Abstract
Primary forms of focal and segmental glomeruloslerosis (FSGS) are driven by circulating factors that cause dysfunction or loss podocytes. Rare genetic forms of FSGS can be caused by mutations in TRPC6, which encodes a Ca2+-permeable cationic channel expressed in mesangial cells and podocytes; and NPHS2, which encodes podocin, a TRPC6-binding protein expressed in podocyte slit diaphragm domains. Here we observed that exposing immortalized mouse podocytes to serum or plasma from recurrent FSGS patients for 24 hr increased the steady-state cell-surface abundance of TRPC6, accompanied by an increase in currents through endogenous TRPC6 channels evoked by a hypoosmotic stretch stimulus. These effects were mimicked by the soluble urokinase receptor (suPAR) and by tumor necrosis factor (TNF), circulating factors implicated in nephrotic syndromes. Most but not all of the recurrent FSGS plasma samples that we examined also caused a loss of podocin over a period of several hours. The loss of podocin was also seen following exposure to suPAR but not TNF. However, TNF increased the effects of suPAR on TRPC6 and podocin, and TNF and suPAR are required for the full effects of one of the recurrent FSGS plasma samples. The actions of FSGS plasma, suPAR and TNF on surface abundance of TRPC6 were blocked by cilengitide, an inhibitor of αvβ3-integrin signaling. These data suggest that primary FSGS is a heterogeneous condition mediated by multiple circulating factors, and support TRPC6 and αvβ3-integrin as potential therapeutic targets.
Keywords: chronic kidney disease, TRPC6, suPAR, TNF, integrin, podocin
1. Introduction
Focal and segmental glomerulosclerosis refers to lesions in which an accumulation of extracellular matrix obliterates varying portions of glomerular capillary tufts. While these lesions have several etiologies, many cases of histologically verified FSGS are primary and idiopathic. Primary FSGS patients who present with nephrotic levels of proteinuria and who do not respond to glucocorticoids have an especially high risk of progressing to renal failure. Moreover, a substantial portion of patients who receive a kidney allograft as a result of steroid-resistant primary FSGS will experience early recurrence of nephrotic range proteinuria and are at high risk for graft failure [1,2].
It is now generally accepted that recurrence of FSGS in an allograft recipient is caused by circulating factors that affect podocytes [3,4]. The identity of the circulating factor(s) that drive primary and recurrent FSGS has been elusive and controversial. One candidate is the soluble urokinase and plasminogen activator receptor (suPAR) [5], a term that encompasses a class of 22–50 kD glycoproteins shed from many cell types, including hematopoietic cells, endothelial cells, fibroblasts, and smooth muscle cells, as a result of proteolytic or phospholipase-mediated cleavage of a glycosylphosphatidylinositol-anchored glycoprotein [6]. It was originally reported that total plasma suPAR levels are elevated in a subset of patients with FSGS, especially patients with recurrent forms of FSGS [5], and this association was subsequently found to be stronger when urine levels of suPAR were measured [7–9]. Beyond the context of FSGS, large longitudinal studies have found that elevated blood suPAR levels in people with normal baseline renal function are associated with future chronic kidney disease and declines in estimated glomerular filtration rate [10,11]. Moreover, there is evidence that elevated serum suPAR levels predict future microalbuminuria in patients at risk for or with newly manifested type 2 diabetes mellitus [12]. In mice there is evidence that the circulating suPAR that drives kidney disease is derived from immature myeloid cells in bone marrow, and that transplantation of myeloid cells secreting high levels of suPAR can induce kidney disease in recipient mice [13].
Other factors have been proposed to mediate recurrent FSGS. A handful of case reports have documented remissions of recurrent FSGS following anti-TNF therapy in pediatric patients [14–16]. In addition, there are reports that circulating TNF is elevated in patients with primary nephrotic syndromes [17], and monocytes isolated from these patients secrete TNF at an order of magnitude greater rate than monocytes from healthy controls [18–20]. Moreover, changes in podocyte cytoskeletal organization evoked by plasma from patients with recurrent FSGS were blocked by inhibitors of TNF signaling [16]. Sustained TNF infusion induces glomerular pathology [21]. It has also been reported that TNF increases the albumin permeability of isolated glomeruli [22]. Other circulating factors, such as cardiotrophin-like cytokine 1, which activates transduction cascades that overlap those of TNF, may also drive recurrent FSGS [23]. It is possible that primary and recurrent FSGS may be driven by different patterns of circulating factors in different patients [24].
Genetic studies have identified a number of genes that are mutated in familial forms of FSGS. Two of these genes encode proteins that are the focus of the present study. Mutations in the NPHS2 gene encoding the hairpin loop protein podocin give rise to severe and typically early-onset autosomal recessive nephrotic syndromes [25]. Most NPHS2 mutations result in non-functional proteins [26–28]. The TRPC6 gene encodes a Ca2+-permeable cation channel (TRPC6) expressed in many different cell types, including mesangial cells and podocytes. FSGS associated with TRPC6 mutations often presents with an adult onset, with an autosomal dominant mode of inheritance, and the mutant proteins frequently have a gain of function or increased surface expression when examined in heterologous expression systems [29–31]. It should be noted, however, that loss of activation by at least some gating modes has been seen with some TRPC6 mutations [32,33], including a dominant-negative mutation that resulted in complete loss of activation by G protein cascades and that occurred with an unusually early disease onset [33].
TRPC6 and podocin are expressed at the slit diaphragm domains of podocytes [30,34,35] and there are functionally significant biochemical interactions between these two proteins [30,35,36]. We have previously reported that podocin differentially regulates the sensitivity of podocyte TRPC6 channels to various activating stimuli [36]. Specifically, podocin enhances activation of TRPC6 induced by G protein signaling pathways or by diacylglycerol [36–38] or eicosanoids [39]. Conversely, podocin suppresses activation of podocyte TRPC6 channels induced by membrane stretch, a process that occurs by poorly understood transduction mechanisms [36]. Therefore, processes that result in a loss in glomerular podocin expression would be expected to exert complex functional effects on TRPC6, but in particular should increase activation by membrane stretch or other mechanical stimuli.
It has been reported that patients with primary FSGS have elevated glomerular expression of TRPC6 [40] as well as reduced expression of podocin [41–43]. Moreover, a loss or internalization of podocin can be recapitulated in vitro by exposing cultured podocytes to serum from patients with recurrent FSGS [44–46]. Therefore, it is possible that dysfunction in one or both of these proteins could contribute to disease progression in “acquired” as opposed to genetic forms of kidney disease.
The primary purpose of the present study is to explore the effects of putative glomerular permeability factors, as well as serum and plasma from patients with recurrent forms of FSGS, on TRPC6 channels in a widely used immortalized podocyte cell line. Since podocin is a TRPC6-interacting protein that affects TRPC6 function, we have also examined this protein. The results support a model in which functionally significant changes in TRPC6 surface expression and increases in its activation occur in response to circulating factors in FSGS patients, in many but not all cases accompanied by a loss of podocin. These data are also consistent with multiple circulating factor models of primary FSGS, in which suPAR, TNF, and probably several other circulating factors produce additive or synergistic effects that converge on podocytes. We will also present evidence that these circulating factors utilize integrin signaling pathways to increase the abundance of TRPC6 channels on the cell surface. If this multiple-factor model is correct, correlations between circulating levels of any one circulating factor and clinical status in primary FSGS (or other glomerular diseases) may not always be seen, even in cases when that factor contributes to the pathology. However, it is possible that downstream targets shared by these circulating factors, such as TRPC6 channels or integrins, might represent effective therapeutic strategies for acquired forms of FSGS.
2. Material and methods
2.1 Podocyte cell culture and glomerular isolation
An immortalized mouse podocyte cell line (MPC-5) was provided by Dr. Peter Mundel of Harvard Medical School and propagated and maintained as described previously [36,47]. Podocyte differentiation and expression of podocyte markers was induced by removal of γ-interferon and temperature switch to 37°C for 14 days. These cells robustly express a wide range of podocyte marker proteins after differentiation, including nephrin, synaptopodin, podocin, and podocalyxin. Some immortalized podocyte cell lines do not express these markers, and cell lines (including this one) lose the ability to express these markers after high passage numbers.
