Abstract
Antipsychotic drugs, such as haloperidol (HAL), are prescribed in the clinic to manage traumatic brain injury (TBI)-induced agitation. While preclinical studies have consistently shown that once-daily administration of HAL hinders functional recovery after TBI in male rats, its effects in females are unknown. Hence, the objective of this study was to directly compare neurobehavioral and histological outcomes in both sexes to determine whether the reported deleterious effects of HAL extend to females. Anesthetized adult female and male rats received either a controlled cortical impact (CCI) or sham injury and then were randomly assigned to a dosing regimen of HAL (0.5 mg/kg, i.p.) or vehicle (VEH; 1 mL/kg, i.p.) that was initiated 24 hours after injury and continued once daily for 19 consecutive days. Motor function was tested using established beam-balance/walk protocols on post-operative days 1–5 and acquisition of spatial learning was assessed with a well-validated Morris water maze task on days 14–19. Cortical lesion volume was quantified at 21 days. No statistical differences were revealed between the HAL and VEH-treated sham groups and thus they were pooled for each sex. HAL only impaired motor recovery in males (p < 0.05), but significantly diminished spatial learning in both sexes (p < 0.05). Females, regardless of treatment, exhibited smaller cortical lesions vs VEH-treated males (p < 0.05). Taken together, the data show that daily HAL does not prohibit motor recovery in females, but does negatively impact cognition. These task-dependent differential effects of HAL in female vs male rats may have clinical significance as they can direct therapy.
Keywords: antipsychotic drug, beam-walking, behavior, controlled cortical impact (CCI), functional recovery, haloperidol, hippocampus, learning and memory, Morris water maze, sex, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is often thought to be a disease of young males. However, females comprise approximately 40% of the TBI population (Guerrero et al., 2000). In the era of personalized medicine, it is critical that we examine questions related to sex and outcomes after TBI, not only from the perspective of spontaneous recovery, but also from the response to treatment. Many pharmacological agents are administered post-TBI, and given the biological differences in males and females, the beneficial or deleterious effects of these pharmacological agents may or may not be equivalent between the sexes.
In general, female patients are more likely to report side effects as a result of a medication, while males may be more sensitive as demonstrated by Morag and colleagues (2012) who compared growth inhibition sensitivities from immortalized human lymphoblastoid cell lines (LCLs) in unrelated females and males to examine sensitivity to common drugs. LCLs can be used to assess inter-individual variability in responses to various drugs, which is important for personalized medicine (Stark et al., 2010; Wheeler and Dolan, 2012). The data showed that males were more responsive and sensitive to the typical and atypical antipsychotic drugs (APDs), haloperidol (HAL) and risperidone, respectively. Females were more affected than males by the SSRI paroxetine (Stark et al., 2010; Wheeler and Dolan, 2012). These findings suggest that there may indeed be differences between how females and males respond to various clinically-relevant drugs, which may be related to the effect of hormones. Regarding the dopaminergic system, Wagner and colleagues reported that female rats exhibited smaller TBI-induced decreases in cortical and striatal dopamine transporter (DAT) expression relative to males (Wagner et al., 2005). Increased DAT density has also been shown in non-TBI female rats (Rivest et al., 1995) and in healthy human females (Lavalaye et al., 2000) relative to males. Thus, sex differences in dopamine transmission exist regardless of whether there is a brain injury or not, and provides a strong rationale for evaluating the effects of dopaminergic drugs, such as HAL in females and males.
APDs are often prescribed in the clinic to manage agitation and aggression, that may be, in part, a consequence of TBI. One of several APDs that are prescribed is HAL. However, HAL has consistently been shown to hinder functional recovery in male rats after experimental TBI when provided daily and compared to vehicle (VEH) controls (Wilson et al., 2003; Kline et al., 2008; Hoffman et al., 2008; Folweiler et al., 2016). Moreover, the deleterious effects of HAL persist for months after drug withdrawal (Phelps et al., 2015). Whether the detrimental effects of HAL extend to females after TBI is not established. The significance of knowing how APDs affect females is that both sexes exhibit comparable levels of agitation post injury (Kayden et al., 2004; Magnotti et al., 2008) and based on current standard practice, both females and males would receive APDs, such as HAL, without consideration for possible differential effects. Hence, the objective of this study is to determine if differences exist between female and male rats in the response to chronic HAL treatment after a controlled cortical impact (CCI) injury of moderate severity.
Materials and methods
Subjects
Seventy-two aged-matched (3 months old) adult female (n=36; 260–290 g) and male (n=36; 300–325 g) Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed in standard steel-wire mesh cages and maintained in a temperature (21 ± 1°C) and light (on 7:00 a.m. to 7:00 p.m.) controlled environment with ad libitum food and water. After one week of acclimatization all rats underwent a single day of beam-walk training, which consisted of 3–5 trials to traverse the entire beam under 5 sec. Following training, the rats were randomly assigned to a the following groups: TBI + VEH (1.0 mL/kg; n=13 female and n=13 male), TBI + HAL (0.5 mg/kg; n=13 female and n=13 male; Sham + VEH (1.0 mL/kg; n=5 female and n=5 male), and Sham + HAL (0.5 mg/kg; n=5 female and n=5 male). All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Every attempt was made to limit the number of rats used and to minimize suffering.
Determination of estrous stage
On the morning of surgery, normal cycling female rats were evaluated for estrous stage using classic cytology. Briefly, following a vaginal smear the epithelial cells were examined by light microscopy and based on traditional distinguishable characteristics of each stage (proestrous = predominantly nucleated epithelial cells with few cornified epithelial cells and leukocytes; estrous = predominantly cornified epithelial cells; diestrous = predominantly leukocytes with some nucleated epithelial cells) the rats were classified accordingly (Wagner et al, 2004; Monaco et al., 2013). Normal cycling females were used as this paradigm more closely mimics clinical TBI (3–4 rats from each group fit the criteria for each stage). Moreover, we have previously shown that estrous stage at the time of injury does not impact subsequent recovery (Wagner et al., 2004; Monaco et al., 2013).
