Abstract
Prior studies have shown that drug-seeking behaviors increase, rather than dissipate, over weeks to months after withdrawal from drug self-administration. This phenomenon - termed incubation - suggests that drug-craving responses elicited by conditioned environmental or discrete cues may intensify over pronged abstinence. While most of this work is conducted in rats with intravenous drug self-administration models, there is less evidence for incubation in mice that have greater utility for molecular genetic analysis and perturbation. We tested whether incubation of cocaine-seeking behavior is evident in C57BL/6J mice following 3 weeks (5 days/week) of cocaine self-administration in 2 h self-administration sessions. We compared cocaine-seeking (drug-paired lever) responses 1, 7, or 28 days after withdrawal from cocaine self-administration, and over similar times following sucrose pellet self-administration. We found that the initial re-exposure to the self-administration test chambers elicited increased reward-seeking behavior in both sucrose and cocaine self-administering mice, with maximal responses found at 7 days compared to 1 or 28 days after self-administration with either reinforcer. However, following extinction training, reinstatement of cocaine seeking reinforced by response-contingent presentation of reward-associated cues (tone/light) was significantly higher after 28 days compared to 1 or 7 days following cocaine self-administration. In contrast, cue-induced reinstatement of sucrose-paired lever pressing did not increase over this time frame, demonstrating a drug-specific incubation effect not seen with a natural reward. Thus, C57BL/6J mice display incubation of cue-induced reinstatement of cocaine seeking similar to findings with rats, but only show a transient incubation of context-induced cocaine seeking.
Keywords: incubation, cocaine, self-administration, drug-seeking, cue-induced reinstatement, extinction, sucrose, sucrose-seeking, mouse, craving
Introduction
Cocaine craving can be elicited by exposure to conditioned environmental cues associated with prior drug use, and drug craving has been shown to be highly predictive of the propensity for relapse after a period of abstinence1. Drug-associated cues also elicit marked electrophysiological responses in human cocaine abusers that intensify after prolonged periods of abstinence2. In rats, a similar time-dependent intensification of cocaine-seeking behavior, often referred to as incubation, is observed following chronic intravenous cocaine self-administration3-13. These rat studies often employ extinction/reinstatement procedures where cocaine seeking is measured by non-reinforced responding at a drug-paired lever after initial return to the self-administration test chambers. Following extinction of the lever-press behavior, exposing animals to the discrete injection cues associated with prior cocaine self-administration also is used to test the propensity for relapse, or reinstatement of cocaine seeking. Using these models, cocaine seeking elicited by both initial return to the self-administration context and cue-induced reinstatement of cocaine seeking progressively increases, rather than dissipates, over weeks to months after withdrawal from self-administration8,14,15 very similarly to the increases in cue reactivity recently described in humans cocaine abusers2. Given this time-dependent exacerbation in cue responsiveness, a delineation of the neurobiological mechanisms underlying the effect may lead to more effective treatments for cocaine addiction, especially over long-term abstinence.
A similar incubation of drug seeking has been shown for other abused drugs including heroin6,16, alcohol17, methamphetamine18-20, and nicotine21,22, suggesting that incubation reflects a pathological process common to drug addiction in general. The incubation phenomenon also occurs with behavior motivated by natural rewards including sucrose, but the enhanced sucrose-seeking behavior fails to persist for more than a few weeks following self-administration training in rats6,10,23. Thus, incubation may reflect a natural process of reward memory intensification over some discrete period that facilitates animal survival, but that drugs of abuse exacerbate and prolong this natural process leading to vulnerability to relapse long after cessation of drug use24.
Studies on the incubation of cocaine-seeking behavior are extensively performed using rat models3-12, but relatively few have employed mouse models25-27. Mouse models of intravenous cocaine self-administration are more difficult to perform, but allow for the use of transgenic and gene deletion models not readily accessible in rats. In this study, we tested whether the C57BL/6J mice display incubation of cocaine seeking over time in response to contextual and discrete injection cues using the extinction/reinstatement paradigm, and compared responses in cocaine-trained mice to responses in mice trained to self-administer sucrose pellets as a natural reward.