2.2 Immunoblot analysis and cell surface biotinylation assays
Methods used for immunoblot analysis from podocyte lysates have been described in detail previously [36,48]. Filters were probed using primary antibodies, washed, incubated with horseradish peroxidase-conjugated secondary antibodies, and visualized using chemiluminescence. Methods for cell surface biotinylation assays to measure steady-state surface abundance of TRPC6 channels were also described previously [37,39,47–49]. Rabbit antibodies against TRPC6 (ACC-017) and TRPC5 (ACC-020) were obtained from Alomone Labs (Jerusalem, Israel), antibodies against podocin (sc-21009) and β3-integrin (sc-14009) were obtained from Santa Cruz (Santa Cruz, CA).
2.3 Patient serum and plasma samples
Studies with serum and plasma samples were done following a protocol approved by the University of Houston Committee for the Protection of Human Subjects. Serum or plasma samples were taken from patients with FSGS that recurred after transplantation following ethical review by panels at the institutions where the patients were seen. Information on the patients is summarized in Table 1. Plasma samples from patients 101 and 356 were provided to us by Drs. Moin Saleem and Dan Henson at the University of Bristol, UK. The plasma samples were collected during relapse and after treatment by plasma exchange, with patient status based on measurements of urine protein/creatinine ratios and blood urea nitrogen (BUN). Patient 101 is an adult female, 44 years old at the time samples were collected. Patient 356 is a male child who was initially diagnosed with steroid-resistant FSGS at the age of 19 months, and who was 12 years old at the time the samples were taken. Samples were taken before and after plasma exchange therapy. Sera from patients 048 and 054 were collected from recurrent FSGS patients seen at the Johns Hopkins University at the time their disease recurred, and were provided by Drs. Sanja Sever and Nada Alachkar. We do not have additional clinical information on those patients. Sera from patients 004, DH, and 011 were provided by Drs. Thomas Benzing and Henning Hagmann of the University of Cologne, Germany, along with information on blood urea nitrogen BUN levels at the time of sampling. The samples from patient DH were collected during relapse and after this patient achieved a temporary improvement as a result of intensive low density lipoprotein (LDL) apharesis therapy using a Miltenyi TheraSorb™ column (Miltenyi Biotec, Gladbach, Germany) with preasborbed sheep antibody against the B-100 component of LDL. The serum samples from patients 021 and 022 were from primary FSGS patients and were sent to us by Dr. Jochen Reiser of Rush University Medical School. We do not have additional information on those patients. All of these plasma and serum samples were stored at −80° for varying periods of time before they were sent to us. Except where indicated, immortalized mouse podocytes were cultured with media containing plasma or serum at 10% (replacing fetal bovine serum) for 24 hr. However, plasma from patient 101 was generally used at 2%. Controls in those cases consisted of sera from healthy humans replacing fetal bovine serum. Serum from healthy controls never produced changes compared to cells cultured in fetal bovine serum. Several of the samples were provided to us in such small volumes that only a small number of analyses could be performed. Cell surface biotinylation assays and electrophysiological analyses require substantial amounts of serum or plasma, which precluded carrying out these analyses for several of the samples.
Table 1.
Clinical features of patients whose serum or plasma samples were used in this study.
| Patient | Diagnosis and features | Renal function tests at time of sample | Source of sample |
|---|---|---|---|
| 101 | Plasma from 44 year old female with recurrent FSGS. Samples were taken before and after transient remission induced by plasma exchange. | NA | Bristol, UK |
| 048 | Serum from patient with recurrent FSGS | NA | Johns Hopkins University, Baltimore, MD |
| 054 | Serum from patient with recurrent FSGS | NA | Johns Hopkins University, Baltimore, MD |
| 356 | Plasma from male child with recurrent FSGS, with initial nephrotic syndrome diagnosis at age of 16 months. Samples taken before and after plasma exchange | Proteinuria noted after transplant and transiently reduced after plasma exchange. | Bristol, UK |
| 021 | Serum from patient with recurrent FSGS | NA | Rush University, Chicago, IL |
| 022 | Serum from patient with recurrent FSGS | NA | Rush University, Chicago, IL |
| 004 | Plasma from adult female with recurrent FSGS at time of sampling. Currently back on dialysis. | BUN = 49 mg/dl | Cologne, Germany |
| DH | Plasma from male child with recurrent FSGS. Samples were taken before and after transient remission was induced by three sessions of LDL apharesis. | BUN = 99 mg/dl during relapse. BUN = 78 mg/dl after LDL apheresis |
Cologne, Germany |
| 011 | Plasma from female with recurrent FSGS, has received two kidney transplants to date. | BUN = 114 mg/dl | Cologne, Germany |
Coded samples of serum or plasma were sent to us from clinician scientists at different university medical centers as indicated. All of these patients have had recurrence of FSGS after a kidney transplant. In some cases we received samples from the same patients at different times in which the severity of the disease appeared different based on clinical tests. We have provided laboratory values where we could obtain the information.
2.4. Recombinant proteins, drugs and ELISA assays
Recombinant TNF was obtained from R&D Systems (Minneapolis, MN). A large recombinant form of suPAR containing domains 1, 2 and 3 was also obtained from R&D Systems. A smaller recombinant form of suPAR containing domains 2 and 3 provided by Dr. Sanja Sever of the Harvard Medical School (Boston, MA) was used in a few experiments. SKF-96365 was obtained from Sigma Aldrich (St. Louis, MO), and cilengitide was obtained from Seleck Chem (Houston, TX, USA). ELISA assay kits for quantifying serum TNF (DTA00C) and suPAR (DUP00) were obtained from R&D Systems. Antibodies used for neutralization of suPAR (AF-807) and TNF (AF-210-NA) in a nephrotic plasma sample were from R&D Systems.
2.5. Electrophysiology
Methods for making whole-cell recordings from podocytes, including the composition of the recording electrode, and preparation of control bath (340 mOsm/liter), and 70% hypoosmotic bath solutions (238 mOsm/liter), are described elsewhere [36]. The bath was perfused at a constant flow rate (0.3 ml/min) and outwardly rectifying currents were periodically evoked by ramp voltage commands (−80 to + 80 mV over 2.5 sec) starting from a holding potential of −40 mV [36,50]. After achieving a stable baseline in control (normosmotic) bath solution, perfusion of 70% hypoosomotic stretch solution causes reversible activation of TRPC6 channels in podocytes. This is seen as an increase in outwardly rectifying current that reverses at 0 mV and stabilizes in 2–3 min, and responses shown here were recorded within that time. In many experiments, bath solutions containing 50 μM La3+ were applied in hypoosmotic saline immediately after the response to stretch stabilized. Micromolar La3+ is useful for the present experiments because it causes rapid blockade of TRPC6 but not TRPC5 [50,51]. We have previously shown that stretch-evoked cationic currents in podocytes are inhibited by TRPC6 knockdown [36] and we show in Supplemental Data Figure 3 that they are blocked by low nanomolar concentrations of 4-(((1R,2R)-2-((R)-3-aminopiperidin-1-yl)-2,3-dihydro-1H-inden-1-yl)oxy)-3-chlorobenzonitrile dihydrochloride (SAR7334), the most selective known inhibitor of TRPC6 [52], which we obtained from MedChem Express (Monmouth Junction, NJ). We note, however, that in some cells, more prolonged exposure to hypoosmotic solutions cause activation of unidentified La3+-resistant currents with current-voltage characteristics distinct from TRPC6. Currents were quantified at +80 mV and bar graphs show average fold-changes ± SEM compared to baseline current in normal external bath saline once stable whole-cell contact was made (prior to application of activating stimuli).
2.6. Statistical analyses
All experiments on immunoblot or cell surface biotinylation assays were performed in triplicate and analyzed by densitometry using Image J™ software (Bethesda, MD). Those data are presented as fold changes relative to the lowest value observed in a control group (as mean ± SD). Data were analyzed by Bonferonni t-test with P < 0.05 considered significant. Electrophysiological data are presented as mean ± SEM and were analyzed by Student’s unpaired t-test or Bonferonni t-test, comparing cells cultured in normal media to cells cultured in presence of recombinant TNF or suPAR or plasma from patients with recurrent FSGS.
