Abstract
IMPORTANCE
Cancer-related fatigue (CRF) remains one of the most prevalent and troublesome adverse events experienced by patients with cancer during and after therapy.
OBJECTIVE
To perform a meta-analysis to establish and compare the mean weighted effect sizes (WESs) of the 4 most commonly recommended treatments for CRF—exercise, psychological, combined exercise and psychological, and pharmaceutical—and to identify independent variables associated with treatment effectiveness.
DATA SOURCES
PubMed, PsycINFO, CINAHL, EMBASE, and the Cochrane Library were searched from the inception of each database to May 31, 2016.
STUDY SELECTION
Randomized clinical trials in adults with cancer were selected. Inclusion criteria consisted of CRF severity as an outcome and testing of exercise, psychological, exercise plus psychological, or pharmaceutical interventions.
DATA EXTRACTION AND SYNTHESIS
Studies were independently reviewed by 12 raters in 3 groups using a systematic and blinded process for reconciling disagreement. Effect sizes (Cohen d) were calculated and inversely weighted by SE.
MAIN OUTCOMES AND MEASURES
Severity of CRF was the primary outcome. Study quality was assessed using a modified 12-item version of the Physiotherapy Evidence-Based Database scale (range, 0–12, with 12 indicating best quality).
RESULTS
From 17 033 references, 113 unique studies articles (11525 unique participants; 78% female; mean age, 54 [range, 35–72] years) published from January 1, 1999, through May 31, 2016, had sufficient data. Studies were of good quality (mean Physiotherapy Evidence-Based Database scale score, 8.2; range, 5–12) with no evidence of publication bias. Exercise (WES, 0.30; 95% CI, 0.25–0.36; P < .001), psychological (WES, 0.27; 95% CI, 0.21–0.33; P < .001), and exercise plus psychological interventions (WES, 0.26; 95% CI, 0.13–0.38; P < .001) improved CRF during and after primary treatment, whereas pharmaceutical interventions did not (WES, 0.09; 95% CI, 0.00–0.19; P = .05). Results also suggest that CRF treatment effectiveness was associated with cancer stage, baseline treatment status, experimental treatment format, experimental treatment delivery mode, psychological mode, type of control condition, use of intention-to-treat analysis, and fatigue measures (WES range, −0.91 to 0.99). Results suggest that the effectiveness of behavioral interventions, specifically exercise and psychological interventions, is not attributable to time, attention, and education, and specific intervention modes may be more effective for treating CRF at different points in the cancer treatment trajectory (WES range, 0.09–0.22).
CONCLUSIONS AND RELEVANCE
Exercise and psychological interventions are effective for reducing CRF during and after cancer treatment, and they are significantly better than the available pharmaceutical options. Clinicians should prescribe exercise or psychological interventions as first-line treatments for CRF.
Cancer-related fatigue (CRF) is one of the most common and disabling adverse effects reported by patients with cancer during and after treatment.1–6 Cancer-related fatigue can persist for years after treatment completion4,6–11 and is exacerbated by co-occurring cancer-related adverse effects such as depression, anxiety, sleep disturbance, and pain.3,4,12–18 Cancer-related fatigue reduces a patient’s ability to complete medical treatments for cancer and participate in essential and valued life activities, thus undermining quality of life and potentially reducing overall survival.6,9,19 Cancer-related fatigue has been designated a high-priority research area by the National Cancer Institute and is 1 of the 5 highest priority research areas designated by the National Cancer Institute Clinical Oncology Research Program in the United States.20
Randomized clinical trials (RCTs) have tested exercise, psychological, exercise plus psychological, and pharmaceutical interventions for the amelioration of CRF.21–35 Results of these RCTs are promising; however, development and implementation of guidelines for clinical practice36–38 are challenging owing to the lack of a direct meta-analytic comparison of these 4 most commonly recommended behavioral and pharmaceutical treatments for CRF. Although clinical practice guidelines exist for the management of CRF,36–38 which mode of treatment is most effective remains unclear.
To our knowledge, no prior review of CRF has applied meta-analytic methods to compare the efficacy of all 4 major types of treatments recommended for managing CRF, nor has any prior review systematically explored factors that are associated with treatment effectiveness (eg, age, type of cancer, during vs completed primary cancer treatment, study quality) when managing CRF. This information can enhance a personalized medicine approach when treating CRF and can inform future research.
The primary purposes of this meta-analysis were to (1) ascertain a more comprehensive and definitive estimate of weighted effect sizes for exercise (ie, aerobic, anaerobic or strength, or both), psychological (ie, cognitive behavioral, psychoeducational, or eclectic), the combination of exercise and psychological, and pharmaceutical interventions used to treat CRF; (2) to determine which of these 4 interventions significantly improves CRF; and (3) to compare the magnitudes of improvement in CRF produced by each intervention type. The secondary purpose was to identify independent variables associated with treatment efficacy for the management of CRF.
Methods
Search Strategy
Methods and reporting for this meta-analysis adhere to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the recommendations of 2 experts (D.M. and S.M.M.) in meta-analytic procedures on the team.39,40 We searched the following electronic databases: PubMed, PsycINFO, CINAHL, EMBASE, and the Cochrane Library. Articles published in English between the inception of each database and May 31, 2016, were searched for controlled-vocabulary terms specific to each database related to CRF, neoplasms, questionnaires, intervention strategies, and study design (eTable 1 in the Supplement).
