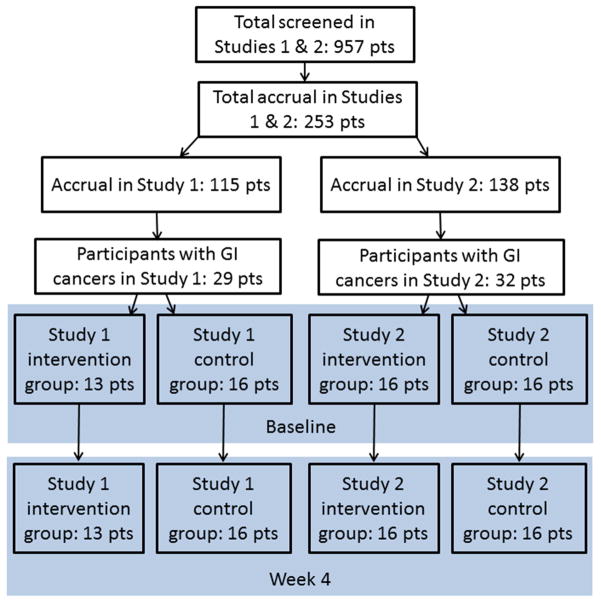
Participants were randomly assigned to either a multidisciplinary QOL-directed intervention or usual medical care arm. The two studies enrolled a total of 253 patients (115 and 138 subjects, respectively), of whom 61 had cancers originating from the GI tract and gallbladder. The proportion of those having GI cancers did not differ significantly between the studies (25% and 23%) or between their intervention and control group assignments. As noted above, data from the two studies were pooled to create a cohort of 61 participants with GI cancer of which 29 received the QOL-directed intervention, and 32 usual medical care.
Intervention
Both trials included structured group sessions, conducted two to three times per week, eight in Study 1 and six in Study 2, led by a psychiatrist or psychologist. Session content has been previously described and is summarized in Table 1.22,23 Each session had a theme that incorporated specific QOL domains and was designed to stand alone. Sessions opened with 20 minutes of gentle stretching and resistive exercise led by a physical therapist and closed with 10–20 minutes of guided relaxation.25 Depending on the theme, sessions were co-facilitated by a social worker, advanced practice nurse or certified hospital chaplain. Educational materials were provided for review and independent study of a session’s content.
Table 1.
Social, Cognitive, and Emotional Components of the Multidisciplinary Quality of Life- Directed Intervention
| Session | Intervention Components | ||
|---|---|---|---|
| Social | Cognitive | Emotional | |
| 1 | Empathy, Positive regard | Adjustment phases | Problem solving |
| 2 | Sources of support | Prioritizing | Goal setting |
| 3 | Communication strategies | Coping, Accessing support | Stress management |
| 4 | Emotional expressiveness | Taking charge | Stress management |
| 5 | Role playing | Lifestyle change | Assertiveness |
| 6 | Interpersonal relationships | Healthy lifestyle choices | Irrational thoughts |
| 7 | Body image and sexuality | Coping | Challenging thoughts |
| 8 | Closure and termination | Goal setting | Relapse prevention |
Data Collection
The outcomes and covariates used in our analyses were obtained from two sources. The first consisted of the demographic and cancer treatment information collected during Studies 1 and 2. The second consisted of information retrospectively abstracted from participants’ Mayo Clinic electronic medical records (EMRs), after completion of Studies 1 and 2, regarding the perioperative courses of participants receiving neoadjuvant treatments, as well as the treatment tolerance of patients receiving both neoadjuvant and adjuvant CR. Retrospective EMR review was performed by two physicians blinded to participants’ group assignment. Disagreements were resolved though a consensus process involving simultaneous EMR review by both physicians. Data abstracted for all participants included:
Hospitalization During CR. A participant was considered to have been hospitalized if an admission note was entered in their EMR by a clinical service and they spent at least one night in the hospital.
-
Completion of the Planned CR Regimen. Participants were considered to have completed CR as planned if there were no reductions in their planned chemotherapy or radiation dosages, or interruptions in scheduled administration because of toxicity or hospitalization.