Surgery
CCI injury was produced as previously described (Dixon et al., 1991; Kline et al., 2010; deWitt et al., 2011; Matter et al., 2011; Bondi et al., 2014). Briefly, surgical anesthesia was induced with 4% isoflurane in 2:1 N2O:O2 and the rats were intubated and secured in a stereotaxic frame. During surgery the rats were maintained at a surgical level with 2% isoflurane and carrier gases. Utilizing aseptic procedures a midline scalp incision was made, the skin and fascia were reflected to expose the skull, and a craniectomy (6−mm in diameter) was made in the right hemisphere (encompassing bregma and lambda and between the sagittal suture and the coronal ridge) with a high speed dental drill. The bone flap was removed and the craniectomy was enlarged further. Subsequently, the impacting rod was extended and the impact tip (6 mm, flat) was centered and lowered through the craniectomy until contact was made with the dura mater, then the rod was retracted and the impact tip was advanced 2.8 mm farther to produce a brain injury of moderate severity (2.8 mm tissue deformation at 4 m/sec). Core body temperature was maintained at 37 ± 0.5°C with a heating blanket. Immediately after the CCI, anesthesia was discontinued and the incision was promptly sutured. The rats were subsequently extubated and assessed for acute neurological outcome. Sham rats underwent similar surgical procedures, but were not subjected to the impact.
Acute neurological evaluation
Hind limb reflexive ability was assessed immediately upon terminating the anesthesia by gently squeezing the rats' paw with forceps every 5 sec and recording the time to elicit a withdrawal response. Return of the righting reflex was determined by the time required to turn from the supine to prone position. These tests are sensitive indicators of injury severity and duration of anesthesia (Dixon et al., 1991; Kline et al., 2010; deWitt et al., 2011; Matter et al., 2011; Bondi et al., 2014).
Drug administration
HAL (Sigma, St. Louis, MO) was prepared daily by dissolving in 1:1 dimethyl sulfoxide (DMSO)/saline, which also served as the VEH. The dose of HAL was chosen because it has been reported to be comparable to that used clinically to control psychosis (Rosengarten and Quartermain, 2002) and has been used in several brain injury studies investigating functional outcome (Feeney et al., 1982; Hovda and Feeney, 1995; Wilson et al., 2003; Kline et al., 2007, 2008; Hoffman et al., 2008; Phelps et al., 2015; Folweiler et al., 2017). Treatments began 24 hr after CCI or sham surgery and were provided intraperitoneally once daily for 19 days. The half-life of HAL using this dose and route is reported to be 2.6 hr (Kapetanovic et al., 1982) and thus it was provided after the daily behavioral assessments to circumvent sedative effects, which may confound the results.
Motor performance
Well established beam-balance and beam-walk tasks were utilized to assess motor performance (Dixon et al., 1991; Kline et al., 2010; deWitt et al., 2011; Matter et al., 2011; Bondi et al., 2014). Briefly, the beam-balance task consists of placing the rat on an elevated (90 cm) narrow beam (1.5 cm wide) and recording the time it remained on for a maximum of 60 sec. The beam-walk task, modified from Feeney and colleagues (Feeney et al., 1982), and used extensively in our laboratory (Kline et al., 2002, 2004, 2007, 2010; Sozda et al., 2010; Bondi et al., 2014), consists of training/assessing rats using a negative-reinforcement paradigm to escape bright light and white noise by traversing an elevated narrow beam (2.5 cm wide × 100 cm long) and entering a darkened goal box at the opposite end. Beam-walk performance was assessed by recording the elapsed time to traverse the beam as well as distance traveled. The scoring criteria for distance traveled is based on a rating scale from 0 to 5, where 0 indicates an inability to ambulate beyond the start point, 1–4 corresponds to distal segments of 20, 40, 60, or 80 cm from the starting point, respectively, and 5 indicates traversal of the entire length of the beam (100 cm) and entrance into the goal box. Rats were trained prior to CCI or sham injury to perform the tasks without errors (i.e., maintain their balance for 60 sec and traverse the beam in under 5 sec). A baseline performance assessment was taken on the day of surgery. Performance on both beam tasks was assessed on post-operative days 1–5 and consisted of three trials (60 sec allotted time per trial) per day on each task. The average daily scores for each subject were used in the statistical analyses.
Cognitive function: acquisition of spatial learning
Spatial learning was assessed in a Morris water maze task (Morris 1984) that has been shown to be sensitive to cognitive function after TBI (Hamm et al., 1992; Scheff et al., 1997; Kline et al., 2010; deWitt et al., 2011; Matter et al., 2011; Folweiler et al., 2017; de la Tremblaye et al., 2016). Briefly, the maze consisted of a plastic pool (180 cm diameter; 60 cm high) filled with tap water (26 ± 1°C) to a depth of 28 cm. The maze was located in a room with salient visual cues that remained constant throughout the study. The platform was a clear Plexiglas stand (10 cm diameter, 26 cm high) that was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat. Spatial learning began on post-operative day 14 and consisted of providing a block of four daily trials (4−min inter-trial interval) for five consecutive days (14–18) to locate the platform when it was submerged 2 cm below the water surface. On day 19 the platform was made visible to the rats by raising it 2 cm above the water surface as a control procedure to determine the contributions of non-spatial factors (e.g., sensory-motor performance, motivation, and visual acuity) on cognitive performance. For each daily block of trials the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the goal within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials. The times of the 4 daily trials for each rat were averaged and used in the statistical analyses.
Cognitive function: probe trial (memory retention)
One day after the final acquisition training session (i.e., day 19), all rats were given a single probe trial to measure memory retention. In this scenario, the platform was removed from the pool and the rats were placed in the maze from the location point distal to the quadrant where the platform was previously situated (i.e., “target quadrant”) and allowed to freely explore the pool for 30 sec. The percent time spent in the target quadrant was used in the statistical analysis.