Methods
Animals
Male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, Maine) at 6-8 weeks of age. Mice weighed on average 25 ± 0.41 g at the beginning of the experiment. The mice were acclimated for 1 week prior to being placed in individual housing in a colony room maintained at 21°C under a 12-hour light/dark cycle (lights on at 6:00 am). Mice had free access to water and rodent chow (2016 Harlan Teklad Global Diet) except during lever-press training for 25 mg sucrose pellets when mice were food restricted for 16 h prior to each test session. All experiments were conducted during the light cycle in accordance with guidelines established by the National Institutes of Health (NIH) and the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Southwestern Medical Center (UTSW).
Drugs
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (NIDA, Research Triangle Park) and dissolved in 0.9% sterile saline.
Lever Press Training
All mice initially were food-restricted and received lever-press training for sucrose pellets in operant test chambers with levers that retract at the end of each session (Med-Associates, St. Albans, VT) on a fixed ratio 1 (FR1) reinforcement schedule in daily 1 h sessions until an acquisition criterion of 30 sucrose pellets was consumed for 3 consecutive test days. Following lever-press training, animals assigned to cocaine self-administration groups were fed ad libitum for at least 3 days prior to surgery. Sucrose self-administering animals were simply returned to their home cages for a week of ad libitum feeding prior to initiating sucrose self-administration.
IV catheter implantation surgery
Animals were anesthetized with 100 mg/kg ketamine/10 mg/kg xylazine (i.p.) prior to surgical implantation of a chronic indwelling intravenous catheter. The catheters consisted of Silastic tubing (0.02 inch i.d. 0.037 inch o.d.; Green Rubber, Woburn, MA) treated with tridodecylmethyl ammonium chloride (TDMAC) heparin (Polysciences Inc., Warrington, PA). Each catheter was secured at the jugular vein with Mersiline surgical mesh (General Medical, New Haven, CT), and passed subcutaneously to exit the back through 22-gauge cannula (Plastics One, Roanoke, VA) embedded in dental cement on a Mersiline surgical mesh base. Following surgery, animals received a prophylactic injection of penicillin (200,000IU/kg/ml, 0.2 ml, i.m.), and antibiotic ointment was applied daily to the exit wound. Catheters were flushed daily with 0.2 ml of heparinized (20 IU/ml) bacteriostatic saline containing gentamycin sulfate (0.33 mg/ml) and 5 mg/ml of enrofloxacin (Baytril®) to prevent clotting and curb infection. Catheter patency was checked weekly using methohexital sodium (5 mg/ml, 0.05ml i.v.).
Cocaine Self Administration
Animals were placed into operant test chambers for self-administration, extinction, and reinstatement testing. Each chamber was enclosed in a ventilated, sound-attenuating box and equipped with an injection pump assembly consisting of a Razel Model A injection pump (Stamford, CT) and a 10-ml glass syringe connected to Teflon® tubing which connects through a swivel mechanism to the animal's catheter exit port. Each operant chamber contained two response levers located 2 cm off the floor; during self-administration testing, a lever-press response on the paired lever delivered an intravenous cocaine injection (0.5 mg/kg/injection) over 2.5 s in a 50 μl volume of sterile saline; lever press responses on the unpaired lever produced no programmed consequence. During the injection period, a cue light (above the lever) was illuminated for 2.5 s paired with a 2.9 kHz tone and the house light was extinguished. A 12.5 s time-out interval followed during when the house lights remained off and responding at the paired lever had no programmed consequences. The illumination of the house light signaled the end of the 15 s injection/time-out interval. Mice self-administered cocaine on a fixed ratio (FR) 1 reinforcement schedule in daily 2-h sessions, 5 days per week, for 3 weeks. Mice had no access to food or water while in the operant chambers. At the conclusion of self-administration, animals were returned to their home cages for the duration of the withdrawal period. Catheters continued to be flushed routinely during the withdrawal period.
