Abstract
Dominant Vγ2Vδ2 T-cell subset recognizes phosphoantigen, and exist only in humans and nonhuman primates. Despite the discovery of γδ T cells for >30 years, a proof-of-concept (POC) study has not been done to prove the principle that Vγ2Vδ2 T-cell subset is protective against M. tuberculosis (Mtb) and other infections. Here, we employed adoptive cell transfer strategy to define protective role for Vγ2Vδ2 T cells in primate TB model. Vγ2Vδ2 T cells for adoptive transfer displayed central/effector memory and mounted effector functions of producing anti-Mtb cytokines and inhibiting intracellular mycobacteria. They also expressed CXCR3/CCR5/LFA-1 trafficking/tissue-resident phenotypes and consistently trafficked to the airway and retained there detectable from 6 hours through 7 days after adoptive transfer. Interestingly, the test group of macaques receiving transfer of Vγ2Vδ2 T cells at weeks 1 and 3 after high-dose 500 CFU Mtb infection exhibited significantly lower levels of Mtb infection burdens in lung lobes and extra-pulmonary organs than the control groups receiving PBL or saline. Consistently, adoptive transfer of Vγ2Vδ2 T cells attenuated TB pathology and contained lesions mostly in the infection-site of right caudal lung lobe, with no or reduced TB dissemination to other lobes, spleens or livers/kidneys whereas the controls showed widespread TB dissemination. The POC finding supports the view that dominant Vγ2Vδ2 T-cell subset may be included for the rational design of TB vaccine or host-directed therapy.
Keywords: γδ T cells, tuberculosis, phosphoantigen, HMBPP, Macaques
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), has become a top killer among infectious diseases worldwide due to epidemics of HIV/AIDS and multi-drug resistant TB (1, 2). In 2014, 9.6 million people fell ill with TB and 1.5 million died from TB(www.who.int). The sole TB vaccine, BCG, inconsistently protects against adult TB (3–7). There is a pressing need to develop a new TB vaccine and/or immunotherapeutics, and this cannot be done without in-depth studies elucidating protective immunity and mechanisms against Mtb infection.
Over the past decades, we have been studying fundamental aspects of the major Mtb-reactive γδ T cell subset, Vγ2Vδ2 T cells in infections. Vγ2Vδ2 T cells remain a single γδ T-cell subset capable of recognizing isoprenoid metabolites such as isopentenyl pyrophosphate (IPP) and (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), which are usually referred to as phosphoantigens(8, 9). Isoprenoid metabolites are produced via two major pathways: the classical mevalonate pathway and the alternative or non-mevalonate pathway. IPP is an intermediate metabolite found in both pathways, whereas HMBPP is only produced in the non-mevalonate pathway by some selected microbes including Mtb and M. bovis BCG(8, 9). Vγ2Vδ2 T-cell subset exists only in humans and nonhuman primates, constitutes 65–90% of total circulating γδ T cells in humans, and contributes to both innate and adaptive immune responses in infections (10–13). Vγ2Vδ2 T cells can mount major expansion and multi-functional responses during infections with Mtb and other pathogens(14–17). Notably, rapid recall-like expansion of Vγ2Vδ2 T cells correlates with detectable immunity against severe TB after Mtb challenge of BCG-vaccinated infant rhesus macaques(14).
A proof-of-concept (POC) study has not been done to fully define protective roles of Vγ2Vδ2 T cells since γδ T cells were discovered 30 years ago. This is largely attributed to the lack of manipulation tools for in vivo studies in primates. It is noteworthy that mouse TB models, though useful, cannot provide an ideal setting in which to define protection by Vγ2Vδ2 T cells due to the fact that mouse γδ T cells cannot recognize HMBPP or other Mtb antigens(18). Recently, we have demonstrated that expansion/differentiation of Vγ2Vδ2 T cells by cHMBPP plus IL-2 treatment can increase immune resistance to TB in macaques(16). While this finding implicates a role of Vγ2Vδ2 T cells, one can argue that IL-2 activation of other immune cells could also contribute to the protection. Virtually, expansion of T effector and Treg cells by IL-2 alone treatment can synergize detectable resistance to TB although the level of IL-2-induced immunity is significantly lower than the protection achieved by cHMBPP plus IL-2 expansion of Vγ2Vδ2 T cells (19). A better strategy is needed to prove the concept that Vγ2Vδ2 T cells are protective against Mtb infection. Ideally, Vγ2Vδ2 TCR knock-out macaques or depleting antibodies would be useful for the POC study. However, these tools have not been available for definitive studies.
To circumvent the lack of manipulating tools, we have employed adoptive cell transfer strategy to conduct a POC study in the primate TB model. Our POC study demonstrated that adoptive transfer of autologous Vγ2Vδ2 T cells could confer detectable protection against Mtb infection and TB pathology in macaques. Findings therefore help to address or narrow the long-standing gap in defining primate γδ T-cell immunity.
MATERIALS AND METHODS
Macaque animals and IACUC approval
Cynomolgus macaques, aged 4–8, were used in the current study. Both female and male macaques were used without selection. All macaques were subjected to initial screening for the ability to expand in response to ex vivo stimulation with Zoledronic Acid/IL-2 prior to recruitment for the study. All macaques in the 3 groups were able to mount ex vivo expansion in response to Zoledronic Acid/IL-2 protocol [Fig.S1.(A)]. The use of macaques and experimental procedures were approved by Institutional Animal Care and Use Committee and Biosafety Committee (Protocol A 13–128), University of Illinois College of Medicine at Chicago (UIC), and we followed the national and international guidelines [International Primatological Society (IPS) International Guidelines for the acquisition, care and breeding of nonhuman primates] regarding “The use of non-human primates in research”.
Peripheral blood mononuclear cell (PBMC) isolation
Freshly collected EDTA anti-coagulated blood was centrifuged and the buffy coat was removed and diluted in phosphate buffered saline (PBS). Diluted buffy coats were layered over Ficoll-Paque Plus (Amersham, Piscataway, NJ) and centrifuged to separate PBMCs from red blood cells (RBCs) and granulocytes. PBMCs were aspirated from top layer of Ficoll-Paque and contaminating RBCs were lysed using RBC lysis buffer (eBioscience). Purified PBMCs were washed twice and counted using Trypan Blue exclusion.
Bronchoalveolar Lavage (BAL) and Bronchoalveolar Lavage Fluid (BALF) collection
BAL was performed as previously described. Briefly, Macaques were sedated with Ketamine (10 mg/kg) and xylazine (1–2 mg/kg), by intramuscular injection. An intratracheal tube was inserted through the Larynx into the trachea and placed at the carina. Saline solution was instilled and harvested from the lungs through the intratracheal tube. A maximum of 10 mL/kg of solution was placed in the lungs of macaques, and the recovery rate was greater than 50% in all cases.
In case of TB infected macaques, BAL and BALF collection were performed using a pediatric bronchoscope. The bronchoscope was inserted into the right caudal lobe (infection lobe) of the animals to allow harvesting of cells that might be involved in anti TB immunity.
Collection of cells for adoptive transfer
All monkeys assigned to participate in the experiments of cell tracking or TB infection were bled 4 times every 2 weeks before the onset of the study. <10% of the total monkey blood volume was collected each bleeding time. Blood was drawn from monkeys’ cephalic or saphenous veins under anesthesia (Ketamine 10 mg/kg IM). This has been shown to be well-tolerated by macaques in past studies as hematocrit, hemoglobin levels and lymphocyte counts stayed within the normal range (20). Cell blood counts were ran at each collection time point to make sure the animals were not anemic (hematocrit < 25%). If these limits were met, sample collection was delayed until the animal returned to normal red blood cell parameters. In case more cells were needed, at least 2 week resting period was taken before starting another round of collection. Monkeys had to rest for >2 weeks before the onset of TB infection.