Selection Strategy
Study selection strategy was rigorously defined. For inclusion, studies met the following criteria: (1) use of an RCT design, (2) adult (≥18 years) participants with cancer, (3) CRF severity measured as an outcome (eTable 2 in the Supplement for fatigue measures), (4) evaluation of CRF severity not solely as an adverse effect of cancer treatment, (5) no report on a pharmaceutical intervention that evaluated an erythropoietin drug because such drugs are used primarily for treating anemia and are not recommended as a stand-alone treatment for CRF due to adverse effects, (6) no report of a complementary and alternative intervention with the exception of exercise-based therapies (ie, yoga, tai chi), and (7) no use of reduced energy, vitality, or vigor as the fatigue outcome measure because these constructs are qualitatively different from CRF.41
Review Strategy
All reviews and data extractions were performed independently by at least 3 raters (includes all authors) considered experts in the field of cancer control and CRF. Data were extractedusing online coding and Excel programs (Microsoft, Inc) designed specifically for this project. The programs produced a list of data abstraction and coding discrepancies among reviewers. All discrepancies were resolved by independent third-party review and consensus; independent review was required for 6 studies, and 100% agreement was obtained for all 113 studies. Study investigators were contacted by standardized email letters at least 3 times to provide information omitted from published articles. To assess the methodologic quality of the studies, a modified 12-item version of the Physiotherapy Evidence-Based Database (PEDro) scale, developed using a Delphi expert consensus technique,42–44 was used because it identifies studies that are generalizable, internally valid, and statistically interpretable. The PEDro scale (range, 0–12, with 12 indicating highest quality) accounts for unique issues regarding blinding of the participant, assessor, or therapist in behavioral trials.42–44 Delineation of exercise interventions as aerobic, anaerobic, or both and psychological interventions as cognitive behavioral, psychoeducational, or eclectic was based on descriptions provided in the published articles.
Statistical Analysis
Effect sizes (Cohen d) were computed as the mean difference in change from pretreatment to posttreatment between the experimental and control groups, divided by the pooled post-intervention SDs. The effect sizes were combined across all intervention types with weights based on a random-effects model (Hedges random effects45) to facilitate generalizability of results and because we expected considerable heterogeneity.46 Owing to the smaller number of studies, we used fixed-effects models to combine effect sizes within each intervention type and to model predictors of intervention effectiveness. Cut points for determining small, moderate, and large effects were defined as 0 to 0.29, 0.30 to 0.59, and 0.60 or greater, respectively.47 Details for the computations are given online in the eMethods of the Supplement. All analyses were performed using the meta for package in R (version 3.2).48
Estimation of Intervention Effectiveness
Tests for significant differences between groups used a fixed-effects model for categorical independent variables. Method of moments estimation was used for analysis of continuous independent variables in the univariate metaregression.47 Variables to be tested for association with intervention effectiveness were selected a priori and included age, sex, cancer type, cancer stage, treatment status at baseline (ie, inpatient, outpatient, or mixed), experimental treatment format (ie, group or individual), primary delivery mode of experimental treatment (ie, in-person only, in-person plus other [eg, telephone calls, mailings, or web], or no in-person contact), exercise mode (ie, aerobic, resistance or nonaerobic, or combined), psychological mode (ie, psychoeducational, cognitive behavioral, or eclectic), type of control comparison (ie, no intervention, standard care, or wait-list vs placebo, time, attention, and education), allocation concealment, intention-to-treat analysis, use of treatment fidelity protocol, PEDro scale quality score, and fatigue scale used.49–53
Sensitivity Analyses
Sensitivity analyses were conducted because of studies with multiple treatment conditions that resulted in 2 or more intervention comparisons (eg, treatment 1 vs control and treatment 2 vs control) from the same study. To detect an artificial reduction of heterogeneity and a bias in the overall mean effect size, we conducted analyses in which we included only 1 comparison per study at a time.
Bias Analyses
Publication bias was tested by examining funnel plots and the trim and fill procedure of Duval and Tweedie.54 To examine stability of the overall effect, fail-safe number was calculated to determine the number of studies with a null effect size that was needed to reduce the overall effect to nonsignificance.55
Results
Studies
We selected more than 17 033 titles and abstracts for initial review. An article was excluded if information in the title and abstract indicated it was not an RCT, it did not assess fatigue, or it used an ineligible intervention method. We selected 351 articles for full review. One hundred seventy-eight articles did not meet inclusion criteria (eg, nonrandomization, assessed vigor rather than fatigue, ineligible intervention method) and were eliminated; 60 of the remaining 173 articles did not provide sufficient data for calculation of effect sizes, even after querying the authors multiple times. Ultimately, we analyzed 113 unique studies (eTable 3 in the Supplement) and calculated 127 effect sizes (14 articles had multiple treatment arms). Of these 127 effect sizes, 69 evaluated exercise interventions, 34 evaluated psychological interventions, 10 evaluated the combination of exercise and psychological interventions, and 14 evaluated pharmaceutical interventions. Figure 1 displays the PRISMA study selection flowchart.56,57
Figure 1. PRISMA Diagram.