Sucrose Self Administration
Food restricted mice received approximately 2 g of chow per day mice and were weighed daily to prevent weight loss of > 5% initial body weight. During the testing period, mice were placed in operant chambers where responding on the paired lever resulted in delivery of a sucrose pellet on a fixed ratio 1 (FR1) reinforcement schedule. Sucrose pellet delivery was paired with a similar cue light/tone cue for 2.5 s while the house light was extinguished to signal the unavailability of further sucrose pellets during the 12.5 s time-out period. At the conclusion of the time-out period, the house light was illuminated to signal the availability of further sucrose pellets. Animals were allowed to self-administer sucrose for a maximum of 60 pellets per session. Mice self-administered sucrose in daily 2-h sessions, 5 days per week, for 3 weeks. Levers retracted and lights extinguished at the end of each session or after 60 pellets were earned, but mice remained in the chamber for the 2 h session in either case. At the end of 15 days of sucrose self-administration, mice we fed ad libitum in their home cages for the remainder of the experiment.
Extinction and Cue-induced Reinstatement
Cocaine self administering animals were assigned to the 1, 7, or 28 day withdrawal groups with cocaine intake balanced across the 3 groups. Likewise, the sucrose self administering groups were balanced based on their latency to self-administer the first 30 sucrose pellets. Following the designated withdrawal period (1, 7, or 28 days), mice were placed back into the operant chambers under extinction conditions when lever presses resulted in neither cocaine nor sucrose pellet reinforcement nor the conditioned stimuli (light and tone). Mice remained in the operant chambers for extinction testing for 6 h. At the conclusion of the 6-h extinction period, animals were presented with 5 non-contingent cocaine- or sucrose-associated cues (light/tone for 2.5 sec) every 2 min for 10 min as a primer. Lever-pressing on the reward-paired lever produced response-contingent light/tone reward-associated cues during both the initial 10 min primer period and throughout the 1 h test.
Statistical Analysis
Responding during the first h of extinction testing and cue-induced reinstatement was analyzed by 2-way ANOVA with repeated measures on lever, or across all 6 h of extinction with repeated measures on time. Bonferroni's tests were performed to identify differences from the 1 d WD group with significance level of p < 0.05.
Results
Self-administration
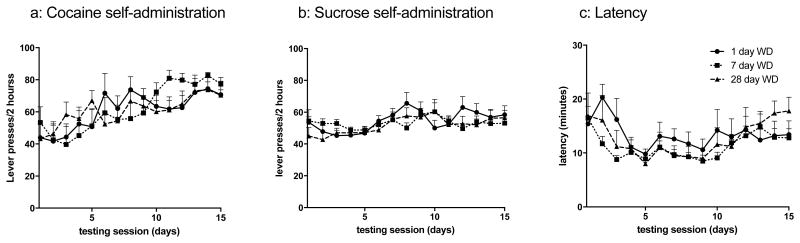
Groups of mice reliably self-administered cocaine starting with an initial average lever pressing of 46.7 ± 3.4 on the first day of self-administration. Over the three week period, all mice increased their lever pressing by approximately 57% to an average of 73.4 ± 2.0 presses/2 h session on the final day of self-administration. Mice were assigned into 3 withdrawal groups evenly matched for lever presses for the 15 days of training (Fig. 1a). A separate cohort of mice self-administered sucrose for 15 days and were balanced based on the number of lever presses (Fig. 1b) and the latency to achieve 30 sucrose pellets per session (Fig. 1c). Mice generally self-administered 30 pellets with a latency of ∼16 minutes (16.3 ± 0.4 min, Fig. 1c).
Figure 1. Cocaine or sucrose self-administration.
a) The number of lever presses for self-administered cocaine infusions (0.5 mg/kg/injection) during 2 h sessions over 15 days of cocaine self-administration training for each withdrawal (WD) group: 1 day WD (n = 9), 7 day WD (n = 11) or 28 day WD (n = 12). The number of b) lever presses for self-administered sucrose pellets (60 max) and c) the latency to earn the first 30 sucrose pellets in 2 h sessions over 15 days of sucrose self-administration training for each WD group: 1 day WD (n=12), 7 day WD (n=12) or 28 day WD (n=11).
