Abstract
We report three unrelated patients with mutations in the GRM6 gene that normally encodes the glutamate receptor mGluR6. This neurotransmitter receptor has been shown previously to be present only in the synapses of the ON bipolar cell dendrites, and it mediates synaptic transmission from rod and cone photoreceptors to this type of second-order neuron. Despite the synaptic defect, best visual acuities were normal or only moderately reduced (20/15 to 20/40). The patients were night blind from an early age, and when maximally dark-adapted, they could perceive lights only with an intensity equal to or slightly dimmer than that normally detected by the cone system (i.e., 2-3 log units above normal). Electroretinograms (ERGs) in response to single brief flashes of light had clearly detectable a-waves, which are derived from photoreceptors, and greatly reduced b-waves, which are derived from the second-order inner retinal neurons. ERGs in response to sawtooth flickering light indicated a markedly reduced ON response and a nearly normal OFF response. There was no subjective delay in the perception of suddenly appearing white vs. black objects on a gray background. These patients exemplify a previously unrecognized, autosomal recessive form of congenital night blindness associated with a negative ERG waveform.
Keywords: bipolar cell, glutamate receptor, retina
The mammalian retina has two fundamental categories of photoreceptor cells, the highly sensitive rods and the less sensitive but faster-responding cones. Both photoreceptor types hyperpolarize in response to light. There are also two fundamental categories of bipolar cells with which the photoreceptors synapse, called ON and OFF bipolar cells (1). In response to a sudden increase of illumination, the ON bipolar cells depolarize while the OFF bipolar cells hyperpolarize. Rods synapse with rod ON bipolar cells, whereas cones synapse with both cone ON bipolar cells and cone OFF bipolar cells (1, 2). Thus, an abrupt increase in illumination will lead to the hyperpolarization of all photoreceptor cells, the depolarization of the rod and cone ON bipolar cells, and the hyperpolarization of the cone OFF bipolar cells. A sudden decrease in illumination (e.g., when a bright light is turned off) will result in the depolarization of the photoreceptors and the cone OFF bipolar cells and the hyperpolarization of the rod and cone ON bipolar cells (2).
Electroretinograms (ERGs) recorded with a contact lens electrode on the surface of the cornea show waveforms in response to flashes of light that correspond to the changes in the polarization of the photoreceptors and the bipolar cells (3, 4). ON and OFF responses are typically recorded from the cone system (2). Light stimuli are presented along with a constant rod-desensitizing background light. Under these conditions, the ON response to the onset of a bright light pulse of long duration (i.e., >50 msec) has an initial cornea-negative a-wave, derived from the hyperpolarization of the cone photoreceptors and possibly the OFF bipolar cells and horizontal cells (5), followed by a cornea-positive b-wave that primarily reflects the depolarization of the cone ON bipolar cells (4). The cessation of the light pulse induces a positive waveform, called the d-wave, which reflects the depolarization of the cone photoreceptors and cone OFF bipolar cells (6); the amplitude and shape of the d-wave is modulated by a concurrent hyperpolarization of the ON bipolar cells (4, 7). If the light-adapted retina is stimulated with a brief (e.g., <1 msec) flash of light rather than a long pulse of light, the ERG will initially show a cornea-negative a-wave that reflects hyperpolarization of the photoreceptors (3) followed by a cornea-positive b-wave that is a summation of potentials from the ON and the OFF bipolar cells and the long-duration component of the photoreceptor response (2, 7-10).
Both rods and cones continually release the neurotransmitter glutamate into their synapses with bipolar cells, with the rate of release reduced according to the level of light-induced hyperpolarization (11, 12). Both rod and cone ON bipolar cells use the same metabotropic glutamate receptor, termed mGluR6, that detects glutamate in the synapse (13, 14). The mGluR6 receptor is expressed by rod and cone ON bipolar cells but nowhere else in the central nervous system (14). Transgenic mice lacking this receptor had no apparent abnormality in the cellular organization of the retina (15). ERGs and electrical recordings from the superior colliculi of these mice indicated a defect in the ON bipolar cell pathway of the retina (15). The mice had normal light-conditioned behavioral responses (i.e., avoidance of a shock preceded by a light) (15) and normal circadian locomotor activity. However, they had no pupillary responses to dim light and had reduced and delayed pupillary responses to bright light (16). Also, optokinetic responses were diminished especially to low-contrast stimuli (16). We under-took this study to identify humans with similar loss-of-function mutations in the homologous human gene and to document their visual function.
Materials and Methods
This study was performed in accord with the tenets of the Declaration of Helsinki, is compliant with the Health Insurance Portability and Accountability Act of 1996, and received approval from the Institutional Review Boards of the Massachusetts Eye and Ear Infirmary (MEEI), Harvard Medical School, and the University of Illinois College of Medicine. Informed consent was obtained from the 26 index patients before donating a blood sample. Index patients had a prior diagnosis of congenital night blindness. The index patients were unrelated to each other as far as could be determined through evaluations of pedigrees; however, family histories were not available for some patients (e.g., those who were adopted). The relatives of patients with GRM6 sequence variations were asked to participate in this study by donating a blood sample. We also evaluated 189 normal control individuals with no history of hereditary retinal degeneration and no known blood relatives with such conditions. DNA was purified from the leukocytes of the patients, relatives, and controls.