Ex vivo expansion of Vγ2Vδ2 T cells
Cryopreserved PBMCs collected overtime from individual macaques were used as a starting cell population for the ex vivo expansion of Vγ2Vδ2 T cells. Cryopreserved vials were immediately placed in a 37°C water bath until thawed completely. Cells were quickly washed 3–4 times with the R10A and then counted using Trypan Blue exclusion. R10A consists of RPMI 1640 medium with 10% FBS and 1X antibiotics/antimycotics (Life Technologies; 100 units/mL of penicillin, 100 µg/mL of streptomycin, and 2.5 µg/mL of Fungizone).
PBMCs were resuspended in R10A containing 5 µM Zoledronic Acid (Sigma Aldrich).
On day zero, cells were resuspended in culture medium at 2×106 cells/ml and seeded in U shaped wells 96 well plates at 100µL/well. Plates were placed in a humidified 37°C, 5% CO2 incubator. On day 3, 100µL of human recombinant IL-2 (1000 IU/ml of R10A) were added to each well. On day 6, depending on the color of the medium and cell density, more IL-2 was added and cells were split. The culture was maintained at a cell density of 0.5–2 × 106 cells/ml. Fresh medium containing human IL-2 (1000 IU/ml) only (without Zometa) was added every 2–3 days, and cells were split as needed. Cells were harvested on day 10–12 and the frequency, phenotype, and functions of γδ T cells was determined by flow cytometry.
Adoptive transfer of autologous cells into macaques
For the IV administration of cells, monkeys were given 10 mg/kg ketamine IM for sedation. An IV catheter was placed in the cephalic or saphenous vein. Cells were washed twice and resuspended in sterile saline containing 100 U/mL heparin (APP pharmaceuticals, LLC) at a concentration of 1X108 cells/mL right before infusion. After cell infusion, the catheter was flushed with 3 ml sterile saline, and then removed.
Phenotyping of PBMC and BAL lymphocytes from macaques
Five antibodies, conjugated to FITC, PE, PECy7, PB and APC, were used for phenotyping of cell surface and intracellular markers of ex vivo expanded Vγ2Vδ2 T cells as well as lymphocytes isolated from blood and BAL samples of monkeys.
Cells were incubated with antibodies against cell surface markers for 15 minutes. In case needed, this was followed by cell permeabilization (FOXP3 Fix/Perm Buffer; eBioscience) (45 minutes, room temperature) and addition of antibodies for intracellular markers (45 minutes, room temperature). Cells were washed and fixed with 2% formalin and analyzed on an LSR Fortessa flow cytometer (BD Biosciences).
For T cell-subtype analysis, cells were washed and stained with surface antibodies; anti-human CD3 (SP34-2; BD Biosciences), anti-human Vγ2 (7A5; Thermo Scientific).
For T cell memory phenotype analysis, isolated cells were stained with anti-human CD3 (SP34-2; BD Biosciences), anti-human Vγ2 (7A5; Thermo Scientific), anti-human CD27 (O323; BioLegend), anti-human CD28 (CD28.2; BioLegend), and anti-human CD45RA (5H9; BD Biosciences) for 15 min at room temperature.
For phenotyping of adhesion molecules and homing receptors, cells were stained with anti-human CXCR3 (G025H7, BioLegend), CXCR2 (48311, R&D Systems), CD62L (SK11, eBioscience), CCR5 (3A9, BD Biosciences), CCR7 (G043H7, BioLegend), LFA-1 (6.7, eBioscience) and anti-non-human primates CXCR5 (87.1, eBioscience). Anti-human PD-1 (EH12.2H7, BioLegend) was used to inspect cell exhaustion. Anti-human Ki67 (Ki-67, BioLegend) was used to inspect cell proliferation. Anti-human CD107a (H4A3, BioLegend) was used to inspect degranulation of cytotoxic molecules.
Intracellular cytokine staining
Intracellular cytokine staining (ICS) was used to examine effector function(s) of ex vivo expanded Vγ2Vδ2 T cells as well as lymphocytes isolated from blood and BAL samples of monkeys. As previously described (21), 3–5×106 cells plus anti-human CD28 (BioLegend, 1µg/ml) and anti-human CD49d (BioLegend, 1µg/ml) were incubated with or without 40ng/mL HMBPP (provided by Dr. Hassan Jomaa, Giessen, Germany) in 150µl final volume of medium for 1 hour at 37°C, 5% CO2 followed by an additional 5 hours in the presence of monensin (GolgiStop, BD Biosciences). Five antibodies, conjugated to FITC, PE, PECy7, PB and APC, were used for staining of cell surface markers and intracellular cytokines. After staining cell surface markers for 15 minutes, cells were permeabilized for 45 minutes (FOXP3 Fix/Perm Buffer; eBioscience) and stained for intracellular cytokines/markers for another 45 minutes. Cells were washed and fixed with 2% formalin and analyzed on an LSR Fortessa flow cytometer (BD Biosciences).
For T helper 1 functions, cells were stained with anti-human IFN-γ (B27, BioLegend) and anti-human TNF-α (Mab11, BioLegend). For cytotoxic functions, cells were stained with anti-human Granzyme A (CB9, BioLegend), Granzyme B (GB11, BioLegend), Granulysin (eBioDH2, eBioscience) and Perforin (Pf-344, MABTECH). For pro-inflammatory functions, cells were stained with anti-human IL-1β (508A3H12, ThermoFisher Scientific), IL-6 (MQ2–6A3, BD Biosciences), IL-8 (E8N1, BioLegend) and IL17 (BL168, BioLegend). For Regulatory Functions, cells were stained with anti-human FoxP3 (259D, Biolgend), IL-10 (JES3–9D7, BioLegend) and TGF-β (TW4-2F8, BioLegend).
Inhibition of Mycobacteria growth assay
The THP1 human monocyte cell line was cultured in RPMI-1640 supplemented with 10 % heat inactivated fetal calf serum. Cells were seeded at 2 × 105 cells per well in 24 well plates and differentiated to macrophages by stimulation with 20 ng/ml phorbol myristate acetate (PMA) for 18 to 20 hours. Cells were subsequently washed with sterile medium and infected with BCG at a dose of 10 bacteria per cell. After 3 hours of incubation at 37°C, supernatants were aspirated and each well was washed three times to remove non-ingested mycobacteria and medium was replaced with 10% FBS containing RPMI-1640 supplemented with 10µg/mL gentamicin to kill all extracellular bacteria. Cells were then harvested using 5mM EDTA in PBS, pH 7.4. Ex vivo expanded macaque Vδ2Vδ2 T cells were used as effector cells and were aliquoted into wells containing target cells at the indicated ratios(Fig.1). The total volume of target effector: target cell mixture was 200 µl in all cases. At 24 hours following co-culture, cells were centrifuged, medium was removed, and cells were lysed using sterile water solution to release intracellular bacteria. Solutions were serially diluted and placed on Middlebrook 7H10 agar plates to determine CFU counts of BCG after 3–4 weeks of incubation at 37°C-5% CO2
Fig.1. Vγ2Vδ2 T cells generated for adoptive transfer exhibited multi-effector functions for producing anti-Mtb cytokines and inhibiting intracellular mycobacteria.

A). Representative flow histograms showing the percentage of Vγ2Vδ2 T cells in total CD3+ T-cell population before and after 12 days of ex vivo expansion using zoledronate and IL-2.
B). Representative flow histograms showing ex vivo expanded Vγ2Vδ2 T cells producing IFN-γ, TNF-α, Granzyme A and Granzyme B after 12-day culture with Zoledronate/IL-2. Panels were gated on CD3+ lymphocytes. Numbers in upper-right quadrant indicate the percentages of cytokine-producing Vγ2+ T cells in total Vγ2+ population.
C). Graph data showing percentages of cytokines-producing Vγ2Vδ2 T-cell subsets in total Vγ2+ population with or without HMBPP stimulation.
D). Representative flow histograms (left) show expression levels of the degranulation marker CD107a by expanded Vγ2Vδ2 T cells in the absence or presence of HMBPP. Graph data (right) demonstrate pool mean percentages of CD107a+Vγ2+ T cells in total Vγ2+ population with or without HMBPP stimulation.
