The article by Sclavi et al. (1) in this issue of PNAS addresses “initiation,” the first step in transcription. Gene transcription is catalyzed in cells by large multisubunit proteins called RNA polymerases (RNAP). The eubacteria holoenzyme of RNAP is composed of five core subunits (α, α2, β, β′, and ω) that contain the amino acid residues required for the enzyme's catalytic activity. A sixth subunit (σ) guides RNAP to specific sequences on the genomic DNA (promoters) that mark the beginning of a gene or group of genes.
The Process of Transcription
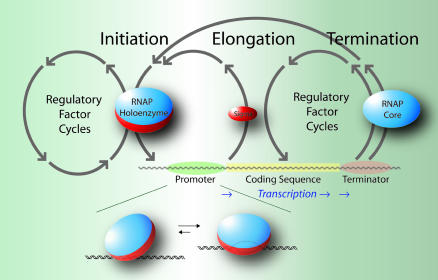
Transcription consists of three phases: initiation, elongation, and termination, with each of these phases being highly regulated by DNA/RNA sequence and/or accessory proteins (Fig. 1). RNAP holoenzyme must first locate a promoter. After binding to the promoter, the RNAP holoenzyme and the bound DNA undergo a series of conformational changes from the closed to the open promoter complex, in which ≈12 base pairs of DNA denature to form a bubble of the two separated strands of DNA.
Fig. 1.
A schematic outline of the transcription cycle phases at the operon level of genomic organization. RNAP holoenzyme binds to the promoter and initiates RNA synthesis. The σ-subunit is released at the onset of elongation. RNA synthesis continues without dissociation of the RNAP core until the terminator sequence is reached when the RNA transcript and polymerase are released. The expanded diagram beneath the schematic depicts RNAP holoenzyme binding to a promoter and its isomerization to an open complex.
RNAP holoenzyme is now poised to initiate RNA synthesis. After the addition of 6-10 RNA nucleotides, the σ-subunit is released, and the RNAP core continues to elongate the RNA chain until reaching a DNA terminator sequence. The terminator induces dissociation of the RNAP core and the completed RNA transcript from the DNA. RNAP core reassociates with the σ-subunit and is poised to again initiate the transcription cycle. Although the promoter binding by RNAP appears to be a simple step, we will see that it, too, is a complex process.
Structural and single-molecule analyses, in conjunction with an extensive biochemical literature, are providing new views of the transcriptional molecular machines that link structure with function during the transcription cycle (2-4). The article by Sclavi et al. (1) quantitatively connects structure with function by monitoring promoter binding by Escherichia coli RNAP during transcription initiation. This connection is established by the real-time direct measurement of the formation of RNAP contacts at specific sites on the promoter DNA.
Time-Resolved Footprinting
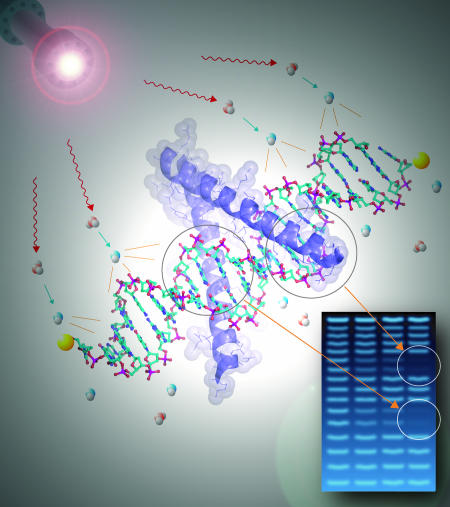
The technique of nucleic acid footprinting at the heart of the approach used by Sclavi et al. (1) is an ensemble approach that typically utilizes the inhibition of nuclease cleavage to report changes in the solvent accessibility of the backbone of DNA or RNA due to conformational changes and/or protein binding. Nuclease cleavage products are sorted by size and separated by electrophoresis; the term “footprint” reflects the decreased intensity of the electrophoretic bands representing nucleotides whose backbone is protected from cleavage (Fig. 2). Footprinting that is carried out following defined protocols yields isotherms or progress curves in thermodynamic and kinetic studies, respectively (5). Thus, quantitative footprinting provides biophysically valid measures identified to specific DNA sequences and/or structures.
Fig. 2.
A schematic outline of synchrotron x-ray •OH footprinting. X-ray radiolysis of water produces •OH that diffuses to a fragment of labeled duplex DNA to allow detection of a unique set of cleavage products. Cleavage by •OH is relatively uniform under single-hit conditions, yielding a series of bands representing molecules with n, n + 1, n + 2... nucleotides that are sized by gel electrophoresis. Protein binding to specific DNA sequences protects the backbone of these nucleotides from •OH cleavage, resulting in decreased density of the electrophoretic bands corresponding to these nucleotides.