Effect of withdrawal time on extinction responding
Following withdrawal time periods of either 1, 7, or 28 days in their home cages, mice returned to the test chambers under extinction conditions without response-contingent reward delivery or reward-associated discrete cues (light/tone) for 6 hours. Responding during the first h of extinction testing, and index of context-elicited cocaine-seeking behavior, was higher at the lever paired with previous cocaine injections when compared with unpaired lever responses in all groups (Fig. 2a left, 2-way ANOVA, effect of lever: F1,29 = 74.78, p < 0.001; withdrawal time × lever interaction: F2,29 = 4.374, p = 0.022). Cocaine-seeking responses at the drug-paired lever were significantly higher 7 days after withdrawal compared to mice tested 1 day after the last cocaine self-administration test session (Fig 2a left, effect of withdrawal time: F2,29 = 4.097, p = 0.027). Drug-paired lever responses in the 28 day withdrawal group displayed a trend (p = 0.068) but failed to significantly differ from the 1 day withdrawal group. This time-dependent increase in drug-paired lever responding after withdrawal from cocaine self-administration produced a significant withdrawal time × h interaction across all 6 h of extinction testing (Fig. 2a right: F10,145 = 3.63, p = 0.002). Here, responses at drug-paired lever significantly increased at both 7 and 28 days compared to 1 day withdrawal times during the first h given the added power of this analysis. However, all groups extinguished to similar low levels by the 6th h (effect of time: F5,145 = 67.33, p < 0.001), with mice meeting an extinction criteria of fewer than 15 lever presses per h.
Figure 2. Both cocaine- and sucrose-seeking behavior peaks after 7 days WD during an extinction session.
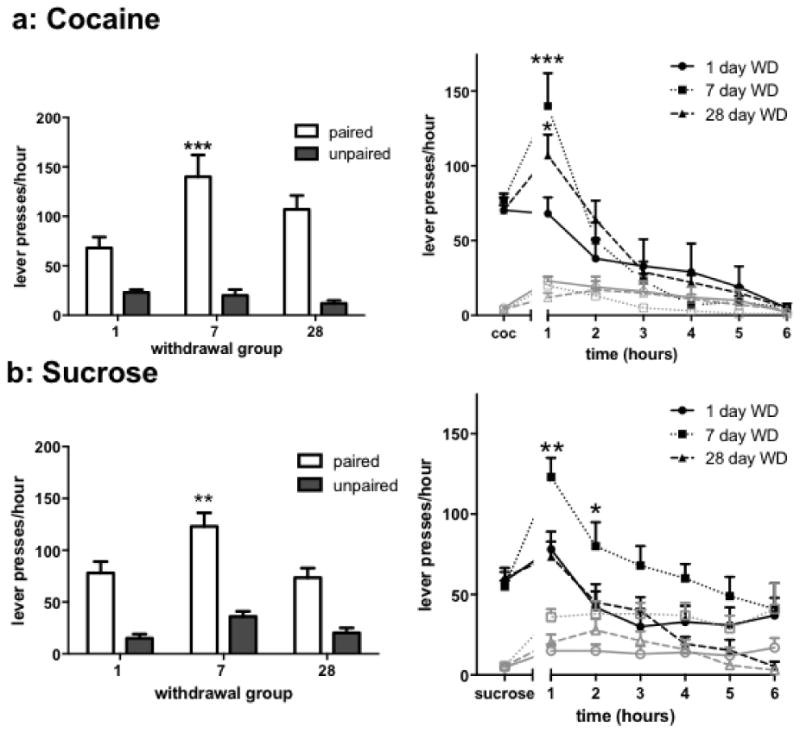
a-left) Cocaine-paired and unpaired lever presses during the first hour of extinction at 1, 7, and 28 days after WD from cocaine self-administration. a-right) Paired (black lines) and unpaired (grey lines) lever pressing over a 6 h extinction session for all cocaine WD groups relative to lever presses on the last day of cocaine self-administration training. b-left) Sucrose-paired and unpaired lever presses during the first hour of extinction after at 1, 7, and 28 days after WD from sucrose self-administration. b-right) Sucrose-paired (black lines) and unpaired lever presses (grey lines) over a 6 hour extinction session for all sucrose self-administration WD groups relative to lever presses on the last day of sucrose self-administration. Differs from 1 day WD *p < 0.05, **p < 0.01, ***p < 0.001 by Bonferroni tests.
