Abstract
Background
Adenoid cystic carcinoma of the head and neck (ACC) is a rare but highly malignant tumor. Cancer testis antigens (CTA) represent an immunogenic family of cancer-specific proteins and thus represent an attractive target for immunotherapy.
Methods
Eighty-four cases of ACC were identified, the CTAs pan-MAGE (M3H67) and NY-ESO-1 (E978) were detected immunohistochemically and correlated with clinical data.
Results
Expression of NY-ESO-1 was found in 48/84 (57.1%) and of pan-MAGE in 28/84 (31.2%). Median OS in NY-ESO-1 positive vs. negative patients was 130.8 and 282.0 months (p=0.223), respectively. OS in pan-MAGE positive vs. negative patients was 105.3 and 190.5 months, respectively (p=0.096). Patients expressing both, NY-ESO-1 and pan-MAGE simultaneously had significantly reduced OS with a median of 90.5 months compared to 282.0 months in negative patients (p=0.047).
Conclusions
A significant fraction of ACC patients show expression of the cancer testis antigens NY-ESO-1 and/or Pan-MAGE with promising immunotherapeutic implications.
Keywords: adenoid cystic carcinoma, head and neck cancer, cancer-testis antigens, MAGE, NY-ESO-1
Introduction
Adenoid cystic carcinoma of the head and neck (ACC) is a rare malignant tumor, which often occurs in the major salivary glands although occurrence in the minor salivary glands, the palate, base of tongue, paranasal sinuses and all other head and neck sites combined is more frequent. Primary therapy consists of radical surgery, and adjuvant radiotherapy is added in cases of nodal involvement, advanced stage or close resection margins1. Locoregional recurrences due to perineural invasion (Pn), or distant failure, most often due to lung metastases, result in poor long-term prognosis. Available chemotherapeutics and targeted agents have shown little effect on this slowly but relentlessly growing entity2,3. This, along with the recent success of immunotherapy in other tumor types, makes identification of new immunotherapeutic targets of significant interest in this disease.
Cancer testis antigens (CTA) represent a highly immunogenic family of proteins that are expressed physiologically in germ-line tissue, but also in certain malignant tumors. In adult patients, CTA expression is highly cancer-specific. Previous studies from Park et al. in 2012 indicate that Melanoma Antigen Gene (MAGE) can be useful in the differentiation of pleomorphic adenoma and adenoid cystic carcinoma because MAGE was expressed in a very high percentage of the tested samples whereas it was not detected in adenomas at all4. The clinical impact of CTA expression in ACC has not been investigated so far. In head and neck squamous cell carcinoma (HNSCC) on the other hand, CTAs of the MAGE-family and NY-ESO-1 (New York esophageal squamous cell carcinoma) have been identified as an independent marker for poor overall survival (OS)5. Because of the high specificity and immunogenicity of these antigens, immunotherapy seems to be a promising approach. Several vaccination trials against NY-ESO-1, another CTA, show first positive therapeutic effects in different solid tumors 6–8.
The aim of this study was to determine the incidence of CTA expression in ACC as a potential target for specific immunotherapy. A secondary goal was the evaluation of CTA expression as a prognostic marker.
Materials and Methods
Eighty-four cases of ACC with available clinical data and paraffin-embedded tissue were identified. The study was endorsed by the local ethics committee (Ethikkommission Ulm, Nr. 304/13) and the institutional review board. Demographical and clinical data was obtained from the electronic health record and pseudonymized. Perineural invasion (Pn1), positive resection margins (R+) and histological growth pattern (solid, tubular, cribriform, mixed) were determined in the surgical specimen of the initial resection.
Overall survival (OS) was calculated from the time of diagnosis until the time of death and progression free survival was calculated from time of diagnosis until disease recurrence, progression or death.
Immunohistochemistry
3μm thick sections were cut from paraffin embedded tissue. After deparaffinization in xylene and descending alcohol grades, HIER (Heat-induced antigen retrieval) was obtained using a steam cooker (Multi Gourmet, Braun™, Germany) for 20 min with 1mM EDTA-buffer (ethylenediaminetetraacetic acid) (pH 8.0). TBST-bath (Tris buffered saline) was used as washing buffer. After encirceling the specimens with Dako Pen (Dako™, Glostrup, Denmark), the slides were treated by Dako EnVision+ System-HRP™ (Dako™, Glostrup, Denmark) for use with primary mouse antibodies (Dako™, Carpinteria, CA, USA). Peroxidase block was used for 5 min, followed by a protein block (Dako „serum-free, Ready-To-Use“, Dako™, Carpinteria, CA, USA) for 10 min. Subsequently, the samples were incubated with the primary antibodies for 30 min. Following antibodies and dilutions were used: NY-ESO-1 (E978) 1:200; pan-MAGE (M3H67) 1:1000, both antibodies were provided by Dr. Gerd Ritter from New York Branch of the Ludwig Institute for Cancer Research (New York, NY, USA). Antigen specificity of the above antibodies has been described previously5.
Subsequently after washing twice with TBST-buffer (5 min each) the secondary antibody conjugated with an HRP labeled polymer was applied for 30 min. After another TBST-buffer bath (5 min) the samples were incubated with DAB-chromogene (3,3′-Diaminobenzidine) (preparation of 1 ml substrate buffer and 1 drop DAB-chromogene, Dako™, Carpinteria, CA, USA) for 15 min. Counterstaining was obtained with HE (Mayer’s™ hematoxylin solution, Sigma-Adlrich, Chemie GmbH, Steinheim, Germany). The samples were fixated by Aquatex™ (Merck™, KGaA, Darmstadt, Germany) and cover glasses.
Human testis specimens served as positive controls. Healthy human mucosal tissue (tongue) and parotid tissue from voluntary donors who underwent surgery for parotid adenoma was used for negative controls.