3. Results
3.1 Effects of sera and plasma from patients with recurrent FSGS on TRPC6 and podocin expression in cultured podocytes
The differentiated cells of an immortalized mouse podocyte cell line (MPC-5 cells) were cultured for 24 hr in the presence of plasma samples from a 44-year old female patient (101) with recurrent FSGS taken while she was in relapse (in September of 2007), and a sample taken after this patient had achieved a temporary remission after plasma exchange (in October of 2007) (Fig. 1a). The clinical status of the patient was assessed by clinicians in the UK based on measurements of serum albumin and urine protein:creatinine ratios. These plasma samples were added to our standard culture media at a concentration of 2% and cells were exposed to this plasma for 24 hr and then examined using various assays. The plasma sample taken while patient 101 was in relapse markedly reduced the abundance of podocin as measured by immunoblot, whereas a sample taken from the same patient after remission was achieved had almost no effect on podocin abundance compared to cells cultured in fetal bovine serum (Fig. 1a). The plasma sample taken during relapse also increased the steady-state surface abundance of TRPC6 compared to the control, as measured by cell surface biotinylation assays, whereas the sample taken during remission produced a barely discernible effect (Fig. 1b). We have seen a similar pattern (increase in surface TRPC6 accompanied by a loss of podocin) using serum or plasma samples from several other patients with active primary FSGS. Effects of serum samples from two these patients (denoted as 048 and 054) are shown in Fig. 1c. Note again the increase in steady-state surface expression of TRPC6 accompanied by a reduction in podocin abundance measured in total cellular lysates. Examples of data using samples from six other primary or recurrent FSGS patients are shown in Supplemental Figure 1a–c. We observed that plasma samples from two recurrent FSGS patients did not fully recapitulate this pattern. For example, plasma from patient 356 had no effect on podocin abundance but induced an increase in the surface abundance of TRPC6. The effect on surface TRPC6 appeared to be slightly greater with the sample taken during the disease relapse (Fig. 2). Serum from patient 011 from the University of Cologne cohort also had no effect on podocin (Supplemental Figure 1d), but we did not have sufficient material to test its effects on TRPC6. Note that the increase in surface abundance of TRPC6 is not an obligatory consequence of the loss of podocin, since podocin knockdown in this cell line actually causes a decrease in steady-state surface levels of TRPC6 (Supplemental Fig. 2).
Figure 1.
Effects of plasma and sera from patients with recurrent FSGS on TRPC6 and podocin in immortalized podocytes. (a) Experiments using plasma from patient 101 sampled during a relapse of recurrent FSGS and later, after remission was achieved following plasma exchange. A representative immunoblot shows total podocin abundance in immortalized mouse podocytes cultured for 24 hr with media containing 2% plasma from 101 taken during relapse and remission, as indicated, as well as in podocytes grown in normal media. Note reduction in total podocin abundance in podocytes cultured with plasma from 101 in relapse. Plasma from 101 taken after remission does not have this activity. This representative immunoblot is shown above bar graph summarizing densitometric analysis of three repetitions of this experiment, presented as mean ± SD of podocin relative to actin. Asterisk indicates P < 0.05 compared to control. (b) Plasma from 101 in relapse also caused marked increase in steady-state cell surface abundance of TRPC6 channels in podocytes as measured by cell-surface biotinylation assays. Bar graph (mean ± SD) summarizes three repetitions of this experiment. Activity is reduced in plasma taken from 101 after remission was achieved. (c) Serum from two other recurrent FSGS patients (048 and 054) caused a reduction in total podocin and an increase in steady-state surface TRPC6. Representative assays are shown to left, and bar graph summaries of three repetitions of these experiments are shown to the right (mean ± SD). Podocytes were treated with media containing 10% of these sera for 24 hr. Asterisks indicate P < 0.05 by Bonferroni t-test.
Figure 2.
Different pattern of activity in plasma from a different patient with recurrent FSGS during relapse and remission. (a) Plasma from patient 356 had no effect on total podocin abundance. (b) Plasma from patient 356 caused modest increase in steady-state surface expression of TRPC6 measured by a biotinylation assay after 24 hr treatment at 10%. Activity was present in both plasma samples from this patient, but there was a trend towards higher activity in the sample taken during relapse. Representative blots are shown above densitometric analysis of three repetitions of this experiment (mean ± SD). Asterisks indicate P < 0.05 by Bonferroni t-test.
3.2 Effects of recombinant putative permeability factors on abundance of podocin and TRPC6
Circulating suPAR occurs in a variety of forms that vary in domain structure and the extent of glycosylation [6]. We have examined two different recombinant preparations of suPAR; a smaller form consisting of protein domains 2 and 3, and a larger commercially available form containing domains 1, 2 and 3. Both preparations of recombinant suPAR produced a similar effect on cultured podocytes at 10 ng/ml, and specifically caused a marked increase in steady-state surface expression of TRPC6 accompanied by a marked reduction in total podocin abundance (Fig. 3a). Treating podocytes with 10 ng/ml TNF for 24 hr also caused an increase in surface abundance of TRPC6 (Fig. 3b). However, in marked contrast to suPAR, exposure to TNF did not produce a robust or consistent effect on podocin abundance, and in this respect TNF differs markedly from suPAR. The effects of suPAR and TNF on surface expression of TRPC6 appear to be additive or greater. To examine this, immortalized podocytes were cultured for 24 hr in presence of 1 ng/ml of either suPAR or TNF, or in the presence of both factors, each at 1 ng/ml. At this low concentration, the effects of suPAR or TNF on the surface abundance of TRPC6 were comparatively small and not always seen. However robust increases in surface expression of TRPC6 were consistently observed when both factors were present at 1 ng/ml (Fig. 3c).
Figure 3.
Effects of recombinant human suPAR and TNF on TRPC6 and podocin in immortalized podocytes. (a) Two isoforms of suPAR at 10 ng/ml for 24 hr caused increase steady-state surface expression of TRPC6 measured by cell surface biotinylation assays, and reduced podocin abundance measured by immunoblot of total cellular lysates. Summary bar graphs (mean ± SD) of three replications of these experiments are shown to the right of representative blots. We could not discern any consistent difference in the activities of these two recombinant preparations at this concentration at the limits of resolution of this in vitro assay. (b) Culturing podocytes with 10 ng/ml TNF for 24 hr causes an increase in the steady-state surface expression of TRPC6 measured by biotinyation assays but has no effect on overall abundance of podocin. (c) TNF and suPAR by themselves at a lower concentration (1 ng/ml) do not cause a notable increase in surface TRPC6 by themselves but a robust increase occurs when both factors are present at 1 ng/ml. Asterisks indicate P < 0.05 by Bonferroni t-test.
While our work in this study focuses on TRPC6, we note here that neither suPAR nor TNF by themselves at 10 ng/ml were able to increase the abundance of TRPC5 subunits on the podocyte cell surface (Supplemental Figure 4a, b). However, a consistent increase in surface TRPC5 was seen when TNF and suPAR were both present at 10 ng/ml (Supplemental Figure 4c), and a similar effect was seen with plasma from patient 101 taken during relapse (Supplemental Figure 4d). Therefore, TRPC5 can be mobilized to the podocyte cell surface by circulating factors implicated in FSGS and other kidney diseases, but this effect may require either a stronger stimulus than is needed for mobilization of TRPC6, or may simply respond preferentially to other circulating factors not studied here.
An earlier study on sera from patients with recurrent FSGS also reported loss of podocin expression in immortalized human podocytes, and those workers reported that the maximum effect required 24 hr of exposure [44]. For comparison, we treated immortalized mouse podocytes with suPAR, TNF, or patient 101 plasma (in relapse) for 3, 6, or 24 hr and then examined podocin abundance. In agreement with that earlier study, we observed that loss of podocin evoked by either 10 ng/ml suPAR or plasma from patient 101 required several hours of exposure, whereas podocin was essentially unaffected by 10 ng/ml TNF even after 24 hr (Fig. 4).
Figure 4.
Time course of circulating factors effects on podocin abundance in podocytes. Cells were treated for times indicated. A loss of podocin required several hours of treatment with suPAR (a) but did not occur with TNF, even with 24-hr exposure (b). The loss of podocin evoked by 101 plasma also required several hours (c). Representative blots are shown above bar graphs (mean ± SD) summarizing results of three repetitions of each experiment. Asterisks indicate P < 0.05 by Bonferroni t-test.