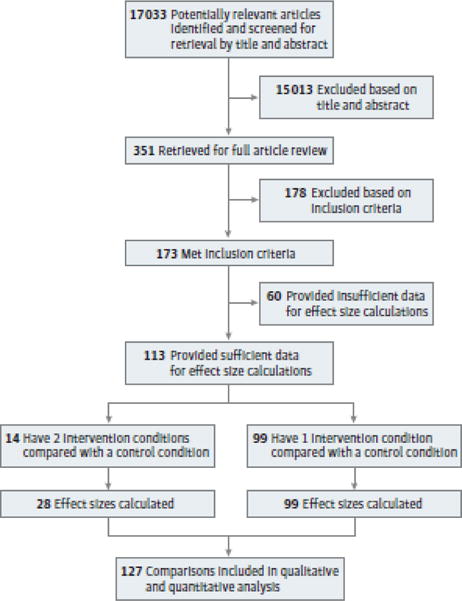
Flow of study screening, final inclusion, and effect size calculations are depicted.
Participants
The 113 included studies yielded a sample of 11 525 unique participants (78% female and 22% male). Fifty-three studies (46.9%) were performed among women with breast cancer and the remaining studies were performed among patients with other cancer types. Fifty-four studies included only women and 10 studies included only men. The mean age of participants was 54 (range, 35–72) years across all studies. Race, educational level, and partner status could not be accurately summarized owing to missing data. With regard to cancer stage, 50 studies (44.2%) enrolled patients with nonmetastatic cancer; 11 studies (9.7%), with metastatic cancer; and 33 studies (29.2%), with metastatic and nonmetastatic cancer. The remaining 19 studies (16.8%) did not provide staging information. With regard to primary treatments, 51 studies (45.1%) enrolled patients receiving primary treatment (defined as surgery, chemotherapy, and/or radiotherapy) during the study intervention, 45 studies (39.8%) enrolled patients who had already completed primary treatments, 15 studies (13.3%) enrolled patients of mixed treatment status (during and after primary treatment), and 2 studies (1.8%) did not provide sufficient information on treatment status. Recruitment for these studies was conducted primarily in medical clinics using systematic screening and mixed recruitment strategies.
Intervention and Control Conditions
Mean (SD) sample size was 102 (95.5) at baseline with 47 (47.3) participants in the control groups and 57 (49.0) participants in the intervention groups at baseline. Mean duration of interventions was 14 (range, 1–60) weeks, included a mean of 43 (range, 1–364) sessions, and sessions lasted a mean of 60 (range, 16–150) minutes. With regard to control interventions, 77 studies (68.1%) used standard cancer care, no intervention, or waitlist control, whereas 36 studies (31.0%) used a placebo, time, attention, or education control. Two pharmaceutical studies tested paroxetine hydrochloride; 4, modafinil or armodafinil; 5, methylphenidate hydrochloride or dexymethylphenidate; 1, dexamphetamine; and 1, methylprednisolone. Thirty-six exercise studies tested aerobic modes of exercise, 13 tested anaerobic modes, and 20 tested a combination of aerobic and anaerobic modes. Nineteen psychological studies tested a cognitive behavioral method, 14 tested a psychoeducational method, and 1 tested an eclectic method (a unique combination of psychotherapeutic methods). Ten studies tested a combined exercise plus psychological intervention. Ninety-nine studies used a traditional 2-arm RCT design (ie, intervention vs control), whereas 14 studies used a 3-arm RCT design (ie, intervention 1 vs intervention 2 vs control). eTable 3 in the Supplement provides a detailed summary of all included studies.
Quality of Studies
The mean PEDro scale score for all studies was 8.2 (range, 5–12), suggesting that the studies were of good quality. In all 113 studies, inclusion and exclusion criteria for study participants were specified; random allocation was used for group assignment, and between-group statistical comparisons were reported for CRF severity. Seventy studies (61.9%) used intention-to-treat analyses; 32 studies (28.3%) concealed allocation from participants or blinded outcome assessors; and 38 studies (32.7%) monitored treatment quality, fidelity, and drift.
Meta-analysis Main Effects
Changes in CRF by Intervention Type
We found significant moderate improvements in CRF (weighted effect size [WES], 0.33; 95% CI, 0.24–0.43; P < .001) across all 113 studies, including all 4 intervention types (ie, exercise [n = 69], psychological [n = 34], exercise plus psychological [n = 10], and pharmaceutical [n = 14] from before to after intervention). Studies that intervened with exercise demonstrated the largest overall improvement in CRF, with significant moderate effects (WES, 0.30; 95% CI, 0.25–0.36; P < .001). Studies using psychological interventions exhibited similar improvements in CRF (WES, 0.27; 95% CI, 0.21–0.33; P < .001). Studies that delivered the combination of exercise plus psychological interventions also exhibited similar improvements in CRF (WES, 0.26; 95% CI, 0.13–0.38; P < .001). Pharmaceutical interventions yielded significant but very small improvements in CRF (WES, 0.09; 95% CI, 0.00–0.19; P = .05). Comparisons across all 4 intervention types revealed that exercise, psychological, and exercise plus psychological interventions produced significantly greater improvements in CRF compared with pharmaceutical interventions, with no other demonstrated differences between intervention types (Figure 2 and eFigure 1 in the Supplement depict forest plots).