Histology: quantification of cortical lesion volume
Three weeks after surgery the rats were anesthetized with Fatal-Plus® (0.3 mL, i.p.) and perfused transcardially with 200 mL 0.1 M phosphate buffered saline (pH 7.4) followed by 300 mL 4% paraformaldehyde. The heads were placed in the perfusate for one week and then the brains were extracted, dehydrated with alcohols, and embedded in paraffin. Coronal sections (7μm thick) were cut at 1−mm intervals through the lesion on a rotary microtome and mounted on Superfrost®/Plus glass microscope slides. After drying at room temperature, the sections were deparaffinized in xylenes, rehydrated, and stained with Cresyl violet. An observer blinded to experimental conditions analyzed cortical lesion volumes (mm3) by calculating the area of the lesion (mm2), which was done by outlining the inferred area of missing cortical tissue for each section (typically 5–7) taken at 1−mm intervals as previously reported (Olsen et al., 2012; Monaco et al., 2013, 2014; Radabaugh et al., 2016).
Statistical analyses
All analyses were performed using Statview 5.0.1 software (Abacus Concepts, Inc., Berkeley, CA) on data collected by blinded experimenters. The motor and cognitive analyses were conducted using repeated-measures analysis of variance (rANOVA). The acute neurological data (i.e., hind limb withdrawal reflex and righting reflex) as well as the data for the visible platform, probe trial, swim speed, and cortical lesion volume were analyzed using one-factor ANOVAs. When the overall ANOVA revealed significant effects, the Newman-Keuls post-hoc test was used to determine specific group differences. The results are expressed as the mean ± standard error of the mean (S.E.M.) and were considered significant when p ≤ 0.05.
Results
Three rats (1 from each the TBI + VEH female, TBI + HAL female, and TBI + VEH male groups) were excluded from the study because of an inability to locate the visible platform, which may be indicative of visual acuity deficits and therefore could be a potential confound given the necessity to see the cues located on the walls to acquire spatial learning. Hence, statistical analyses are based on sixty-nine rats. There were no significant differences in any assessment among the sham control groups, regardless of treatment (VEH or HAL), and thus the data were pooled into a single sham group for each sex (i.e., Female SHAM and Male SHAM). Moreover, determination of estrous stage yielded only a few rats in each category, which precluded including the separate stages in the statistical analyses due to limited power. However, no trend was revealed with a cursory sub-analysis of the estrous groups.
Acute neurological assessments
The TBI females regained hind limb reflexive ability quicker than their male counterparts (range for right limb: 137.5 ± 5.0 – 139.2 ± 5.1 sec vs. 149.9 ± 3.6 – 157.2 ± 5.6 sec, females vs. males, respectively [p < 0.05]; range for left limb: 141.7 ± 5.0 – 144.2 ± 4.8 sec vs. 154.6 ± 3.6 – 161.7 ± 5.5 sec, females vs. males, respectively, [p < 0.05]). Albeit the females righted themselves faster than males, no statistical differences were revealed (range = 322.8 ± 16.5 −334.2 ± 10.1 sec vs. 356.0 ± 14.8 − 363.2 ± 20.2 sec, females vs. males, respectively, [p > 0.05]).
Motor function: beam-balance
There were no pre-surgical differences between any of the groups as all rats were able to balance on the beam for the maximum allotted time of 60 sec (Fig. 1). However, following the CCI injury, the rANOVA revealed significant Group [F5,63 = 12.758, p < 0.0001] and Day [F5,315 = 50.262, p < 0.0001] differences, as well as a significant Group × Day interaction [F25,315 = 6.629, p < 0.0001]. The post-hoc analysis revealed that all TBI groups were significantly impaired compared to the SHAM groups, which were generally able to maintain balance for the full 60 sec [p's < 0.05]. Among the TBI groups, both the VEH and HAL-treated female groups performed better than the VEH and HAL males [p < 0.05], but did not differ from each other [p > 0.05]. In contrast, the HAL-treated male group performed worse than the males administered VEH [p < 0.05].
Fig. 1.
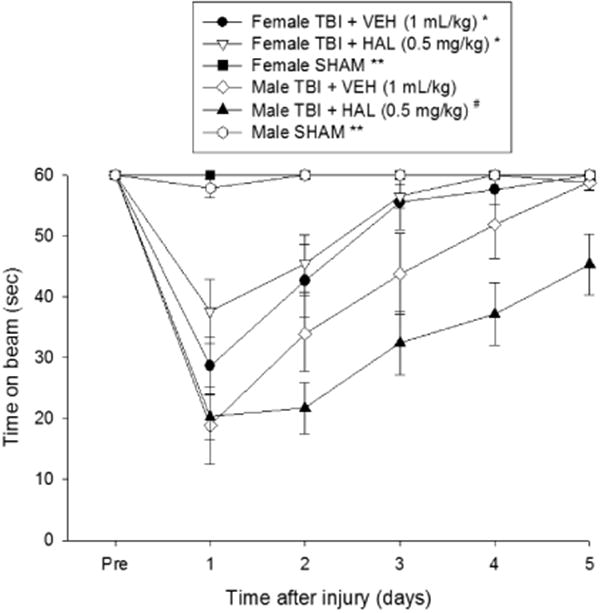
Mean (± S.E.M.) time (sec) balancing on an elevated narrow beam prior to, and after, TBI or sham injury. There were no significant differences among the sham groups and thus the data were pooled for each sex (Female SHAM and male SHAM). * p < 0.05 vs. Male TBI + VEH and Male TBI + HAL [p < 0.05]. # p < 0.05 vs. Male TBI + VEH. ** p < 0.05 vs. all TBI groups, regardless of treatment. No other comparisons were significant.