Interpretation of the data was done by light microscopy. All slides were evaluated by three independent experienced specialists and blinded control was done by a consultant of the pathology department.
A well-established immunohistochemical staining score was gathered from staining intensity and fraction of positive tumor cells. The staining intensity was evaluated separately in nuclear and cytoplasmatic staining and was scored as follows: 0, no staining; 1+, weak staining, 2+, mild staining, 3+, strong staining. Percentage of antigen-positive cells were categorized in ≤10%; >10% and ≤70% and in >70%. The final score was defined as “negative” if staining intensity was 0, or 1+ in ≤10% of cells; as “weakly positive” if the intensity was 1+ in >10% and ≤70% of tumor cells, or 2+ in ≤30%. Tumor specimens were considered “moderately positive” with a staining intensity of 1+ in >70% of cells, of 2+ in >30% and ≤70%, or a staining intensity of 3+ in ≤30% of tumor cells. “Strongly positive” was obtained if the staining intensity showed 2+ in >70% of cells, or 3+ in >30%. An overall positive score was built from the staining results if tumor was at least weakly positive in at least 10% of the tumor cells.
Statistical and graphical analysis was done with SPSS™ (Statistical Package for the Social Sciences) Version 21 by IBM™ (International business machines, Armonk, NY, USA). Median values were used due to irregular distribution. For correlations, Pearson’s test was used. Progression free survival (PFS) and overall survival (OS) were analyzed and depicted with Kaplan-Meier-Charts. Significance levels were tested with log-rank-test, significance was presumed with p ≤ 0.05.
Results
Patient cohort
All 84 patients underwent surgery, the majority (66/84) received adjuvant radiotherapy. 38/84 (45.2%) tumors were localized in the major salivary glands, 26/84 (31%) of these in the parotid, 9/84 (10.7%) in the submandibular and 3/84 (3.6%) in the sublingual gland. Other primary sites were found in 46/84 (54.8%). These included the paranasal sinuses in 17/84 (21.4%), the palate in 8/84 (9.5%), the floor of the mouth in 4/84 (4.8%) and other localizations like the midface or lacrimal glands in 13/84 (15.5%). The subsites can be found in table 1. Median age at primary diagnosis was 54 years, 44 patients were female and 40 female. Median PFS and OS were 36.1 and 82.3 months, respectively. Further patient characteristics can be found in table 2.
Table 1.
Primary tumor sites.
| Sites and subsites | No. of patients | Percentage [%] |
|---|---|---|
| Major salivary glands | 38 | 45.2 |
| Parotid gland | 26 | 31 |
| Submandibular gland | 9 | 10.7 |
| Sublingual gland | 3 | 3.6 |
| Other locations | 46 | 54.8 |
| Paranasal sinuses | 18 | 21.4 |
| Palate | 8 | 9.5 |
| Floor of mouth | 4 | 4.8 |
| Tongue | 3 | 3.6 |
| Others* | 13 | 15.5 |
Carcinomas of other head and neck regions, e.g. midface, auditory meatus and lacrimal gland
Table 2.
Clinical and demographical details of patient cohort
| No. of patients§ | Percent % | ||
|---|---|---|---|
| sex | female | 44 | 47.6 |
| male | 40 | 42.4 | |
| pT* | 1 | 13 | 15.5 |
| 2 | 15 | 17.9 | |
| 3 | 12 | 14.3 | |
| 4 | 30 | 35.7 | |
| n.a.¶ | 14 | 16.7 | |
| pN† | 0 | 56 | 66.7 |
| 1 | 8 | 9.5 | |
| 2 | 6 | 7.1 | |
| n.a. | 14 | 16.7 | |
| cM‡ | 0 | 65 | 77.4 |
| 1 | 3 | 3.6 | |
| n.a. | 16 | 19 | |
| resection margins | negative | 37 | 44 |
| positive | 37 | 42.9 | |
| n.a. | 10 | 11.9 | |
| perineural invasion | negative | 18 | 21.4 |
| positive | 42 | 50 | |
| n.a. | 24 | 28.6 | |
| histologic growth pattern | solid | 17 | 20.2 |
| tubular | 31 | 36.9 | |
| cribriform | 20 | 23.8 | |
| mixed | 16 | 19 |
: pathological tumor classification
: pathological nodal classification
: clinical classification of distant metastases
: total number of patients
: not available.
Prognostic markers
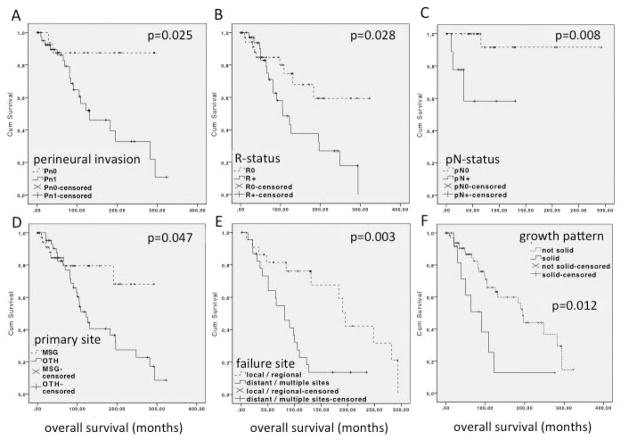
Perineural invasion (Pn1) was a highly significant prognosticator for OS. Median OS for Pn1 patients was 130.8 months, whereas in Pn0 patients median OS was not reached (p=0.025, figure 1A). Median PFS for Pn1 vs. Pn0 was 42 and 267.3 months, respectively (p=0.039). Positive resection margins were associated with reduced OS (R+=105.3 months, R0=median OS not reached; p=0.028, figure 1B), but did not significantly influence PFS (R+=39.5, R0=85.0 months; p=0.103). Positive nodal status was associated with reduced OS (median OS not reached; p=0.008, figure 1C), but did not significantly impact PFS (pN+: 30.3, pN0=86.8 months; p=0.287). A primary tumor outside of the major salivary glands went along with reduced OS (major salivary gland primary= median OS not reached, other primary= 122.5 months; p=0.047, figure 1D). Differences in PFS were not significant (major salivary gland primary= 83.3, other primary= 42.0 months; p=0.301). The site of treatment failure was another significant prognosticator. Distant failure compared to local and/or regional failure resulted in a reduced median OS of 81.3 vs. 195.3 months respectively (p=0.003, figure 1E). PFS was not significantly different with 21 vs. 27 months respectively. Solid growth pattern of ACC is another parameter, which is associated with reduced survival. We transformed the histologic growth pattern (tubular, cribriform, mixed, solid) into a bivariate variable (not solid vs. solid). OS was significantly reduced for solid compared to not solid tumors with 96.6 months compared to 190.1 months respectively (p=0.012) as shown in figure 1F. PFS difference did not reach significance with 59.5 months and 125.4 months respectively (p=0.086).