Figure 2. Forest Plot of Weighted Effect Sizes (WESs).
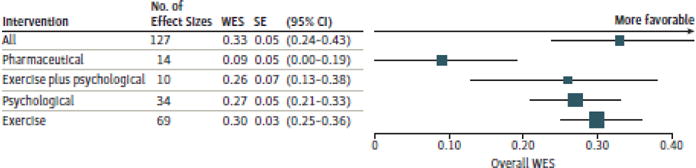
Overall WES across all interventions, exercise interventions, psychological interventions, exercise plus psychological interventions, and pharmaceutical interventions. Different sizes of markers indicate weight.
Independent Variables Associated With Intervention Effectiveness
We tested whether each of 15 variables listed in the Methods section was associated with the effectiveness of all 4 intervention types for improving CRF per their WES (for all data and P values, see Table). Results suggest that intervention effectiveness is associated with the following 8 variables: cancer stage (nonmetastatic, metastatic, or mixed), treatment status at baseline (during primary treatment, after primary treatment, and mixed), experimental treatment format (group or individual), primary delivery mode of experimental treatment (in-person, in-person plus other, or no in-person contact), psychological mode (psychoeducational, cognitive behavioral, or eclectic), type of control condition, use of intention-to-treat analysis, and fatigue scale used. Although improvements in CRF were reported by all patients and survivors, patients with early-stage (ie, nonmetastatic) disease and patients who had completed primary treatments (ie, surgery, chemotherapy, or radiotherapy) reported the greatest benefit. Interventions were the most effective for reducing CRF when delivered using group-based and in-person formats. The most effective type of psychological intervention for reducing CRF was cognitive behavioral therapy, and these interventions were most effective when implemented among survivors after primary treatment. Participants were also more likely to report the greatest reductions in CRF when it was measured using the Piper Fatigue Scale,49 the use of intention-to-treat analysis was not clearly stated, and the control condition was standard care. However, exercise and psychological interventions produced significant improvements in CRF, even when a rigorous specific-component (behavioral placebo) control comparison was used.
Table.
Factors Associated With Intervention Effectiveness on CRF
| Variablea | Overall WES (95% CI) | P Value | No. of Effect Sizes | ||||
|---|---|---|---|---|---|---|---|
| Cancer stage at baseline, all interventions | |||||||
| Only nonmetastatic | 0.37 (0.31 to 0.42) | <.001 | 59 | ||||
| Only metastatic | 0.29 (0.16 to 0.41) | <.001 | 11 | ||||
| Mix of nonmetastatic and metastatic | 0.10 (0.04 to 0.17) | .001 | 35 | ||||
| Treatment status at baseline, all interventions | |||||||
| After primary treatment | 0.29 (0.23 to 0.36) | <.001 | 53 | ||||
| Mix during and after primary treatment | 0.30 (0.19 to 0.40) | <.001 | 15 | ||||
| During primary treatment | 0.22 (0.17 to 0.27) | <.001 | 57 | ||||
| Experimental treatment format, all interventions | |||||||
| Group-based | 0.38 (0.31 to 0.46) | <.001 | 35 | ||||
| Individual-based | 0.23 (0.18 to 0.27) | <.001 | 79 | ||||
| Individual-, couple-, and family-based | 0.23 (−0.64 to 1.09) | .61 | 1 | ||||
| Individual- and group-based | 0.02 (−0.13 to 0.17) | .77 | 5 | ||||
| Primary delivery mode of experimental treatment, all interventions | |||||||
| Web | 0.99 (0.21 to 1.78) | .01 | 1 | ||||
| Telephone and print | 0.46 (0.04 to 0.89) | .03 | 1 | ||||
| Telephone | 0.30 (0.19 to 0.41) | <.001 | 6 | ||||
| In-person | 0.28 (0.23 to 0.32) | <.001 | 103 | ||||
| In-person and telephone | 0.006 (−0.11 to 0.25) | .47 | 7 | ||||
| In-person and print | −0.03 (−0.22 to 0.15) | .72 | 6 | ||||
| In person, telephone, and print | −0.36 (−1.12 to 0.40) | .35 | 1 | ||||
| −0.91 (−1.53 to −0.30) | .004 | 1 | |||||
| Psychological mode, only psychological interventions | |||||||
| Eclectic | 0.78 (0.29 to 1.27) | .002 | 1 | ||||
| Cognitive behavioral therapy | 0.37 (0.28 to 0.47) | <.001 | 17 | ||||
| Behavioral | 0.32 (0.13 to 0.50) | .001 | 3 | ||||
| Cognitive | 0.28 (−0.02 to 0.58) | .07 | 2 | ||||
| Psychoeducational | 0.17 (0.08 to 0.26) | <.001 | 17 | ||||
| Motivational interviewing | 0.10 (−0.17 to 0.37) | .47 | 2 | ||||
| Cognitive behavioral stress management | 0.10 (−0.18 to 0.38) | .48 | 1 | ||||
| Control condition, only exercise and psychological | |||||||
| Standard cancer care, wait-list control | 0.31 (0.26 to 0.35) | <.001 | 88 | ||||
| Specific component (ie, time, attention, education) | 0.24 (0.17 to 0.31) | <.001 | 23 | ||||
| Use of intention-to-treat analysis, all interventions | |||||||