Additional data collected for the 37 participants who underwent surgical tumor resection following neoadjuvant CR treatment included:
Length of Surgical Stay. Length of stay for participants hospitalized following CR for resection of residual tumor was based on their hospital discharge summary and included both the day of admission and discharge.
Thirty-Day Readmission. Hospitalization within thirty days after post-surgical hospital discharge was assessed by reviewing participants’ Mayo Clinic EMRs and querying surviving participants and caregivers of decedents regarding hospitalization in non-Mayo facilities.
Post-Operative Complications. Participants were considered to have experienced postoperative complications if they were transferred to the intensive care unit, required an unplanned specialist consultation, or if their course was described as “complicated” in their hospital discharge summary.
-
Complete Pathological Response (CPR). Participants’ were considered to have a CPR if the pathology report from their post-CR surgery included the phrase “complete pathological response.”
Vital status was verified through death certificates, the Mayo Clinic EMR, next-of-kin reports, the Mayo Clinic Tumor Registry and the Social Security Death Index website.
Statistical Analyses
Descriptive statistics for the total study cohort, treatment arms, and cancer-based subgroups were calculated using proportions for categorical and means for continuous variables. Inter-group differences were assessed with the X2 test for binary and independent t-tests for continuous variables. Logistic regression was utilized to estimate inter-group differences in hospitalization during CR and completion of the planned CR regimen for all participants. Among the subgroup of participants receiving neoadjuvant CR, logistic regression also was used to evaluate intergroup differences in the presence of postoperative complications, 30-day readmissions and the presence of a CPR. Ordinary least square regression was used to compare length of hospital stay between the groups. Cox proportional hazard models were used to compare survival between the study groups. Conventional model diagnostics were performed. All analyses were performed with Linux SAS v 9.3.
Results
Table 2 lists the demographic and cancer characteristics of the study cohort. In summary, the pooled subgroup from the two studies did not differ significantly with respect to treatment arm assignment (P = 0.93). Participants were, on average, 62 years old and roughly half were female. While almost twice as many participants in the control group received cisplatinum and 5- Fluorouracil during CR, this difference did not reach statistical significance, P = 0.09. Forty- five participants underwent neoadjuvant CR and 37 went on to undergo surgical tumor resection, 17 (81.0%) intervention and 20 (83.3%) control group, P = 0.84.
Table 2.
Demographic and Clinical Characteristics of the Study Population
| Intervention | Control | p value | |
|---|---|---|---|
| Total Study Cohort | 29 | 32 | |
| Study 1, n (%) | 13 (48.2%) | 16 (47.1%) | 0.93 |
| Age, mean (SD) | 61.2 (10.9) | 61.9 (11.5) | 0.82 |
| Female, n (%) | |||
| Cancer | 0.188 | ||
| Colorectal, n (%) | 9 (33.3%) | 4 11.8%) | |
| Esophageal/GE* junction, n (%) | 13 (48.15) | 22 (64.7%) | |
| Gastric, n (%) | 2 (7.4%) | 5 (14.7%) | |
| Other*, n (%) | 3 (11.1%) | 3 (8.8%) | |
| Chemotherapy | 0.23 | ||
| 5-FU (+/− leucovorin), n (%) | 14 (51.9%) | 14 (41.2%) | |
| cisplatinum + 5-FU | 9 (34.6%) | 17 (50.0%) | |
| Other† | 5 (18.5%) | 3 (8.8%) | |
| Deceased | 17 (63.0%) | 22 (64.7%) | |
| Neoadjuvant chemoradiation, n (%) | 21 (77.8%) | 24 (71.0%) | 0.53 |
Other = cholagiocarcinoma, ampullary, and gallbladder
Other = “McDonald protocol” & capecitabine; capecitabine; Alimta & carboplatin; FOLFOX & Avastin; cisplatinum, docetaxel, & 5-FU; carboplatinum & premetrexed; carboplatinum, paclitaxel & 5-FU.
