Abstract
Background and Aims
While effective in the treatment of eosinophilic esophagitis (EoE) in children, limited data exists on long-term safety and efficacy of swallowed topical corticosteroids.. We investigated if long-term use of swallowed fluticasone in children with EoE leads to sustained reduction in esophageal eosinophils, and endoscopic and clinical improvement.
Methods
In an open-label, prospective, single center study, we offered pediatric patients with active EoE fluticasone 2 puffs to swallow twice a day (strengths in μg/puff: 2–4 years: 44, 5–11 years: 110, ≥12 years: 220). Clinical, endoscopic and histological assessments were performed at baseline and shortly after therapy. If histological remission was seen, fluticasone was continued with clinical follow-ups every 4 months, and endoscopic and histological follow-ups yearly. Clinical scores were derived from 8 symptoms (abdominal pain, nausea, vomiting, regurgitation, chest pain, dysphagia, food impaction and early satiety). Endoscopic scores were derived from 6 features (rings, exudates, furrows, edema, stricture and shearing). Scores were expressed as ratio (features present/total). In addition to peak eosinophils/HPF (primary outcome), histological features (eosinophilic microabscesses, degranulation, superficial layering, basal zone hyperplasia, dilated intercellular spaces and lamina propria fibrosis) were assessed. Median clinical and endoscopic scores and individual histologic features were compared over 4 time intervals: <4 months, 4–12 months, 13–24 months and >24 months. Growth and adverse effects were monitored.
Results
We enrolled 54 patients, 80% male, median age 6.5 years (range 2–17 years), 85% atopic (57% asthma, 68% allergic rhinitis, 31% atopic dermatitis) and 74% with food allergy. Mean follow-up was 20.4 months, the longest being 68 months (5.7 years). Esophageal eosinophils counts significantly decreased (median peak eosinophils/HPF at baseline 72, <4 months: 0.5, 4–12 months: 1.75, 13–24 months: 10, and >24 months: 12, all p<0.01). All histological features significantly decreased from baseline to all follow-up time points (all p<0.01). Lamina propria fibrosis significantly decreased (% patients with fibrosis at baseline 92, <4 months: 41, 4–12 months: 50, 13–24 months: 45, and >24 months: 39, all p<0.01). Endoscopic features improved (score at baseline 0.37, <4 months: 0.17, 4–12 months: 0.17, 13–24 months: 0, and >24 months: 0.1, all p<0.01 except at >24 months: p<0.05). Symptoms improved (score at baseline 0.22, <4 months: 0, 4–12 months: 0.11, 13–24 months: 0.11, and >24 months: 0.11, all p<0.05 except at >24 months: p=0.05). Asymptomatic esophageal candidiasis was seen in 3 children but resolved with anti-fungal therapy. Height and weight Z-scores followed expected growth curves.
Conclusion
We demonstrate that swallowed fluticasone is effective as a long-term maintenance therapy for children with EoE, without growth impediment or serious side effects.
Keywords: Eosinophilic esophagitis, fluticasone, pediatric, long-term outcome, corticosteroids
Introduction
Eosinophilic esophagitis (EoE) is a chronic immune-mediated inflammatory disease of the esophagus1, 2. EoE is characterized clinically by symptoms of esophageal dysfunction including abdominal pain, regurgitation, vomiting, dysphagia and esophageal food impactions3, 4. Many children with EoE fail to thrive unless treated successfully 4. Esophageal features that can be identified endoscopically and are indicative of clinical symptoms include fixed esophageal rings (trachealization), strictures, longitudinal furrows, exudates (white plaques) and edema5. The histological hallmark of the disease is infiltration of the esophagus by eosinophils (≥15 eosinophils/HPF in the maximally involved area)2. Per definition, both clinical and histopathological features are refractory to therapy with proton pump inhibitors (PPI)2. EoE is associated with histological evidence of esophageal remodeling, even in young children6, and if left untreated, can lead to esophageal dysmotility and strictures7.
The prevalence of EoE in children has been increasing, currently approaching that of pediatric Crohn’s disease and ulcerative colitis8–11. Goals of therapy currently include improvement of clinical symptoms, remission of histopathological features and avoidance of long-term complications. However, no FDA- approved medical therapies for EoE exist to date12. Available therapeutic approaches include various dietary restrictions and off-label use of topical corticosteroids to the esophagus2. Given the chronic nature of EoE, its tendency to develop progressive fibrosis over time that is reversible with therapy in children13, and its tendency to relapse within a few weeks to months following discontinuation of therapy14, long-term therapy for EoE is needed. While dietary restriction therapies can be used chronically in children with EoE15–20, their long-term acceptance by patients has been a challenge21. Topical corticosteroids in the form of fluticasone propionate (FP) to swallow14, 22–28 or oral viscous budesonide29–32 have been shown to be effective in children, as demonstrated by several case series and short-term randomized controlled trials, but their efficacy or safety for chronic use has not yet been established. The longest treatment duration reported is 6 months in children28, with some efficacy of the drug still demonstrated and with minimal side effects. Previous work has shown that swallowed fluticasone improves EoE in the short term, but if this effect is sustained beyond six months is unknown..
Our objective was to investigate the long-term efficacy and safety of swallowed FP in children with EoE. We hypothesized that in pediatric patients with EoE, maintenance therapy with swallowed FP leads to a sustained improvement of eosinophilic infiltration of the esophagus, as well as endoscopic and clinical features. We also investigated the safety of long-term swallowed FP administration in children.
Methods
Design and Participants
We conducted an open-label, prospective, single center study of long-term swallowed FP as a maintenance therapy for children with EoE. All pediatric patients (age 2–18 years) with confirmed active EoE seen at the Mount Sinai Center for Eosinophilic Disorders between June 2006 and August 2012 were offered FP. The choice of this therapy was made by participants and their families. Options offered to everyone included an empiric elimination diet consisting of dietary removal of common EoE food triggers, an elemental diet consisting of exclusive feeding of an amino acid-based formula with one food, or FP to swallow. Each option was offered for long-term use if proven effective clinically and histologically after short-term therapy (Figure 1). All participants provided consent, and assent when applicable, to have all their data analyzed, following approval from the Institutional Review Board at our institution. Active EoE was defined as presence of esophageal symptoms and histological evidence of ≥15 eosinophils/HPF in the maximally involved areas of the esophagus on esophagogastroduodenoscopy with biopsies despite prior therapy with a proton pump inhibitor per current consensus recommendations1, 2. Patients with evidence of concomitant eosinophilic gastritis and/or enteritis, active Helicobacter pylori infection, or parasitic infection were excluded.
Figure 1.
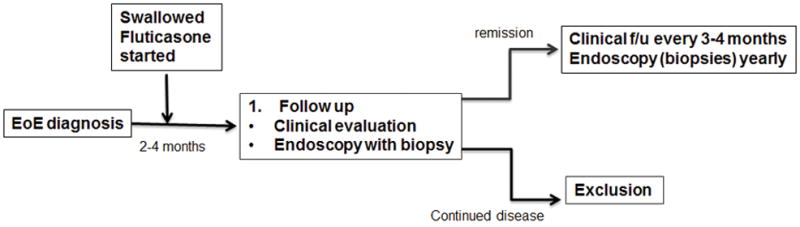
Flow chart of study design and decision tree.