| None | 0.34 (0.27 to 0.40) | <.001 | 46 | ||||
| Used | 0.22 (0.17 to 0.26) | <.001 | 79 | ||||
| Fatigue scale, all interventions | |||||||
| Piper Fatigue Scale | 0.64 (0.49 to 0.80) | <.001 | 10 | ||||
| Brief Fatigue Inventory | 0.31 (0.19 to 0.42) | <.001 | 12 | ||||
| Multidimensional Fatigue Inventory | 0.26 (0.13 to 0.39) | <.001 | 9 | ||||
| Functional Assessment of Chronic Illness Therapy | 0.22 (0.15 to 0.29) | <.001 | 31 | ||||
| European Organization for Research and Treatment of Cancer Quality of Life Questionnaire | 0.12 (0.02 to 0.22) | .02 | 13 | ||||
| Treatment status at baseline, separated by intervention type | |||||||
| During primary: exercise | 0.34 (0.25 to 0.42) | <.001 | 31 | ||||
| During primary: psychological | 0.23 (0.15 to 0.31) | <.001 | 18 | ||||
| During primary: exercise and psychological | 0.01 (−0.26 to 0.28) | .95 | 2 | ||||
| During primary: pharmaceutical | 0.04 (−0.07 to 0.32) | .51 | 6 | ||||
| After primary: exercise | 0.26 (0.18 to 0.34) | <.001 | 29 | ||||
| After primary: psychological | 0.42 (0.29 to 0.55) | <.001 | 13 | ||||
| After primary: exercise and psychological | 0.32 (0.17 to 0.47) | <.001 | 7 | ||||
| After primary: pharmaceutical | 0.08 (−0.17 to 0.32) | .55 | 4 | ||||
| Type of control condition, separated by intervention type | |||||||
| Standard: exercise | 0.32 (0.26 to 0.38) | <.001 | 57 | ||||
| Standard: psychological | 0.27 (0.19 to 0.36) | <.001 | 25 | ||||
| Standard: exercise and psychological | 0.31 (0.13 to 0.49) | <.001 | 6 | ||||
| Standard: pharmaceutical | 0 | ||||||
| Specific component: exercise | 0.22 (0.09 to 0.35) | .001 | 12 | ||||
| Specific component: psychological | 0.27 (0.17 to 0.36) | <.001 | 8 | ||||
| Specific component: exercise and psychological | 0.16 (−0.05 to 0.37) | .13 | 3 | ||||
| Specific component: pharmaceutical | 0.09 (0.00 to 0.19) | .05 | 14 | ||||
Abbreviations: CRF, cancer-related fatigue; WES, weighted effect size.
Variables shown in the table were statistically significantly associated with the WES across all intervention types. The following variables were not associated with the WES: age, sex, cancer type, exercise mode, allocation concealment, treatment fidelity protocol, or Physiotherapy Evidence-Based Database scale quality score (all P > .10).
The following variables were not associated with intervention effectiveness: age, sex, cancer type (breast vs others), exercise mode (aerobic, resistance or anaerobic, or combined), allocation concealment, treatment fidelity protocol, or PEDro scale quality score. Patients of all ages and with all types of cancer equally experienced improvements in CRF. Aerobic and anaerobic exercise interventions were equally effective for treating CRF. However, exercise was most effective when prescribed for patients receiving primary treatment, whereas the combination of exercise plus psychological interventions was most effective when delivered in survivors after they received the primary treatment. Participants were also equally likely to report improvements in CRF regardless of allocation concealment, use of a treatment fidelity protocol, and PEDro scale quality score.
Publication Bias and Sensitivity Analyses
We found no evidence of publication bias per the funnel plot (eFigure 2 in the Supplement) and the trim and fill methods. The fail-safe analysis indicated that 6264 RCTs with null findings for CRF would have to be included in this meta-analysis to alter the reported conclusions. Sensitivity analyses revealed no substantial change in overall WES or WES by intervention type, indicating no artificial reduction of heterogeneity or bias when multiple intervention comparisons from the same study were included in the analyses.
Discussion
To our knowledge, this meta-analysis is the most comprehensive and rigorous conducted to date to examine the influence of exercise, psychological, exercise plus psychological, and pharmaceutical interventions on CRF. This meta-analysis is also, to our knowledge, the first to calculate WES across more than 110 well-designed RCTs testing the efficacy of the 4 most recommended treatment intervention types for CRF. Our results demonstrate that exercise, psychological, and exercise plus psychological interventions are effective for improving CRF during and after primary treatment, whereas pharmaceutical interventions, as studied to date, are not. Exercise and psychological interventions are significantly more effective for improving CRF compared with pharmaceutical interventions overall.