Motor function: beam-walk
No pre-surgical differences in time to traverse the beam were revealed among groups as all rats were well trained and reached the escape box in approximately 5 sec (Fig. 2). After CCI injury, the rANOVA revealed significant Group [F5,63 = 114.331, p < 0.0001] and Day [F5,315 = 184.130, p < 0.0001] differences, as well as a significant Group × Day interaction [F25,315 = 19.913, p < 0.0001]. The post-hoc analysis revealed that all TBI groups were significantly impaired relative to the SHAM groups [p < 0.05]. No overall difference was detected between the VEH and HAL-treated female groups [p > 0.05], but the latter did perform better than both the VEH and HAL-treated TBI males [p < 0.05]. Additionally, no overall difference was observed between the VEH and HAL-treated male groups [p > 0.05], but a single day analysis did show that the HAL-treated males were more impaired than the VEH-treated males on the last day of testing [p < 0.05]. Lastly, no differences were detected between the VEH-treated TBI females and the TBI males, regardless of treatment [p > 0.05].
Fig. 2.
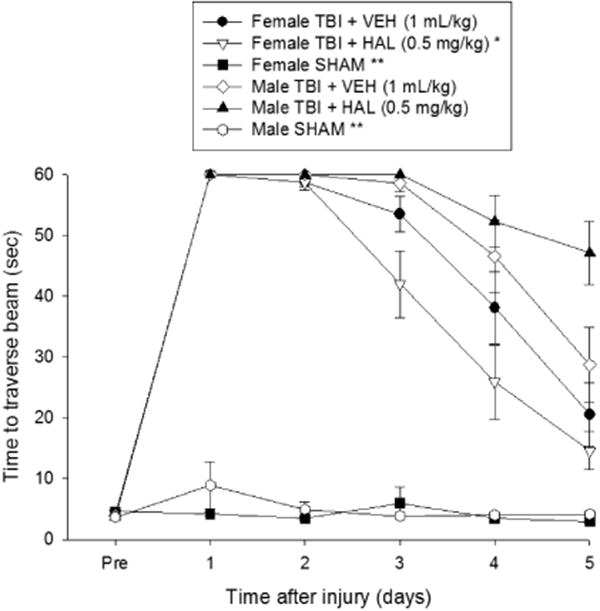
Mean (± S.E.M.) time (sec) to traverse an elevated narrow beam after TBI or sham injury. There were no significant differences among the sham groups and thus the data were pooled for each sex (Female SHAM and Male SHAM). * p < 0.05 vs. Male TBI + VEH and Male TBI + HAL. ** p < 0.05 vs. all TBI groups, regardless of treatment. No other overall comparisons were significant. However, a single day analysis did show that the HAL-treated males were more impaired than the VEH-treated males on the last day of testing [p < 0.05].
Motor function: beam-walk (distance)
Prior to TBI or sham injury, each rat traversed the entire length of the beam to reach the goal box (Fig. 3). After surgery, the statistical analysis revealed significant Group [F5,63 = 80.500, p < 0.0001] and Day [F5,315 = 150.866, p < 0.0001] differences, as well as a significant Group × Day interaction [F25,315 = 17.162, p < 0.0001]. The post-hoc analysis revealed that all TBI groups were significantly impaired relative to the SHAM groups [p < 0.05]. The TBI + VEH female group performed better than the TBI + HAL male group [p < 0.05]. Additionally, the TBI + HAL females performed better than the TBI + VEH males and TBI + HAL males [p < 0.05]. Moreover, the TBI + HAL males performed worse than the TBI + VEH males [p < 0.05].
Fig. 3.
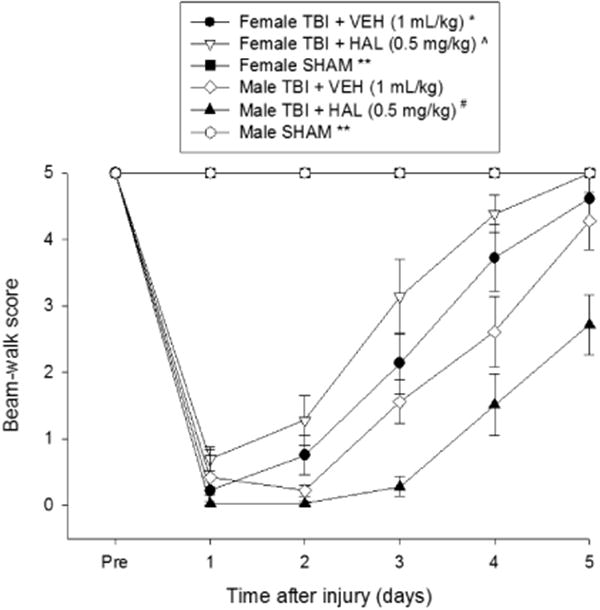
Mean (± S.E.M.) distance traveled score along an elevated narrow beam before and after TBI or sham injury. There were no significant differences among the sham groups and thus the data were pooled for each sex (Female SHAM and Male SHAM). * p < 0.05 vs. Male TBI + HAL. ^ p < 0.05 vs. Male TBI + VEH and Male TBI + HAL. # p < 0.05 vs. Male TBI + VEH. ** p < 0.05 vs. all TBI groups, regardless of treatment. No other comparisons were significant.
Cognitive function: acquisition of spatial learning
The rANOVA on the spatial learning data revealed significant Group [F5,63 = 30.849, p < 0.0001] and Day [F4,252 = 36.393, p < 0.0001] differences. The post-hoc analysis revealed that the SHAM groups were significantly better than all TBI groups [p < 0.05; Fig. 4], but did not differ from one another [p > 0.05]. Among the TBI groups, the HAL-treated females were significantly impaired relative to the VEH-treated females [p < 0.05]. Similarly, the HAL-treated males were impaired vs. the VEH-treated males [p < 0.05]. Additionally, the females receiving HAL were impaired relative to the males receiving VEH [p < 0.05] and the males administered HAL were impaired when compared to the VEH-treated females [p < 0.05]. No differences were observed between the females and males receiving VEH [p > 0.05] or between the females and males receiving HAL [p > 0.05].