E). Ex vivo expanded Vγ2Vδ2 T cells could kill/inhibit intracellular BCG(left) and Mtb(right) bacilli. Expanded Vγ2Vδ2 T cells were co-cultured with BCG-infected ThP1 monocytes at an effector to target ratio of 10:1 in the presence of blocking antibodies against IFN-γ, TNF-α, or Granzyme A or mouse IgG isotype. Cultured cells were lysed and lysates were serially diluted and plated on Middlebrook 7H10 agar plates. For the intracellular Mtb inhibition assay, expanded Vγ2Vδ2 T cells were similarly incubated for 72 hrs with autologous monocytes/macrophages infected with Mtb at a MOI of 10:1. Cells were then lysed, and lysates were plated on Middlebrook 7H11 agar.
Data in (A) to (E) are derived from 3–5 independent experiments. Values are expressed as means ± SEM. P values are results from non-parametric t test. *P < 0.05; **P < 0.01; ***P < 0.001. Similar trends of p values seen via ANOVA analysis.
The inhibition assay for restricting intracellular Mtb growth was done as we previously described (16). Similarly, zoledronate-expanded Vγ2Vδ2 T cells were incubated with autologous monocytes/ macrophages infected with Mtb at a MOI of 10:1. After 72 hrs incubation, cells were lysed, and lysates were plated on Middlebrook 7H11 agar. CFU were counted at 4 weeks.
Bacterial Strains
Mycobacterium bovis Karlson and Lessel (ATCC® 35748™) or strain TMC 1108 [BCG Pasteur SM-R] was used to infect monocytes for the inhibition of bacterial intracellular growth assay. The Erdman Strain of Mycobacterium tuberculosis (Mtb) was used for bronchoscope-guided infection in this study. This was a gift from Dr William Jacobs (Albert Einstein College of Medicine).
Mtb infection of cynomolgus macaques
Cynomolgus macaques were sedated with Ketamine (10mg/kg) and xylazine (1–2 mg/kg) by intramuscular injection. A pediatric bronchoscope was inserted into the right caudal lobe of the animals, and 500 CFUs of Mtb Erdman strain was injected in 3 mL of saline followed by a 3 mL bolus of air to ensure full dose administration. The inoculation dose for infection was confirmed as previously described (19).
Determining bacterial colony forming unit (CFU) counts in organ tissue homogenates
Details were described previously(19). Briefly, 1/3–1/2 tissues harvested from right caudal, right middle, and left caudal lung lobes and liver, spleen, and kidney of necropsied macaques were taken after gross pathological analysis was completed. If there were gross TB lesions in the respective lobe, a half of the lung tissue containing 50% lesions and 50% healthy tissue was taken. If no visible lesions were present in the respective lobe, a random piece of tissue was taken for tissue homogenization. Tissue homogenates were made using a homogenizer (PRO 200, PRO Scientific INC, CT) and diluting the homogenate in sterile PBS+0.05% Tween-80. 5-fold serial dilutions of samples were plated on Middlebrook 7H11 plate (BD). CFU counts on plates were measured at weeks 3–4 after the culture.
Gross pathologic analysis of TB lesions
The standard procedures were essentially the same as we previously described. Briefly, two months following infection, macaques were euthanized with pentobarbital 37.5 mg/kg IV) and immediately necropsied in a cabinet within a biosafety level 3 (BSL-3) facility. A pathologist and assistants performed a blinded gross pathologic evaluation using standard procedures. Each step was documented and reported in details and pictures were taken. Organs including lung lobes, bronchial, mesenteric, axillary and inguinal lymph nodes, tonsils, liver, spleen, kidneys, intestines and others were collected and labeled. Multiple tissue specimens were collected from all organs with gross lesions and also from organs showing no apparent lesions. For apparently infected organs, presence, number, location, size, distribution and consistency were recorded. A standard scoring system (19) was used to calculate gross pathology scores for TB lesions. All scorings of infected lungs and other tissues were performed in a blinded fashion.
Statistical analysis
Statistical analysis was done using paired or nonparametric t test and ANOVA using Graphpad software (Prism, La Jolla, CA) as we previously described(19).
Results
Vγ2Vδ2 T cells generated for adoptive transfer exhibited central/effector memory and tissue-trafficking phenotypes
As an initial effort, we validated our protocol of large-scale expansion of Vγ2Vδ2 T cells for transfer and characterized immune functions of these γδ T cells. Our protocol modified from human cancer immunotherapy (22–25) could reproducibly expand macaque Vγ2Vδ2 T cells in frozen peripheral blood mononuclear cells (PBMC) to a large scale, with purity of up to 91–94% (Fig.1A, Fig.S1.(A), although expansion magnitude and purity varied in individual macaques (Fig.S1). In fact, Vγ2Vδ2 T cells from most of ~60 macaques that we tested could undergo apparent expansion in response to Zoledronate/IL-2 stimulation(Fig.S1.(A). We also found that age and sex of macaques did not impact expansion outcome[Fig.S1.(A), (26)].
We then examined if Vγ2Vδ2 T cells generated for adoptive transfer could express memory and trafficking phenotypes relevant to effector functions and homing destinies in vivo (27–29). Almost all Vγ2Vδ2 T cells for transfer indeed expressed the CD45RA-CD27+ memory, not naïve or terminally-differentiated (TEMRA), phenotype [Supplemental Fig.S1.(B)]. ~80% of them polarized toward the effector memory (TEM) linked to effector function producing cytokines (27–29), and ~20% exhibited the CD28+ central memory(TCM) phenotype [Supplemental Fig.S1.(B)].
Concurrently, we examined homing potential of these Vγ2Vδ2 T cells generated for adoptive transfer. We focused on the potential expression of the following trafficking-related molecules: CXCR2 as homing for inflamed tissues(30), CXCR5, CCR7 and CD62L as tissue-homing (31–33), CXCR3 as trafficking/residence for Th1 cells (34), CCR5 as tissue-homing for CD4+ T cells(35), and LFA-1 as surrogate T cell-APC interaction in tissues (36, 37). In agreement with the TEM phenotype, almost all of these expanded Vγ2Vδ2 T cells exclusively expressed high levels of CXCR3, CCR5 and LFA-1, but not CXCR2, CXCR5, CCR7 or CD62L [Supplemental, Fig.S1.(C)].
Thus, Vγ2Vδ2 T cells generated for adoptive transfer expressed CD27+CD28+ effector memory and CD27-CD28+ central memory markers, with tissue trafficking phenotypes of CXCR3, CCR5 and LFA-1. Expression of these phenotypes appeared to reflect the potential ability of activated Vγ2Vδ2 T cells to traffic to and accumulate in pulmonary compartment after Mtb infection (16).
Vγ2Vδ2 T cells generated for adoptive transfer could produce anti-Mtb cytokines and inhibited intracellular mycobacteria
We then sought to examine whether Vγ2Vδ2 T cells generated for adoptive transfer could produce anti-Mtb cytokines, IFN-γ and TNF-α (38–40) as well as killing cytokines perforin, granulysin, and granzyme A and B (41, 42). We found that up to 61% of these Vγ2Vδ2 T cells could produce IFN-γ or TNF-α in response to HMBPP stimulation in culture, but only few of them expressed IL-17, IL-1β, IL-6, IL-8, IL-10 or TGF-β [Fig.1B, 1C, & Supplemental Fig.S1.(D)]. Moreover, up to 98% of these Vγ2Vδ2 T cells “constitutively” produced cytotoxic granule molecules granzyme B and A, respectively, with detectable low frequencies of granulysin or perforin production (Fig.1B, 1C). Interestingly, HMBPP triggering of TCR appeared to de-granulate or release granzymes A and B from cytotoxic granules (29), as numbers of the γδ T cells producing these two cytokines decreased notably upon HMBPP stimulation (Fig.1B, 1C). Consistently, HMBPP stimulation led to increases in numbers of Vγ2Vδ2 T cells producing CD107a (Fig.1D), which was considered a degranulation marker for releasing cascades of cytotoxic granules (43, 44),
The cytotoxic phenotype of these Vγ2Vδ2 T cells prompted us to assess them for the ability to inhibit or kill intracellular mycobacteria. To this end, Vγ2Vδ2 T cells were co-cultured with BCG-infected THP1 macrophages/monocytes. As shown in Fig.1E, at an effector: target ratio of 10:1, Vγ2Vδ2 T cells were able to induce ~70% inhibition of intracellular BCG growth. Anti-cytokine mAb-based neutralization assays showed that neutralization of TNF-α, IFN-γ or granzyme A could reduce the ability of Vγ2Vδ2 T cells to inhibit intracellular mycobacteria (Fig.1E).