Figure 1.
Analysis of overall survival (OS) of ACC patients based on known prognosticators:
A: Perineural invasion: Pn1 = positive, Pn0 = negative (Pn). B: Resection margin status: R+ = positive resection margins, R0 = negative resection margins. C: Lymph node involvement: pN+ = positive lymph nodes, pN0 = negative lymph node involvement. D: Anatomical primary site of the tumor: MSG = primary tumor in the major salivary glands, OTH = primary tumor in a site other than the major salivary glands. E: Site of treatment failure: distant vs. local and/or regional. F: Histological growth pattern: Solid growth pattern vs. non-solid growth pattern (including tubular, cribriform and mixed histologic growth pattern).
Pn1 was significantly correlated with positive resection margins (p<0.0001) and a primary site outside of the major salivary glands (p=0.02). Positive resection margins were also correlated with a primary site outside of the major salivary glands (p=0.036). Positive lymph node status and the site of treatment failure were not correlated with any of the other clinical parameters. A solid growth pattern was significantly associated with treatment failure at distant/multiple sites (p=0.024).
Immunohistochemical expression of CTAs
Positive staining was exclusively observed in tumor cells, not in non-tumoral surrounding tissue or negative controls. Immunohistochemical staining showed NY-ESO-1 expression in 48/84 (57.1%) According to the IHC-score described in the methods section, weak staining intensity was found in 28/84 (43.5%)%, moderate staining in 12/84 (14.3%) and strong intensity in 7/84 (8.3%).
Pan-MAGE-positivity was observed in 28/84 (31.2%). According to the IHC score, a weak staining intensity was found in 12/84 (14.3%), moderate intensity in 11/84 (13.1%) and strong intensity in 5/84 (6.0%). Representative staining examples for NY-ESO-1 and pan-MAGE are presented in figure 2 and figure 3 respectively.
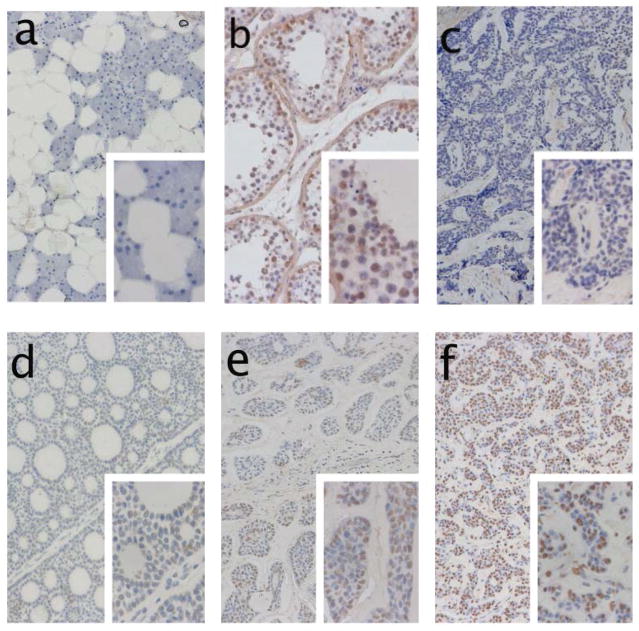
Figure 2.
Immunohistochemical staining of NY-ESO-1 (antibody E978): a) Negative control of parotid gland tissue. b) positive control staining of testicular tissue. c) negative staining of tumor tissue. d) weakly positive staining of tumor tissue with tubular growth pattern. e) moderately strong staining of ACC with solid growth pattern. f) strong expression of NY-ESO-1 in 50% of tumor tissue, stroma tissue is negative.
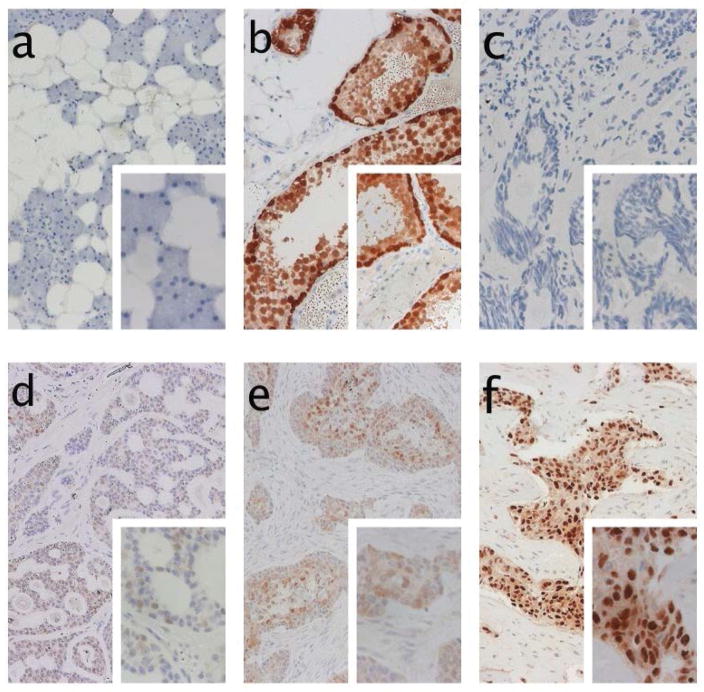
Figure 3.