The hydroxyl radical (•OH) is a valuable nucleic acid footprinting probe by virtue of its small size and promiscuous reactivity (6, 7). Because •OH is of comparable size to water, its reactivity pattern closely reflects solvent accessibility. Reactivity patterns can be generated and analyzed with resolution as fine as a single nucleotide. Chemical and radiolytic methods have been used to generate •OH for static, equilibrium, and time-resolved nucleic acid footprinting (6, 8-11). A singular advantage to the use of a synchrotron x-ray beam [such as that used by Sclavi et al. (1)] for radiolytic generation of •OH is that millisecond exposure yields sufficient concentrations of radicals for footprinting. When coupled to a rapid mixer, synchrotron footprinting can follow the evolution of changes in the solvent-accessible surface of nucleic acids with resolution as fine as single-nucleotide at times as short as milliseconds. B. Sclavi (1) participated in the development of this technique, principally in its application to RNA folding (11-13). While synchrotron footprinting has also been applied to the kinetics of protein-DNA interactions (14), the study by Sclavi et al. (1) breaks new ground in the complexity of the reaction being explored, the analyis approach being used, and the technical implementation of the experiment.
Connecting Function with Structure
Initiation frequency depends on the competing among promoters for RNAP holoenzyme. Promoter strength is governed by the ability of RNAP holoenzyme to bind and then undergo the conformational changes that lead from a closed to an open promoter complex. Two conserved regions that are 10 and 35 nucleotides upstream of the start site of RNA synthesis generally define promoters in E. coli. However, DNA sequences farther upstream (up regions) of the start site of transcription can dramatically affect the formation of a catalytically competent open complex. The conformational changes that lead to this open complex have been characterized by kinetic studies and footprinting of static complexes as a function of temperature. These studies show formation of two intermediate complexes that subsequently isomerize to the open complex (15, 16).
Sclavi et al. (1) use time-resolved synchrotron •OH footprinting to follow the conformational changes that lead to the formation of an open complex on a promoter containing an up region. Unlike previous footprinting studies of RNAP, real-time footprinting does not rely on trapping intermediate states. These experiments reveal that at least five distinct conformational states are populated before formation of the open complex. RNAP holoenzyme appears to bind to DNA in three different conformations that are in equilibrium. In these initial conformations, RNAP interacts primarily with the up region of this promoter (base pairs -60 to -30 relative to the start site of transcription). These intermediates subsequently isomerize to two additional conformations that contact the promoter DNA from -60 to +1. It is the latter intermediates, which contact the promoter DNA over ≈60 base pairs, which ultimately isomerize to the open complex.
A feature of Sclavi et al.'s (1) model is that the formation of the open complex is not simply a sequential progression of conformational changes. RNAP-promoter binding follows multiple pathways, reminiscent of a folding funnel (17). Another feature of the model is the key role of the up region in open complex formation. RNAP holoenzyme contacts the up region of the promoter DNA upon its first encounter. These contacts are maintained as the complex progresses to the open complex with a lessening of the extent of up-region contact in the final open complex. Recent kinetic studies show that up regions accelerate open complex formation (18, 19).
Figure 5 of ref. 1 shows the importance of DNA bending for the ultimate formation of the open promoter complex. Although there is no sequence conservation in the up region, it is often A-rich, which can facilitate the bending of DNA duplexes. DNA wrapping around RNAP during initiation appears to be a common feature of both prokaryotic and eukaryotic RNAPs (18, 20). The resulting distortion of the bound DNA likely facilitates open complex formation. This mechanism is reminiscent of replication initiation in which DNA wrapping around a complex of initiation proteins precedes formation of the replicative open complex (21).
Analysis and Technology
Sclavi et al. (1) are the first to explicitly test footprinting progress curves against formal kinetic models. The development of the structural-kinetic model shown in figure 5 of ref. 1 depends on the assignment of specific •OH reactivity changes to kinetic intermediates and subsequently with known structures. Globally testing possible models against the entire data set engenders confidence that the models are consistent with all available data. Two notable technological innovations to synchrotron footprinting are introduced (1). Protocols for using an automated sequencer to detect the •OH cleavage products using fluorescently tagged DNA were developed by balancing •OH-induced degradation of the detection fluorophore against the necessary cleavage of the target DNA. This protocol facilitates tedious sample processing and data reduction and allows the ready exploitation of single-nucleotide resolution. Development of a semiautomated rapid mixer allows efficient acquisition of multiple time points. Although multiplexing was accomplished at the expense of mixing dead time, the development of automated mixers will facilitate many types of kinetics experiments, including footprinting.