Collectively, these results demonstrated that Vγ2Vδ2 T cells for transfer exhibited appreciable effector functions for producing anti-Mtb cytokines IFN-γ, TNF-α, granzyme A and inhibiting intracellular mycobacteria. Findings also consisted with the notion that IFN-γ, TNF-α or granzyme A produced by these “to-be-transferred” γδ T cells may help to enhance anti-mycobacterium activity(45, 46).
Transferred Vγ2Vδ2 T-cell subset could traffic to the airway and retain there detectable from 6 hours through 7 days after the infusion in naïve uninfected macaques
Next, we performed adoptive transfer experiments to determine whether infused Vγ2Vδ2 T effector cells could readily traffic to the pulmonary compartment and how long they could accumulate there. We feel that these two questions could be addressed more readily in naïve macaques without infection, as high-dose Mtb infection would rapidly expand non-transferred Vγ2Vδ2 T cells, making it difficult to determine transferred and non-transferred sources of autologous γδ T cells in infected macaques.
In the adoptive transfer experiments, ~6–10×10^7 PBMC were collected and frozen 4–5 times from 3 naïve macaques; each time drawing ~30–40 ml blood. PBMC frozen from single individual macaques were then pooled after thaw, and expanded to a large scale for autologous infusion. To facilitate immuno-kinetic studies, each macaque was infused with 50% fluorescently-labeled and 50% unlabeled Vγ2Vδ2 T cells. The fluorescent lipophilic dye PKH26 was used to label cells despite its toxic potential, since PKH26, but not CSFE, does not interfere with the fluorescence of FITC-conjugated anti-Vγ2 Ab during flow cytometry-based tracking of γδ T cells. Both the labeled and unlabeled Vγ2Vδ2 T-cell subpopulations were tracked in blood and BALF over time after infusion of macaques.
Interestingly, transferred Vγ2Vδ2 T-cell subset appeared to traffic to the airway as early as 6 hours, and retain there detectable through 7 days after the infusion in naïve uninfected macaques. PKH26-labeled Vγ2Vδ2 T cells in BALF increased >500 folds from baseline <0.003% to 2.43% at 6 hr, sustained 500–1000-folds at 24 hr and 48 hr respectively, and remained detectable through 7 days after the adoptive transfer (Fig.2B, bottom panel). Consistently, major increases in all Vγ2Vδ2 T cells in the airway were detected as well, constituting up to 47% of Vγ2Vδ2 T cells in the whole CD3+ T-cell population in BALF at days 1–7 after the adoptive transfer (Fig.2B, top panel; 2C). Such increases were most apparent for the macaque CN8365 infused with 3×10^8 cells/kg of Vγ2Vδ2 T cells (Fig.2A, 2C). After adoptive transfer, αβ T populations or speculating macrophages /neutrophils did not undergo such striking increases as Vγ2Vδ2 T cells did in BAL fluid (data not shown). Of note, although there were only subtle increases in all Vγ2Vδ2 T cells in the blood samples after the infusion(Fig.S2.), PKH26-labeled Vγ2Vδ2 T cells in blood significantly increased at 5 minutes through 48 hours(Supplemental Fig.S2). Notably, comparative analysis showed that the frequencies of PKH26-labeled Vγ2Vδ2 T cells and the whole Vγ2Vδ2 T-cell subpopulation in the airway were 10 times greater than those detected in the blood at 24 – 48 hours after infusion (Fig.2B–C, Supplemental Fig.S2).
Fig.2. Transferred Vγ2Vδ2 T-cell subset could traffic to the airway and retain there detectable from 6 hours through 7 days after the infusion in naïve uninfected macaques.

A). Three naïve macaques were infused with variable numbers of ex vivo expanded Vγ2Vδ2 T cells per kilogram, half of which were fluorescently labelled with PKH26.
B). Representative flow histograms showing the percentages of Vγ2+ cells in BALF samples collected from CN 8365 (3×108 cells/Kg) overtime after infusion. Upper panels show percentages of Vγ2+ cells out of CD3+ T cells. Lower panels show the percentage of PKH26+ Vγ2+ T cells out of CD3+ T cells overtime after infusion.
C). Graph data showing percentages of Vγ2+ cells of the CD3+ T-cell population in BALF samples collected from 3 monkeys overtime after infusion.
D). Representative flow histograms showing that infused Vγ2+ cells were still able to produce TNF-α in response to HMBPP stimulation at 24 and 48 hours post infusion. Numbers in upper-right quadrants indicate the percentages of cytokine-producing Vγ2+ cells out of total Vγ2+ population.
Because there were not enough lymphocytes in most BALF samples collected from non-inflamed lungs of these un-infected macaques, we were only able to perform phenotypic staining analysis, but not standard intracellular cytokine staining (ICS) assays that require large cell numbers. However, some extra BALF cells from the macaque (CN8365) infused with the largest number of γδ T cells allowed us to run ICS assays. The limited ICS analysis showed that Vγ2Vδ2 T cells in BALF collected at 24 hr and 48 hr after infusion were still able to produce TNF-α in response to HMBPP re-stimulation in culture(Fig.2D). Such detection at 24–48 hrs appeared to partially recapitulate TNF-α production seen before adoptive transfer (Fig.1B, 1C).
Collectively, these results suggest that transferred Vγ2Vδ2 T cells may rapidly traffic to the airway as early as 6 hours after adoptive transfer, and remain detectable in the pulmonary compartment for at least 7 days in uninfected macaques.
Adoptive transfer of autologous γδ T cells after Mtb infection led to early increases in pulmonary Vγ2Vδ2 T-cell subset with proliferating phenotype
Given that Vγ2Vδ2 T cells for adoptive transfer displayed anti-Mtb effector functions and underwent pulmonary trafficking after transfer, we sought to examine whether adoptively-transferred Vγ2Vδ2 T cells could attenuate Mtb infection. To this end, we infused considerably-pure autologous Vγ2Vδ2 T cells back to respective seven macaques two times after Mtb infection (Table 1). For evaluation of anti-Mtb function, we did not transfer bio-labeled Vγ2Vδ2 T cells in Mtb-infected macaques because bio-labeling reagents could potentially impact viability and function of macaque T cells(47). Two control groups were included in the study. One group of macaques similarly received infusion of autologous peripheral blood lymphocytes (PBL) generated in cultures containing IL-2 only, whereas another control group received saline injection. Since this was a POC study, we elected to adoptively transfer unlabeled Vγ2Vδ2 T cells at days 3 and 14 after high-dose (500 CFU) Mtb infection. These early time points would help to determine potential early protection by transferred Vγ2Vδ2 T cells, since non-transferred Vγ2Vδ2 T cells could usually start to increase in the airway at 2–3 weeks after pulmonary infection of macaques with high-dose Mtb and other pathogens (14, 48). In addition, we adoptively transferred ~108 Vγ2Vδ2 T cells per monkey (kg), taking considerations of macaque’s blood volume and the numbers of cells infused in mice [≥106 cells, (49, 50)] and in cancer patients [ ≥109 cells, (22–24)]. To minimize variations, we performed adoptive transfer and evaluation in two separate experiments, each one involving 3–4 macaques for the test group and control groups, respectively.