Immunohistochemical staining of pan-MAGE (M3H67 antibody): a) Negative control of parotid gland tissue. b) positive control staining of testicular tissue. c) negative staining of tumor tissue. d) weakly positive staining of pan-MAGE. e) moderately strong expression of pan-MAGE in tumor tissue with solid growth pattern, stroma tissue is negative. f) example of strong expression of pan-MAGE in >70% of ACC tumor cells.
Prognostic impact of CTA expression
Using the chi2 test and a Pearson correlation, we found that NY-ESO-1 expression was more frequent in primary sites other than the major salivary glands (p=0.037, table 3). Pan-MAGE expression was more frequent in patients with positive lymph nodes (p=0.045, table 3).
Table 3.
Cross table analysis of different clinical and pathological parameters and antigen expression of CTA (NY-ESO-1 or Pan-MAGE) (Pearson’s correlation and chi-square test, significance level <0.05 (2-sided).
| antigen | NY-ESO-1 | p | |||
| − | + | Σ | (2-sided) | ||
| perineural invasion | − | 8 | 10 | 18 | 0.464 |
| + | 23 | 19 | 42 | ||
| Σ | 31 | 29 | 60 | ||
| resection margins | − | 18 | 19 | 37 | 0.483 |
| + | 15 | 22 | 37 | ||
| Σ | 33 | 41 | 74 | ||
| nodal status | − | 16 | 9 | 25 | 0.307 |
| + | 4 | 5 | 9 | ||
| Σ | 20 | 14 | 34 | ||
| primary site | MSG* | 21 | 17 | 38 | 0.037 |
| OTH† | 15 | 31 | 46 | ||
| Σ | 36 | 48 | 84 | ||
| site of failure | LR‡ | 9 | 13 | 22 | 0.595 |
| DIS§ | 8 | 16 | 24 | ||
| Σ | 17 | 29 | 46 | ||
| histologic growth pattern | non solid | 30 | 37 | 67 | 0.486 |
| solid | 6 | 11 | 17 | ||
| Σ | 36 | 48 | 84 | ||
| antigen | Pan-MAGE | p | |||
| − | + | Σ | (2-sided) | ||
| perineural invasion | − | 12 | 6 | 18 | 0.859 |
| + | 27 | 15 | 42 | ||
| Σ | 39 | 21 | 60 | ||
| resection margins | − | 27 | 10 | 37 | 0.144 |
| + | 21 | 16 | 37 | ||
| Σ | 48 | 26 | 74 | ||
| nodal status | − | 20 | 5 | 25 | 0.045 |
| + | 4 | 5 | 9 | ||
| Σ | 24 | 10 | 34 | ||
| primary site | MSG* | 28 | 10 | 38 | 0.215 |
| OTH† | 28 | 18 | 46 | ||
| Σ | 26 | 28 | 84 | ||
| site of failure | LR‡ | 14 | 8 | 22 | 0.936 |
| DIS§ | 15 | 9 | 24 | ||
| Σ | 29 | 17 | 46 | ||
| histologic growth pattern | non solid | 45 | 22 | 67 | 0.850 |
| solid | 11 | 6 | 17 | ||
| Σ | 56 | 28 | 84 | ||
= major salivary glands
= other
= local and/or regional failure
= distant failure
Σ=sum, −=negative, +=positive.
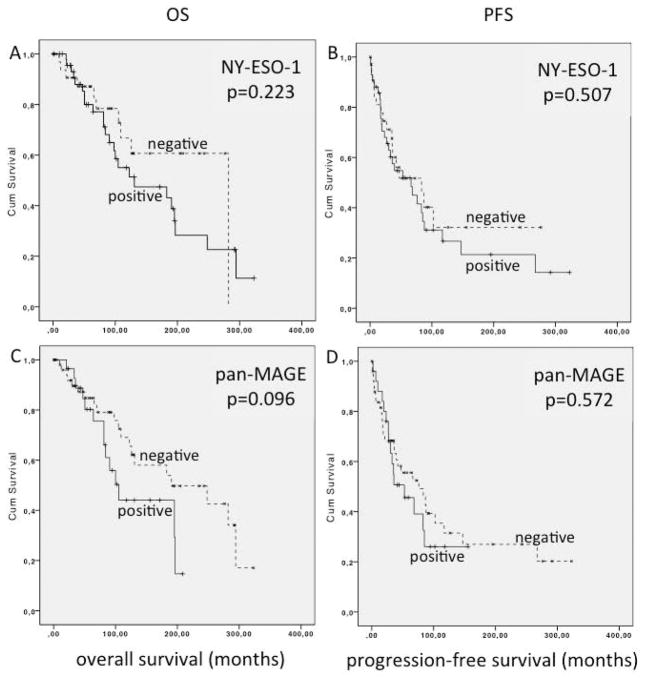
Median OS in NY-ESO-1 positive patients was 130.8 months compared to 282.0 months (p=0.223) in patients lacking NY-ESO-1 expression (figure 4A). In NY-ESO-1 positive patients PFS was 66.3 compared to 88.3 months in negative cases (p=0.507) (figure 4B).
Figure 4.
Analysis of overall survival (OS) and progression free survival (PFS) of HNACC patients in based on CTA expression status: A: OS of NY-ESO-1 positive vs. negative patients, B: PFS of NY-ESO-1 positive vs. negative patients, C: OS of pan-MAGE positive vs. negative patients, D: PFS of pan-MAGE positive vs. negative patients.