For all subjects, baseline clinical, endoscopic and histological assessments were obtained prior to therapy with fluticasone.
Pharmacological Treatment
Therapy with FP metered dose inhaler was given as two puffs to swallow twice a day, specifically after breakfast and at bedtime. All patients were instructed to puff the medication into their mouth without synchronous deep inhalation or the use of a spacer. They were also instructed not to eat, drink, rinse their mouth or brush their teeth for an hour in the morning and then all night at bedtime after taking fluticasone. The dose of swallowed FP was modeled according to age specific treatment doses for asthma. Three different strengths of FP were used according to age: 2–4 years old: 44 μg/puff, 5–11 years old: 110 μg/puff, and ≥12 years old: 220 μg/puff. The dose was unchanged over time, unless disease relapse without an identifiable cause was observed. Correct administration was demonstrated, and proper adherence was discussed by instructing the subjects and their families to check the reading on the counter that is fitted to the back of the inhaler. At study entry, diets were liberalized for any patients who were on dietary restriction therapy for EoE.
After short-term therapy with fluticasone, patients were instructed to follow up at our Center for repeat clinical, endoscopic and histological evaluations. If patients achieved disease remission with the above therapy, defined as complete resolution of their main symptom and histological clearance of esophageal eosinophils to <15/HPF in the maximally involved area on biopsies, swallowed FP was continued at the same dose with clinical follow-up assessments every 4 months and endoscopic and histological follow-up assessments yearly. Additional unscheduled clinical, endoscopic and histological evaluations were done in the event of re-emergence of esophageal symptoms at any time during their therapy. Clinical data that was collected at the time of endoscopic assessment was included in the analysis. Proper use of FP was demonstrated at every follow-up visit. Adherence to FP was also monitored at every follow-up visit by reminding the subjects and their families to check the reading on the counter that is fitted to the back of the inhaler, and when needed, by calling the dispensing pharmacy to check on the number of refills obtained. Potential side effects were investigated at every follow-up visit.
While a systematic follow-up schedule was designed for the study as described above, some patients followed individualized schedules based on personal schedules and dictated by disease activity (Supplemental Figure 1). Patients had clinical, endoscopic and histologic data at all reported time-points. However, single clinical symptoms or endoscopic or histologic features were missing at few time-points in some patients. Scores were calculated based on available data and missing data was accounted for.
Study endpoints
The primary endpoint of the study was reduction over time of the peak esophageal eosinophil count in the maximally involved area of the esophagus, achieved with swallowed FP. We also reported reduction of peak eosinophil count in the distal and proximal esophagus respectively. Secondary endpoints were as follows: (1) determination of histological remission over time, as measured by reduction in peak eosinophil counts to < 15, <10, and <5 eosinophils/HPF (all 3 endpoints were considered since histological remission is currently not yet defined)1, 12; (2) improvement of esophageal histologic features other than the intraepithelial eosinophil count; (3) resolution of esophageal remodeling as determined by presence of sub-epithelial lamina propria fibrosis; (4) improvement of endoscopic features; and (5) improvement of clinical symptoms.
Histological evaluation
Hematoxylin and eosin (H&E)-stained slides were blindly evaluated by two pathologists (M.G.H. and M.S.M.). All esophageal biopsies were scanned at 100× power. Four distal esophageal, 4 proximal esophageal, 2 gastric antral, 2 gastric fundic, and 4–6 duodenal biopsies were obtained at each procedure for evaluation, in addition to any sites with macroscopically abnormal areas. From the area of maximum intraepithelial eosinophilic density, the number of intraepithelial eosinophils was then reported for the 400× HPF (0.237 mm2) with the highest concentration of eosinophils (peak count). Eosinophils were counted both when the entire cell including the nucleus was evident, and/or when a discrete cluster of eosinophilic granules was identified. The highest eosinophil count found in the distal and proximal esophageal biopsies was recorded for analysis. The following additional histological features were recorded6: presence of eosinophilic microabscesses (defined as a cluster of ≥ 4 eosinophils), presence and extent of eosinophilic degranulation (graded under HPF as absent/mild= 0 to few extracellular granules; or extensive= moderate to marked degranulation), superficial layering of intraepithelial eosinophils, epithelial basal zone thickness in well-oriented sections (graded from 0 to 3 based on its proportion of the total epithelial thickness; 0= ≤20% total epithelial layer thickness, 1= 20% to 1/3 thickness, 2= 1/3 to 2/3 thickness, 3= ≥2/3 to full thickness), presence of dilated intercellular spaces, and presence of lamina propria fibrosis. Edges of the biopsy specimen and areas with crush artifact were excluded from evaluation. Histological features were assessed over time.
Endoscopic evaluation
All pre- and post-treatment endoscopies were performed by the same gastroenterologist (M.C.) who recorded 6 macroscopic features (rings, white plaques, furrows, decreased vascular pattern (edema), stricture, and esophageal shearing). These were then graded using the modified classification and grading system published by Hirano et al for adults with EoE5. A final score was calculated for each patient at every endoscopy by summing up the grades given for every feature, and dividing it by the number of features assessed (6 features). Double weight was given to rings and strictures in the final summation score, since their rare presence in the pediatric population indicates severe and advanced disease6. Median endoscopy scores were compared over time.
Clinical evaluation
Clinical evaluations included a history and physical examination with measurements of height and weight. Up to 8 clinical symptoms (abdominal pain, nausea, vomiting, regurgitation, chest pain, dysphagia, food impaction and early satiety) were assessed for their presence by parent and patient. A symptom score was calculated at each visit by dividing the number of clinical symptoms present per patient by the total number of symptoms assessed at that visit (up to 8 symptoms). Median clinical scores were compared over time.
Peripheral Eosinophil Counts
Eosinophils in peripheral blood were measured on the same day of every endoscopy and reported as cells per μL.
Safety
Since candida infections and growth delay have been subjects of concern with topical corticosteroid use in children with EoE, side effects were monitored by clinical history, physical examination, and following of growth by calculating the Z score for height and weight for all patients over time using the SAS program for the CDC 2000 growth charts (2 to ≤20 years) [http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm]. Occurrence of oropharyngeal or esophageal candida infection was recorded and treated without interruption of FP maintenance therapy.
Alteration of the HPA-axis is a concern with long-term steroid use. Morning serum cortisol levels were collected when prompted by parental anxiety regarding chronic use of steroids.
Statistical Analysis
Intention to treat analysis was performed. Statistical analysis was done by use of SAS statistical software package, version 9.3. Follow-up time points were grouped by timing of endoscopies into <4 months, 4–12 months, 13–24 months and >24 months intervals. Depending on individual initial treatment timing and follow-up schedules, participants had none, one or multiple endoscopies per time period at the time of data analysis. Comparison for all outcomes was made from baseline (before treatment) to the 4 different follow up intervals on therapy. Statistical significance between groups of data was determined by use of the paired t-test for continuous variables. Secondary histological outcome evaluating different cutoffs for remission (<15, <10 and <5 eosinophils/HPF) was calculated using McNemar’s test for categorical variables. The correlation coefficient between the endoscopic score and individual histological features was calculated adjusting for intra-class correlation. Mean Z-scores for height and weight at baseline of all patients were compared to all follow up times using paired t-tests. For comparisons of continuous variables between patients who started FP as first therapy and those switching from diet, Mann-Whitney U test for non-parametric continuous data was used. Statistical significance was determined at p<0.05.