A View of the Future
Initiation is the first step in the synthesis of RNA. Transcription elongation and termination are obvious targets for time-resolved footprinting studies. Much of our understanding of these processes comes from the analysis of stalled or blocked complexes; it is important to remember that such complexes are off-pathway rather being true synthesis intermediates and that multiple synthesis pathways have been identified (22). Time-resolved footprinting analysis of RNAP movement along the DNA will complement transient-state kinetic studies and single-molecule force measurements in determining the mechanism of transcription.
Conceptual and technological advances will facilitate time-resolved footprinting studies. Brighter x-ray beams will make larger complexes and shorter timescales accessible. Improved analytical and kinetic modeling approaches will allow more robust and detailed information to be extracted about the preferred reaction pathways, their intermediates, and the rates that connect them. Last, the development of synchrotron protein footprinting will allow protein-DNA interactions to be followed in real time from the point of view of both macromolecules (23).
See companion article on page 4706.
Sayan Gupta prepared the figures.
References
- 1.Sclavi, B., Zaychikov, E., Rogozina, A., Walther, F., Buckle, M. & Heumann, H. (2005) Proc. Natl. Acad. Sci. USA 102, 4706-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Browning, D. F. & Busby, S. J. (2004) Nat. Rev. Microbiol. 2, 57-65. [DOI] [PubMed] [Google Scholar]
- 3.Guthold, M. & Erie, D. A. (2001) ChemBioChem 2, 167-170. [DOI] [PubMed] [Google Scholar]
- 4.Kane, C. M. (2002) Biochim. Biophys. Acta 1577, 173-174. [DOI] [PubMed] [Google Scholar]
- 5.Petri, V. & Brenowitz, M. (1997) Curr. Opin. Biotechnol. 8, 36-44. [DOI] [PubMed] [Google Scholar]
- 6.Tullius, T. D. & Dombroski, B. A. (1986) Proc. Natl. Acad. Sci. USA 83, 5469-5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latham, J. A. & Cech, T. R. (1989) Science 245, 276-282. [DOI] [PubMed] [Google Scholar]
- 8.Hayes, J. J., Kam, L. & Tullius, T. D. (1990) Methods Enzymol. 186, 545-549. [DOI] [PubMed] [Google Scholar]
- 9.King, P. A., Jamison, E., Strahs, D., Anderson, V. E. & Brenowitz, M. (1993) Nucleic Acids Res. 21, 2473-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampel, K. J. & Burke, J. M. (2001) Methods 23, 233-239. [DOI] [PubMed] [Google Scholar]
- 11.Sclavi, B., Woodson, S., Sullivan, M., Chance, M. R. & Brenowitz, M. (1997) J. Mol. Biol. 266, 144-159. [DOI] [PubMed] [Google Scholar]
- 12.Sclavi, B., Woodson, S., Sullivan, M., Chance, M. & Brenowitz, M. (1998) Methods Enzymol. 295, 379-402. [DOI] [PubMed] [Google Scholar]
- 13.Sclavi, B., Sullivan, M., Chance, M. R., Brenowitz, M. & Woodson, S. A. (1998) Science 279, 1940-1943. [DOI] [PubMed] [Google Scholar]
- 14.Dhavan, G. M., Crothers, D. M., Chance, M. R. & Brenowitz, M. (2002) J. Mol. Biol. 315, 1027-1037. [DOI] [PubMed] [Google Scholar]
- 15.Buc, H. & McClure, W. R. (1985) Biochemistry 24, 2712-2723. [DOI] [PubMed] [Google Scholar]
- 16.Roe, J. H., Burgess, R. R. & Record, M. T., Jr. (1985) J. Mol. Biol. 184, 441-453. [DOI] [PubMed] [Google Scholar]
- 17.Dill, K. A. & Chan, H. S. (1997) Nat. Struct. Biol. 4, 10-19. [DOI] [PubMed] [Google Scholar]
- 18.Davis, C. A., Capp, M. W., Record, M. T., Jr., & Saecker, R. M. (2005) Proc. Natl. Acad. Sci. USA 102, 285-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross, W. & Gourse, R. L. (2005) Proc. Natl. Acad. Sci. USA 102, 291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coulombe, B. (1999) Biochem. Cell Biol. 77, 257-264. [PMC free article] [PubMed] [Google Scholar]
- 21.Kornberg, A. & Baker, T. A. (1992) DNA Replication (Freeman, New York), 2nd Ed.
- 22.Erie, D. A. (2002) Biochim. Biophys. Acta 1577, 224-239. [DOI] [PubMed] [Google Scholar]
- 23.Kiselar, J. G., Janmey, P. A., Almo, S. C. & Chance, M. R. (2003) Mol. Cell Proteomics 2, 1120-1132. [DOI] [PubMed] [Google Scholar]