Table 3 presents the results of logistic regression models for the study cohort as a whole. Significantly more members of the intervention than the control group completed CR treatment as planned (77.8 vs. 38.2%, P = 0.003). The significance of this finding was robust to adjustment for chemotherapy regimen. Similarly, while the results only approached statistical significance, a lower proportion of those in the intervention group (5 of 29) than in the control group (14 of 32) (P = 0.063) were hospitalized during CR.
Table 3.
Results of Logistical Regression Models Estimating the Associations of Group Assignment with Completion of Chemoradiation as Planned and Hospitalization During Chemoradiation, Both Unadjusted and Adjusted for Chemotherapy Regimen
| Odds ratio | SE | p value | 95% Confidence Interval | |
|---|---|---|---|---|
| Hospitalized during chemotherapy | ||||
| Univariate logistic regression model | ||||
| Group | 0.325 | 0.197 | 0.063 | 0.099 – 1.064 |
| Multivariate logistic regression model | ||||
| Group | 0.337 | 0.208 | 0.078 | 0.100 – 1.131 |
| Other (referent) | ||||
| Cisplatinum & 5-FU | 1.243 | 1.188 | 0.82 | 0.191 – 8.097 |
| 5-FU (+/− leucovorin) | 1.051 | 0.995 | 0.958 | 0.164 – 6.723 |
| Completion of CR as planned | ||||
| Univariate logistic regression model | ||||
| Group | 0.177 | 0.103 | 0.003 | 0.057 – 0.554 |
| Multivariate logistic regression model | ||||
| Group | 0.150 | 0.096 | 0.003 | 0.043 – 0.528 |
| Other (referent) | ||||
| Cisplatinum & 5-FU | 3.906 | 4.699 | 0.257 | 0.369 – 41.278 |
| 5-FU (+/− leucovorin) | 9.107 | 11.000 | 0.067 | 0.854 – 97.150 |
Among the 45 participants undergoing neoadjuvant CR, those in the intervention group were significantly less likely to be hospitalized during CR; 14.3% (n=3) vs. 50.0% (n=12), P = 0.011, and more likely to complete CR as planned; 81.0% (n=17) vs. 37.5% (n=9), P = 0.005. The associations of study group with hospitalization and CR completion as planned were robust to adjustment by chemotherapy regimen as presented in Table 4. Study group assignment was not significantly associated with characteristics of participants’ hospitalizations for tumor resection after neoadjuvant CR including length of stay, presence of complications, or 30-day readmission. The rate of CPR did not differ between the groups.
Table 4.
Results of Logistical Regression Models, Among Participants Who Received Neoadjuvant Chemoradiation, Estimating the Associations of Group Assignment with Completion of Chemoradiation as Planned and Hospitalization During Chemoradiation, Both Unadjusted and Adjusted for Chemotherapy Regimen
| Odds ratio | SE | p value | 95% Confidence Interval | |
|---|---|---|---|---|
| Hospitalized during chemotherapy | ||||
| Univariate logistic regression model | ||||
| Group | 0.167 | 0.124 | 0.016 | 0.039 – 0.718 |
| Multivariate logistic regression model | ||||
| Group | 0.130 | 0.107 | 0.013 | 0.026 – 0.647 |
| Cisplatinum & 5-FU | 0.796 | 0.835 | 0.828 | 0.102 – 6.217 |
| 5-FU (+/- leucovorin) | 1.794 | 2.025 | 0.605 | 0.196 – 16.392 |
| Completion of CR as planned | ||||
| Univariate logistic regression model | ||||
| Group | 0.141 | 0.098 | 0.005 | 0.036 – 0.554 |
| Multivariate logistic regression model | ||||
| Group | 0.130 | 0.107 | 0.013 | 0.026 – 0.647 |
| Cisplatinum & 5-FU | 0.796 | 0.835 | 0.828 | 0.110 – 6.217 |
| 5-FU (+/− leucovorin) | 1.794 | 2.025 | 0.605 | 0.196 – 16.392 |
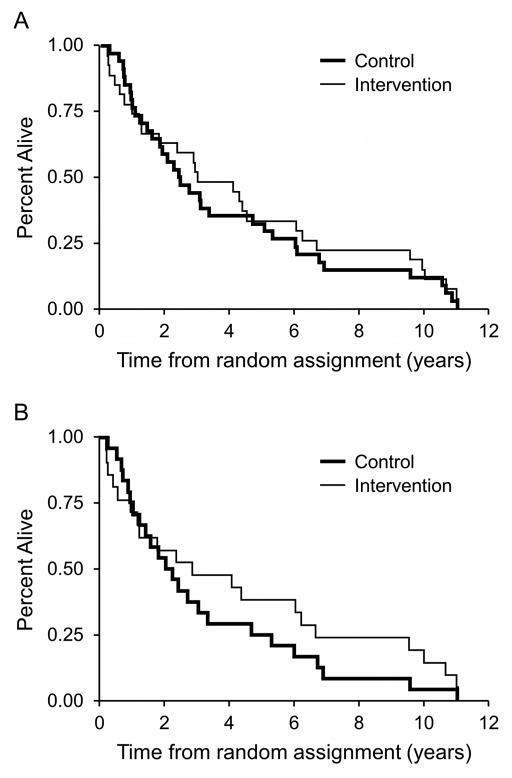