Mixed linear regression model
We fitted a mixed linear regression (negative binomial) model to test the hypothesis that treatment initiation would lead to a sustained decrease in the histological outcome, the peak eosinophil count, our response variable, while accounting for correlation of repeated observations in the same patient in a per patient analysis.
Very briefly, in our models, we modeled the population averaged peak eosinophil count response with fixed effects using the predictors treat (indicating treatment initiation) and further follow-up (indicating subsequent follow-up observations), controlling for the potential confounders Diet (prior diet modification) and PPI (prior proton inhibitor treatment) and allowing for individual patient (random) intercepts and further follow-up (to account for the correlation between repeated measurement in the same patient and the variability in patient response). Overdispersion led us to prefer a negative binomial over a poisson model. The most parsimonious model was selected based on the expected log predicted density (ELPD), but inclusion of potential confounders and or omission of the random effect to allow for individual patient long term effects (further follow-up) did not affect the inferences.
We fitted our mixed models in the statistical software environment R33. We used the R package rstanarm34 as an interface to the Stan statistical programming language35 to implement our model with Stan’s Hamiltonian Monte Carlo algorithms. We used the R package shinystan36 to explore the Monte Carlo Markov Chain output, render some graphs and confirm model convergence. We relied on Rhat as a convergence diagnostic37.
Results
Participants
Fifty-four patients with a diagnosis of active EoE chose FP to swallow as treatment option and were enrolled in the study. Patients were predominantly male (80%) with a median age of 6.5 years (range 2–17 years). The majority of patients (85%) had other atopic conditions like asthma (57%), allergic rhinitis (68%) and/or atopic dermatitis (31%). 74% had history of IgE-mediated food allergy (Table 1). Since patients with EoE often have an atopic background, including IgE-mediated food allergy, atopic dermatitis, allergic rhinitis and asthma, the question remains if EoE disease severity and progression is influenced by an atopic predisposition15, 16, 25, 38. The primary outcome (peak eosinophil count) was compared for these atopic conditions. An underlying diagnosis of an atopic disease in a patient (asthma, allergic rhinitis, atopic dermatitis, food allergy) did not correlate with severity of EoE measured as peak esophageal eosinophil count (p-values >0.4 for all atopic features examined). Only 3 patients continued PPI therapy throughout their treatment with swallowed FP.
Table 1.
Patient characteristics of all patients at time of inclusion into the study.
| All Patients (n=54) | |
|---|---|
|
| |
| Gender, male/female | 43/11 |
|
| |
| Age (years): median (range) | 6.5 (2–17) |
|
| |
| Atopy: No. patients (%) | 46 (85%) |
| Asthma | 31 (57%) |
| Allergic rhinitis | 37 (68%) |
| Atopic Dermatitis | 17 (31%) |
|
| |
| IgE-mediated food allergy: No. patients (%) | 40 (74%) |
|
| |
| Esophageal peak eosinophil count/HPF: median (range) | 72 (1–220) |
|
| |
| Blood Eosinophil count (cells/μL): median (range) | 400 (100–1500) |
FP was the first treatment modality chosen by 15 patients; an additional 39 children had been on dietary restriction therapy before opting for swallowed FP, their diets were fully liberalized at study entry. All but 2 patients that had been on a diet prior to changing to FP had failed dietary restriction therapy. Baseline characteristics were comparable for those two subgroups (Table 2).
Table 2.
Patient characteristics at inclusion for patients who switched from dietary restriction therapy to FP compared to patients who started swallowed FP as their first treatment modality.
| Dietary restriction therapy prior to fluticasone (n=39) | Fluticasone as first therapy modality (n=15) | p-value | |
|---|---|---|---|
|
| |||
| Gender, male/female | 30/9 | 13/2 | |
|
| |||
| Age (years): median (range) | 6 (2–17) | 7 (2–17) | 0.4211 |
|
| |||
| Atopy: No. patients (%) | 33 (85%) | 2 (87%) | |
| Asthma | 21 (54%) | 10 (67%) | |
| Allergic rhinitis | 28 (72%) | 9 (60%) | |
| Atopic Dermatitis | 15 (38%) | 2 (13%) | |
|
| |||
| IgE-mediated food allergy: No. patients (%) | 29 (74%) | 11 (73%) | |
|
| |||
| Esophageal peak eosinophil count/HPF: median (range) | 75 (38–145) | 72 (1–220) | 0.7869 |
|
| |||
| Blood Eosinophil count (cells/μL): median (range) | 400 (200–700) | 500 (100–1500) | 0.9127 |
Of the 54 participants, 2 were lost to follow up and 8 withdrew from therapy with swallowed FP, 7 due to lack of histological response at various time points during therapy and 1 for parental concern over chronic steroid use. 44 patients remained on treatment at the time of data analysis. All 54 patients were included in the data analysis.
Mean duration of follow-up of patients after initiation of swallowed FP was 20.4 months (1.7 years), with the longest being 68 months (5.7 years).
Efficacy
Peak esophageal eosinophil count (primary endpoint)
Treatment with swallowed FP significantly decreased the maximum number of eosinophils/HPF in the most severely affected area of the esophagus at all follow up time points compared to baseline (median 72 eosinophils/HPF at baseline compared to median 0.5 eosinophils/HPF at follow up within 4 months; and 1.75 eosinophils/HPF, 10 eosinophils/HPF and 12 eosinophils/HPF at 4–12 months, 13–24 months and >24 months follow ups respectively, all p<0.0001) (Figure 2a). Separate analysis of peak eosinophils in the distal and proximal esophagus showed a comparable reduction of peak eosinophils at all time-points respectively. In the distal esophagus, median 60 eosinophils/HPF at baseline was present, compared to median 0.5 eosinophils/HPF at follow up within 4 months, p<0.0001, and 1.0 eosinophils/HPF, 8 eosinophils/HPF and 12 eosinophils/HPF at 4–12 months, 13–24 months and >24 months follow ups respectively (all p-values between <0.0001 and 0.0002). As for the proximal esophagus, median 55.5 eosinophils/HPF at baseline was present, compared to median 0 eosinophils/HPF at follow up within 4 months, p<0.0001, and 0 eosinophils/HPF, 0.5 eosinophils/HPF and 0.5 eosinophils/HPF at 4–12 months, 13–24 months and >24 months follow ups respectively (all p-values between <0.0001 and 0.0004) (Figure 2 b, c).
Figure 2.