3.3. Putative permeability factors and recurrent FSGS plasma increase stretch-evoked activation of podocyte TRPC6 channels
In these experiments, we utilized a 70% hypoosmotic stretch stimulus to examine whether biochemical changes in the abundance of TRPC6 and podocin noted above are associated with functional changes in ionic currents. Outwardly rectifying cationic currents were recorded during application of a ramp voltage command (−80 to +80 mV) before and during application of a 70% hypoosmotic bath solution [36]. Stretch-evoked currents in podocytes are inhibited by the highly selective TRPC6 inhibitor SAR7334 with an EC50 of around 10 nM, comparable to that observed with recombinant TRPC6 (Supplemental Figure 3). By comparison, SAR7334 blocks TRPC3 with an EC50 of greater than 180 nM and has no effect on TRPC5 at 10 μM [52]. Stretch-evoked currents in podocytes are also blocked by very low concentrations 50 μM La3+ which blocks TRPC6 but not TRPC5 at these low concentration [36,51]. Here we note that La3+-sensitive stretch-evoked currents were substantially larger relative to the pre-stretch baseline in podocytes cultured for 24 hr in recombinant human suPAR (10 ng/ml) (Fig. 5a). A smaller but still significant increase was also observed in podocytes cultured for 24 hr in 10 ng/ml TNF (Fig 5b). Increases in stretch-evoked currents were also seen in podocytes cultured with plasma from patient 101 sampled during a relapse (Fig. 6). The plasma sample taken from 101 during a remission did not produce large increases in stretch-evoked currents (Fig. 6). Thus, the biochemical effects of circulating factors are associated with functional changes in podocyte physiology.
Figure 5.
Recombinant circulating factors increase stretch-evoked cationic current in mouse podocytes. Cells were cultured for 24 hr in either control medium, or medium containing suPAR or TNF as indicated, after which whole-cell recordings were made. (a) Representative examples of currents evoked shortly after making whole-cell contact (left), in the same cell 2–3 minutes after switching to a 70% hypoosmotic external medium (center) and after bath application of hypoosmotic medium containing 50 μm La3+ (right), which inhibits TRPC6-mediated currents in podocytes. Currents shown were recorded during application of a ramp voltage command (−80 mV to + 80 mV over 2.5 sec). Note increase in amplitude of stretch-evoked currents in cells that had been cultured in suPAR or TNF compared to cell cultured in control medium. (b) Mean fold increases in current at +80 mV recorded in hypoosmotic stretch solution relative to baseline current in normal bath solution for cells cultured in control medium or suPAR. Data are mean ± SEM with 10 cells in each group. (c) Mean fold increase in stretch-evoked current ± SEM in cells that had been cultured in TNF for 24 hr compared to cells cultured in control media. Asterisks indicate P < 0.05 (Student’s unpaired t-test).
Figure 6.
Plasma from a recurrent FSGS patient increases stretch-evoked cationic current in mouse podocytes. Cells were cultured for 24 hr in either control medium, or medium containing 2% of the plasma of patient 101 taken during relapse or remission as indicated. (a) Representative examples of currents evoked in cells in normal media shortly after making whole-cell contact (left), in the same cell 2–3 minutes after switching to a 70% hypoosmotic external medium (center) and after bath application of hypoosmotic medium containing 50 μm La3+ (right), and then after a return to the normosmotic medium. Note increase in amplitude of stretch-evoked currents in cells cultured in plasma from patient 101 taken during relapse. (b) Mean fold increases ± SEM in current at +80 mV recorded in stretch solution relative to normosmotic baseline current for cells cultured in normal medium or in plasmas from patient 101. Asterisk indicates P < 0.05 (Bonferonni t-test).
3.3 Effects of neutralizing antibodies on actions of recurrent FSGS plasma
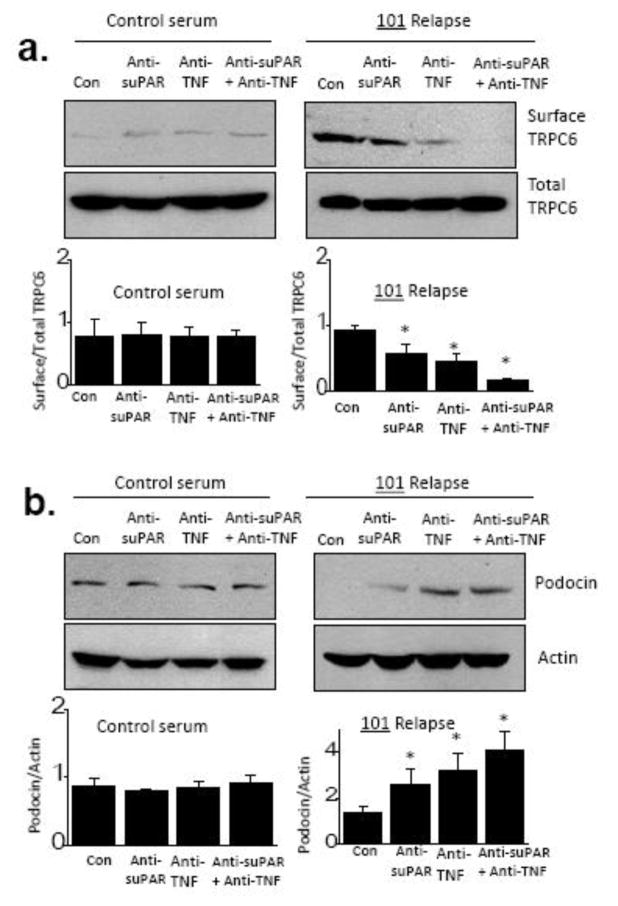
The previous results showing additive or synergistic effects of TNF and suPAR also indicate that the response of podocytes to one circulating factor can depend on the concentrations of other circulating factors. Therefore, we examined whether a multiple-factor hypothesis based on TNF and suPAR could explain some of the effects of a recurrent FSGS plasma on cultured podocytes. Obviously our focus on those two factors in this plasma sample does not exclude possible contributions from any number of other circulating factors [24]; indeed our ELISA measurements of suPAR and TNF concentrations in several serum and plasma samples that we received suggest that a consideration of just these two factors is probably inadequate to account for all of our observations (Table 1). To test this idea we used commercial neutralizing antibodies raised against suPAR (anti-suPAR, 2 μg/ml) and TNF (anti-TNF, 0.5 μg/ml). In pilot biochemical experiments we observed that these antibodies could completely block the actions of recombinant forms of their target proteins at 10 ng/ml (Supplemental Figure 5). The experiments in Fig. 7 show the effects of these antibodies on actions of plasma taken from patient 101 during relapse. The plasma was incubated with anti-TNF, anti-suPAR, or both for 3 hr at 37°. The mixture was then added to cultured podocytes for 24 hr. As with previous experiments, we observed a marked increase in steady-state surface abundance of TRPC6 (Fig. 7a) and a loss of podocin (Fig. 7b) when podocytes were exposed to plasma from patient 101. Those effects were slightly reduced in plasma pre-incubated with anti-suPAR, whereas pre-incubation with anti-TNF was more effective. However, the combination of anti-suPAR and anti-TNF abolished the effects of 101 plasma on TRPC6, and markedly reduced its effects on podocin. These antibodies had no effect by themselves or in combination on TRPC6 levels in podocytes cultured in control conditions. These data suggest that multiple bioactive factors including suPAR and TNF are circulating in this patient and possibly in other patients with recurrent forms of FSGS. In this regard, we detected quite high concentrations of both factors in the plasma from patient 101, especially TNF, both of which were reduced in the sample taken after remission was achieved (Table 2).
Figure 7.
Effects of neutralizing antibodies to TNF and suPAR on activities in plasma from patient 101 taken during relapse. (a) Effects of 101 plasma on steady-state surface expression of TRPC6 are reduced by either anti-suPAR or by anti-TNF, but are essentially abolished by the combination of those antibodies. (b) A similar pattern is seen in measurements of podocin abundance, indicating that both factors contribute to the overall activity of this patient’s plasma sample. Note that this pattern does not exclude possible contributions from other circulating factors. Representative blots are shown above bar graphs summarizing three repetitions of this experiment. Data are mean ± SD and asterisks indicate P < 0.05 (Bonferonni t-test).
Table 2.