Median OS in pan-MAGE positive patients was 105.3 months compared to 190.5 months in negative patients (p=0.096; figure 4C). Median PFS was 52.8 months compared to 76.0 months in pan-MAGE-negative cases (p=0.572; figure 4D).
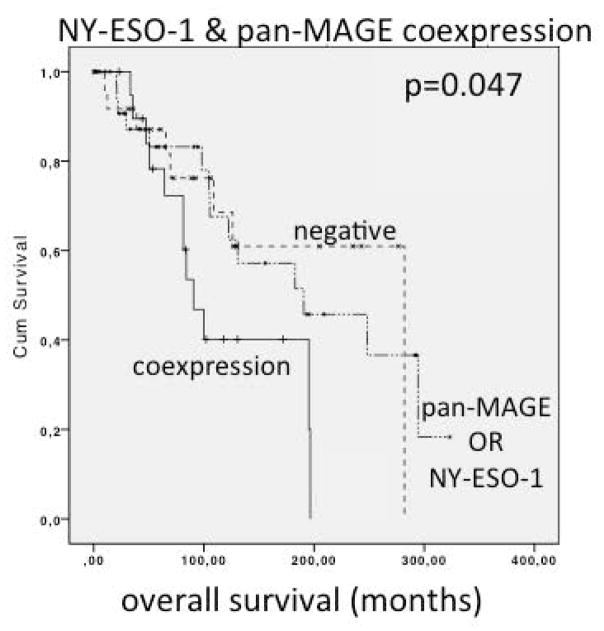
Patients expressing both, NY-ESO-1 and pan-MAGE simultaneously had significantly reduced survival with a median of 90.5 months compared to 282.0 months in negative patients (p=0.047) and 190.5 months in patients either expressing only NY-ESO-1 or only pan-MAGE (p=0.063). The survival difference between negative patients and NY-ESO1 OR pan-MAGE only patients was not statistically significant (p=0.867). Survival curves are depicted in figure 5. There was no significant correlation to other prognostically relevant markers in this subset.
Figure 5.
Analysis of overall survival (OS) of patients with head and neck adenoid cystic carcinoma (HNACC) based on CTA expression status. Patients positive for both pan-Melanoma antigen (pan-MAGE) and New York esophageal squamous cell carcinoma (NY-ESO-1) show significantly worse (p=0.047) OS than patients with negative expression status
Discussion
To our knowledge, this is the largest cohort of ACC that has been analyzed for CTA expression and its potential prognostic impact. Due to the low incidence of ACC the gathering of relevant patient numbers and survival data is cumbersome and therefore requires a multi-institutional approach.
In this retrospective study we focused on the expression CTAs in ACC. CTA expression is by definition restricted to malignant tumors and not found in normal tissues with the exception of testis and fetal placenta 9. The antibodies used (E978 for NY-ESO-1, M3H67 for pan-MAGE) have been employed by multiple groups for the detection of CTAs and are well established for IHC 5,9–13. IHC results have been reported to correspond well with mRNA analysis 14. We have previously shown that M3H67 detects multiple MAGE-family antigens, but not control peptides 5. A risk of false positive staining cannot be excluded, but has not been reported for these antibodies. This is the first study analyzing the prognostic impact of CTA expression in ACC. Specimens were collected from four different treatment sites. Thus, it was possible to assemble a cohort of 84 patients with relevant follow up and clinical data.
In order to determine the validity of our cohort, we analyzed known prognostic markers for OS such as perineural invasion, positive resection margins, positive lymph nodes, the primary tumor site, the pattern of treatment failure and the histological growth pattern. All of these factors were, as expected, associated with worse outcome.
Furthermore we determined that the site of the primary tumor was associated with OS. Patients with a primary tumor site other than the major salivary glands were more likely to be Pn-1 and positive margin resection. Due to common anatomic proximity to fundamental structures like the orbit and skull base, the most frequent other primary sites, such as the nasal sinuses, the midface and the lacrimal glands with close proximity confront the treating surgeon to functional problems. This may explain the higher rate of positive resection margins at primary resection in this group of patients. Another reason may be the higher rate of perineural invasion, which also reduces the probability of complete surgical resection.
ACC is a rare, but highly malignant disease that challenges the clinician in effective cancer therapy. Even in the case of successful primary surgery with curative intent, loco-regional control is often unsatisfactory. Treatment failure, whether loco-regional or distant, occurred in approximately 60% of our patient cohort, as also seen in the literature. If a disease relapse is non-resectable or occurs as distant metastasis, the treatment options for patients are very limited and include radiotherapy or systemic treatment such as chemotherapy or the use of molecular targeting agents. For either of these options treatment results from clinical trials have been frustrating 1–3.
Immunotherapy has the advantage of a systemic treatment potential that is independent of the site of failure. Recent developments in the field of immune checkpoint inhibition, especially in malignant melanoma, e.g. CTLA-4 or PD-1/PD-L1 blockade have moved immunotherapy into the spotlight 15–22.