Table 1. Numbers and purity of Vγ2Vδ2 T cells infused in test group of Mtb-infected macaques.
| Monkey ID Vγ2Vδ2 group |
Day 3 # Cells Infused / Purity |
Day 18 # Cells Infused / Purity |
Total Vγ2Vδ2 cells infused/Kg |
|---|---|---|---|
| CN 7826 | 1.25 X 108 / 93% | 2.85 X 108 / 91% | 1.02 X 108 |
| CN 8343 | 1.25 X 108 / 80% | 2.27 X 108 / 83% | 0.83 X 108 |
| CN 8345 | 1.25 X 108 / 89% | 3.91 X 108 / 82% | 1.12 X 108 |
| CN 8346 | 1.25 X 108 / 90% | 4.85 X 108 / 86% | 0.55 X 108 |
| CN 8730 | 1.31 X 108 / 76% | 0.98 X 108 / 78% | 1.6 X108 |
| CN 8732 | 1.13 X 108 / 90% | 1.33 X 108 / 88% | 1.77 X 108 |
| CN 8738 | 1.69 X 108 / 75% | 1.99 X 108 / 81% | 2.58 X 108 |
Similar to the results in naïve macaques(Fig.2), up to 4.5-fold increases in percentage numbers of Vγ2Vδ2 T cells were detected in BALF collected at 48 hr after the first infusion (1 week after Mtb) compared to baseline in the test group, but no increases were seen in the control groups (Fig3.A). Consistently, the test group showed >10-fold increases in percentages of Vγ2Vδ2 T effector cells expressing Ki67, the marker for cell proliferating function (51), in the airway BALF (Fig.3.B) and blood at 1 week, supporting the notion of pulmonary trafficking of transferred cells. However, at 3 weeks and later after high-dose Mtb infection, the test and control groups exhibited comparable numbers of Vγ2Vδ2 T cells and Ki67+ Vγ2Vδ2 T cells in the airway (Fig.3). This might be explained by the possibility that progressive high-dose Mtb infection at week 3 and later times in the control groups induced pulmonary trafficking and accumulation of non-transferred Vγ2Vδ2 T cells, whereas these TB-driven increases might not be apparent in the test group due to potential early reduction of Mtb burdens by transferred Vγ2Vδ2 T cells. This might also result from greater extents of inflammation and lesions in lung tissues of the control groups. Consistent with this scenario, no significant differences in Vγ2Vδ2 T cells producing cytokines were found either in BALF and blood between the test and control groups at 3 weeks and later times after the high-dose Mtb infection (Supplemental Fig.S3), and data not shown). As described above, ICS analysis of cells in BALF at 1 week could not be performed due to a shortage of airway lymphocytes in BALF in such very early Mtb infection.
Fig.3. Adoptive transfer of autologous γδ T cells after Mtb infection led to early increases in pulmonary Vγ2Vδ2 T-cell subset with proliferating phenotype.

A). Representative flow histograms on left show the percentage of Vγ2+ T cells in BALF samples from the test group infused with Vγ2Vδ2 T cells and the control groups infused with PBL or saline overtime after Mtb infection. Numbers in histograms indicate the percentages of Vγ2+ T cells in CD3+ T-cell population. Vγ2 T cells were interpreted as Vγ2Vδ2 T cells as described in our previous publications. Graph data on right show fold changes in the percentages of Vγ2+ T cells in BALF samples from the test group and the control groups.
(B). Representative flow cytometry histograms(left) and graph data(right) show that the test group, but not control groups, exhibited >10-fold increases in percentages of Vγ2Vδ2 T cells expressing Ki67, the marker for cell proliferation, in airway BALF collected at 1 week after infusion. Similar increases were seen for test and control groups at 3 weeks and latter time points in airway BALF. Similar trends of results were seen in the blood(data not shown)
Collectively, the results in naïve and Mtb-infected macaques suggest that adoptively-transferred Vγ2Vδ2 T cells after high-dose Mtb infection were able to traffic to the pulmonary compartment and retain a surrogate marker of proliferation in early infection phase.
Adoptive transfer of Vγ2Vδ2 T cells in Mtb-infected macaques led to decreases in Mtb infection burdens in lungs and extra-pulmonary organs, without an apparent loss of body-weights
We next sought to examine whether the adoptive transfer of Vγ2Vδ2 T cells after high-dose Mtb infection could lead to changes in Mtb infection burdens and clinical status. While the control groups exhibited clinical deterioration featured by a progressive loss (>15%) of body-weights during Mtb infection course, no apparent decreases in body weights were noted in the test group receiving adoptive transfer of Vγ2Vδ2 T cells(Fig.4a). Measurements of Mtb infection burdens demonstrated that the test group exhibited significantly lower numbers of bacilli CFU counts in the right caudal lung lobe (infection site), right middle lobe and left caudal lobe than the control groups (Fig.4B). The reduced Mtb burdens in lung lobes were consistent with early increases in transferred Vγ2Vδ2 T cells in the airway after adoptive transfer (Figs.2–3; Figs.S2–S2). Furthermore, detectable increases in Vγ2Vδ2 T cells early after adoptive transfer were also found coincident with subsequent decreases in Mtb dissemination to extra-pulmonary organs since the test group showed significant lower Mtb bacilli CFU counts in the spleen, liver and kidney than the control groups (Fig.4B).
Fig.4. Adoptive transfer of Vγ2Vδ2 T cells in Mtb-infected macaques led to decreases in Mtb infection burdens in lungs and extra-pulmonary organs, without apparent losses of body weights.

A). Graph data showing percentage of body weight losses in the three groups of macaques at indicated time points after Mtb infection. Values are expressed as means ± SEM.
B). and C). Graph data showing bacterial burdens (CFU counts) in homogenized tissues (1 cm3) of different lung lobes (B) and extra-pulmonary organs (C) collected at the necropsy time from the test and control groups. Values are expressed as means ± SEM. P values are results from non-parametric t test. *P < 0.05; **P < 0.01. Similar trends were seen via ANOVA analysis.
Thus, these results suggest that adoptive transfer of Vγ2Vδ2 T cells in Mtb-infected macaques could reduce pulmonary Mtb infection and limit extra-pulmonary Mtb dissemination, containing TB-driven loses of body weights.
Adoptive transfer of Vγ2Vδ2 T cells in Mtb-infected macaques attenuated TB pathology and contained lesions mostly within the right caudal lobe (infection site)
Finally, we sought to determine whether the adoptive transfer of Vγ2Vδ2 T cells in Mtb-infected macaques could impact TB pathology/lesions and TB dissemination. We conducted complete necropsy study at the end point to comparatively examine gross TB pathology or lesions between the test and control groups. Notably, most macaques in PBL and saline control groups exhibited widespread caseating TB granulomas/lesions in right caudal, middle, and cranial lobes, with coalescing granulomas often extending to left lung lobes (Fig.5A). Moreover, TB lesions in most control groups of macaques readily disseminated to the thoracic pleura and extra-pulmonary organs including the spleen, liver, and kidney (Fig.S4).
Fig.5. Adoptive transfer of Vγ2Vδ2 T cells in Mtb-infected macaques could result in attenuation of TB lesions and containment of TB in the right caudal lobe(infection site).
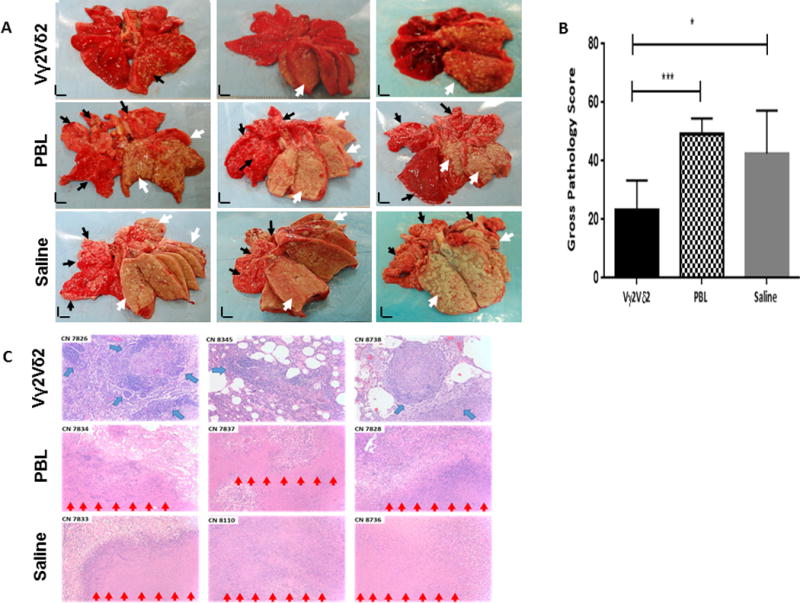
A). Shown are representative digital pictures of cut sections of all lung lobes from the test control groups. The right caudal lung lobe, the Mtb infection site, was displayed in the bottom right side of each photo. Lungs and other organs were obtained in necropsy at week 9 after Mtb infection. The test group of macaques showed apparent decreases in severity of TB lesions and extent of TB dissemination to the upper right lung lobes, left lung lobes and extra-pulmonary organs compared to the PBL and saline control groups. Extent and severity of the TB lesions could be adjudged based on the examples pointed by white arrows indicating caseation pneumonia or extensive coalescing granulomas and by small arrows demonstrating less-coalescing or non-coalescing granulomas. Vertical/horizontal bars at bottom-left represent the 1-cm scale derived from the fluorescence rulers of each original photo. Comparative gross pathology of extra-pulmonary organs was shown in Supplemental Fig.S4.(A),(B).