Interestingly, sucrose-trained mice also showed greater initial sucrose-paired lever responding 7 days following sucrose self-administration compared to mice tested 1 day after self-administration training ended (Fig. 2b left, 2-way ANOVA, effect of withdrawal time: F2,32 = 6.945, p = 0.003). However, responding returned to the early withdrawal baseline 28 days after sucrose self-administration. Responding was substantially biased towards the sucrose-paired lever compared to the unpaired lever (effect of lever: F1,32 = 60.00, p < 0.001). Extinction responses over 6 h showed that mice in the 7 day withdrawal group responded at high rates at the sucrose-paired lever throughout extinction testing (Fig. 2b right, 2-way ANOVA, effect of withdrawal time: F2,32 = 5.391, p = 0.010), but only mice in the 28 day withdrawal group met an extinction criteria of < 15 responses at the sucrose-paired lever by the 6th hour of extinction testing.
Effect of withdrawal time on cue-induced reinstatement of reward-seeking behavior
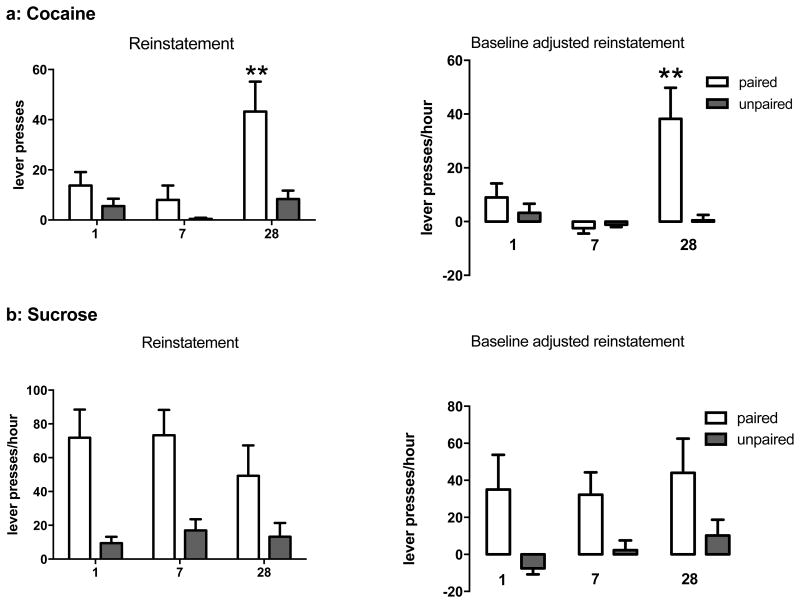
After 6 h of extinction training, mice were presented with 5 non-contingent light and tone cues that previously were paired with receipt of reward, and subsequent responding was reinforced by the compound cue in a 1 h reinstatement test. In cocaine-trained mice, only mice tested 28, but not 7, days after withdrawal from self-administration significantly increased responding at the drug-paired lever compared to mice tested 1 day after cocaine (Fig. 3a left, 2-way ANOVA, effect of withdrawal time: F2,29 = 5.18, p = 0.012), indicating a late forming incubation of cocaine seeking in response to discrete cues. A main effect of lever reflects responding biased at the drug-paired lever (F1,29 = 12.36, p = 0.002). When reinstatement responses were adjusted for baseline response rates in the 6th h of extinction testing (reinstatement responses minus extinction responses), a similar increase was observed from 1 to 28 days withdrawal from cocaine self-administration (Fig. 3a right, 2-way ANOVA, effect of withdrawal time: F2,29 = 5.275, p = 0.011), and lever pressing was again preferential for the drug-paired lever (effect of lever: F1,29 = 10.74, p = 0.003; lever × withdrawal time interaction: F2,29 = 5.00, p = 0.014). In contrast to cocaine self-administering mice, sucrose-trained mice showed similar cue-induced reinstatement of responding at the sucrose-paired lever at all 3 time points (Fig. 3b left) with lever pressing preferential for the paired lever (2-way ANOVA, effect of lever: F1,32 = 42.29, p < 0.001). The lack of incubation in cue-induced sucrose seeking remained even when responses were adjusted for differences in baseline responding during the 6th h of extinction testing (Fig. 3b right), and only a main effect of lever was significant (2-way ANOVA: F1,32 = 20.67, p < 0.001), although positive response rates indicate that discrete cues effectively increased sucrose-paired lever responding from extinction baselines.