All these agents have one trait in common: They reconstitute the efficacy of pre-existing immune responses in patients. Since immune checkpoint modulators are known to release the brake of the immune system 23,24 the presence of an existing immune response is a necessary requirement for the success of such immune checkpoint modulators. Data from melanoma treated with immune checkpoint modulators suggest that a high mutational load is associated with a good clinical response to immune checkpoint blockade 25. This is explained by a higher propability of mutations leading to the expression of so-called mutational neoantigens which can be recognized by the immune system 25–27. But the average number of mutations found in adenoid cystic carcinoma is low compared to other cancer types 2,28,29. The prevalence of neoantigens in ACC is yet unknown, but we demonstrate here a frequent expression rate of MAGE family and NY-ESO-1 CTAs as potential immunologic targets. In the studies demonstrating immune responses to neoantigens in patients treated with immune checkpoint blockade, other non-mutational antigens such as CTAs were, due to the methodology of the antigen screening process, neglected thus far. Nevertheless, the addition of an antigen-specific treatment in the form of a vaccine may increase the rate of responders to immune checkpoint targeted therapy. It has been shown that vaccines against MAGE-A330–34 as well as NY-ESO-1 can successfully induce the development of specific T-cell responses35–38. Because of their high tumor-specificity and their immunogenicity, these antigens may represent a good target for specific immunotherapy of ACC. In melanoma patients treated with ipilimumab an increased rate of NY-ESO-1 specific immunity has been associated with improved clinical benefit of treatment, especially in patients developing both NY-ESO-1 specific antibodys and specific CD8 T cells 39. A clinical trial with a multipeptide vaccine containing gp100, MART-1, and NY-ESO-1 with Montanide ISA 51 VG combined with nivolumab has been carried out in high-risk melanoma in an adjuvant setting and yielded an increase of antigen-specific CD8 T cells after treatment 40.
Since CTAs are to be considered self-antigens, spontaneous immune responses may be hampered by thymic tolerance. A detailed analysis of immune responses to CTA in ACC and immune modulatory components may help to identify a promising approach to reduce immunosuppression of CTA specific immunity in ACC. The analysis of specific cellular immune responses and the tumor microenvironment may reveal which kind of immune checkpoint modulation could synergize with a vaccination approach in ACC.
Tumor-infiltrating immune cells (TIC) are often discussed as a sign of immunologic recognition of the tumor 41. TIC have been shown to be important prognosticators in many cancer types, including colorectal cancer 42–46 and head & neck squamous cell carcinoma 47–51. The composition of TIC (different T cell types, B cells, macrophages, regulatory immune cells), their location (intratumoral, tumor margin, tumor stroma) and density are all important factors 42,47,52. There is still an intensive debate which markers need to be measured, which tumor compartments to evaluate and which method to use for quantification of TIC. Currently there are no such data on tumor infiltrating immune cells available for ACC. This is an important task and will be the subject of a future project of our group.
In this patient cohort, NY-ESO-1 expression was found in 48/84 (57.1%) and pan-MAGE expression in 28/84 (31.2%). Our findings differ from the results of Park et al., who described MAGE-A (Santa Cruz Biotechnology, Santa Cruz, CA, dilution 1:200) and MAGE-A4 (ABGENT, San Diego, CA, 1:200) in 100% in a cohort of 31 of ACC of the parotid gland. One reason for this discrepancy may lie in the cutoff for CT antigen expression of 10% positive cells in our study and 1% in the work of Park4. Furthermore the analysis of Park et al. was undertaken in a small cohort of 31 patients with ACC and solely parotid gland tumors were included. In a subgroup analysis of the 26 parotid tumors in our cohort we found NY-ESO-1 in 12/26 and pan-MAGE in 8/26 patients.
In HNSCC, the expression of CTAs of the MAGE-family and NY-ESO-1 have been determined as independent prognostic factors for poor survival5. The results from our data also show a trend toward worse OS in NY-ESO-1 and pan-MAGE positive patients, although this difference was not statistically significant. The trend to reduced survival in this cohort may be a result of the significant correlation with other prognostic markers. In the case of NY-ESO-1, it was the primary tumor site outside of the major salivary glands. Pan-MAGE expression was also associated with positive lymph node status. But the simultaneous expression of NY-ESO-1 and pan-MAGE found in 20 patients was associated with significantly reduced OS and was not associated with other known prognosticators.
Another potential explanation for the reduced survival may lie in the intrinsic function of these proteins. CTA have been suggested to support malignant transformation by preventing apoptosis 53,54 or by the promotion of cell proliferation 55,56. Whereas the function of NY-ESO-1 in both healthy and malignant cells is still poorly characterized, the MAGE family of proteins has previously been functionally analyzed. They also have been described to modify p53 function 57,58. Additionally MAGE seems to promote DNA damage repair in malignant cells 59. In summary, there is some evidence that CTA expression may promote malignancy and may be a sign of an aggressive tumor. It has been previously reported for HNSCC that the expression of more than one CTA is associated with reduced survival 60.
The high rate of CTA expression in ACC leads to more evidence for possible immunotherapeutic approaches in this entity. Before the conduction of clinical trials further understanding of the specific immunological responses in the tumor and its microenvironment are mandatory. In order to conduct prospective clinical trials in this rare tumor entity, multi-center approaches of cancer centers are needed.
Conclusion
A significant fraction of ACC patients show expression of the cancer testis antigens NY-ESO-1 and pan-MAGE. Positivity for both antigens simultaneously is associated with reduced OS. Thus, this subgroup of patients with a poor prognosis carries antigens targetable by specific immunotherapy. In recurrent and metastatic disease, trials of antigen-specific immunotherapy potentially combined with immune-checkpoint blockade seem warranted. In order to establish a strong rationale for this treatment approach, additional studies regarding the frequency of immune responses to these antigens and the most promising targets for immune checkpoint modulation are needed.
Acknowledgments
We thank the medical staff at the participating centers for helping to search and prepare the paraffin embedded tissue samples and gather the clinical information of the follow up status.
Footnotes
The work was presented at the annual meeting of the American Head and Neck Society (AHNS) in July 2014 in New York City and part of the work was presented at the annual DGHNO meeting in Dortmund, Germany in Mai 2014.