Survival did not differ significantly between the groups when considering the total study cohort or the subgroup receiving neoadjuvant CR. The hazard ratio (HR) favored the intervention group as a whole, HR = 0.85, particularly the subgroup undergoing neoadjuvant CR, HR = 0.74; however, these findings were not statistically significant. Fig. 2, Panel A presents a Kaplan Meier curve by group for the cohort as a whole, and Panel B, for the subgroup undergoing neoadjuvant CR.
Fig. 2.
Kaplan Meier curves by group for the cohort as a whole, Panel A, and for the subgroup undergoing neoadjuvant chemoradiation, Panel B.
Discussion
This study is, to the best of our knowledge, the first to suggest that participation in a multidisciplinary QOL-directed intervention may be associated with an improved likelihood of patients with cancer completing their prescribed CR regimen without interruptions or dose reductions. Our findings are particularly notable in two regards. First, the magnitude of the intervention’s effect is clinically meaningful. Over three-quarters of intervention participants relative to only one-third of their control group counterparts completed their CR course as scheduled. Second, it is also clinically significant that among participants receiving neoadjuvant CR, members of the intervention group were significantly less likely to be hospitalized during treatment. The apparent benefit to this subgroup is noteworthy given the high levels of treatment toxicity associated with this approach. Our findings suggest that future investigators should further explore the impact of QOL on adherence to cancer treatment recommendations and health care utilization.
It should be noted that differences were not found in a number of our secondary outcome measures. For example, the benefits of the intervention for the subgroup of participants that received neoadjuvant CR did not extend to their post-CR hospitalizations for tumor resection. Specifically, members of the intervention group did not differ from the control group in terms of rates of post-operative complications, CPR, 30-day readmission, or in hospital lengths of stay.
The few prior studies that have examined health care utilization in trials of QOL-directed interventions, whether in cancer or in other conditions, have seldom noted benefit.26 However, unlike our study, most trials enrolled patients in the far advanced/palliative stages of illness. Two additional distinctions may be important. The first may be the time horizon in that participants in this study were examined over a relatively brief interval, 4–6 weeks. The second is that they were receiving intensive CR for locally advanced GI cancers, a treatment regimen known to be associated with particularly high levels of morbidity, symptom burden and rehospitalization.10–13 The results of this study suggest that there may be a high yield, at least in terms of reducing utilization, in focusing QOL-directed activities at times of high patient vulnerability and treatment intensity.
Whereas the economic importance of reducing the frequency of hospital admissions is indisputable, the clinical relevance of completing CR as planned is less clear. The survival benefits of achieving a CPR have been established,27 and a causal association between treatment intensity and CPR has been proposed but not empirically validated.28 In this study, it is interesting that although not statistically significant, more participants who completed CR as planned achieved a CPR, 77% versus 23%. Completing CR as planned may be a surrogate for treatment tolerance or the extent/severity of patients’ symptom burden, clearly important constructs, but this interpretation remains unestablished.