Figure 3. Incubation of cue-induced reinstatement of cocaine- but not sucrose-seeking behavior after 28 days WD.
a-left) Cocaine-paired and unpaired lever responding during cue-induced reinstatement immediately after 6 hours of extinction training (Fig. 2). a-right) Reinstatement of cocaine-paired and unpaired lever responding after subtraction of baseline extinction response rates during the 6th hour of extinction training. b-left) Sucrose-paired and unpaired lever responding during cue-induced reinstatement immediately after 6 hours of extinction training. b-right) Reinstatement of sucrose-paired and unpaired lever responding after subtraction of baseline extinction response rates during the 6th hour of extinction training. Differs from 1 day WD, **p < 0.01 by Bonferroni tests
Discussion
This study found prominent and late forming incubation of discrete cue-induced reinstatement after 28, but not 1 or 7 days, following withdrawal from cocaine self-administration in C57BL6/J mice, one of the most commonly used genetic background strains for analysis of genetic deletion or transgenic over-expression. Our findings are consistent with previous studies in rats demonstrating that discrete cue-induced reinstatement of cocaine seeking incubates over time8. In contrast to cue-induced reinstatement, initial re-exposure to the self-administration context during extinction testing elicited only transient increases in cocaine or sucrose seeking after a 7 day withdrawal period that declined after 28 days to levels observed 1 day after self-administration training. These extinction findings differ from studies in rats that generally find prominent and long-lasting enhancement in initial context-induced cocaine seeking after longer withdrawal periods3,8. One possibility is that the incentive properties of contextual memories are longer lived in rats when compared to mice28, while the incentive properties of discrete cues gain salience over time. Another possibility is that since discrete cues are more closely associated with drug delivery, this association may survive for longer times compared to contextual cues that are less temporally associated with the receipt of individual cocaine injections6.
A similar transient enhancement (7 days) in the response elicited by the environmental/contextual cues associated with a natural reinforcer, sucrose was found in C57BL6/J mice. However, there was no evidence of incubation in discrete cue-induced sucrose seeking as reinstatement responses remained elevated at all 3 withdrawal times even when corrected for differences in extinction baselines. These results are somewhat similar to studies using rats where responding for sucrose-paired cues dissipate quickly over time6,10,16,23, or are not observed25, while drug-paired cues progressively increase over months in rats24. However, some studies do show an incubation with both cocaine and sucrose in rats, although these studies examined responses at earlier times (day 15 or 21 withdrawal)10,11,29,30, or do not dissociate the initial drug-seeking in extinction from the reinstatement induced by discrete-cues20,30,31. As discussed earlier, this transient incubation of initial context-induced sucrose seeking may represent a natural phenomenon to promote and enhance memories essential for survival by facilitating reward-related cues over a brief time period. However, if animals can survive beyond this time, the incentive salience evoked by these memories returns to normal levels. There are many reports of conditioned responses to natural appetitive stimuli that either remain relatively constant or decay over time32,33.