B). Graph data of mean gross pathology scores between the test and control groups. Gross pathology scoring was performed blindly as previously reported in our serial publications. Values are expressed as means ± SEM. P values are results from non-parametric t test. *P < 0.05; ***P < 0.001. Similar p values were seen via ANOVA analysis.
C). Histology evaluation of tissue sections of right caudal lung lobes from the test and control groups. Shown are H&E stained sections taken from three representative macaques for each group, with macaque ID and magnification indicated for each image. Note that the PBL and saline controls displayed extensive necrotic TB lesions (red arrows), with inflammatory cells and edema fluid presenting in the peripheries of TB granulomas in the right caudal and other lung lobes. TB granulomas identified in the right caudal lobes from the test group usually exhibited more lymphocytic and less necrotic histopathology than those from the control groups. Most macaques in the test group showed small and well-contained TB granulomas in right caudal lobes, with numerous infiltrating lymphocytes forming cuffs (blue arrows) in the peripheries of TB granulomas. More comparative histopathology data are shown in Supplemental Fig.S4.(A),(B).
In contrast, the test group receiving adoptive transfer of Vγ2Vδ2 T cells exhibited less extensive TB granulomas and contained severe TB lesions mostly within the infection site, right caudal lobe (Fig.5A). In fact, two of seven macaques in the test group only showed small less-coalescing TB granulomas that were confined to the right caudal lung lobe (infection site) without any involvements in other lung lobes(Fig.5A). Other 5 animals in the test group showed coalescing or less-coalescing granulomas in the right caudal lobe, with no or very few small non-coalescing TB granulomas in the right middle or cranial lobe, but did not display any apparent TB lesions in left lung lobes (Fig.5A). Furthermore, adoptive transfer of Vγ2Vδ2 T cells in Mtb-infected macaques led to a lack of extra-pulmonary TB dissemination to the spleens [Fig.S4.(A)], with no or reduced occurrence of TB dissemination to livers or kidneys [Fig.S4.(B)]. Virtually, only two of seven macaques in the test group showed 1–2 tiny gross granulomas in the liver or kidney, which were barely seen by in situ close examination of the organs, but not readily revealed in picture [Fig.S4.(A)]. When gross TB pathology was comparatively evaluated between the test and control groups using the scoring criteria as repeatedly described by us and others, the mean pathology score in the test group was significantly lower than that in each of the control groups (Fig.5B).
Overall, histopathology analysis appeared to be consistent with the gross pathology findings. The control groups showed extensive necrotic TB lesions, with inflammatory cells and edema fluid presenting in the peripheries of necrotic TB granulomas in right caudal and other lung lobes (Fig.5C). On the contrary, the test group usually exhibited less necrotic and more lymphocytic histopathology for TB granulomas even in the right caudal lobe than the control groups (Fig.5C). Most macaques in the test group showed well-contained TB granulomas in the right caudal lobe, with numerous infiltrating lymphocytes that form cuffs in the peripheries of TB granulomas (Fig.5C).
The histopathology findings were in agreement with the in situ analysis of γδ T-cell cellular components in lung sections. We observed a consensus that Vγ2 T cells expressing CXCR3 and Ki67 were more readily detected in the lung sections from the test group compared to the control groups (Fig.6).
Fig.6. Lung resident Vγ2Vδ2 T cells in the infection site.

Representative in situ confocal microscopic images (63x NA) of Vγ2 T effector cells that express the proliferating marker Ki67 and tissue-resident marker CXCR3 in tissue sections of the right caudal lung lobes from the test group infused with Vγ2Vδ2 T cells(top panel,CN8738) and the control group infused with PBL(bottom panel,CN7837). The Vγ2 TCR (green) in the test group appear to co-express with Ki67 (red) and CXCR3 (yellow) in the merged images (co-localization marked by arrows) in lung tissue sections. The bottom panel shows very few Vγ2+ cells or Ki67+ cells detected in the right caudal lung section from the representative PBL control group. References of image sizes were marked in bottom right corner. Similar results for Vγ2 T effector cells were seen in other test and control macaques. IgG isotype controls did not give rise to any immune staining in the lung TB tissue sections [see Supplemental Fig.S4.(C)].
Taken together, the results implicated that adoptive transfer of Vγ2Vδ2 T cells in Mtb-infected macaques attenuated TB pathology and contained lesions mostly in the infection site of caudal lung lobe, with no or reduced TB dissemination to other lobes, spleens or livers/kidneys.
Discussion
The current study employs adoptive transfer strategy to prove the concept of protection for the major Vγ2Vδ2 T-cell subset in Mtb infection. This proof-of-concept (POC) study presents the first experimental evidence that adoptive transfer of phosphoantigen-specific γδ T-effector subset can attenuate high-dose Mtb infection in a macaque model. The comparative evaluation between groups shows appreciable protection at three levels: control of TB-induced weight loss; decreases in Mtb infection burdens in the lung and extra-pulmonary organs; attenuation and containment of TB lesions mostly without dissemination to left lung lobes. These findings from the POC study therefore suggest that Vγ2Vδ2 T effector cells, the sole T-cell subset capable of recognizing Mtb phosphoantigen, could confer detectable protection against Mtb infection.
The POC observation may have been long awaited in the field since γδ T cells were discovered 30 years ago. Despite that mouse models of Mtb infection are useful, it has been argued that data in δ-TCR knock-out mice(18) may not be relevant due to the lack of evidence that mouse γδ T cells can recognize HMBPP or other Mtb antigens. On the other hand, protective human studies cannot be done due to ethical and technical issues. Although nonhuman primates are highly relevant to humans, the field has been wrestling with a lack of useful tools to generate γδ T cell-deficient macaques or depleting antibodies for in-depth studies.
The POC protection against Mtb infection by Vγ2Vδ2 T-cell subset is not totally unexpected. In vitro studies in humans and non-human primates have demonstrated that Vγ2Vδ2 T cells can lyse Mtb-infected cells and inhibit intracellular mycobacteria in monocytes/macrophages(52). In addition, anti-Mtb effector function producing IFN-γ/TNF-α by these γδ T cells has long been documented by the studies in both humans and macaques(53–55). These anti-Mtb activities seen in vitro likely represent the protective nature of transferred Vγ2Vδ2 T cells. Furthermore, recent in vivo study also showed that early expansion/differentiation of Vγ2Vδ2 T cells by cHMBPP plus IL-2 treatment can increase resistance to Mtb infection(16), although one cannot eliminate the possibility that IL-2 itself also contributes to the protection. Now the current POC study provides compelling data demonstrating for the first time that Vγ2Vδ2 T-cell effector subset can mediate protection against high-dose Mtb infection.