Measurements of TNF and suPAR concentrations in serum and plasma samples used in this study.
| Sample | TNF (pg/ml) | suPAR (pg/ml) |
|---|---|---|
| Fetal bovine serum | 21.2 ± 4.76 | 541.8 ± 4.63 |
| Control human serum | 45.5 ± 2.42 | 792 ± 6.26 |
| 101 relapse | 1211.0 ± 38.71 | 3363.5 ± 41.16 |
| 101 remission | 85.9 ± 5.49 | 2186.5 ± 15.71 |
| DH relapse | 201.7 ± 8.50 | 3178.8 ± 15.66 |
| DH remission | 49.2 ± 1.68 | 1063.0 ± 5.33 |
| 356 relapse | 51.6 ± 2.46 | 4169.0 ± 36.86 |
| 356 remission | 50.5 ± 6.21 | 2629.6 ± 20.31 |
| 004 relapse | 48.3 ± 3.52 | 1018.0 ± 5.26 |
Concentrations measured by commercial triplicate ELISA assays (R&D Systems, Minneapolis, MN). Data are presented as mean ± SD. FBS refers to fetal bovine serum. Plasma from patient 101 was collected in relapse and after remission was achieved following plasma exchange. Plasma from patient DH was collected during relapse and after a temporary remission achieved following LDL apharesis.
3.4 Role of integrin signaling in modulation of podocyte TRPC6 by circulating factors and FSGS plasma
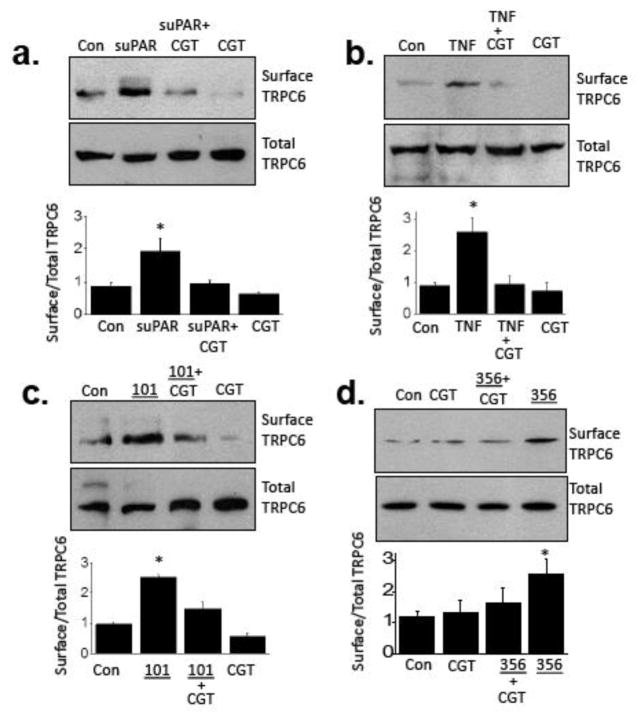
Studies of integrin signaling in podocytes formed the original basis for the proposal of suPAR as a circulating glomerular “permeability factor” in nephrotic syndromes [53]. Specifically, it was observed that induction of uPAR, the membrane-anchored form of suPAR, caused activation of αvβ3-integrin, and that this was required for various stimuli to cause foot process effacement in vivo, or increases in podocyte motility in vitro. Moreover, gene delivery of constitutively active β3-integrin into podocytes was sufficient to cause proteinuria [53]. Cilengitide selectively inhibits signaling through integrins that contain αv subunits [54], such as αvβ3-integrin by locking it into an inactive configuration. Note that αvβ3-integrin is heavily expressed in podocytes [55]. Previous studies have shown that cilengitide can reduce proteinuria evoked by infusion of lipopolysaccharide [5] and also blocks several of the effects of recombinant suPAR on podocytes [5,56]. We observed that 1 μM cilengitide markedly reduced the effects of suPAR, TNF, and plasma from patients 101 and 356 on the surface abundance of TRPC6 in cultured podocytes (Fig. 8). It is worth noting that 24 hr exposure to TNF, suPAR, and several of the FSGS patient samples are able to evoke increases in the overall abundance of β3-integrin detected in cultured mouse podocytes. These effects are also completely blocked by concurrent exposure to 1 μM cilengitide (Supplemental Figure 6).
Figure 8.
Role of integrin signaling in effects of circulating factors. Representative cell surface biotinylation assays are shown above data summaries showing that the effects of suPAR (a), TNF (b), plasma from patient 101 (c) and plasma from patient 356 (d) are blocked by 1 μM cilentigide, an inhibitor of signaling through αv-containing integrins. Representative blots are shown above bar graphs summarizing three repetitions of this experiment. Data are mean ± SD and asterisks indicate P < 0.05 (Bonferonni t-test).
4. Discussion
The primary observation of this study is that exposing cultured podocytes to serum or plasma from patients with primary and recurrent FSGS, or to putative glomerular permeability factors, results in increases in the steady-state surface expression of TRPC6 channels, either through increases in forward trafficking of TRPC6 to the cell surface, decreased endocytosis, or both. The majority of the patient samples examined here also caused a substantial loss of podocin in cultured podocytes, consistent with previous reports [44,46]. Similar effects on TRPC6 and podocin were evoked by suPAR, a circulating factor that has been suggested to drive primary and recurrent FSGS [5,57] and which markedly increases risk for future chronic kidney disease [10–12]. However, not all of the recurrent FSGS plasma samples showed this pattern. For example, the samples from patients 356 and 011 had no effect on podocin, although 356 induced modest increases in the surface expression of TRPC6 in cultured podocytes. The pattern seen with patient 356 is similar to that seen with recombinant TNF, although we note that TNF concentration does not appear to be unusually high in that sample. While these results suggest that primary FSGS is a heterogeneous condition, conclusions about patient heterogeneity from our experiments should be tempered by the fact that the plasma samples used in this study were not collected recently; and while they have been stored in similar conditions (indeed 356 and 101 were sent to us from the same group in the UK), we cannot exclude that there has been differential degradation in the biological activities. This is an issue that impacts almost any molecular study based on banked or archival blood or urine specimens.
Adding to the complexity, the sera and plasma samples from recurrent FSGS patients that were tested here may contain more than one molecular factor capable of affecting podocyte TRPC6 channels. We received sufficient plasma from patient 101 to address this question directly. The effects of this plasma on podocin and TRPC6 were reduced by neutralizing antibodies against either suPAR or TNF (notably with a larger inhibition produced by anti-TNF), and nearly complete inhibition was seen when both antibodies were used. Moreover, we could detect both factors at relatively high concentrations in this sample, although it remains possible that the commercial ELISA assay does not accurately report concentrations of more pathogenic forms. Indeed, the fact that plasma from patient 101 can stimulate TRPC6 even when it is diluted to a final concentration of 2% in media suggests that suPAR and TNF activities are augmented by additional factors in this plasma. If this situation is common in primary FSGS, then the circulating levels of any one putative permeability factor might not show a strong correlation with proteinuria or disease severity, but it could still be an important factor driving the disease process. In other words, the effect of a factor like suPAR could be conditional, and might require the potentiating actions of a second (or third) circulating factor such as TNF, CLCF1, IL-13, hemopexin, syndecan-4, or even an entire cytokine milieu [4,24]. This milieu could also include protective factors [58]. Overall activity may depend on other factors such as serum lipid content, and may also vary according to prior treatments, whether the patients currently harbor an infection, etc. Moreover, pathogenic factors may also be produced locally within glomeruli (as with TNF and suPAR) and might not be captured in measurements from serum or plasma (but might be captured in urine measurements).
In this regard, even the original report on circulating suPAR noted a subset of patients with primary and even recurrent FSGS with serum levels well below the cutoff level of 3,000 pg/ml and well within the range seen in healthy people [5]. Moreover, some patients with high total serum suPAR levels from other causes, such as in certain cancers, do not show signs of glomerular dysfunction [57]. It is possible that in a generally inflammatory milieu, for example in various forms of glomerulonephritis, suPAR signaling would be more deleterious as compared to children or young adults with primary nephrotic syndromes in which inflammation is generally not present. This view accords well with large longitudinal clinical studies in which serum suPAR levels predicted future incident kidney disease and declines in estimated glomerular filtration rate [10,11].