There are other experimental caveats that could contribute to differences between cocaine and sucrose reinstatement. For instance, although we capped the maximum number of sucrose reinforcers available to avoid satiety and approximate the number of cocaine reinforcers mice self-administer under similar conditions in 2 h sessions, mice self-administer most of the sucrose pellets within the first 20 min of the session. In contrast, mice self-administer cocaine infusions more evenly spaced over the 2 h session. Another notable difference is that the overall rates of cue-induced reinstatement in the sucrose-trained group were much higher than in cocaine-trained animals, despite no evidence for incubation of the cue response. The lack of food availability in the operant chambers may have contributed to this higher response in the 7 h extinction/reinstatement sessions. The palatability of the food pellets may also be a factor, since other studies using rats have shown incubation in animals trained with liquid sucrose solutions 10,23,30. To our knowledge, incubation of liquid sucrose seeking has not been shown in mice, and a lack of incubation with food pellets is consistent with the current mouse literature 25. Finally, it is important to note that the present findings could be unique to the C57BL/6J strain of inbred mice, and may not be representative of all other mouse strains.
There are relatively few previous studies showing incubation of cocaine seeking in mice in response to various combinations of discrete and contextual cues. One study found incubation of cue-induced reinstatement in unspecified wild-type littermates of GluR1 knockout mice 66 days after cocaine self-administration26, although this study tested the same mice for cue-induced reinstatement at different times following a single extinction session. Thus, this effect could reflect spontaneous recovery rather than incubation. Two other studies have shown incubation of cocaine seeking when mice are initially returned to the self-administration test chambers but with a combination of both contextual/environmental and response-contingent presentation of discrete injection cues during extinction25,27. Our approach to test contextual and discrete cues separately suggest that this effect may be entirely due incubation in the response to discrete cocaine injection cues (light/tone) and not contextual factors.
Given that incubation of drug seeking in rats also is found with self-administration of heroin6,16, alcohol17, methamphetamine18-20, and nicotine21,22, it is likely that similar incubation could be found in mice self-administering these abused drugs. Mouse incubation models could be used to study strain-specific differences in the genetics underlying addictive disorders, or to study the effects of cell-specific genetic deletion or transgenic expression on the incubation phenomenon, similar to their pivotal use in understanding other aspects of addiction including reinstatement26,34,35. Since incubation of drug craving can be observed in humans2,36, non-human primates37, rats3-13, and mice25-27; future studies in mouse incubation models may have high translational impact to improve treatment strategies in abstinence that to decrease relapse rates in human addicted populations.
Highlights.
Incubation of cue-induced reinstatement of cocaine seeking in C57BL/6J mice
Transient incubation of context-induced cocaine or sucrose seeking
No incubation of cue-induced reinstatement of sucrose seeking
Acknowledgments
All experimental procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and were approved by the UT Southwestern Medical Center Institutional Animal Care and Use Committee (IACUC). The National Institute on Drug Abuse generously provided cocaine.
This work was supported by NIDA grants R01-DA026482 and T32-DA7290.
This work was supported by NIH grant DA-10460, T32-DA7290, and by the Wesley Gilliland Professorship in Biomedical Research. Beyond this, the authors declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Footnotes
Disclosure/conflict of interest: ALN, EMA, EBL, and DWS have no disclosures/conflicts of interest to declare.