From a mechanistic standpoint, the POC documentation of protective Vγ2Vδ2 T cells appears to be linked to early trafficking and accumulation of these Mtb-specific γδ T-cell subset in the pulmonary compartment after the adoptive transfer. This notion is supported by our data collected in BALF versus blood at early time points after the adoptive transfer of γδ T cells in both naïve uninfected macaques and Mtb-infected animals. Nevertheless, there were no significant differences in Vγ2Vδ2 T cells in BALF between the test and control groups at 3 weeks and later after Mtb infection. This could be explained by the interpretation that progressive high-dose Mtb infection at 3 weeks and later could stimulate apparent expansion of non-transfer Vγ2Vδ2 T cells, overshadowing the airway-trafficking of transferred γδ T-cell subset. This interpretation might also help to explain why at later time points there were no significant differences in cytokine-producing Vγ2Vδ2 T effector cells in BALF between the groups when ICS assays were performed using in vitro HMBPP re-stimulation of BALF cells. In this context, initial Mtb infection at one week drove few cells to the airway BALF, leading to unsuccessful ICS measuring of cytokine-producing γδ T effector cells. It is worth to mention that airway γδ T cells in BALF were not the same as those lung tissue-resident Vγ2Vδ2 T cells since these airway γδ T cells had migrated to the airway out of vessel endothelia and lung epithelia, and their migration/retention in the airway could be influenced by lung inflammation/lesions or other factors.
Significant numbers of adoptively-transferred Vγ2Vδ2 T cells might reside in the lung tissue/parenchyma after infusion, and these tissue-resident γδ T cells could contribute to the detectable protection. This presumption appeared to be consistent with the in-situ confocal imaging data demonstrating that CXCR3+Ki67+Vγ2Vδ2 T cells were more readily detected in the lung of test group infused with γδ T cells compared to the control groups. However, proving this presumption would require either the future use of nontoxic long-live labeling of transferred γδ T cells or the multiple-time euthanizing of macaques for in-depth studies of lung-resident γδ T cells from early through late time points after Mtb infection. While the nontoxic long-live labeling is not possible now, multiple euthanasia for assessing lung tissues were not initially designed in the current study due to IACUC ethics and budget constraints. These intensive experiments appear to be beyond the scope of the current POC study.
Presumably, anti-Mtb responses might be taking place early and efficiently in lung tissues of macaques transferred with γδ T cells, given the possibility that adoptively transferred Vγ2Vδ2 T effector cells could actively attack Mtb-infected cells upon Mtb encounter via the action of IFN-γ/TNF-α or perforin/granzymes. Of note, Mtb CFU were also significantly reduced in the caudal lobe where Mtb was initially introduced, suggesting that in addition to blocking dissemination, transferred Vγ2Vδ2 T cells also directly kill or inhibit Mtb at the initial locus of infection. This notion is consistent with the findings that Vγ2Vδ2 T cells generated for adoptive transfer clearly exhibited such anti-Mtb effector functions of producing cytokines and killing/inhibiting intracellular mycobacteria.
In summary, adoptive transfer strategy in the current POC study helps to prove the principle that adoptive transfer of Vγ2Vδ2 T effector cells would protect against Mtb infection. Findings suggest that rational TB vaccine development or BCG boosting may consider inclusion of the major Vγ2Vδ2 T-cell subset.
Supplementary Material
Acknowledgments
We thank other Chen Lab staff for technical assistance.
This work was supported by the NIH R01 grants(RO1OD015092, RO1HL64560, RO1HL129887.
Footnotes
All authors do not have any competing financial Interests.
Author contributions: AQ, DH and CYC contributed equally, sharing 1st authorship. AQ, DH, CYC, LS, ZWC analyzed data. AQ, LS, ZWC wrote manuscript. Other co-authors provide scientific input or comments for this long-term project.
References
- 1.Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, Castro KG, Weyer K. HIV Infection and Multidrug-Resistant Tuberculosis—The Perfect Storm. Journal of Infectious Diseases. 2007;196:S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 2.Lawn SD, Zumla AI. Tuberculosis. The Lancet. 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA, Brewer TF, Berkey CS, Mosteller F. Efficacy of bcg vaccine in the prevention of tuberculosis: Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 4.Kaufmann HSE, Gengenbacher M. Recombinant live vaccine candidates against tuberculosis. Current Opinion in Biotechnology. 2012;23:900–907. doi: 10.1016/j.copbio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 5.RODRIGUES LC, DIWAN VK, WHEELER JG. Protective Effect of BCG against Tuberculous Meningitis and Miliary Tuberculosis: A Meta-Analysis. International Journal of Epidemiology. 1993;22:1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 6.Trunz BB, Fine PEM, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. The Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 7.Yuk J-M, Jo E-K. Host immune responses to mycobacterial antigens and their implications for the development of a vaccine to control tuberculosis. Clin Exp Vaccine Res. 2014;3:155–167. doi: 10.7774/cevr.2014.3.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belmant C, Espinosa E, Poupot R, Peyrat MA, Guiraud M, Poquet Y, Bonneville M, Fournie JJ. 3-Formyl-1-butyl pyrophosphate A novel mycobacterial metabolite-activating human gammadelta T cells. J Biol Chem. 1999;274:32079–32084. doi: 10.1074/jbc.274.45.32079. [DOI] [PubMed] [Google Scholar]
- 9.Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett. 2003;544:4–10. doi: 10.1016/s0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 10.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZW. Immune biology of Ag-specific gammadelta T cells in infections. Cell Mol Life Sci. 2011;68:2409–2417. doi: 10.1007/s00018-011-0703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davey MS, Lin CY, Roberts GW, Heuston S, Brown AC, Chess JA, Toleman MA, Gahan CG, Hill C, Parish T, Williams JD, Davies SJ, Johnson DW, Topley N, Moser B, Eberl M. Human neutrophil clearance of bacterial pathogens triggers anti-microbial gammadelta T cell responses in early infection. PLoS pathogens. 2011;7:e1002040. doi: 10.1371/journal.ppat.1002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meraviglia S, Caccamo N, Salerno A, Sireci G, Dieli F. Partial and ineffective activation of V gamma 9V delta 2 T cells by Mycobacterium tuberculosis-infected dendritic cells. Journal of immunology (Baltimore, Md:1950) 2010;185:1770–1776. doi: 10.4049/jimmunol.1000966. [DOI] [PubMed] [Google Scholar]
- 14.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao S, Huang D, Chen CY, Halliday L, Zeng G, Wang RC, Chen ZW. Differentiation, distribution and gammadelta T cell-driven regulation of IL-22-producing T cells in tuberculosis. PLoS Pathog. 2010;6:e1000789. doi: 10.1371/journal.ppat.1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CY, Yao S, Huang D, Wei H, Sicard H, Zeng G, Jomaa H, Larsen MH, Jacobs WR, Jr, Wang R, Letvin N, Shen Y, Qiu L, Shen L, Chen ZW. Phosphoantigen/IL2 expansion and differentiation of Vgamma2Vdelta2 T cells increase resistance to tuberculosis in nonhuman primates. PLoS Pathog. 2013;9:e1003501. doi: 10.1371/journal.ppat.1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang D, Chen CY, Ali Z, Shao L, Shen L, Lockman HA, Barnewall RE, Sabourin C, Eestep J, Reichenberg A, Hintz M, Jomaa H, Wang R, Chen ZW. Antigen-specific Vgamma2Vdelta2 T effector cells confer homeostatic protection against pneumonic plaque lesions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7553–7558. doi: 10.1073/pnas.0811250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. The Journal of experimental medicine. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CY, Huang D, Yao S, Halliday L, Zeng G, Wang RC, Chen ZW. IL-2 Simultaneously Expands Foxp3+ T Regulatory and T Effector Cells and Confers Resistance to Severe Tuberculosis (TB): Implicative Treg-T Effector Cooperation in Immunity to TB. The Journal of Immunology. 2012;188:4278–4288. doi: 10.4049/jimmunol.1101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams CR, Halliday LC, Nunamaker EA, Fortman JD. Effects of Weekly Blood Collection in Male and Female Cynomolgus Macaques (Macaca fascicularis) Journal of the American Association for Laboratory Animal Science. 2014;53:81–88. [PMC free article] [PubMed] [Google Scholar]