From a therapeutic perspective, the complex and possibly intractable nature of the circulating and/or locally produced factors that drive primary FSGS may not be crucial if their actions converge onto a small subset of targetable proteins in podocytes. Two targets suggested by the present results are TRPC6 channels and αvβ3-integrin. The later protein functions as a receptor or co-receptor for suPAR [59]. Here we observed that cilengitide, an inhibitor of signaling through αv-containing integrins such as αvβ3-integrin [54], completely blocked the increases in surface abundance of TRPC6 evoked by all of these treatments, including suPAR, TNF, and plasmas from patients 101 and 356. TNF acts on a distinct family of its own receptors but there is evidence that it can induce inside-out activation of integrins [60]. In addition, an experiment with a design similar to ours showed that plasma from a recurrent FSGS patient caused TNF-dependent activation of β3-integrin signaling in cultured podocytes [16], and TNF has also been recently reported to contribute to NFATc1-dependent injury in podocytes [61]. Both of those results are entirely consistent with present observations. In this regard, we recently demonstrated that the ectodomain of syndecan-4 (Sdc4) can also cause mobilization of podocyte TRPC6 channels through cascades that are blocked by cilengitide [49]. As with TNF, application of Sdc4 did not reduce the expression of podocin [49]. Therefore, loss of podocin expression is not an inevitable consequence of integrin signaling in podocytes. Regardless of how αvβ3-integrin is activated by circulating factors, several studies have shown efficacy of αvβ3-integrin antagonists in animal models of proteinuric diseases [5,62,63]. As an aside, we should note here that human TNF is only able to activate the type 2 TNF receptor in mice [64], but that receptor is already thought to play a role in driving kidney disease [65].
The primary observation of this study is that all of these treatments converge to increase functional TRPC6 channels in podocytes. This markedly expands the significance of observations made more than a decade ago that well-characterized genetic forms of FSGS occur as a result of mutations that lead to a gain of TRPC6 function [29–33] or a loss of functional podocin [25,27,28]. Podocin and TRPC6 are functionally linked in foot processes where they are endogenously co-localized. Thus, our recent study on TRPC6 gating in podocytes demonstrated direct interactions between cytosolic domains near the carboxy terminals of podocin and TRPC6, and showed that large increased in TRPC6 currents evoked by membrane stretch occur after podocin knockdown [36]. In other words, podocin normally suppresses mechanical activation of TRPC6. Podocyte foot processes are attached to a mechanically dynamic matrix and are subjected to measurable pulsations driven by the cardiac cycle [66] or through expansion of the sub-podocyte space [67]. It is possible that pathological changes that alter the structure and composition of cell membranes, such as a loss of podocin and/or an increase in TRPC6, could cause sustained and excessive Ca2+ influx. Moreover, the forces impacting podocytes would be expected to increase during chronic kidney disease as the number of functional nephrons declines [68].
Most of the macroscopic ionic current in podocytes that is activated by native G protein signaling or other stimuli can be attributed to TRPC6-containing channels on the basis of sensitivity to TRPC6 knockdown, La3+ and SKF-96365 [36,38,39] and SAR7334 (the present study). However, TRPC5 channels are also expressed in podocytes [48,51] and have been proposed to play a role in the progression of kidney disease [69], although the factors that normally cause them to become active are not known. We observed that strong stimuli, such as the plasma taken from patient 101 during relapse, and the combined presence of higher concentrations of suPAR and TNF, can stimulate increased mobilization of TRPC5 subunits to the cell surface. It is possible that TRPC5 channels may also contribute to some cases of severe primary FSGS, or alternatively that a subset of patients have a pattern of circulating factors that exert a preferential effect on TRPC5.
5. Conclusions
We have shown that serum and plasma from patients with recurrent FSGS in relapse can increase the steady-state surface expression of podocyte TRPC6 channels, an affect accompanied in most cases by a loss of podocin. These effects are closely recapitulated by recombinant suPAR. However, some recurrent FSGS patient samples, and recombinant TNF, do not affect podocin but still stimulate an increase in surface expression of TRPC6. The effects of TNF and suPAR on TRPC6 are at least additive and possibly synergistic. Therefore, the overall effects of any one circulating factor on glomerular and podocyte function may depend on which other factors are present in the circulation or within the glomerulus. The effects of all of the treatments studied here were blocked by an inhibitor of αvβ3-integrin signaling, which may form the basis for therapeutic strategies.
Supplementary Material
Highlights.
Circulating factors in patients with recurrent FSGS modulate TRPC6 channels in cultured podocytes, and many but not all of these samples also induce a loss of podocin over a period of 24 hours.
These effects are mimicked by suPAR, a circulating factor implicated in chronic kidney disease, especially FSGS.
The effects of suPAR are increased in the presence of TNF.
Modulation of TRPC6 by suPAR, TNF, and circulating factors in FSGS patients require signaling through alphaV-containing integrins.
TRPC6 and αVβ-integrin may be useful therapeutic targets in glomerular diseases.
Acknowledgments
We are grateful to Dr. Peter Mundel, formerly of Harvard Medical School (Boston, MA) for providing the MPC-5 immortalized podocyte cell line used in these studies; to Drs. Moin Saleem and Dan Henson (University of Bristol, UK), Henning Hagmann and Thomas Benzing (University of Cologne, Germany), Nada Alachkar (Johns Hopkins University, Baltimore MD) and Dr. Sanja Sever of Harvard Medical School, and Jochen Reiser (Rush University, Chicago, IL) for sending us patient serum and plasma samples; and to Dr. Sanja Sever of Harvard Medical School for providing a sample of recombinant suPAR.
This work was supported by National Institutes of Health grant R01-DK104708
Abbreviations
- BUN
blood urea nitrogen
- Cyto D
cytochalasin D
- DAPI
4,6-diamidino-2-phenylindole
- FSGS
focal and segmental glomerulosclerosis
- LDL
low sensity lipoprotein
- suPAR
soluble urokinase receptor
- TNF
tumor necrosis factor
- TRPC6
canonical transient receptor potential-6 channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Hoyer JR, Vernier RL, Najarian JS, Raij L, Simmons RL, Michael AF. Recurrence of idiopathic nephrotic syndrome after renal transplantation. Lancet. 1972;2:343–8. doi: 10.1016/s0140-6736(72)91734-5. [DOI] [PubMed] [Google Scholar]
- 2.Leca N. Focal segmental glomerulosclerosis recurrence in the renal allograft. Adv Chronic Kidney Dis. 2014;21:448–52. doi: 10.1053/j.ackd.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:2115–21. doi: 10.2215/CJN.03800609. [DOI] [PubMed] [Google Scholar]
- 4.Davin JC. The glomerular permeability factors in idiopathic nephrotic syndrome. Pediatr Nephrol. 2016;31:207–215. doi: 10.1007/s00467-015-3082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–60. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blasi F, Sidenius N. The urokinase receptor: focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett. 2010;584:1923–30. doi: 10.1016/j.febslet.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Huang J, Liu G, Zhang YM, Cui Z, Wang F, Liu XJ, Chu R, Zhao MH. Urinary soluble urokinase receptor levels are elevated and pathogenic in patients with primary focal segmental glomerulosclerosis. BMC Med. 2014;12:81. doi: 10.1186/1741-7015-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco Palacios CR, Lieske JC, Wadei HM, Rule AD, Fervenza FC, Voskoboev N, Garovic VD, Zand L, Stegall MD, Cosio FG, Amer H. Urine but not serum soluble urokinase receptor (suPAR) may identify cases of recurrent FSGS in kidney transplant candidates. Transplantation. 2013;96:394–9. doi: 10.1097/TP.0b013e3182977ab1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto K, Imura J, Atsumi H, Matsui Y, Adachi H, Okuyama H, Yamaya H, Yokoyama H. Clinical significance of serum and urinary soluble urokinase receptor (suPAR) in primary nephrotic syndrome and MPO-ANCA-associated glomerulonephritis in Japanese. Clin Exp Nephrol. 2015;19:804–14. doi: 10.1007/s10157-014-1067-x. [DOI] [PubMed] [Google Scholar]