Fig. 4.
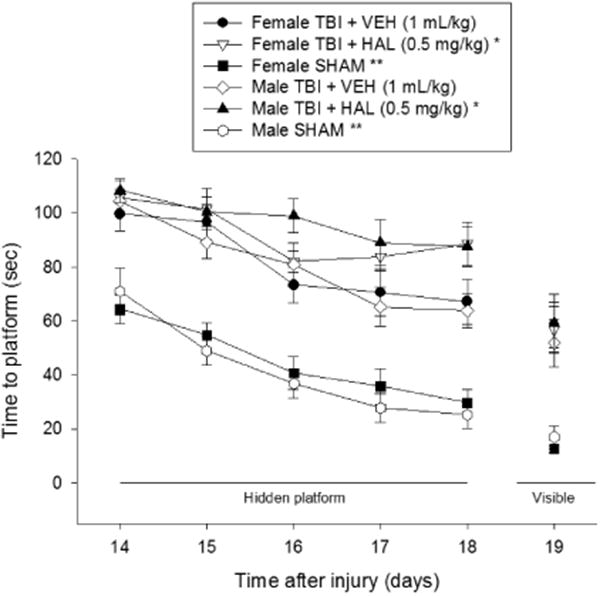
Mean (± S.E.M.) time (sec) to locate a hidden and visible platform in the water maze. There were no significant differences among the sham groups and thus the data were pooled for each sex (Female SHAM and Male SHAM). For the hidden platform assessments, * p < 0.05 vs. Female TBI + VEH and Male TBI + VEH. ** p < 0.05 vs. all TBI groups, regardless of treatment. For the visible platform assessment, ** p < 0.05 vs. all TBI groups. No other comparisons were significant.
No significant differences in swim speed (range = 24.6 ± 0.8 cm/sec to 29.7 ± 1.2 cm/sec) were observed among any of the TBI or SHAM groups [p > 0.05]. Regarding the visible platform data, the SHAM control groups performed better than all TBI groups, regardless of treatments [p < 0.05], and did not differ from each other [p > 0.05]. No differences in visible platform performance were revealed among the TBI groups [p > 0.05].
Cognitive function: probe trial
Analysis of the probe data revealed significant memory retention in the both SHAM groups as evidenced by a greater percentage of the allotted time spent in the target quadrant (40.7 ± 2.9 % and 42.4 ± 3.2 %, for females and males, respectively) vs. the TBI + VEH females (23.3 ± 2.8 %), TBI + HAL females (26.7 ± 2.5 %), TBI + VEH males (28.6 ± 2.7 %), and TBI + HAL males (28.3 ± 3.4 %) [p < 0.05]. No significant differences were revealed between the SHAM controls [p > 0.05] or among the TBI groups [p > 0.05; data not graphically presented].
Histology: quantification of cortical lesion volume
Analysis of the cortical lesion volume data revealed a significant difference between the VEH-treated males (43.0 ± 3.6 mm3) vs. the VEH-treated females (28.4 ±1.1 mm3) and the HAL-treated females (29.6 ± 2.3 mm3) [p < 0.05]. In contrast, the HAL-treated males (38.6 ± 4.3 mm3) did not differ from any TBI group [p > 0.05]. Additionally, the female groups, regardless of treatment, did not differ from each other [p > 0.05; Fig. 5].
Fig. 5.
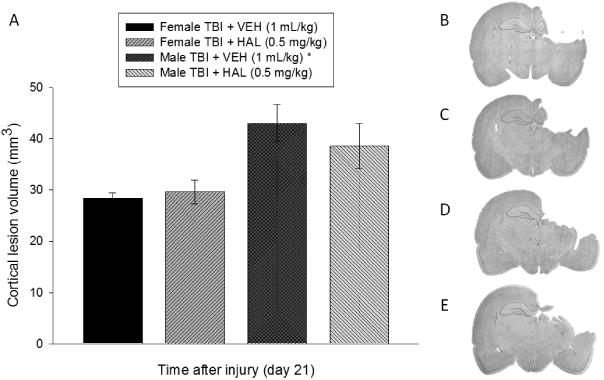
A. Mean (± S.E.M.) cortical lesion volume (mm3) 3 weeks after controlled cortical impact injury. B, C, D, and E depict average size lesions, stained with Cresyl violet, at the level of the dorsal hippocampus for Female TBI + VEH, Female TBI + HAL, Male TBI + VEH, and Male TBI + HAL, respectively. * p < 0.05 vs. Female TBI + VEH and Female TBI + HAL. No other comparisons were significant [p > 0.05].
Discussion
Several studies have shown that HAL prevents recovery of cognitive function in male rats after TBI (Wilson et al., 2003; Kline et al., 2007, 2008; Hoffman et al., 2008; Phelps et al., 2015; Folweiler et al., 2016), but empirical research is lacking regarding the drug's effect on neurobehavioral or cognitive performance in female rats. Understanding the effect of APDs in females sustaining a TBI is crucial as they are just as likely as males to become agitated and subsequently prescribed APDs to reduce and manage that maladaptive behavior. Hence, the goal of this study was to shed light on whether once daily HAL administration after CCI injury produces the same detrimental effects in females as it does in males. The results suggest, in part, that females do appear to respond differently to HAL after TBI, but only in response to less sensitive motor tasks where HAL did not impede recovery in females, but did so in TBI males.
Specifically, regarding motor function, there were no pre-injury differences between male and female rats in either the beam-balance or beam-walk tasks, which was expected as all rats were trained to criterion before surgery. Also expected, and confirmed, is the finding that the SHAM controls performed better than the TBI groups, regardless of treatment. Following TBI, the motor data vary as female rats performed better than the males on the beam-balance task when both were administered VEH, but did not differ on the beam-walk task. These findings suggest that with respect to motor function, CCI may not impair females as severely as males on some gross motor functions. Indeed, there is support for this finding as reported by Wagner and colleagues (2004) who showed less motor deficits in females after a comparable CCI injury.