In this meta-analysis, studies using the combination of exercise plus psychological interventions produced inconsistent results. In the studies we reviewed, the combination of the 2 interventions is sometimes equivalent to or inferior to a single modality. These combinations could be counterproductive owing to insufficient psychological content or exercise prescriptions and doses and added complexity and time demands leading to reduced adherence. These combinations also could be synergistic and provide patients with much needed motivation, specific and reasonable goals, and assistance with trouble-shooting barriers. With only 10 studies of exercise plus psychological interventions, we cannot determine whether these inconsistent results stem from inferior study quality or dose dilution effects. Additional research is needed to draw definitive conclusions.
This meta-analysis is the first to demonstrate that the effectiveness of CRF interventions is related to cancer stage, baseline treatment status, experimental treatment format, experimental treatment delivery mode, psychological mode, type of control condition, use of intention-to-treat analysis, and fatigue scale used. This meta-analysis is also the first to demonstrate that the effectiveness of behavioral interventions, specifically exercise and psychological interventions, is not attributable to time, attention, and education. In addition, this meta-analysis is also the first to show that certain intervention modes may be more effective for treating CRF at different points in the cancer treatment trajectory. For example, exercise may be the most effective treatment for patients receiving primary treatment, whereas psychological and exercise plus psychological interventions may be most effective for survivors who have completed primary treatment.
Strengths and Limitations
This meta-analysis has several strengths, including the large number of studies included (113 studies and 127 effect sizes), a rigorous literature search by a team specializing in treatment or/and research of CRF; abstracting and consensus building of the data by highly qualified, experienced, and independent raters; adherence to stringent inclusion criteria and analytic methods; use of standard and valid measures of CRF severity; and examination of variables associated with intervention effectiveness. This meta-analysis also has limitations, most of which stem from the study designs and reporting methods in the published literature. For example, less than half of the studies provided detailed information on race, educational level, socioeconomic status, and other demographic factors, which limits the accuracy of the description of study participants and prohibits definitive conclusions regarding the generalizability of the results. Most of the studies are among patients with breast cancer or breast cancer survivors. Most studies did not screen for a specific level of fatigue as part of inclusion criteria or clearly designate the fatigue severity outcome as primary or secondary; moreover, these trials were not registered (eg, ClinicalTrials.gov) to provide reporting transparency. Only small numbers of published RCTs examined the combination of exercise plus psychological interventions and pharmaceutical interventions. Few studies use an appropriate control condition for specific components. Some studies were excluded because they were not written in English or because they did not include the basic statistics needed to calculate an effect size. Long-term follow-up (eg, 1–12 months) on continued adherence to the behavioral changes stemming from the interventions and their resultant effectiveness in treating CRF could not be examined owing to the lack of follow-up assessments and the inconsistency of assessment timing. Finally, as a limitation inherent to meta-analyses, residual confounding may result when combining WES across studies owing to distinct eligibility or other factors across studies (ie, participants were randomized within each study but not across studies).
Future RCTs need to provide demographic, medical, and statistical data to enable meta-analysis (means and SDs at each point). Future RCTs needto register trials; implement CRF severity screening criteria; identify CRF as a primary outcome; design studies to test independent variables associated with intervention effectiveness; use appropriate specific-component control conditions for comparisons; identify biomarkers of CRF; identify biological and psychosocial mechanisms of CRF and its treatment; identify new drugs, exercise, psychological, and combination interventions to test; and implement longer-term follow-up assessments at consistent times. Although the results of this meta-analysis are very informative, conducting more high-quality, phase 3 RCTs to test new treatment options and directly compare treatments with known efficacy for managing CRF is of critical importance.
Conclusions
These findings demonstrate that exercise and psychological interventions are effective for improving CRF during and after primary treatment, whereas pharmaceutical interventions are not. More research is needed to better understand the effectiveness of interventions that combine exercise and psychological treatments for CRF. Clinicians should prescribe exercise and psychological interventions as first-line therapy for patients experiencing CRF.
Supplementary Material
Key Points.
Question
Which of the 4 most commonly recommended treatments for cancer-related-fatigue—exercise, psychological, the combination of exercise and psychological, and pharmaceutical—is the most effective?
Findings
This meta-analysis of 113 unique studies (11525 unique participants) found that exercise and psychological interventions and the combination of both reduce cancer-related fatigue during and after cancer treatment. Reduction was not due to time, attention, or education. In contrast, pharmaceutical interventions do not improve cancer-related fatigue to the same magnitude.
Meaning
Clinicians should prescribe exercise and/or psychological interventions as first-line treatments for cancer-related fatigue.
Acknowledgments
Funding/Support: This study was supported by grants CA102618B, 1R01CA181064, R21CA185678, and NCI UGCA189961 (Dr Mustian), CA102618B (Drs Heckler and Peppone), CA189961 and R25 CA102618 (Dr I. R. Kleckner), and R01CA181659 and R21CA185678 (Dr Palesh) from the National Cancer Institute.
Role of Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Mustian and Heckler had full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Mustian, Alfano, A. S. Kleckner, Mohr, Piper, Scarpato, Smith, Miller.
Acquisition, analysis, or interpretation of data: Mustian, Alfano, Heckler, A. S. Kleckner, I. R. Kleckner, Leach, Mohr, Palesh, Peppone, Piper, Scarpato, Smith, Sprod.