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rohsenow DJ, Martin RA, Eaton CA, Monti PM. Cocaine craving as a predictor of treatment attrition and outcomes after residential treatment for cocaine dependence. J Stud Alcohol Drugs. 2007;68:641–648. doi: 10.15288/jsad.2007.68.641. [DOI] [PubMed] [Google Scholar]
- 2.Parvaz MA, Moeller SJ, Goldstein RZ. Incubation of Cue-Induced Craving in Adults Addicted to Cocaine Measured by Electroencephalography. JAMA psychiatry. 2016;73:1127–1134. doi: 10.1001/jamapsychiatry.2016.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran-Nguyen LT, et al. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- 4.Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- 5.Alleweireldt AT, Weber SM, Neisewander JL. Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacology, biochemistry, and behavior. 2001;69:555–560. doi: 10.1016/s0091-3057(01)00573-1. [DOI] [PubMed] [Google Scholar]
- 6.Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47(Suppl 1):202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- 8.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimm JW, et al. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm JW, Shaham Y, Hope BT. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol. 2002;13:379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickens CL, et al. Neurobiology of the incubation of drug craving. Trends in neurosciences. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 13.Lee BR, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nature neuroscience. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koya E, et al. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- 17.Bienkowski P, et al. Time-dependent changes in alcohol-seeking behaviour during abstinence. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2004;14:355–360. doi: 10.1016/j.euroneuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biological psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 20.Krasnova IN, et al. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2008–2016. doi: 10.1038/npp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diergaarde L, de Vries W, Raaso H, Schoffelmeer AN, De Vries TJ. Contextual renewal of nicotine seeking in rats and its suppression by the cannabinoid-1 receptor antagonist Rimonabant (SR141716A) Neuropharmacology. 2008;55:712–716. doi: 10.1016/j.neuropharm.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Funk D, et al. Role of Central Amygdala Neuronal Ensembles in Incubation of Nicotine Craving. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:8612–8623. doi: 10.1523/JNEUROSCI.1505-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- 25.Halbout B, Bernardi RE, Hansson AC, Spanagel R. Incubation of cocaine seeking following brief cocaine experience in mice is enhanced by mGluR1 blockade. The Journal of neuroscience : the official journal of the Society for Neuroscience. 34:1781–1790. doi: 10.1523/JNEUROSCI.1076-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mead AN, Zamanillo D, Becker N, Stephens DN. AMPA-receptor Glu R1 subunits are involved in the control over behavior by cocaine-paired cues. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:343–353. doi: 10.1038/sj.npp.1301045. [DOI] [PubMed] [Google Scholar]
- 27.Terrier J, Luscher C, Pascoli V. Cell-Type Specific Insertion of GluA2-Lacking AMPARs with Cocaine Exposure Leading to Sensitization, Cue-Induced Seeking, and Incubation of Craving. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:1779–1789. doi: 10.1038/npp.2015.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 29.Uejima JL, Bossert JM, Poles GC, Lu L. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res. 2007;181:292–296. doi: 10.1016/j.bbr.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Counotte DS, Schiefer C, Shaham Y, O'Donnell P. Time-dependent decreases in nucleus accumbens AMPA/NMDA ratio and incubation of sucrose craving in adolescent and adult rats. Psychopharmacology. 2014;231:1675–1684. doi: 10.1007/s00213-013-3294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darling RA, Dingess PM, Schlidt KC, Smith EM, Brown TE. Incubation of food craving is independent of macronutrient composition. Sci Rep. 2016;6:30900. doi: 10.1038/srep30900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riccio DC, Ackil J, Burch-Vernon A. Forgetting of stimulus attributes: methodological implications for assessing associative phenomena. Psychol Bull. 1992;112:433–445. doi: 10.1037/0033-2909.112.3.433. [DOI] [PubMed] [Google Scholar]
- 33.Trowill JA, Panksepp J, Gandelman R. An incentive model of rewarding brain stimulation. Psychol Rev. 1969;76:264–281. doi: 10.1037/h0027295. [DOI] [PubMed] [Google Scholar]
- 34.Mameli M, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nature neuroscience. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 35.Graham DL, et al. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nature neuroscience. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 36.Bedi G, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biological psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weerts EM, Goodwin AK, Kaminski BJ, Hienz RD. Environmental cues, alcohol seeking, and consumption in baboons: effects of response requirement and duration of alcohol abstinence. Alcohol Clin Exp Res. 2006;30:2026–2036. doi: 10.1111/j.1530-0277.2006.00249.x. [DOI] [PubMed] [Google Scholar]