When both sexes received HAL, the beam-balance data still favored the females over their male counterparts as there were no differences between the VEH and HAL-treated females, but both were significantly better than the males, regardless of treatment. Moreover, the HAL-treated males performed worse than those receiving VEH. Regarding the beam-walk task, HAL did not worsen outcome for either the females or males relative to their VEH-treated controls. Additionally, the females administered VEH did not differ from the males, regardless of their treatment, which suggests that for this more sensitive motor test, there was no behavioral sparing for the females. However, the females administered HAL performed better than both male groups. Support for the TBI male rats faring worse on motor performance after HAL treatment comes from unilateral sensorimotor cortex lesion studies by Feeney and colleagues (1982) and Goldstein and Bullman (2002). These preclinical findings support clinical data that human males are more sensitive to HAL than females (Morag et al., 2013).
With respect to cognitive function, which entailed acquisition of spatial learning, all TBI groups, regardless of treatment, were impaired as they required significantly more time to locate the hidden escape platform relative to the sham controls. Moreover, HAL significantly impaired recovery in both the female and male groups relative to VEH treatment. In contrast to the motor outcomes, there was no statistical difference between HAL-treated females and HAL-treated males, indicating that HAL was equally deleterious for both sexes. The differences in spatial learning between the VEH and HAL-treated females is seen despite similar cortical lesion volumes. Moreover, the males receiving VEH had larger lesions than the HAL-treated females, yet the latter group was significantly impaired. These data replicate findings that histological outcomes and behavioral performance do not always correlate (Lyeth et al., 1990; Kline et al., 2016; Radabaugh et al., 2016). Overall, these data confirm previous studies showing that daily HAL treatment significantly inhibits cognition in males after CCI injury (Kline et al., 2007, 2008; Hoffman et al., 2008; Phelps et al., 2014; Folweiler et al., 2017), and illustrate for the first time that HAL impairs spatial learning in females as well. Moreover, the impairment seen in the females is comparable to that seen in males in previous reports (Wilson et al., 2003; Kline et al., 2007, 2008; Hoffman et al., 2008; Phelps et al., 2015; Folweiler et al., 2016).
The clinical literature describing gender differences in functional outcomes suggest that females fare better on the Glasgow Coma Scale (Slewa-Younan et al., 2004) and the Glasgow Outcome Scale (Brazinova et al., 2010), proposing they have less severe injuries initially and continue to have lower levels of disability chronically. This general conclusion parallels the data from the current study that revealed shorter latencies to withdraw the affected hind limb after a brief paw pinch, which is used as an acute neurological marker of injury severity (Cheng et al., 2008, 2012; Kline et al., 2010; deWitt et al., 2011; Matter et al., 2011; Folweiler et al., 2017; de la Tremblaye et al., 2016). The differences in injury severity, which is observed despite both sexes undergoing the same injury parameters (i.e., tissue deformation and impact speed) may explain, at least in part, why females did not exhibit significant motor deficits, and may be mediated by endogenous hormones. Indeed, there is support for hormonal protection as evidenced by smaller cortical lesions in normal cycling female rats that received a TBI during proestrous or non-proestrous, relative to males and ovariectomized females (Bramlett and Dietrich, 2001). Additionally, progesterone and/or estrogen have been shown to limit edema, reduce cell death, maintain cerebral blood flow, and lessen mortality after experimental TBI (Roof et al., 1994; Roof and Hall, 2000; Galani et al., 2001; Shear et al., 2002; Djebaili et al., 2004). However, it is important to note that despite a neuroprotective effect in some endpoints in the current study, the females were not spared from the deleterious effects of HAL on cognition as they and the males exhibited comparable impairment that was protracted by HAL treatment.
These findings are important because of the potential implications for rehabilitation. That HAL inhibits motor recovery significantly more in males than in females suggests the possibility of diminished occupational and physical therapy benefits for males who sustain TBI. Furthermore, the HAL-induced hindrance in cognitive recovery after TBI in both sexes could lead to further decline in a patient's cognitive status after TBI. It is likely that increased and more-intensive neurorehabilitation may be required for the patients to reach the same milestones they might achieve if not for being treated with HAL or other high affinity D2 receptor antagonists.
Despite the current, and previously published data showing that HAL is deleterious to the recovery process after TBI (Wilson et al., 2003; Kline et al., 2007, 2008; Hoffman et al., 2008; Phelps et al., 2015; Folweiler et al., 2016), APDs are still necessary for managing agitated patients. Fortunately, recent studies have emerged showing that APDs with different mechanisms of action do not impede recovery after TBI. Specifically, intermittent administration (i.e., once every other day for 3 weeks) of quetiapine did not hinder motor or cognitive performance after CCI injury in male rats (Weeks et al., 2016). Moreover, aripiprazole also did not hamper recovery at either of the doses provided, but did lead to enhanced spatial learning with the lowest dose (Phelps et al., 2017). The different outcomes may be attributed to the mechanism of action in that quetiapine is a weaker D2 receptor antagonist and aripiprazole is a D2 receptor agonist and a 5-HT1A partial agonist. Studies from our laboratory have shown that various pharmacotherapies with these mechanisms of action benefit functional recovery after TBI (for excellent reviews see Bales et al., 2009; Bondi et al., 2015; Cheng et al., 2016), and thus suggest that patients can be treated efficiently for TBI-induced agitation and aggression without restricting their ability to recover spontaneously after TBI.
Highlights.