Drafting of the manuscript: Mustian, Alfano, Heckler, A. S. Kleckner, I. R. Kleckner, Leach, Mohr, Palesh, Peppone, Scarpato, Smith, Sprod.
Critical revision of the manuscript for important intellectual content: Mustian, Alfano, Heckler, A. S. Kleckner, I. R. Kleckner, Leach, Palesh, Peppone, Piper, Scarpato, Smith, Sprod, Miller.
Statistical analysis: Mustian, Alfano, Heckler, A. S. Kleckner, I. R. Kleckner, Leach, Palesh, Peppone, Smith.
Obtained funding: Mustian.
Administrative, technical, or material support: Mustian, A. S. Kleckner, Mohr, Piper, Scarpato, Smith, Sprod.
Study supervision: Mustian, Mohr, Piper, Miller.
Conflict of Interest Disclosures: None reported.
Contributor Information
Karen M. Mustian, Department of Surgery, Wilmot Cancer Institute, University of Rochester Medical Center, Rochester, New York.
Catherine M. Alfano, Behavioral Medicine Research Center, American Cancer Society, Washington, DC.
Charles Heckler, Department of Surgery, Wilmot Cancer Institute, University of Rochester Medical Center, Rochester, New York.
Amber S. Kleckner, Department of Surgery, Wilmot Cancer Institute, University of Rochester Medical Center, Rochester, New York.
Ian R. Kleckner, Department of Surgery, Wilmot Cancer Institute, University of Rochester Medical Center, Rochester, New York.
Corinne R. Leach, Behavioral Medicine Research Center, American Cancer Society, Washington, DC.
David Mohr, Department of Preventive Medicine, Northwestern University, Rochester, New York.
Oxana G. Palesh, Department of Psychiatry and Behavioral Sciences, Stanford Cancer Institute, Stanford University, Stanford, California.
Luke J. Peppone, Department of Surgery, Wilmot Cancer Institute, University of Rochester Medical Center, Rochester, New York.
Barbara F. Piper, Department of Nursing, School of Health and Human Services, National University, San Diego, California.
John Scarpato, Department of Psychosocial and Biobehavioral Medicine, Fox Chase Cancer Center, Philadelphia, Pennsylvania.
Tenbroeck Smith, Behavioral Medicine Research Center, American Cancer Society, Washington, DC.
Lisa K. Sprod, School of Health and Applied Human Sciences, University of North Carolina Wilmington.
Suzanne M. Miller, Department of Psychosocial and Biobehavioral Medicine, Fox Chase Cancer Center, Philadelphia, Pennsylvania.
References
- 1.Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Panel National Institutes of Health State-of-the-Science Conference Statement: symptom management in cancer: pain, depression, and fatigue, July 15–17, 2002. J Natl Cancer Inst. 2003;95(15):1110–1117. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- 2.Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment. Support Care Cancer. 2008;16(7):791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 3.Kuhnt S, Ernst J, Singer S, et al. Fatigue in cancer survivors—prevalence and correlates. Onkologie. 2009;32(6):312–31. doi: 10.1159/000215943. [DOI] [PubMed] [Google Scholar]
- 4.Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. Eur J Cancer. 2002;38(1):27–43. doi: 10.1016/s0959-8049(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;(32):40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 6.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue. Oncologist. 2007;12(suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 7.Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? Breast Cancer Res Treat. 2008;112(1):5–13. doi: 10.1007/s10549-007-9831-1. [DOI] [PubMed] [Google Scholar]
- 8.Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: a critical appraisal. Eur J Cancer. 2006;42(7):846–863. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116(24):5740–5748. doi: 10.1002/cncr.25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cella D, Davis K, Breitbart W, Curt G, Fatigue Coalition Cancer-related fatigue. J Clin Oncol. 2001;19(14):3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 11.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4):751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter JS, Elam JL, Ridner SH, Carney PH, Cherry GJ, Cucullu HL. Sleep, fatigue, and depressive symptoms in breast cancer survivors and matched healthy women experiencing hot flashes. Oncol Nurs Forum. 2004;31(3):591–598. doi: 10.1188/04.onf.591-598. [DOI] [PubMed] [Google Scholar]
- 13.Stepanski EJ, Walker MS, Schwartzberg LS, Blakely LJ, Ong JC, Houts AC. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. J Clin Sleep Med. 2009;5(2):132–136. [PMC free article] [PubMed] [Google Scholar]
- 14.Roscoe JA, Kaufman ME, Matteson-Rusby SE, et al. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12(suppl 1):35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 15.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors. J Clin Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 16.Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45(3):384–392. doi: 10.1016/j.ejca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan KA, Jacobsen PB. Fatigue, depression, and insomnia. Semin Oncol Nurs. 2007;23(2):127–135. doi: 10.1016/j.soncn.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Beck SL, Dudley WN, Barsevick A. Pain, sleep disturbance, and fatigue in patients with cancer. Oncol Nurs Forum. 2005;32(3):542. doi: 10.1188/04.ONF.E48-E55. [DOI] [PubMed] [Google Scholar]
- 19.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients. Oncologist. 2000;5(5):353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 20.Symptom Management and Quality of Life Steering Committee. National Cancer Institute. Strategic Priorities: Symptom Management and Quality of Life Steering Committee. 2015:2015. https://www.cancer.gov/about-nci/organization/ccct/steering-committees/2015-SxQoLSC-StrategicPriorities. Accessed August 8, 2016.