The mechanism by which a QOL-oriented intervention can reduce the frequency of hospitalization remains speculative. Proposed explanations include: 1) enhanced patient self-efficacy and communication; 2) reduction of stress induced by symptoms such as insomnia, fatigue and lack of appetite; 3) lower levels of stress-induced circulating inflammatory cytokines, and 4) more proactive detection and treatment of problematic signs and symptoms. Unfortunately, we were not able to explore these possibilities in this investigation. Future studies would need to adopt clear theoretical frameworks with discoverable mediating factors if we are to enhance the effectiveness of QoL-directed interventions.
This study, as do all, has its strengths and weaknesses. Its novel findings and focus on QOL, treatment adherence and utilization of patients receiving CR for advanced cancer are among its strengths. Its principal limitation arises from the fact that even with the pooling of two very similar studies, the post hoc and exploratory nature of these analyses can legitimately be perceived as a limitation. Additionally, only GI cancer was examined, constraining the generalizeability of our findings. The decision to include patients with GI cancers was largely determined by our interest in the intervention’s impact on participants undergoing CR. Participants undergoing treatment for head and neck cancer comprised the only other subgroup in which more than half of participants were receiving radiation and parenteral chemotherapy. These participants had all recently undergone surgical resection of oropharyngeal tumors with uni- or bilateral neck dissections, making their morbidity risk profile distinct from that of other participants. Further, hospitalization for dehydration occurs frequently during CR for head and neck cancer.24 We hypothesized that this subgroup’s utilization would be less amenable to reduction through the QOL-directed intervention and consequently did not include them in the study. While we agree that a larger cohort would have been preferable, our sample of 61 was not miniscule and our findings, at least with respect to CR regimen adherence and rehospitalizations, were robust. The imbalance in the number of esophago-gastric malignancies and the type of CR received (Cisplatin-5FU) may be responsible, to some extent, for the differences in therapy completion. Additional studies, with more diverse samples, powered to more clearly define the impact of QOL-directed interventions on survival outcomes appear warranted.
Conclusion
A structured multidisciplinary QOL-directed intervention delivered to patients undergoing CR for advanced GI cancers may increase the proportion of those who complete treatment as planned and reduce unplanned hospital admissions.
Fig. 1.
CONSORT diagram.
Acknowledgments
This study was funded by the Linse Bock Foundation.
Footnotes
Disclosures : The authors have no financial disclosures or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glasgow RE, Ilson DH, Hayman JA, et al. Modern approaches to localized cancer of the esophagus. J Natl Compr Canc Netw. 2011;9:902–911. doi: 10.6004/jnccn.2011.0074. [DOI] [PubMed] [Google Scholar]
- 2.Hu CY, Delclos GL, Chan W, Du XL. Assessing the initiation and completion of adjuvant chemotherapy in a large nationwide and population-based cohort of elderly patients with stage-III colon cancer. Med Oncol. 2011;28:1062–1074. doi: 10.1007/s12032-010-9644-7. [DOI] [PubMed] [Google Scholar]
- 3.Repetto L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol. 2003;1:18–24. [PubMed] [Google Scholar]
- 4.Hill A, Kiss N, Hodgson B, Crowe TC, Walsh AD. Associations between nutritional status, weight loss, radiotherapy treatment toxicity and treatment outcomes in gastrointestinal cancer patients. Clin Nutr. 2011;30:92–98. doi: 10.1016/j.clnu.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 6.Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–509. doi: 10.1016/s0959-8049(97)10090-9. [DOI] [PubMed] [Google Scholar]