References
- 1.Ho K, Lin H, Ann DK, Chu PG, Yen Y. An overview of the rare parotid gland cancer. Head Neck Oncol. 2011;3:40. doi: 10.1186/1758-3284-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Büchsenschütz K, Veit JA, Schuler PJ, et al. Molecular approaches to systemic therapy of adenoid cystic carcinoma of the head and neck area. Laryngorhinootologie. 2014;93(10):657–64. doi: 10.1055/s-0034-1382024. [DOI] [PubMed] [Google Scholar]
- 3.Bell D, Hanna EY. Head and neck adenoid cystic carcinoma: what is new in biological markers and treatment? Curr Opin Otolaryngol Head Neck Surg. 2013;21(2):124–9. doi: 10.1097/MOO.0b013e32835c05fd. [DOI] [PubMed] [Google Scholar]
- 4.Park JH, Do NY, Han SI, Lim S-C. Usefulness of the melanoma antigen gene (MAGE) in making the differential diagnosis between pleomorphic adenoma and adenoid cystic carcinoma. J Otolaryngol Head Neck Surg. 2012;41(1):20–9. [PubMed] [Google Scholar]
- 5.Laban S, Atanackovic D, Luetkens T, et al. Simultaneous cytoplasmic and nuclear protein expression of melanoma antigen-A family and NY-ESO-1 cancer-testis antigens represents an independent marker for poor survival in head and neck cancer. Int J Cancer. 2014;135(5):1142–52. doi: 10.1002/ijc.28752. [DOI] [PubMed] [Google Scholar]
- 6.Adams S, O’Neill DW, Nonaka D, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181(1):776–84. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada H, Sato E, Uenaka A, et al. Analysis of peripheral and local anti-tumor immune response in esophageal cancer patients after NY-ESO-1 protein vaccination. Int J Cancer. 2008;123(10):2362–9. doi: 10.1002/ijc.23810. [DOI] [PubMed] [Google Scholar]
- 8.Kageyama S, Wada H, Muro K, et al. Dose-dependent effects of NY-ESO-1 protein vaccine complexed with cholesteryl pullulan (CHP-NY-ESO-1) on immune responses and survival benefits of esophageal cancer patients. J Transl Med. 2013;11:246. doi: 10.1186/1479-5876-11-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jungbluth AA, Chen YT, Stockert E, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92(6):856–60. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 10.Figueiredo DLA, Mamede RCM, Spagnoli GC, et al. High expression of cancer testis antigens MAGE-A, MAGE-C1/CT7, MAGE-C2/CT10, NY-ESO-1, and gage in advanced squamous cell carcinoma of the larynx. Head Neck. 2011;33(5):702–7. doi: 10.1002/hed.21522. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann A-K, Imig J, Klar A, et al. Expression of MAGE-C1/CT7 and selected cancer/testis antigens in ovarian borderline tumours and primary and recurrent ovarian carcinomas. Virchows Arch. 2013;462(5):565–74. doi: 10.1007/s00428-013-1395-3. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan HA, Svobodova S, Macgregor D, et al. Immunohistochemical and molecular analysis of human melanomas for expression of the human cancer-testis antigens NY-ESO-1 and LAGE-1. Clin Cancer Res. 2004;10(24):8396–404. doi: 10.1158/1078-0432.CCR-04-0809. [DOI] [PubMed] [Google Scholar]
- 13.Inaoka RJ, Jungbluth AA, Baiocchi OC, et al. An overview of cancer/testis antigens expression in classical Hodgkin’s lymphoma (cHL) identifies MAGE-A family and MAGE-C1 as the most frequently expressed antigens in a set of Brazilian cHL patients. BMC Cancer. 2011;11(1):416. doi: 10.1186/1471-2407-11-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Wu X-J, Zhao A-L, et al. Cancer/testis antigen expression and autologous humoral immunity to NY-ESO-1 in gastric cancer. Cancer Immun. 2004;4:11. [PubMed] [Google Scholar]
- 15.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postow MA, Harding J, Wolchok JD. Targeting immune checkpoints: releasing the restraints on anti-tumor immunity for patients with melanoma. Cancer J. 2012;18(2):153–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Robert C, Long GV, Brady B, et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N Engl J Med. 2014;372(4):141116004513004. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 19.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolchok JD, Chan TA. Cancer: Antitumour immunity gets a boost. Nature. 2014;515(7528):496–8. doi: 10.1038/515496a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermott D, Lebbé C, Hodi FS, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev. 2014;40(9):1056–64. doi: 10.1016/j.ctrv.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarhini A, Lo E, Minor DR. Releasing the brake on the immune system: ipilimumab in melanoma and other tumors. Cancer Biother Radiopharm. 2010;25(6):601–13. doi: 10.1089/cbr.2010.0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryan LJ, Gordon LI. Releasing the Brake on the Immune System: The PD-1 Strategy for Hematologic Malignancies. Oncology (Williston Park) 2015;29(6):431–9. [PubMed] [Google Scholar]
- 25.Snyder A, Makarov V, Merghoub T, et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med. 2014;371(23):141119140020009. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA4 blockade in metastatic melanoma. Science. 2015 doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–81. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho AS, Kannan K, Roy DM, et al. The mutational landscape of adenoid cystic carcinoma. Nat Genet. 2013;45(7):791–8. doi: 10.1038/ng.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephens PJ, Davies HR, Mitani Y, et al. Whole exome sequencing of adenoid cystic carcinoma. J Clin Invest. 2013;123(7):2965–8. doi: 10.1172/JCI67201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adam V, Wauters I, Vansteenkiste J. Melanoma-associated antigen-A3 vaccination in the treatment of non-small-cell lung cancer. Expert Opin Biol Ther. 2014;14(3):365–76. doi: 10.1517/14712598.2014.880421. [DOI] [PubMed] [Google Scholar]
- 31.Atanackovic D, Altorki NK, Cao Y, et al. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci. 2008;105(5):1650–5. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atanackovic D, Altorki NK, Stockert E, et al. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol. 2004;172(5):3289–96. doi: 10.4049/jimmunol.172.5.3289. [DOI] [PubMed] [Google Scholar]