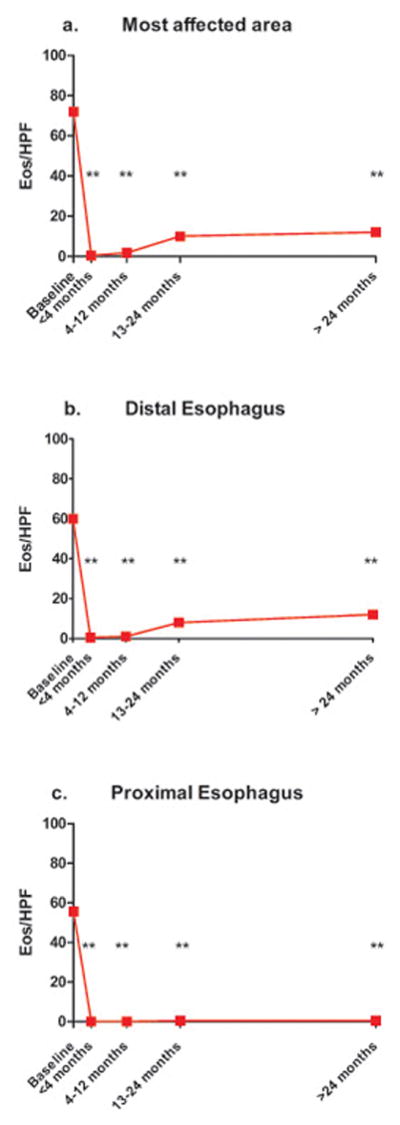
Primary outcome: median peak eosinophils/HPF at baseline compared to 4 follow-up intervals, (a) most affected area of the esophagus, (b) distal esophagus, (c) proximal esophagus, ** p<0.001.
Other histologic Findings
While the peak number of eosinophils/HPF to diagnose EoE is clearly defined at ≥15, no consensus on criteria for histologic remission exists to date12. To better understand treatment response and histologic remission, resolution of eosinophilic infiltration was evaluated applying 3 different criteria (<15 eosinophils/HPF, <10 eosinophils/HPF and <5 eosinophils/HPF).
The percentage of patients that achieved remission when analyzed at the various intervals (<4 months, 4–12 months, 13–24 months and >24 months) were as follows: <15 eosinophils/HPF in the most severely affected area: 83%, 84%, 59%, 63%; <10 eosinophils/HPF in the most severely affected area: 75%, 78%, 51%, 58%; <5 eosinophils/HPF in the most severely affected area: 63%, 66%, 48%, 58%. (Supplemental Table 1)
When distal and proximal esophageal peak eosinophil counts were analyzed separately, similar results were seen with respect to the remission rate. Distal esophagus: (<4 months, 4–12 months, 13–24 months and >24 months): <15 eosinophils/HPF: 83%, 88%, 63%, 63%, <10 eosinophils/HPF: 75%, 84%, 56%, 58%; <5 eosinophils/HPF: 63%, 69%, 48%, 58%). Proximal esophagus: (<4 months, 4–12 months, 13–24 months and >24 months): <15 eosinophils/HPF: 88%, 84%, 81%, 79%, <10 eosinophils/HPF: 88%, 81%, 81%, 79%; <5 eosinophils/HPF: 88%, 72%, 81%, 74%.
Of the 43 patients that achieved remission at the first follow-up endoscopy, a total of 13 patients had relapse during their follow-up course, 6 of whom had multiple relapses, for a total of 25 relapse occurrences for all patients (Supplemental Figure 1). Eighteen of 25 relapse occurrences were noted to mainly affect the distal esophagus; median eosinophils/HPF 37 in the distal esophagus (range 15–182) and 2 in the proximal esophagus (range 0–99). Reasons for relapse in these patients were non-adherence to swallowed FP therapy (4 patients), incorrect technique when the children took over the responsibility for medication administration as they got older (3 patients), and unidentified cause (6 patients, one of whom responded to an increase in the dose as he grew into the next dosing category, and another responded to addition of a proton pump inhibitor).
When present, each individual histologic feature showed significant improvement compared to baseline (Table 3a). Peak eosinophil count significantly correlated with the presence of secondary histologic features (eosinophilic microabscesses, eosinophil superficial layering, dilated intercellular spaces, basal zone hyperplasia, eosinophilic degranulation and lamina propria fibrosis). Lamina propria adequate for evaluation was present in 75% of biopsies. At baseline, fibrosis of the lamina propria was noted in 91.5% of patients. The percentage of patients with LP fibrosis decreased to 41% and remained similarly low at all follow-up periods (Table 3a).
Table 3.
Secondary outcomes: Percent of patients with presence of (a) histologic, (b) endoscopic, and (c) and clinical features at baseline and at follow-up intervals. The features are also summarized in a score (median/range) for endoscopic and clinical data at baseline and follow-up intervals.
| a | Baseline (n=54) | <4 months (n=24) | 4–12 months (n=33) | 13–24 months (n=27) | >24 months (n=20) |
|---|---|---|---|---|---|
| % Patients with histologic feature | |||||
| Eosinophilic microabcesses | 53.85 | 8.33** | 6.25** | 7.41** | 21.05** |
| Eosinophil superficial layering | 44.23 | 0.00 | 9.38** | 14.81** | 21.05** |
| Dilated intercellular spaces | 84.62 | 17.39** | 15.63** | 25.93** | 21.05** |
| Basal zone hyperplasia | 92.31 | 33.33** | 31.25** | 44.44** | 47.37** |
| Eosinophilic degranulation | 53.85 | 4.17** | 6.25** | 11.11** | 21.05** |
| Lamina propria fibrosis | 91.49 | 41.18** | 50.00** | 45.45** | 38.89** |
| b | Baseline | <4 months | 4–12 months | 13–24 months | >24 months |
|---|---|---|---|---|---|
| % Patients with endoscopic feature | |||||
| Fixed Rings | 10.20 | 0.00 | 0.00 | 3.70 | 0.00 |
| White Plaques | 58.33 | 13.04* | 24.24 | 29.63 | 30.00 |
| Furrows | 75.51 | 26.09** | 12.12** | 18.52** | 30.00* |
| Edema | 67.44 | 56.52 | 46.67* | 14.81* | 26.32 |
| Stricture | 1.85 | 0.00 | 0.00 | 0.00 | 0.00 |
| Shearing | 4.17 | 0.00 | 0.00 | 0.00 | 0.00 |
| Endoscopic symptom score: median (range) | 0.37 (0–1.33) | 0.17 (0–0.67)** | 0.17 (0–0.67)** | 0.00 (0–0.67)** | 0.10 (0–0.67)* |
| c | Baseline | <4 months | 4–12 months | 13–24 months | >24 months |
|---|---|---|---|---|---|
| % Patients with clinical symptom | |||||
| Abdominal pain | 38.89 | 16.67 | 34.38 | 26.92 | 30.00 |
| Nausea | 14.81 | 8.33 | 9.38 | 15.38 | 15.00 |
| Vomiting | 18.52 | 0.00 | 6.25 | 11.54 | 25.00 |
| Regurgitation | 38.89 | 0.00 | 12.50 | 23.08 | 10.00 |
| Chest pain | 18.52 | 12.50 | 12.50 | 7.69 | 5.00 |
| Dysphagia | 35.19 | 12.50 | 12.50 | 15.38 | 5.00 |
| Food Impaction | 22.22 | 12.50 | 0.00 | 15.38 | 5.00 |
| Early Satiety | 18.52 | 0.00 | 0.00 | 3.85 | 0.00 |
| Clinical symptom score: median (range) | 0.22 (0–0.67) | 0.00 (0–0.44)** | 0.11 (0–0.44)* | 0.11 (0–0.33)* | 0.11 (0–0.33) |
p<0.05,
p<0.001
Endoscopic Findings
The majority of children had white plaques (58.3%), furrows (75.5%), and edema (67.4%) on endoscopy. White plaques and furrows rapidly and significantly responded to treatment. Resolution of edema was less pronounced short-term but showed a significant reduction between 4 and 24 months of treatment. Shearing was present in 4.2% of patients at baseline and quickly resolved with therapy. Fixed rings were found in 10.2% of patients, resolved after 4–12 months of FP treatment and did not recur except in one patient in his second year of treatment with FP in the absence of esophageal eosinophilia. Rings cleared again on subsequent follow-ups. A stricture was found in 1/54 (1.85%) of patients at baseline. After 7 months of treatment with FP, the stricture resolved and did not recur.