Females exhibit less severe motor deficits after TBI relative to males
HAL increases beam-walk deficits in males, but not females
HAL equally impairs cognitive recovery in females and males
Acknowledgments
This work was supported, in part, by the National Institutes of Health grants NS060005, HD069620, HD069620-S1, NS084967 (AEK), NS094950, NS099683 (COB), and the University of Pittsburgh Physicians/UPMC Academic Foundation (COB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bales JW, Wagner AK, Kline AE, Dixon CE. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci Biobehav Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Cheng JP, Tennant HM, Monaco CM, Kline AE. Old dog, new tricks: the attentional set-shifting test as a novel cognitive behavioral task after controlled cortical impact injury. J Neurotrauma. 2014;31:926–937. doi: 10.1089/neu.2013.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Semple BD, Noble-Haeusslein LJ, Osier ND, Carlson SW, Dixon CE, Giza CC, Kline AE. Found in translation: Understanding the biology and behavior of experimental traumatic brain injury. Neurosci Biobehav Rev. 2015;58:123–146. doi: 10.1016/j.neubiorev.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 2001;18:891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- Brazinova A, Mauritz W, Leitgeb J, Wilbacher I, Majdan M, Janciak I, Rusnak M. Outcomes of patients with severe traumatic brain injury who have Glasgow Coma Scale scores of 3 or 4 and are over 65 years old. J Neurotrauma. 2010;27:1549–1555. doi: 10.1089/neu.2010.1315. [DOI] [PubMed] [Google Scholar]
- Cheng JP, Hoffman AN, Zafonte RD, Kline AE. A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav Brain Res. 2008;194:79–85. doi: 10.1016/j.bbr.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JP, Leary JB, Sembhi A, Edwards CM, Bondi CO, Kline AE. 5-hydroxytryptamine1A (5-HT1A) receptor agonists: A decade of empirical evidence supports their use as an efficacious therapeutic strategy for brain trauma. Brain Res. 2016;1640:5–14. doi: 10.1016/j.brainres.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JP, Shaw KE, Monaco CM, Hoffman AN, Sozda CN, Olsen AS, Kline AE. A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits. J Neurotrauma. 2012;29:2684–2688. doi: 10.1089/neu.2012.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Tremblaye PB, Bondi CO, Lajud N, Cheng JP, Radabaugh HL, Kline AE. Galantamine and environmental enrichment enhance cognitive recovery after experimental traumatic brain injury but do not confer additional benefits when combined. J Neurotrauma. 2016 doi: 10.1089/neu.2016.4790. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witt BW, Ehrenberg KM, McAloon RL, Panos AH, Shaw KE, Raghavan PV, Skidmore ER, Kline AE. Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury. Neurorehabil Neural Repair. 2011;25:343–350. doi: 10.1177/1545968310390520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Meth. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- Folweiler KA, Bondi CO, Ogunsanya EA, LaPorte MJ, Leary JB, Radabaugh HL, Monaco CM, Kline AE. Combining the antipsychotic drug haloperidol and environmental enrichment after traumatic brain injury is a double-edged sword. J Neurotrauma. 2017;34:451–458. doi: 10.1089/neu.2016.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani R, Hoffman SW, Stein DG. Effects of the duration of progesterone treatment on the resolution of cerebral edema induced by cortical contusions in rats. Restor Neurol Neurosci. 2001;18:161–166. [PubMed] [Google Scholar]
- Goldstein LB, Bullman S. Differential effects of haloperidol and clozapine on motor recovery after sensorimotor cortex injury in rats. Neurorehabil Neural Repair. 2001;16:321–325. doi: 10.1177/154596830201600402. [DOI] [PubMed] [Google Scholar]
- Guerrero JL, Thurman DJ, Sniezek JE. Emergency department visits associated with traumatic brain injury: United States, 1995-1996. Brain Inj. 2000;14:181–186. [PubMed] [Google Scholar]
- Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Cheng JP, Zafonte RD, Kline AE. Administration of haloperidol and risperidone after neurobehavioral testing hinders the recovery of traumatic brain injury-induced deficits. Life Sci. 2008;83:602–607. doi: 10.1016/j.lfs.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovda DA, Feeney DM. Haloperidol blocks amphetamine induced recovery of binocular depth perception after bilateral visual cortex ablation in cat. Proc West Pharmacol Soc. 1985;28:209–211. [PubMed] [Google Scholar]
- Kadyan V, Mysiw WJ, Bogner JA, Corrigan JD, Fugate LP, Clinchot DM. Gender differences in agitation after traumatic brain injury. Am J Phys Med Rehabil. 2004;83:747–752. doi: 10.1097/01.phm.0000140790.30468.f4. [DOI] [PubMed] [Google Scholar]
- Kapetanovic IM, Sweeney DJ, Rapoport SI. Age effects on haloperidol pharmacokinetics in male, Fischer-344 rats. J Pharmacol Exp Ther. 1982;221:434–438. [PubMed] [Google Scholar]
- Kline AE, Hoffman AN, Cheng JP, Zafonte RD, Massucci JL. Chronic administration of antipsychotics impede behavioral recovery after experimental traumatic brain injury. Neurosci Lett. 2008;448:263–267. doi: 10.1016/j.neulet.2008.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Leary JB, Radabaugh HL, Cheng JP, Bondi CO. Combination therapies for neurobehavioral and cognitive recovery after experimental traumatic brain injury: is more better? Prog Neurobiol. 2016;142:45–67. doi: 10.1016/j.pneurobio.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Ma X, Zafonte RD, Dixon CE. Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J Neurotrauma. 2004;21:1712–1722. doi: 10.1089/neu.2004.21.1712. [DOI] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Marion DW, Dixon CE. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Zafonte RD, Dixon CE, DeFeo JR, Rogers EH. Differential effects of single versus multiple administrations of haloperidol and risperidone on functional outcome after experimental brain trauma. Crit Care Med. 2007;35:919–924. doi: 10.1097/01.CCM.0000256722.88854.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, McAloon RL, Henderson KA, Bansal UK, Ganti BM, Ahmed RH, Gibbs RB, Sozda CN. Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J Neurotrauma. 2010;27:2021–2032. doi: 10.1089/neu.2010.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med. 2000;27:867–869. doi: 10.1007/s002590000279. [DOI] [PubMed] [Google Scholar]