- 10.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, Altintas MM, Wei C, Hotton AL, French AL, Sperling LS, Lerakis S, Quyyumi AA, Reiser J. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–25. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz C-A, Persson M, Christensson A, Hindy G, Almgren P, Nilsson PM, Melander O, Engström, Orho-Melander M. Soluble urokinase-type plasminogen activator receptor (suPAR) and impaired kidney function in the population-based Malmö diet and cancer study. Kidney Int Rep. 2017 doi: 10.1016/j.ekir.2016.11.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guthoff M, Wagner R, Randrianarisoa E, Hatziagelaki E, Peter A, Häring H-U, Fritsche A, Heyne N. Soluble urokinase receptor (suPAR) predicts microalbuminuria in patients at risk for type 2 diabetes mellitus. Sci Rep. 2017;7:40627. doi: 10.1038/srep40627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahm E, Wei C, Fernandez I, Li J, Tardi NJ, Tracy M, Wadhwani S, Cao Y, Peev V, Zloza A, Lusciks J, Hayek SS, O’Connor C, Bitzer M, Gupta V, Sever S, Sykes DB, Scadden DT, Reiser J. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat Med. 2017;23:100–106. doi: 10.1038/nm.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raveh D, Shemesh O, Ashkenazi YJ, Winkler R, Barak V. Tumor necrosis factor-alpha blocking agent as a treatment for nephrotic syndrome. Pediatr Nephrol. 2004;19:1281–4. doi: 10.1007/s00467-004-1573-2. [DOI] [PubMed] [Google Scholar]
- 15.Leroy S, Guigonis V, Bruckner D, Emal-Aglae V, Deschênes G, Bensman A, Ulinski T. Successful anti-TNFalpha treatment in a child with posttransplant recurrent focal segmental glomerulosclerosis. Am J Transplant. 2009;9:858–61. doi: 10.1111/j.1600-6143.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- 16.Bitzan M, Babayeva S, Vasudevan A, Goodyer P, Torban E. TNFα pathway blockade ameliorates toxic effects of FSGS plasma on podocyte cytoskeleton and β3 integrin activation. Pediatr Nephrol. 2012;27:2217–26. doi: 10.1007/s00467-012-2163-3. [DOI] [PubMed] [Google Scholar]
- 17.Suranyi MG, Guasch A, Hall BM, Myers BD. Elevated levels of tumor necrosis factor-alpha in the nephrotic syndrome in humans. Am J Kidney Dis. 1993;21:251–9. doi: 10.1016/s0272-6386(12)80742-6. [DOI] [PubMed] [Google Scholar]
- 18.Bustos C, González E, Muley R, Alonso JL, Egido J. Increase of tumour necrosis factor alpha synthesis and gene expression in peripheral blood mononuclear cells of children with idiopathic nephrotic syndrome. Eur J Clin Invest. 1994;240:799–805. doi: 10.1111/j.1365-2362.1994.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 19.Lama G, Luongo I, Tirino G, Borriello A, Carangio C. Salsano MET-lymphocyte populations, cytokines in childhood nephrotic syndrome. Am J Kidney Dis. 2002;39:958–65. doi: 10.1053/ajkd.2002.32769. [DOI] [PubMed] [Google Scholar]
- 20.Bakr A, Shokeir M, El-Chenawi F, El-Husseni F, Abdel-Rahman A, El-Ashry R. Tumor necrosis factor-alpha production from mononuclear cells in nephrotic syndrome. Pediatr Nephrol. 2003;18:516–20. doi: 10.1007/s00467-003-1122-4. [DOI] [PubMed] [Google Scholar]
- 21.Bertani T, Abbate M, Zoja C, Corna D, Perico N, Ghezzi P, Remuzzi G. Tumor necrosis factor induces glomerular damage in the rabbit. Am J Pathol. 1989;134:419–30. [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy ET, Sharma R, Sharma M, Li JZ, Ge XL, Dileepan KN, Savin VJ. TNF-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol. 1998;9:433–8. doi: 10.1681/ASN.V93433. [DOI] [PubMed] [Google Scholar]
- 23.Sharma M, Zhou J, Gauchat JF, Sharma R, McCarthy ET, Srivastava T, Savin VJ. Janus kinase 2/signal transducer and activator of transcription 3 inhibitors attenuate the effect of cardiotrophin-like cytokine factor 1 and human focal segmental glomerulosclerosis serum on glomerular filtration barrier. Transl Res. 2015;166:384–98. doi: 10.1016/j.trsl.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van den Berg JG, Weening JJ. Role of the immune system in the pathogenesis of idiopathic nephrotic syndrome. Clin Sci (Lond) 2004;107:125–136. doi: 10.1042/CS20040095. [DOI] [PubMed] [Google Scholar]
- 25.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–54. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 26.Zhang SY, Marlier A, Gribouval O, Gilbert T, Heidet L, Antignac C, Gubler MC. In vivo expression of podocyte slit diaphragm-associated proteins in nephrotic patients with NPHS2 mutation. Kidney Int. 2004;66:945–54. doi: 10.1111/j.1523-1755.2004.00840.x. [DOI] [PubMed] [Google Scholar]
- 27.Roselli S, Moutkine I, Gribouval O, Benmerah A, Antignac C. Plasma membrane targeting of podocin through the classical exocytic pathway: effect of NPHS2 mutations. Traffic. 2004;5:37–44. doi: 10.1046/j.1600-0854.2003.00148.x. [DOI] [PubMed] [Google Scholar]
- 28.Schurek EM, Völker LA, Tax J, Lamkemeyer T, Rinschen MM, Ungrue D, Kratz JE, 3rd, Sirianant L, Kunzelmann K, Chalfie M, Schermer B, Benzing T, Höhne M. A disease-causing mutation illuminates the protein membrane topology of the kidney-expressed prohibitin homology (PHB) domain protein podocin. J Biol Chem. 2014;289:11262–71. doi: 10.1074/jbc.M113.521773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–4. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 30.Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–44. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heeringa SF, Möller CC, Du J, Yue L, Hinkes B, Chernin G, Vlangos CN, Hoyer PF, Reiser J, Hildebrandt F. A novel TRPC6 mutation that causes childhood FSGS. PLoS One. 2009;4:e7771. doi: 10.1371/journal.pone.0007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson C, Dryer SE. A mutation in TRPC6 channels abolishes their activation by hypoosmotic stretch but does not affect activation by diacylglycerol or G protein signaling cascades. Am J Physiol Renal Physiol. 2014;306:F1018–25. doi: 10.1152/ajprenal.00662.2013. [DOI] [PubMed] [Google Scholar]
- 33.Riehle M, Büscher AK, Gohlke BO, Kaßmann M, Kolatsi-Joannou M, Bräsen JH, Nagel M, Becker JU, Winyard P, Hoyer PF, Preissner R, Krautwurst D, Gollasch M, Weber S, Harteneck C. TRPC6 G757D Loss-of-function mutation associates with FSGS. J Am Soc Nephrol. 2016;27:2771–83. doi: 10.1681/ASN.2015030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attié T, Gubler MC, Antignac C. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol. 2002;160:131–9. doi: 10.1016/S0002-9440(10)64357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber TB, Schermer B, Müller RU, Höhne M, Bartram M, Calixto A, Hagmann H, Reinhardt C, Koos F, Kunzelmann K, Shirokova E, Krautwurst D, Harteneck C, Simons M, Pavenstädt H, Kerjaschki D, Thiele C, Walz G, Chalfie M, Benzing T. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci U S A. 2006;103:17079–86. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson M, Kim EY, Hagmann H, Benzing T, Dryer SE. Opposing effects of podocin on the gating of podocyte TRPC6 channels evoked by membrane stretch or diacylglycerol. Am J Physiol Cell Physiol. 2013;305:C276–89. doi: 10.1152/ajpcell.00095.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim EY, Anderson M, Wilson C, Hagmann H, Benzing T, Dryer SE. NOX2 interacts with podocyte TRPC6 channels and contributes to their activation by diacylglycerol: essential role of podocin in formation of this complex. Am J Physiol Cell Physiol. 2013;305:C960–71. doi: 10.1152/ajpcell.00191.2013. [DOI] [PubMed] [Google Scholar]