The cumulative score of endoscopic features significantly improved with swallowed FP at all time-points (Table 3b). Furthermore, the endoscopic score significantly correlated with esophageal eosinophil counts and other histological features (correlation coefficient for peak eosinophils <15/HPF: −0.60476, p<0.0001; eosinophilic microabcesses: 0.64566, p<0.0001; eosinophilic degranulation: 0.62673, p<0.0001; eosinophil superficial layering: 0.61638, p<0.0001; dilation of intercellular spaces: 0.63599, p<0.0001; basal zone hyperplasia: 0.68110, p<0.0001; lamina propria fibrosis: 0.51159, p<0.0001).
Clinical findings
At baseline, most patients (88.9%) had at least 1 of 8 assessed symptoms (abdominal pain, nausea, vomiting, regurgitation, chest pain, dysphagia, food impaction and early satiety). With swallowed FP treatment, patients experienced a significant decrease in the symptom score at all follow-up periods up to 2 years (Table 3c).
Per patient analysis and modeling
In our linear mixed effects model, initiation of treatment with swallowed fluticasone led to a statistically significant and sustained decrease in peak eosinophil counts. We present the detailed regression results of the most parsimonious model in Supplemental Table 2 and explain the findings with a representative patient with a starting peak eosinophil count of 80/HPF, treatment initiation may suppress the peak eosinophil count to 10 at the first endoscopy appointment and stays in remission at further follow up appointments.
Numerically, the regression coefficient for treatment (2.1) equals the difference of the log of a starting peak eosinophil count (80 for a representative patient), minus the log of the peak eosinophil count at the first appointment (10 for typical patient): log(80) – log(10) = 2.1 [regression coefficient for treatment]. More formally stated, in our mixed effects negative binomial regression model, the difference in the logs of expected peak eosinophil counts is expected to change by the respective regression coefficient, given the other predictor variables in the model are held constant.
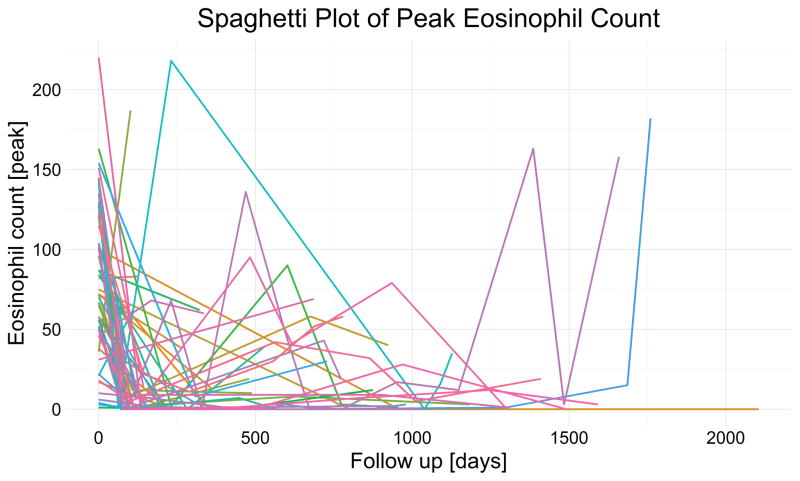
Our model specifically investigated the hypothesis of sustained effect by contrasting the first follow up versus subsequent follow-up. The estimate of the posterior median the regression coefficient for further follow up supports the hypothesis of sustained reduction of peak eosinophil count in our cohort. For our exemplary patient, the peak eosinophil count would remain below 15. But the model estimated wide credible intervals for sustained effect; together with significant patient variability, this mean the effect of swallowed fluticasone is not consistent for all patients. Inter-patient variability may best explain the inter-individual differences (as seen in Figure 4) in patient responses, as a mixed effects model allowing patient specific trajectories fit our data better than the simpler model with only fixed effects. Our models showed that diet modification and prior treatment with proton pump inhibitors do not affect our inferences.
Figure 4.
The peak eosinophil count [peak eosinophils/HPF] is plotted against patient follow-up [in days] with individual patient trajectories indicated by colored lines. After treatment initiation with swallowed fluticasone, the peak eosiophil count decreases and remains low, with a minimal increase into the second year (possibly due to either decreasing adherence or treatment efficacy) and increasing uncertainty as fewer patients were followed beyond two years.
Peripheral eosinophil counts
Median peripheral eosinophil count was 400/μL (range 100–1500/μL) at baseline. No significant change in peripheral eosinophil counts was noted at any follow-up time point (median 300 – 350/μL at all follow-up time points, all p-values between 0.05 and 0.52).
Safety
Asymptomatic candidal esophageal infection was seen in 3 patients at 4–12, 13–24 and >24 months respectively. Esophageal candidiasis resolved with fluconazole therapy as confirmed by repeat endoscopy with biopsies, while FP therapy was continued.
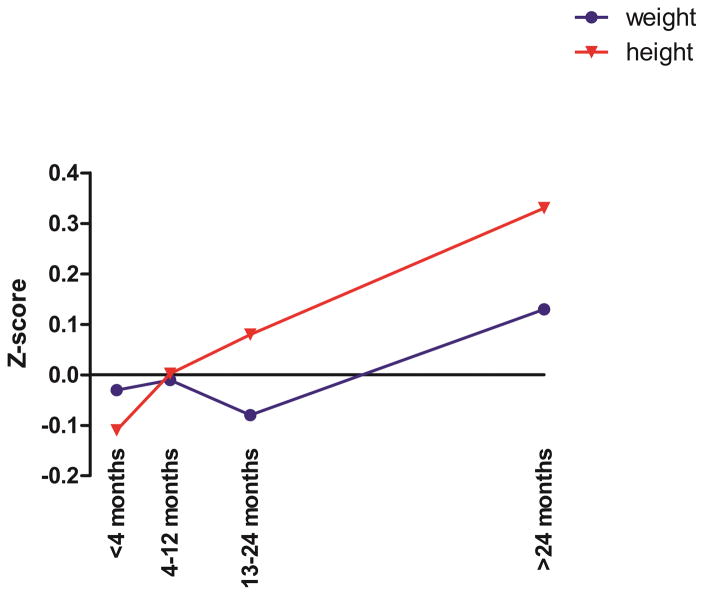
Comparison of mean baseline height z-scores with mean height z-scores recorded at the 4 follow up time periods revealed no significant deviation from the projected growth curves (p-values 0.1479, 0.9602, 0.2263 and 0.1703 at 4–12, 13–24 and >24 months respectively). A similar result was seen for weight z-scores of patients while taking swallowed FP (p= 0.7685, 0.9270, 0.4708, 0.5670 at 4–12, 13–24 and >24 months respectively) (Figure 3).