- 7.Cheng KK, Lee DT. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol. 2011;78:127–137. doi: 10.1016/j.critrevonc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Wikman A, Johar A, Lagergren P. Presence of symptom clusters in surgically treated patients with esophageal cancer: implications for survival. Cancer. 2014;120:286–293. doi: 10.1002/cncr.28308. [DOI] [PubMed] [Google Scholar]
- 9.Imdahl A, Schoffel U, Ruf G. Impact of neoadjuvant therapy of perioperative morbidity in patients with esophageal cancer. Am J Surg. 2004;187:64–68. doi: 10.1016/j.amjsurg.2002.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Ooi BS, Tjandra JJ, Green MD. Morbidities of adjuvant chemotherapy and radiotherapy for resectable rectal cancer: an overview. Dis Colon Rectum. 1999;42:403–418. doi: 10.1007/BF02236362. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 2013;14:317–326. doi: 10.1016/S1470-2045(13)70021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurmuzlu M, Aarstad HJ, Aarstad AK, Hjermstad MJ, Viste A. Health-related quality of life in long-term survivors after high-dose chemoradiotherapy followed by surgery in esophageal cancer. Dis Esophagus. 2011;24:39–47. doi: 10.1111/j.1442-2050.2010.01104.x. [DOI] [PubMed] [Google Scholar]
- 13.Courrech Staal EF, Aleman BM, Boot H, et al. Systematic review of the benefits and risks of neoadjuvant chemoradiation for oesophageal cancer. Br J Surg. 2010;97:1482–1496. doi: 10.1002/bjs.7175. [DOI] [PubMed] [Google Scholar]
- 14.Dobie SA, Baldwin LM, Dominitz JA, et al. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98:610–619. doi: 10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 16.Newton MJ, Hayes SC, Janda M, et al. Safety, feasibility and effects of an individualised walking intervention for women undergoing chemotherapy for ovarian cancer: a pilot study. BMC Cancer. 2011;11:389. doi: 10.1186/1471-2407-11-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Dodd MJ, Dibble SL, Abrams DI. Nausea at the end of adjuvant cancer treatment in relation to exercise during treatment in patients with breast cancer. Oncol Nurs Forum. 2008;35:830–835. doi: 10.1188/08.ONF.830-835. [DOI] [PubMed] [Google Scholar]
- 18.Mason H, DeRubeis MB, Foster JC, Taylor JM, Worden FP. Outcomes evaluation of a weekly nurse practitioner-managed symptom management clinic for patients with head and neck cancer treated with chemoradiotherapy. Oncol Nurs Forum. 2013;40:581–586. doi: 10.1188/13.ONF.40-06AP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92:715–727. doi: 10.1097/PHM.0b013e31829b4afe. [DOI] [PubMed] [Google Scholar]
- 20.Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150:505–514. doi: 10.1016/j.surg.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 21.Courrech Staal EF, Aleman BM, van Velthuysen ML, et al. Chemoradiation for esophageal cancer: institutional experience with three different regimens. Am J Clin Oncol. 2011;34:343–349. doi: 10.1097/COC.0b013e3181dbbafe. [DOI] [PubMed] [Google Scholar]
- 22.Clark MM, Rummans TA, Atherton PJ, et al. Randomized controlled trial of maintaining quality of life during radiotherapy for advanced cancer. Cancer. 2013;119:880–887. doi: 10.1002/cncr.27776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rummans TA, Clark MM, Sloan JA, et al. Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: a randomized controlled trial. J Clin Oncol. 2006;24:635–642. doi: 10.1200/JCO.2006.06.209. [DOI] [PubMed] [Google Scholar]
- 24.Givens DJ, Karnell LH, Gupta AK, et al. Adverse events associated with concurrent chemoradiation therapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1209–1217. doi: 10.1001/archoto.2009.174. [DOI] [PubMed] [Google Scholar]
- 25.Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2012;45:811–821. doi: 10.1016/j.jpainsymman.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA. 2008;299:1698–1709. doi: 10.1001/jama.299.14.1698. [DOI] [PubMed] [Google Scholar]
- 27.Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87:392–398. doi: 10.1016/j.athoracsur.2008.11.001. discussion 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crehange G, Bonnetain F, Peignaux K, et al. Preoperative radiochemotherapy for resectable localised oesophageal cancer: a controversial strategy. Crit Rev Oncol Hematol. 2010;75:235–242. doi: 10.1016/j.critrevonc.2009.11.007. [DOI] [PubMed] [Google Scholar]