- 21.Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev. 2009;(1):CD006953. doi: 10.1002/14651858.CD006953.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peuckmann V, Elsner F, Krumm N, Trottenberg P, Radbruch L. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2010;(11):CD006788. doi: 10.1002/14651858.CD006788.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM. The effect of physical exercise on cancer-related fatigue during cancer treatment. Clin Oncol (R Coll Radiol) 2010;22(3):208–221. doi: 10.1016/j.clon.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 25.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors. Cancer Epidemiol Biomarkers Prev. 2011;20(1):123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 26.van Vulpen JK, Peeters PH, Velthuis MJ, van der Wall E, May AM. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue. Maturitas. 2016;85:104–111. doi: 10.1016/j.maturitas.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors. BMC Cancer. 2015;15:77. doi: 10.1186/s12885-015-1069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of supervised multimodal exercise interventions on cancer-related fatigue. BioMed Res Int. 2015;2015:328–636. doi: 10.1155/2015/328636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puetz TW, Herring MP. Differential effects of exercise on cancer-related fatigue during and following treatment. Am J Prev Med. 2012;43(2):e1–e24. doi: 10.1016/j.amepre.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;(11):CD006145. doi: 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duijts SF, Faber MM, Oldenburg HS, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors. Psychooncology. 2011;20(2):115–126. doi: 10.1002/pon.1728. [DOI] [PubMed] [Google Scholar]
- 32.Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007;26(6):660–667. doi: 10.1037/0278-6133.26.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du S, Hu L, Dong J, et al. Patient education programs for cancer-related fatigue: a systematic review. Patient Educ Couns. 2015;98(11):1308–1319. doi: 10.1016/j.pec.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst. 2008;100(16):1155–1166. doi: 10.1093/jnci/djn250. [DOI] [PubMed] [Google Scholar]
- 35.Gong S, Sheng P, Jin H, et al. Effect of methylphenidate in patients with cancer-related fatigue. PLoS One. 2014;9(1):e84391. doi: 10.1371/journal.pone.0084391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice. Clin J Oncol Nurs. 2014;18(suppl):38–58. doi: 10.1188/14.CJON.S3.38-58. [DOI] [PubMed] [Google Scholar]
- 37.Bower JE, Bak K, Berger A, et al. American Society of Clinical Oncology Screening, assessment, and management of fatigue in adult survivors of cancer. J Clin Oncol. 2014;32(17):1840–1850. doi: 10.1200/JCO.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berger AM, Mooney K, Alvarez-Perez A, et al. NCCN Clinical Practice Guidelines in Oncology: Cancer-Related Fatigue. 2016 https://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf. Published December 19, 2016. Accessed February 8, 2017.
- 39.Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology. 2011;22(1):128. doi: 10.1097/EDE.0b013e3181fe7825. [DOI] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W264. [DOI] [PubMed] [Google Scholar]
- 41.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue. Psychol Bull. 2008;134(5):700–741. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 42.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 43.Bhogal SK, Teasell RW, Foley NC, Speechley MR. The PEDro scale provides a more comprehensive measure of methodological quality than the Jadad scale in stroke rehabilitation literature. J Clin Epidemiol. 2005;58(7):668–673. doi: 10.1016/j.jclinepi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro) Aust J Physiother. 2002;48(1):43–49. doi: 10.1016/s0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- 45.Hedges LV. A random effects model for effect sizes. Psychol Bull. 1983;93(2):388–395. [Google Scholar]
- 46.Higgins JPT, Green S. Cochrane Collaboration: Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: Wiley-Blackwell; 2008. [Google Scholar]
- 47.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-analysis. Chichester, England: John Wiley & Sons; 2009. [Google Scholar]
- 48.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 49.Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale. Oncol Nurs Forum. 1998;25(4):677–684. [PubMed] [Google Scholar]
- 50.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 51.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6(3):143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 52.FACIT.org. Questionnaires. http://www.facit.org/FACITOrg/Questionnaires. Accessed February 7, 2017.
- 53.EORTC Quality of Life Department. EORTC QLQ-C30. http://groups.eortc.be/qol/eortc-qlq-c30. Accessed February 7, 2017.
- 54.Duval S, Tweedie R. The trim and fill method. In: Rothstein HR, Sutton AJ, Borenstein M, editors. Publication Bias in Meta-analysis: Prevention, Assessment and Adjustments. Chichester, England: Wiley; 2005. [Google Scholar]
- 55.Rosenberg MS. The file-drawer problem revisited. Evolution. 2005;59(2):464–468. [PubMed] [Google Scholar]
- 56.Saint-Raymond A, Hill S, Martines J, Bahl R, Fontaine O, Bero L. CONSORT 2010. Lancet. 2010;376(9737):229–230. doi: 10.1016/S0140-6736(10)61134-8. [DOI] [PubMed] [Google Scholar]
- 57.Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT 2010 Statement. J Clin Epidemiol. 2010;63(8):834–840. doi: 10.1016/j.jclinepi.2010.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