- 33.Rapoport AP, Aqui NA, Stadtmauer EA, et al. Combination immunotherapy after ASCT for multiple myeloma using MAGE-A3/Poly-ICLC immunizations followed by adoptive transfer of vaccine-primed and costimulated autologous T cells. Clin Cancer Res. 2014;20(5):1355–65. doi: 10.1158/1078-0432.CCR-13-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zandberg DP, Rollins S, Goloubeva O, et al. A phase I dose escalation trial of MAGE-A3- and HPV16-specific peptide immunomodulatory vaccines in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN) Cancer Immunol Immunother. 2015;64(3):367–79. doi: 10.1007/s00262-014-1640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J-L, Dawoodji A, Tarlton A, et al. NY-ESO-1 specific antibody and cellular responses in melanoma patients primed with NY-ESO-1 protein in ISCOMATRIX and boosted with recombinant NY-ESO-1 fowlpox virus. Int J Cancer. 2015;136(6):E590–601. doi: 10.1002/ijc.29118. [DOI] [PubMed] [Google Scholar]
- 36.Sabado RL, Pavlick A, Gnjatic S, et al. Resiquimod as an Immunologic Adjuvant for NY-ESO-1 Protein Vaccination in Patients with High-Risk Melanoma. Cancer Immunol Res. 2015;3(3):278–87. doi: 10.1158/2326-6066.CIR-14-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuji T, Sabbatini P, Jungbluth AA, et al. Effect of Montanide and poly-ICLC adjuvant on human self/tumor antigen-specific CD4+ T cells in phase I overlapping long peptide vaccine trial. Cancer Immunol Res. 2013;1(5):340–50. doi: 10.1158/2326-6066.CIR-13-0089. [DOI] [PubMed] [Google Scholar]
- 38.Wada H, Isobe M, Kakimi K, et al. Vaccination with NY-ESO-1 overlapping peptides mixed with Picibanil OK-432 and montanide ISA-51 in patients with cancers expressing the NY-ESO-1 antigen. J Immunother. 2014;37(2):84–92. doi: 10.1097/CJI.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 39.Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108(40):16723–8. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibney GT, Kudchadkar RR, DeConti RC, et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res. 2015;21(4):712–20. doi: 10.1158/1078-0432.CCR-14-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 42.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 43.Halama N, Michel S, Kloor M, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71(17):5670–7. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 44.Halama N, Michel S, Kloor M, et al. The localization and density of immune cells in primary tumors of human metastatic colorectal cancer shows an association with response to chemotherapy. Cancer Immun. 2009;9:1. [PMC free article] [PubMed] [Google Scholar]
- 45.Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the “Immunoscore” in the classification of malignant tumours. J Pathol. 2014;232(2):199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29(6):610–8. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 47.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12(2):465–72. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 48.Balermpas P, Rödel F, Rödel C, et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG) Int J Cancer. 2015 doi: 10.1002/ijc.29683. [DOI] [PubMed] [Google Scholar]
- 49.Nordfors C, Grün N, Tertipis N, et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer. 2013;49(11):2522–30. doi: 10.1016/j.ejca.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Wansom D, Light E, Thomas D, et al. Infiltrating lymphocytes and human papillomavirus-16--associated oropharyngeal cancer. Laryngoscope. 2012;122(1):121–7. doi: 10.1002/lary.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward MJ, Thirdborough SM, Mellows T, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110(2):489–500. doi: 10.1038/bjc.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balermpas P, Rödel F, Liberz R, et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br J Cancer. 2014;111(8):1509–18. doi: 10.1038/bjc.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nardiello T, Jungbluth AA, Mei A, et al. MAGE-A inhibits apoptosis in proliferating myeloma cells through repression of Bax and maintenance of survivin. Clin Cancer Res. 2011;17(13):4309–19. doi: 10.1158/1078-0432.CCR-10-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atanackovic D, Hildebrandt Y, Jadczak A, et al. Cancer-testis antigens MAGE-C1/CT7 and MAGE-A3 promote the survival of multiple myeloma cells. Haematologica. 2010;95(5):785–93. doi: 10.3324/haematol.2009.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cappell KM, Sinnott R, Taus P, Maxfield K, Scarbrough M, Whitehurst AW. Multiple cancer testis antigens function to support tumor cell mitotic fidelity. Mol Cell Biol. 2012;32(20):4131–40. doi: 10.1128/MCB.00686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Por E, Byun H-J, Lee E-J, et al. The cancer/testis antigen CAGE with oncogenic potential stimulates cell proliferation by up-regulating cyclins D1 and E in an AP-1- and E2F-dependent manner. J Biol Chem. 2010;285(19):14475–85. doi: 10.1074/jbc.M109.084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marcar L, Maclaine NJ, Hupp TR, Meek DW. Mage-A cancer/testis antigens inhibit p53 function by blocking its interaction with chromatin. Cancer Res. 2010;70(24):10362–70. doi: 10.1158/0008-5472.CAN-10-1341. [DOI] [PubMed] [Google Scholar]
- 58.Peche LY, Scolz M, Ladelfa MF, Monte M, Schneider C. MageA2 restrains cellular senescence by targeting the function of PMLIV/p53 axis at the PML-NBs. Cell Death Differ. 2012;19(6):926–36. doi: 10.1038/cdd.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhatia N, Xiao TZ, Rosenthal KA, et al. MAGE-C2 promotes growth and tumorigenicity of melanoma cells, phosphorylation of KAP1, and DNA damage repair. J Invest Dermatol. 2013;133(3):759–67. doi: 10.1038/jid.2012.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuffel C, Rivals J-P, Zaugg Y, et al. Pattern and clinical significance of cancer-testis gene expression in head and neck squamous cell carcinoma. Int J Cancer. 2011;128(11):2625–34. doi: 10.1002/ijc.25607. [DOI] [PubMed] [Google Scholar]