Figure 3.
Mean z-scores for height and weight at baseline for all patients were compared to mean z-scores at all follow-up periods. A positive z-score indicates net growth/ weight gain above the predicted growth curves, negative z-scores indicate less growth/ weight gain as predicted by predicted growth curves. All times points were not significant compared to baseline.
Data on morning serum cortisol measurements was collected on 9 patients. Morning cortisol levels ranged from 5.1–23.6 μL (median 10.1 μL). Two patients were found to be at the lower and higher end of the normal range for their age. These patients had no clinical findings, had normal growth, and a complete endocrine work-up was negative.
Discussion
This is an important clinical trial that demonstrates that swallowed FP leads to a sustained long-term remission of EoE in children, defined as reduction in peak eosinophil counts in the most affected area of the esophagus; this long-term oral steroid treatment was well tolerated and safe. This is particularly encouraging considering that to date no satisfying alternative long-term treatment options other than significant dietary modifications are available1, 2.
In our prospective study of pediatric patients with eosinophilic esophagitis, swallowed FP led to sustained improvement of histopathological, endoscopic and clinical markers of disease severity. Treatment response of all disease markers in all patients was most pronounced at the first short-term follow up (at <4 months), but was maintained at subsequent follow up time points for several months; the longest being 68 months. In addition, using a linear mixed effects model, we also found that initiation of treatment with swallowed fluticasone led to a statistically significant and sustained decrease in peak eosinophil counts. Using the model, our confidence in the sustained effect of swallowed fluticasone obviously diminishes for later follow-ups where we have less data. We have less data beyond the third follow-up, not because of attrition, (we lost only 6 out of 54 patients), but because patients were enrolled consecutively and not all have had years of follow-up yet. An update with additional long-term results as these become available in our ongoing trial is planned.
While other pediatric studies have shown short-term response to FP using comparable dose ranges22–27 this study adds to the literature by showing that this short-term effect can be maintained safely in pediatric patients for at least 2 years. Our findings concur with and surpass previous studies analyzing the efficacy of topical corticosteroids in inducing remission and histopathological, endoscopic and symptom improvement with short-term treatment22, 23, 25, 26, 39, 40. Various factors likely played into this favorable study outcome. Throughout the study, correct administration was demonstrated at length, strategies to ensure and enhance adherence were reviewed and discussed at every follow-up visit by the treating physician (M.C.), containers were checked when necessary and pharmacies were called to verify refills, and treatment was supervised by the caregivers in most patients. In addition, the design of our study, allowing patient and parent choice of therapy (diet versus swallowed FP) likely improved the outcomes, as families were sharing the responsibility in the treatment process.
Clinical symptoms were recorded and tracked throughout the study, however symptom severity measures were not used and symptom report was based on patient and parent re-collection. While this is a clear limitation of the study it also highlights the need for a standardized and validated clinical symptoms questionnaire designed for pediatric patients with EoE.
While open label design is always a limitation, the fact that the families chose the therapy modality was not reflected in their characteristics. Atopy and food allergy were comparable between patients who started swallowed FP as first treatment modality versus those who were on dietary restriction therapy first (Table 2). Any change or restriction in diet was regarded as dietary modification in our study. The degree of modification was highly variable in our patients, and included elemental diet, empiric 6-food elimination diet, or test-directed elimination diets). These dietary therapies were done at various points, including in the distant past before fluticasone was offered, and the diet was already liberalized by then. Dietary therapy is known to be extremely difficult to maintain and adhere to, especially as patients get older and become more independent. Inconsistent adherence to diet was found to be a problem in some of our patients. All diets were fully liberalized at the start of swallowed fluticasone.
Enrollment into our study started in 2006, before understanding of the role PPI’s play in EoE, and before recommendations re: PPI use in the consensus parameters were available. Three of our patients continued PPI after start of swallowed FP while all other patients discontinued PPI at enrollment into the study. Exclusion of those 3 patients did not change the primary and secondary outcomes. The patients remained in the group for the intention to treat analysis. A recent publication by Rajan et al. also did not find influence of PPI on eosinophilia or tissue remodeling41.
Since histological definition of remission is not yet established, we assessed different peak eosinophil counts (<15, <10 and <5 eosinophils/HPF) for histologic remission as one of our secondary endpoints to validate established practice18, 25, 42–45. Applying stringent criteria for definition of remission, the majority of patients reached and maintained peak eosinophil counts of <5 eosinophils/HPF after initiation of treatment to the longest follow-up period. At all follow-up time points, 75% of patients in remission had a peak of <5 eosinophils/HPF suggesting that true resolution of disease with swallowed FP might be associated with close to absent eosinophils in tissue biopsies, and indicating that the doses we used, though generally not very high, were very effective.
We have demonstrated the effect of swallowed FP on fibrosis of the lamina propria in a previous study13. In this study we were again able to demonstrate long-term complete resolution of lamina propria fibrosis in more than 50% of treated patients, starting as early as <4 months (Table 3a). As discussed above, lamina propria adequate for evaluation was present in 75% of biopsies. These results are not typically seen in adult patients with EoE45. It is possible that esophageal fibrosis in adult patients, since it has often developed over a longer period of time and undergone multiple disease remission and relapse cycles, is less responsive to treatment than in pediatric patients.
A recently published study investigating the long-term control of esophageal remodeling in patients with EoE supports the observation that while esophageal remodeling is present in children and is associated with inflammation, a substantial and sustainable response to swallowed topical steroids can be found. The authors described the main effect of swallowed topical steroids on fibrosis being exerted primarily via control of inflammation, as lower fibrosis scores correlated with decreased number of esophageal eosinophils, rather than with treatment duration and intensity of swallowed topical steroids41. This finding supports our observation that a decrease in peak esophageal eosinophil levels is significantly correlated with LP fibrosis reversal, with the underlying mechanism likely being inflammation control.
Interestingly, relapse was more pronounced and frequent in the distal portion of the esophagus. All patients with relapse in the distal esophagus had decreased amounts of swallowed FP intake, either because of non-adherence, poor technique or outgrown doses. It is possible that this observation is due to less contact time distally in the esophagus with smaller doses, as topical corticosteroid therapies were demonstrated to exert their function locally in the esophagus46. Our results demonstrate the necessity for optimal treatment adherence and dosing. We also demonstrate that transition of medication administration from caregiver to child may affect efficacy of the drug, and should be addressed during visits.
Our study design did not plan for dose increases as children grew. To evaluate if growth might lead to outgrowing of the dose and resulting relapses, we analyzed all relapse occurrences for all patients. We found that only one of the 6 patients with unidentified reasons for relapse responded to an increase in dose. We can therefore conclude that the relatively lower FP dose resulting from growth and weight gain is enough to maintain remission in most patients if good adherence and technique is achieved.
As for the 4 patients with disease relapse over time without clear etiology, it is possible that they have developed resistance to steroids over time. Rajan et al hypothesize that there may be distinct EoE phenotypes including “transient responders”, which may help explain our observation41.