- Lyeth BG, Jenkins LW, Hamm RJ, Dixon CE, Phillips LL, Clifton GL, Young HF, Hayes RL. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- Magnotti LJ, Fischer PE, Zarzaur BL, Fabian TC, Croce MA. Impact of gender on outcomes after blunt injury: a definitive analysis of more than 36,000 trauma patients. J Am Coll Surg. 2008;206:984–991. doi: 10.1016/j.jamcollsurg.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Matter AM, Folweiler KA, Curatolo LM, Kline AE. Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats. Neurorehabil Neural Repair. 2011;25:558–564. doi: 10.1177/1545968310397206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco CM, Mattiola VV, Folweiler KA, Tay JK, Yelleswarapu NK, Curatolo LM, Kline AE. Environmental enrichment promotes robust functional and histological benefits in female rats after controlled cortical impact injury. Exp Neurol. 2013;247:410–418. doi: 10.1016/j.expneurol.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco CM, Gebhardt KM, Chelbowski SM, Shaw KE, Cheng JP, Henchir JJ, Zupa MF, Kline AE. A combined therapeutic regimen of buspirone and environmental enrichment is more efficacious than either alone in enhancing spatial learning in brain-injured pediatric rats. J Neurotrauma. 2014;31:1934–1941. doi: 10.1089/neu.2014.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morag A, Oved K, Gurwitz D. Sex differences in human lymphoblastoid cells sensitivities to antipsychotic drugs. J Mol Neurosci. 2013;49:554–558. doi: 10.1007/s12031-012-9852-z. [DOI] [PubMed] [Google Scholar]
- Morris R. Development of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Olsen AS, Sozda CN, Cheng JP, Hoffman AN, Kline AE. Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A receptor agonist buspirone. J Neurotrauma. 2012;29:1898–1907. doi: 10.1089/neu.2012.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps TI, Bondi CO, Ahmed RH, Olugbade YT, Kline AE. Divergent long-term consequences of chronic treatment with haloperidol, risperidone, and bromocriptine on traumatic brain injury-induced cognitive deficits. J Neurotrauma. 2015;32:590–597. doi: 10.1089/neu.2014.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps TI, Bondi CO, Mattiola VV, Kline AE. Relative to typical antipsychotic drugs, aripiprazole is a safer alternative for alleviating behavioral disturbances after experimental brain trauma. Neurorehabil Neural Repair. 2017;31:25–33. doi: 10.1177/1545968316650281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radabaugh HL, Carlson LJ, O'Neil DA, LaPorte MJ, Monaco CM, Cheng JP, de la Tremblaye PB, Lajud N, Bondi CO, Kline AE. Abbreviated environmental enrichment confers neurobehavioral, cognitive, and histological benefits in brain-injured female rats. Exp Neurol. 2016;286:61–68. doi: 10.1016/j.expneurol.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest R, Falardeau P, Di Paolo T. Brain dopamine transporter: gender differences and effect of chronic haloperidol. Brain Res. 1995;692:269–272. doi: 10.1016/0006-8993(95)00611-s. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129:64–69. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Estrogen-related gender differences in survival rate and cortical blood flow after impact-acceleration head injury in rats. J Neurotrauma. 2000;17:1155–1169. doi: 10.1089/neu.2000.17.1155. [DOI] [PubMed] [Google Scholar]
- Rosengarten H, Quartermain D. The effect of chronic treatment with typical and atypical antipsychotics on working memory and jaw movements in three-and eighteen-month-old rats. Prog Neuro-psychopharmacol Bio Psychiat. 2002;26:1047–1054. doi: 10.1016/s0278-5846(02)00221-x. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178:59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- Slewa-Younan S, Green AM, Baguley IJ, Gurka JA, Marosszeky JE. Sex differences in injury severity and outcome measures after traumatic brain injury. Arch Phys Med Rehabil. 2004;85:376–379. doi: 10.1016/j.apmr.2003.05.007. [DOI] [PubMed] [Google Scholar]
- Sozda CN, Hoffman AN, Olsen AS, Cheng JP, Zafonte RD, Kline AE. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J Neurotrauma. 2010;27:1047–1057. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark AL, Zhang W, Mi S, Duan S, O'Donnell PH, Huang RS, Dolan ME. Heritable and non-genetic factors as variables of pharmacologic phenotypes in lymphoblastoid cell lines. Pharmacogenomics J. 2010;10:505–512. doi: 10.1038/tpj.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Chen X, Kline AE, Li Y, Zafonte RD, Dixon CE. Gender and environmental enrichment impact dopamine transporter expression after experimental traumatic brain injury. Exp Neurol. 2005;195:475–483. doi: 10.1016/j.expneurol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Willard LA, Kline AE, Wenger MK, Bolinger BD, Ren D, Dixon CE. Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 2004;998:113–121. doi: 10.1016/j.brainres.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Weeks JJ, Carlson LJ, Radabaugh HL, de la Tremblaye PB, Bondi CO, Kline AE. Intermittent treatment with haloperidol or quetiapine does not disrupt motor and cognitive recovery after experimental brain trauma. Behav Brain Res. 2016 doi: 10.1016/j.bbr.2016.09.049. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler HE, Dolan ME. Lymphoblastoid cell lines in pharmacogenomic discovery and clinical translation. Pharmacogenomics J. 2012;13:55–70. doi: 10.2217/pgs.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Gibson CJ, Hamm RJ. Haloperidol, but not olanzapine, impairs cognitive performance after traumatic brain injury in rats. Am J Phys Med Rehabil. 2003;82:871–879. doi: 10.1097/01.PHM.0000091982.33232.CB. [DOI] [PubMed] [Google Scholar]