- 21.Ali Z, Yan L, Plagman N, Reichenberg A, Hintz M, Jomaa H, Villinger F, Chen ZW. γδ T Cell Immune Manipulation during Chronic Phase of Simian HIV Infection Confers Immunological Benefits. The Journal of Immunology. 2009;183:5407–5417. doi: 10.4049/jimmunol.0901760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi H, Tanaka Y, Yagi J, Minato N, Tanabe K. Phase I/II study of adoptive transfer of gammadelta T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer immunology, immunotherapy : CII. 2011;60:1075–1084. doi: 10.1007/s00262-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakajima J, Murakawa T, Fukami T, Goto S, Kaneko T, Yoshida Y, Takamoto S, Kakimi K. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2010;37:1191–1197. doi: 10.1016/j.ejcts.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 24.Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, Nieda M. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. British journal of cancer. 2011;105:778–786. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo M, Izumi T, Fujieda N, Kondo A, Morishita T, Matsushita H, Kakimi K. Expansion of Human Peripheral Blood γδ T Cells using Zoledronate. 2011:e3182. doi: 10.3791/3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roux A, Mourin G, Larsen M, Fastenackels S, Urrutia A, Gorochov G, Autran B, Donner C, Sidi D, Sibony-Prat J, Marchant A, Stern M, Sauce D, Appay V. Differential Impact of Age and Cytomegalovirus Infection on the γδ T Cell Compartment. The Journal of Immunology. 2013;191:1300–1306. doi: 10.4049/jimmunol.1202940. [DOI] [PubMed] [Google Scholar]
- 27.Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A. Differentiation of Effector/Memory Vδ2 T Cells and Migratory Routes in Lymph Nodes or Inflammatory Sites. The Journal of Experimental Medicine. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunological Reviews. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 29.Pang DJ, Neves JF, Sumaria N, Pennington DJ. Understanding the complexity of gammadelta T-cell subsets in mouse and human. Immunology. 2012;136:283–290. doi: 10.1111/j.1365-2567.2012.03582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJB, Sansom OJ. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. The Journal of clinical investigation. 2012;122:3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt N, Bentebibel S-E, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends in Immunology. 35:436–442. doi: 10.1016/j.it.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hengel RL, Thaker V, Pavlick MV, Metcalf JA, Dennis G, Yang J, Lempicki RA, Sereti I, Lane HC. Cutting Edge: L-Selectin (CD62L) Expression Distinguishes Small Resting Memory CD4+ T Cells That Preferentially Respond to Recall Antigen. The Journal of Immunology. 2003;170:28–32. doi: 10.4049/jimmunol.170.1.28. [DOI] [PubMed] [Google Scholar]
- 34.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barmania F, Pepper MS. C-C chemokine receptor type five (CCR5): An emerging target for the control of HIV infection. Applied & Translational Genomics. 2013;2:3–16. doi: 10.1016/j.atg.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu Y. LFA-1: more than just T cell Velcro. Nature immunology. 2003;4:1052–1054. doi: 10.1038/ni1103-1052. [DOI] [PubMed] [Google Scholar]
- 37.Verma NK, Kelleher D. Adaptor regulation of LFA-1 signaling in T lymphocyte migration: Potential druggable targets for immunotherapies? European journal of immunology. 2014;44:3484–3499. doi: 10.1002/eji.201344428. [DOI] [PubMed] [Google Scholar]
- 38.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis Associated with Infliximab, a Tumor Necrosis Factor α-Neutralizing Agent. New England Journal of Medicine. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 39.Roach DR, Bean AGD, Demangel C, France MP, Briscoe H, Britton WJ. TNF Regulates Chemokine Induction Essential for Cell Recruitment, Granuloma Formation, and Clearance of Mycobacterial Infection. The Journal of Immunology. 2002;168:4620–4627. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 40.Abebe F. Is interferon-gamma the right marker for bacille Calmette-Guérin-induced immune protection? The missing link in our understanding of tuberculosis immunology. Clinical & Experimental Immunology. 2012;169:213–219. doi: 10.1111/j.1365-2249.2012.04614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spencer CT, Abate G, Sakala IG, Xia M, Truscott SM, Eickhoff CS, Linn R, Blazevic A, Metkar SS, Peng G, Froelich CJ, Hoft DF. Granzyme A produced by gamma(9)delta(2) T cells induces human macrophages to inhibit growth of an intracellular pathogen. PLoS pathogens. 2013;9:e1003119. doi: 10.1371/journal.ppat.1003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodworth JS, Wu Y, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J Immunol. 2008;181:8595–8603. doi: 10.4049/jimmunol.181.12.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolint P, Betts MR, Koup RA, Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J Exp Med. 2004;199:925–936. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barber DL, Wherry EJ, Ahmed R. Cutting Edge: Rapid In Vivo Killing by Memory CD8 T Cells. The Journal of Immunology. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 45.Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–4627. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 46.Spencer CT, Abate G, Sakala IG, Xia M, Truscott SM, Eickhoff CS, Linn R, Blazevic A, Metkar SS, Peng G, Froelich CJ, Hoft DF. Granzyme A Produced by γ<sub>9</sub>δ<sub>2</sub> T Cells Induces Human Macrophages to Inhibit Growth of an Intracellular Pathogen. PLoS pathogens. 2013;9:e1003119. doi: 10.1371/journal.ppat.1003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li P, Zhang R, Sun H, Chen L, Liu F, Yao C, Du M, Jiang X. PKH26 can transfer to host cells in vitro and vivo. Stem cells and development. 2013;22:340–344. doi: 10.1089/scd.2012.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frencher JT, Shen H, Yan L, Wilson JO, Freitag NE, Rizzo AN, Chen CY, Chen ZW. HMBPP-deficient Listeria mutant immunization alters pulmonary/systemic responses, effector functions, and memory polarization of Vgamma2Vdelta2 T cells. Journal of leukocyte biology. 2014;96:957–967. doi: 10.1189/jlb.6HI1213-632R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu W, Zheng J, Liu Y, Sia SF, Liu M, Qin G, Ng IH, Xiang Z, Lam KT, Peiris JS, Lau YL. The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a gammadelta T cell population in humanized mice. J Exp Med. 2011;208:1511–1522. doi: 10.1084/jem.20110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beck BH, Kim HG, Kim H, Samuel S, Liu Z, Shrestha R, Haines H, Zinn K, Lopez RD. Adoptively transferred ex vivo expanded gammadelta-T cells mediate in vivo antitumor activity in preclinical mouse models of breast cancer. Breast cancer research and treatment. 2010;122:135–144. doi: 10.1007/s10549-009-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shedlock DJ, Talbott KT, Morrow MP, Ferraro B, Hokey DA, Muthumani K, Weiner DB. Ki-67 staining for determination of rhesus macaque T cell proliferative responses ex vivo. CytometrypPart A. 2010;77A:275–284. doi: 10.1002/cyto.a.20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dieli F, Troye-Blomberg M, Ivanyi J, Fournie JJ, Krensky AM, Bonneville M, Peyrat MA, Caccamo N, Sireci G, Salerno A. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. The Journal of infectious diseases. 2001;184:1082–1085. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- 53.Dieli F, Sireci G, Caccamo N, Di Sano C, Titone L, Romano A, Di Carlo P, Barera A, Accardo-Palumbo A, Krensky AM, Salerno A. Selective depression of interferon-gamma and granulysin production with increase of proliferative response by Vgamma9/Vdelta2 T cells in children with tuberculosis. J Infect Dis. 2002;186:1835–1839. doi: 10.1086/345766. [DOI] [PubMed] [Google Scholar]
- 54.Liuzzi AR, Kift-Morgan A, Lopez-Anton M, Friberg IM, Zhang J, Brook AC, Roberts GW, Donovan KL, Colmont CS, Toleman MA, Bowen T, Johnson DW, Topley N, Moser B, Fraser DJ, Eberl M. Unconventional Human T Cells Accumulate at the Site of Infection in Response to Microbial Ligands and Induce Local Tissue Remodeling. J Immunol. 2016 doi: 10.4049/jimmunol.1600990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen H, Wang Y, Chen CY, Frencher J, Huang D, Yang E, Ryan-Payseur B, Chen ZW. Th17-related cytokines contribute to recall-like expansion/effector function of HMBPP-specific Vgamma2Vdelta2 T cells after Mycobacterium tuberculosis infection or vaccination. Eur J Immunol. 2015;45:442–451. doi: 10.1002/eji.201444635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