While short-term efficacy of swallowed FP has been previously demonstrated14, 22–28 significant uncertainty regarding long-term safety and efficacy remained concerning this off-label use of an otherwise very well established and safe medication. Two main concerns of long-term treatment with swallowed FP often raised are recurrent candida infections and alteration of the hypothalamic pituitary adrenal (HPA)-axis with suppressed growth47. Our safety data showed a very low rate of local esophageal candida infections (3 patients) that resolved with treatment without recurrence and without the need to discontinue swallowed FP. No impact on growth was noted in our patients. To better address questions about effects on the HPA-axis, measurement of serum morning cortisol was started after completion of the study period. Cortisol levels were within the normal range for their age. None of the children showed clinical signs of adrenal insufficiency or delayed growth, and endocrine work-up was negative. In a recent publication by Butz et al28, saliva cortisol levels did not show significant alteration in patients treated with high dose swallowed FP. Swallowed FP in the doses used in this study can be considered of low risk for long-term treatment of EoE in children, although further studies evaluating the effect of swallowed FP on the HPA-axis, such as with an ACTH stimulation test, are warranted.
Other limitations to our study are its open-label, non-randomized design and the lack of a control group. However, a recently published randomized, double-blind placebo-controlled trial evaluating treatment with high dose swallowed FP did not detect a significant placebo effect, validating our approach28. In addition, a concern with lack of randomization is selection bias that may limit the strength of our conclusions. However, given clinical evidence from randomized trials in adults48–52 and children22, 23, 25–28, equipoise among experts in the field regarding the effectiveness of swallowed FP for EoE in children may have been eroded to the point that a randomized trial could be ethically problematic, especially in minors53. Furthermore, randomization of our patients into diet versus swallowed FP, rather than giving them the choice, would have led to low adherence in several patients, making comparisons of therapy difficult. As detailed above, even though we allowed the families the choice of therapy, our analysis showed similar population characteristics among those that chose swallowed FP versus diet as their first treatment modality. Therefore, we believe that our trial offers the first and best available evidence in support of long-term chronic use of swallowed FP to treat EoE in children, and will allow for the first time comfort in prescribing swallowed FP as a long-term therapeutic option for children with EoE, given its long-term efficacy and safety profile. Further investigation using larger multi-center trials is warranted to confirm optimal long-term dosing regimens while providing maintenance of disease remission and minimizing potential side effects in children with EoE.
Supplementary Material
Supplemental Table 1. Number and percentage of patients that had eosinophil counts <15 eos/HPF at the respective follow-up time points. Also seen is the number of patients with eosinophil counts <10 eos/HPF and <5 eos/HPF. Patients with <5 eos/HPF are included in the groups <10 eos/HPF and <15 eos/HPF, and patients with <10 eos/HPF are included in the group <15 eos/HPF.
Supplemental Table 2. Posterior median estimates (50%) for fixed effects and exemplary random effects regression coefficients are shown above with their corresponding 95% upper and lower bounds, followed by an exemplary random effect for patient 1. For our mixed effects negative binomial regression model, the difference in the logs of expected peak eosinophil counts is expected to change by the respective regression coefficient, given the other predictor variables in the model are held constant. In a representative patient with a starting peak eosinophil count of 80, this might mean a reduction to a peak eosinophil count of 10, which is mostly sustained in further follow-ups. Numerically, −2.1, the reduction in log Eos count estimated by the regression coefficient for treatment initiation, equals the difference between log(80) and log(10). The consistently low Rhat values below 1.1 suggest adequate model convergence.
Flow diagram detailing remission and relapse rates over the study period.
Study Highlights.
What is current knowledge?
Swallowed steroids have been shown to be safe and effective as a short-term therapy in children with EoE.
However, their efficacy for chronic use has not been established.
What is new here?
Swallowed steroids are safe and effective when used as a long-term maintenance therapy in children with EoE.
Treatment positively affects histologic, endoscopic and clinical features of EoE.
Acknowledgments
Funding: Supported in part by grants UL1TR000067 and 5KL2TR001071-03 from the National Center for Advancing Translational Sciences, National Institutes of Health.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- EoE
Eosinophilic Esophagitis
- FP
Fluticasone Propionate
- HPA-axis
Hypothalamic pituitary adrenal axis
- HPF
High Power Field
- PPI
Proton Pump Inhibitor
Footnotes
Disclosures
Doerthe A Andreae, Matthew G Hanna, Margret S Magid, Stefano Malerba, Michael Andreae, Emilia Bagiella and Mirna Chehade do not report any conflicts relevant to the manuscript.
Author Contributions
Doerthe A Andreae: acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
Matthew G Hanna: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Margret S Magid: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Stefano Malerba: statistical analysis; critical revision of the manuscript for important intellectual content
Michael H Andreae: statistical analysis; and interpretation of data; critical revision of the manuscript for important intellectual content
Emilia Bagiella: statistical analysis, critical revision of the manuscript for important intellectual content
Mirna Chehade: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; administrative, technical, or material support; study supervision
References
- 1.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20.e6. doi: 10.1016/j.jaci.2011.02.040. quiz 21–2. [DOI] [PubMed] [Google Scholar]
- 3.Straumann A, Bussmann C, Zuber M, et al. Eosinophilic esophagitis: analysis of food impaction and perforation in 251 adolescent and adult patients. Clin Gastroenterol Hepatol. 2008;6:598–600. doi: 10.1016/j.cgh.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Liacouras CA, Spergel J, Gober LM. Eosinophilic esophagitis: clinical presentation in children. Gastroenterol Clin North Am. 2014;43:219–29. doi: 10.1016/j.gtc.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Hirano I, Moy N, Heckman MG, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62:489–95. doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 6.Chehade M, Sampson HA, Morotti RA, et al. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:319–28. doi: 10.1097/MPG.0b013e31806ab384. [DOI] [PubMed] [Google Scholar]
- 7.Dellon ES, Kim HP, Sperry SL, et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79:577–85.e4. doi: 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–1. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 9.Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055–61. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hruz P, Straumann A, Bussmann C, et al. Escalating incidence of eosinophilic esophagitis: a 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol. 2011;128:1349–1350.e5. doi: 10.1016/j.jaci.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Dellon ES, Jensen ET, Martin CF, et al. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol. 2014;12:589–96.e1. doi: 10.1016/j.cgh.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothenberg ME, Aceves S, Bonis PA, et al. Working with the US Food and Drug Administration: progress and timelines in understanding and treating patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2012;130:617–9. doi: 10.1016/j.jaci.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieberman JA, Morotti RA, Konstantinou GN, et al. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: a historical cohort. Allergy. 2012;67:1299–307. doi: 10.1111/j.1398-9995.2012.02881.x. [DOI] [PubMed] [Google Scholar]