- 38.Roshanravan H, Dryer SE. ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: role of podocin and reactive oxygen species. Am J Physiol Renal Physiol. 2014;306:F1088–9. doi: 10.1152/ajprenal.00661.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roshanravan H, Kim EY, Dryer SE. 20-Hydroxyeicosatetraenoic acid (20-HETE) modulates canonical transient receptor potential-6 (TRPC6) channels in podocytes. Front Physiol. 2016;7:351. doi: 10.3389/fphys.2016.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Möller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- 41.Koop K, Eikmans M, Baelde HJ, Kawachi H, De Heer E, Paul LC, Bruijn JA. Expression of podocyte-associated molecules in acquired human kidney diseases. In situ evaluation of podocin in normal and glomerular diseases. Kidney Int. 2003;64:2092–9. doi: 10.1097/01.asn.0000078803.53165.c9. [DOI] [PubMed] [Google Scholar]
- 42.Horinouchi I, Nakazato H, Kawano T, Iyama K, Furuse A, Arizono K, Machida J, Sakamoto T, Endo F, Hattori S. In situ evaluation of podocin in normal and glomerular diseases. J Am Soc Nephrol. 2003;14:2063–71. doi: 10.1046/j.1523-1755.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- 43.Agrawal V, Prasad N, Jain M, Pandey R. Reduced podocin expression in minimal change disease and focal segmental glomerulosclerosis is related to the level of proteinuria. Clin Exp Nephrol. 2013;17:811–8. doi: 10.1007/s10157-013-0775-y. [DOI] [PubMed] [Google Scholar]
- 44.Doublier S, Musante L, Lupia E, Candiano G, Spatola T, Caridi G, Zennaro C, Carraro M, Ghiggeri GM, Camussi G. Direct effect of plasma permeability factors from patients with idiopatic FSGS on nephrin and podocin expression in human podocytes. Int J Mol Med. 2005;16:49–58. [PubMed] [Google Scholar]
- 45.Coward RJ, Foster RR, Patton D, Ni L, Lennon R, Bates DO, Harper SJ, Mathieson PW, Saleem MA. Nephrotic plasma alters slit diaphragm-dependent signaling and translocates nephrin, Podocin, and CD2 associated protein in cultured human podocytes. J Am Soc Nephrol. 2005;16:629–37. doi: 10.1681/ASN.2004030172. [DOI] [PubMed] [Google Scholar]
- 46.Babayeva S, Miller M, Zilber Y, El Kares R, Bernard C, Bitzan M, Goodyear P, Torban E. Plasma from a case of recurrent idiopathic FSGS perturbs non-muscle myosin IIA (MYH9 protein) in human podocytes. Pediatr Nephrol. 2011;26:1071–81. doi: 10.1007/s00467-011-1831-z. [DOI] [PubMed] [Google Scholar]
- 47.Kim EY, Choi KJ, Dryer SE. Nephrin binds to the COOH terminus of a large-conductance Ca2+-activated K+ channel isoform and regulates its expression on the cell surface. Am J Physiol Renal Physiol. 2008;295:F235–46. doi: 10.1152/ajprenal.00140.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim EY, Anderson M, Dryer SE. Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am J Physiol Renal Physiol. 2012;302:F298–307. doi: 10.1152/ajprenal.00423.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim EY, Roshanravan H, Dryer SE. Syndecan-4 ectodomain evokes mobilization of podocyte TRPC6 channels and their associated pathways: An essential role for integrin signaling. Biochim Biophys Acta. 2015;1853:2610–20. doi: 10.1016/j.bbamcr.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Anderson M, Roshanravan H, Khine J, Dryer SE. Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J Cell Physiol. 2014;229:434–42. doi: 10.1002/jcp.24461. [DOI] [PubMed] [Google Scholar]
- 51.Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstädt H, Hsu HH, Schlondorff J, Ramos A, Greka A. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010;3:ra77. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maier T, Follmann M, Hessler G, Kleemann HW, Hachtel S, Fuchs B, Weissmann N, Linz W, Schmidt T, Löhn M, Schroeter K, Wang L, Rütten H, Strübing C. Discovery and pharmacological characterization of a novel potent inhibitor of diacylglycerol-sensitive TRPC cation channels. Br J Pharmacol. 2015;172:3650–60. doi: 10.1111/bph.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 54.Mas-Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med Chem. 2010;10:753–68. doi: 10.2174/187152010794728639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schordan S, Schordan E, Endlich K, Endlich N. AlphaV-integrins mediate the mechanoprotective action of osteopontin in podocytes. Am J Physiol Renal Physiol. 2011;300:F119–32. doi: 10.1152/ajprenal.00143.2010. [DOI] [PubMed] [Google Scholar]
- 56.Alfano M, Cinque P, Giusti G, Proietti S, Nebuloni M, Danese S, D’Alessio S, Genua M, Portale F, Lo Porto M, Singhal PC, Rastaldi MP, Saleem MA, Mavilio D, Mikulak J. Full-length soluble urokinase plasminogen activator receptor down-modulates nephrin expression in podocytes. Sci Rep. 2015;5:13647. doi: 10.1038/srep13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kronbichler A, Saleem MA, Meijers B, Shin JI. Soluble urokinase receptors in focal segmental glomerulosclerosis: A review on the scientific point of view. J Immunol Res. 2016;2016:2068691. doi: 10.1155/2016/2068691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Musante L, Candiano G, Zennaro C, Bruschi M, Carraro M, Artero M, Ghiggeri GM. Humoral permeability factors in the nephrotic syndrome: a compendium and prospectus. J Nephrol. 2001;14(Suppl 4):S48–50. [PubMed] [Google Scholar]
- 59.Tarui T, Mazar AP, Cines DB, Takada Y. Urokinase-type plasminogen activator receptor (CD87) is a ligand for integrins and mediates cell-cell interaction. J Biol Chem. 2001;276:3983–90. doi: 10.1074/jbc.M008220200. [DOI] [PubMed] [Google Scholar]
- 60.Bouaouina M, Blouin E, Halbwachs-Mecarelli L, Lesavre P, Rieu P. TNF-induced beta2 integrin activation involves Src kinases and a redox-regulated activation of p38 MAPK. J Immunol. 2004;173:1313–20. doi: 10.4049/jimmunol.173.2.1313. [DOI] [PubMed] [Google Scholar]
- 61.Pedigo CE, Ducasa GM, Leclercq F, Sloan A, Mitrofanova A, Hashmi T, Molina-David J, Ge M, Lassenius MI, Forsblom C, Lehto M, Groop PH, Kretzler M, Eddy S, Martini S, Reich H, Wahl P, Ghiggeri G, Faul C, Burke GW, 3rd, Kretz O, Huber TB, Mendez AJ, Merscher S, Fornoni A. Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J Clin Invest. 2016;126:3336–50. doi: 10.1172/JCI85939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee HW, Khan SQ, Faridi MH, Wei C, Tardi NJ, Altintas MM, Elshabrawy HA, Mangos S, Quick KL, Sever S, Reiser J, Gupta V. A Podocyte-based automated screening assay identifies protective small molecules. J Am Soc Nephrol. 2015;26:2741–52. doi: 10.1681/ASN.2014090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maile LA, Busby WH, Gollahon KA, Flowers W, Garbacik N, Garbacik S, Stewart K, Nichols T, Bellinger D, Patel A, Dunbar P, Medlin M, Clemmons D. Blocking ligand occupancy of the αVβ3 integrin inhibits the development of nephropathy in diabetic pigs. Endocrinology. 2014;155:4665–75. doi: 10.1210/en.2014-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Wong GH, Chen EY, Goeddel DV. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci U S A. 1991;88:2830–4. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bruggeman LA, Drawz PE, Kahoud N, Lin K, Barisoni L, Nelson PJ. TNFR2 interposes the proliferative and NF-κB-mediated inflammatory response by podocytes to TNF-α. Lab Invest. 2011;91:413–25. doi: 10.1038/labinvest.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brenner BM, Troy JL, Daugharty TM. The dynamics of glomerular ultrafiltration in the rat. J Clin Invest. 1971;50:1776–80. doi: 10.1172/JCI106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neal CR. Podcytes… What’s under yours? (Podocytes and foot procceses and how they change in nephropathy. Front Endocrinol (Lausanne) 2015;6:9. doi: 10.3389/fendo.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kriz W, Lemley KV. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol. 2015;26:258–69. doi: 10.1681/ASN.2014030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, Ahn W, Wallentin H, Heid H, Hopkins CR, Lindsley CW, Riccio A, Buvall L, Weins A, Greka A. Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest. 2013;123:5298–309. doi: 10.1172/JCI71165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.