- 14.Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198–206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 15.Kelly KJ, Lazenby AJ, Rowe PC, et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503–12. doi: 10.1016/0016-5085(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 16.Spergel JM, Andrews T, Brown-Whitehorn TF, et al. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol. 2005;95:336–43. doi: 10.1016/S1081-1206(10)61151-9. [DOI] [PubMed] [Google Scholar]
- 17.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097–102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 18.Kagalwalla AF, Shah A, Li BU, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr. 2011;53:145–9. doi: 10.1097/MPG.0b013e31821cf503. [DOI] [PubMed] [Google Scholar]
- 19.Spergel JM, Brown-Whitehorn TF, Cianferoni A, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130:461–7.e5. doi: 10.1016/j.jaci.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Arias A, Gonzalez-Cervera J, Tenias JM, et al. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. 2014;146:1639–48. doi: 10.1053/j.gastro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Lucendo AJ. Meta-Analysis-Based Guidance for Dietary Management in Eosinophilic Esophagitis. Curr Gastroenterol Rep. 2015;17:464. doi: 10.1007/s11894-015-0464-y. [DOI] [PubMed] [Google Scholar]
- 22.Faubion WA, Jr, Perrault J, Burgart LJ, et al. Treatment of eosinophilic esophagitis with inhaled corticosteroids. J Pediatr Gastroenterol Nutr. 1998;27:90–3. doi: 10.1097/00005176-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Teitelbaum JE, Fox VL, Twarog FJ, et al. Eosinophilic esophagitis in children: immunopathological analysis and response to fluticasone propionate. Gastroenterology. 2002;122:1216–25. doi: 10.1053/gast.2002.32998. [DOI] [PubMed] [Google Scholar]
- 24.Noel RJ, Putnam PE, Collins MH, et al. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2004;2:568–75. doi: 10.1016/s1542-3565(04)00240-x. [DOI] [PubMed] [Google Scholar]
- 25.Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381–91. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 26.Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008;6:165–73. doi: 10.1016/j.cgh.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Boldorini R, Mercalli F, Oderda G. Eosinophilic oesophagitis in children: responders and non-responders to swallowed fluticasone. J Clin Pathol. 2013;66:399–402. doi: 10.1136/jclinpath-2012-201253. [DOI] [PubMed] [Google Scholar]
- 28.Butz BK, Wen T, Gleich GJ, et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology. 2014;147:324–33.e5. doi: 10.1053/j.gastro.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aceves SS, Bastian JF, Newbury RO, et al. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102:2271–9. doi: 10.1111/j.1572-0241.2007.01379.x. quiz 2280. [DOI] [PubMed] [Google Scholar]
- 30.Dohil R, Newbury R, Fox L, et al. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139:418–29. doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Rubinstein E, Lee JJ, Fried A, et al. Comparison of 2 delivery vehicles for viscous budesonide to treat eosinophilic esophagitis in children. J Pediatr Gastroenterol Nutr. 2014;59:317–20. doi: 10.1097/MPG.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 32.Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13:66–76.e3. doi: 10.1016/j.cgh.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 33.R Core Team. R 2.15. 2: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. http://www.R-project.org. [Google Scholar]
- 34.Gabry J, Goodrich B. rstanarm: Bayesian Applied Regression Modeling via Stan. 2016 [Google Scholar]
- 35.Stan Development Team. Stan: A C++ Library for Probability and Sampling, Version 2.5.0. 2014 [Google Scholar]
- 36.Gabry J. shinystan: Interactive Visual and Numerical Diagnostics and Posterior Analysis for Bayesian Models. 2016 [Google Scholar]
- 37.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. Journal of computational and graphical statistics. 1998;7:434–455. [Google Scholar]
- 38.Liacouras CA, Wenner WJ, Brown K, et al. Primary eosinophilic esophagitis in children: successful treatment with oral corticosteroids. J Pediatr Gastroenterol Nutr. 1998;26:380–5. doi: 10.1097/00005176-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139:1526–37. 1537.e1. doi: 10.1053/j.gastro.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 40.Arora AS, Perrault J, Smyrk TC. Topical corticosteroid treatment of dysphagia due to eosinophilic esophagitis in adults. Mayo Clin Proc. 2003;78:830–5. doi: 10.4065/78.7.830. [DOI] [PubMed] [Google Scholar]
- 41.Rajan J, Newbury RO, Anilkumar A, et al. Long-term assessment of esophageal remodeling in patients with pediatric eosinophilic esophagitis treated with topical corticosteroids. J Allergy Clin Immunol. 2016;137:147–156.e8. doi: 10.1016/j.jaci.2015.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–12. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Gonsalves N, Yang GY, Doerfler B, et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology. 2012;142:1451–9.e1. doi: 10.1053/j.gastro.2012.03.001. quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 44.Lucendo AJ, Arias A, Gonzalez-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol. 2013;131:797–804. doi: 10.1016/j.jaci.2012.12.664. [DOI] [PubMed] [Google Scholar]
- 45.Straumann A, Conus S, Degen L, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400–9.e1. doi: 10.1016/j.cgh.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Dellon ES, Sheikh A, Speck O, et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology. 2012;143:321–4.e1. doi: 10.1053/j.gastro.2012.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gulliver T, Morton R, Eid N. Inhaled corticosteroids in children with asthma: pharmacologic determinants of safety and efficacy and other clinical considerations. Paediatr Drugs. 2007;9:185–94. doi: 10.2165/00148581-200709030-00007. [DOI] [PubMed] [Google Scholar]
- 48.Remedios M, Campbell C, Jones DM, et al. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63:3–12. doi: 10.1016/j.gie.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 49.Lucendo AJ, Pascual-Turrion JM, Navarro M, et al. Endoscopic, bioptic, and manometric findings in eosinophilic esophagitis before and after steroid therapy: a case series. Endoscopy. 2007;39:765–71. doi: 10.1055/s-2007-966738. [DOI] [PubMed] [Google Scholar]
- 50.Peterson KA, Thomas KL, Hilden K, et al. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig Dis Sci. 2010;55:1313–9. doi: 10.1007/s10620-009-0859-4. [DOI] [PubMed] [Google Scholar]
- 51.Alexander JA, Jung KW, Arora AS, et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10:742–749.e1. doi: 10.1016/j.cgh.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 52.Moawad FJ, Veerappan GR, Dias JA, et al. Randomized controlled trial comparing aerosolized swallowed fluticasone to esomeprazole for esophageal eosinophilia. Am J Gastroenterol. 2013;108:366–72. doi: 10.1038/ajg.2012.443. [DOI] [PubMed] [Google Scholar]
- 53.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141–5. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Number and percentage of patients that had eosinophil counts <15 eos/HPF at the respective follow-up time points. Also seen is the number of patients with eosinophil counts <10 eos/HPF and <5 eos/HPF. Patients with <5 eos/HPF are included in the groups <10 eos/HPF and <15 eos/HPF, and patients with <10 eos/HPF are included in the group <15 eos/HPF.
Supplemental Table 2. Posterior median estimates (50%) for fixed effects and exemplary random effects regression coefficients are shown above with their corresponding 95% upper and lower bounds, followed by an exemplary random effect for patient 1. For our mixed effects negative binomial regression model, the difference in the logs of expected peak eosinophil counts is expected to change by the respective regression coefficient, given the other predictor variables in the model are held constant. In a representative patient with a starting peak eosinophil count of 80, this might mean a reduction to a peak eosinophil count of 10, which is mostly sustained in further follow-ups. Numerically, −2.1, the reduction in log Eos count estimated by the regression coefficient for treatment initiation, equals the difference between log(80) and log(10). The consistently low Rhat values below 1.1 suggest adequate model convergence.
Flow diagram detailing remission and relapse rates over the study period.