DNA from the 10 exons (17) of the GRM6 gene were amplified in a total of 9 amplicons by using PCR and the following primers (sense/antisense): exon 1, ggttcggcgcttgtaaga/acgctgtgtgaattcgagtg; exons 2 and 3, cccttaccctccctctcttg/gtgtgtaaggtggcgatgtg; exon 4, gagcaggggagatggataga/ggcaccaattacacagatgc; exon 7, agctcctcctttctcctgct/caggctgcacagagacga; exon 8 (5′ region), gcagagaccctcagactgga/aggtgatggcgtagatgagg; exon 8 (3′ region), cttcgtgcggtacaacaaca/ctttgtaacgttgcggacag; exon 9, cctcaaggggatcctgac/caaaagcacgaacaagcatt; exon 10, atgtggtgaggactgtgtgg/actccctgccactgactgtt. Exons 5 and 6 were amplified by using the sense primer for exon 4 and the antisense primer for exon 7 and then sequenced with the following primers: taccctggaacctccagttg/tcatgtctttggcactcagc. Products of the PCR were directly sequenced with the primers used for amplification (except for exons 5 and 6 as indicated above) on an ABI sequencer (Model 3100, Applied Biosystems). The numbering of the bases of the cDNA sequence in this work is according to GenBank database accession number NM_000843.
Ophthalmic examinations were performed at the Department of Ophthalmology and Visual Sciences at the University of Illinois at Chicago (UIC) or at the Berman-Gund Laboratory for the Study of Retinal Degenerations at MEEI. One index patient was evaluated at both testing sites. Methods for the ophthalmic evaluations are described in refs. 18 and 19. At UIC, full-field ERGs were recorded to test stimuli presented in a Nicolet Ganzfeld apparatus after 30 min of dark adaptation. Responses to brief, short-wavelength flashes (0.003 cd·sec/m2) presented at 15-sec intervals were recorded first, with responses to two flashes averaged. Dark-adapted responses were then recorded to white xenon flashes (5.1 cd·sec/m2) presented at 15-sec intervals, with responses to three flashes averaged. Next, light-adapted ERGs were elicited in response to white test flashes (5.1 cd·sec/m2) presented against a rod-densensitizing adapting field (15 cd/m2) after 10 min of light adaptation, and to a white stimulus (2.5 cd·sec/m2) flickering at 32 Hz presented against the same adapting field. ERGs also were recorded in response to 6.25-Hz middle-wavelength (peak λ = 512 nm) sawtooth stimuli with a mean luminance of 100 cd/m2 and a contrast of 100% presented against a short-wavelength (peak λ = 468 nm) rod-desensitizing adapting field (12.6 cd/m2), generated by a Ganzfeld light-emitting diode stimulator (Espion ColorDome, Diagnosys, Littleton, MA). The sawtooth stimuli were either “rapid on” (abrupt increment in luminance, followed by a linear decrease in luminance) or “rapid off” (abrupt decrement in luminance, followed by a linear increase in luminance), with responses to five sweeps averaged in each case. Dark-adapted thresholds were obtained after 45 min of dark adaptation with the use of a Tübinger perimeter (Oculus, Wetzlar, Germany) by following a procedure described in ref. 20. The test stimulus had a diameter of 1.7° and a duration of 500 msec. Wavelengths of 500 and 656 nm were used to determine whether dark-adapted thresholds were mediated by the rod or cone system. Test locations ranged from 45° in the nasal to 45° in the temporal visual field. Kinetic perimetry was performed by using a Goldmann perimeter. The test targets were moved from nonseeing to seeing areas.
At the MEEI, the time course of dark adaptation and the final visual thresholds after 45 min of dark adaptation were measured with a Goldmann-Weekers adaptometer with an 11° white test light projected 7° below fixation. Kinetic perimetry was performed with the Goldmann perimeter, bringing the test light from nonseeing to seeing areas. Dark-adapted full-field ERGs were elicited to 0.5-Hz flashes of dim blue light (0.1 cd·sec/m2 before attenuation by Wratten 47, 47A, and 47B filters to give λmax = 440 nm), 0.5-Hz flashes of white light (0.4 cd·sec/m2), and 30-Hz white flashes (0.2 cd·sec/m2) in a Ganzfeld dome. Light-adapted full-field ERGs were elicited with 0.5-Hz flashes of white light (0.4 cd·sec/m2) with a background light sufficient in intensity (34 cd/m2) to eliminate the rod contribution to the ERG (21). Responses were recorded without computer averaging. Amplitudes were measured from the trough of the a-wave (or from the baseline, if the a-wave was absent) to the peak of the b-wave for responses to 0.5-Hz light flashes, and from trough to peak for the responses to 30-Hz flashes (22, 23).
Contrast sensitivity was evaluated with the Pelli-Robson chart (24) at 1 m under photopic conditions with optimum spectacle correction and without pupillary dilation. For testing for a subjective delay in the perception of white vs. black objects, the subject viewed a laptop computer monitor at a distance of ≈0.5 m. The monitor had a uniform 50% gray background except for a black “x” at the center where the patient was instructed to hold fixation. A white letter “o” and a black letter “o” appeared simultaneously on opposite sides of the fixation point separated from it by ≈1 cm either horizontally or vertically. For each appearance of the pair of letters, the patient was asked to report which letter appeared first. Similar testing with one of the two letters appearing after a 0.5-sec delay also was conducted.
Results
DNA Sequence Changes Found in GRM6. Our search for individuals with mutations in the GRM6 gene focused on 26 patients with a prior diagnosis of congenital stationary night blindness. We chose patients with this diagnosis because published brief-flash ERGs of mGluR6-deficient mice (15) are similar to those found in many patients with stationary night blindness in that they are electronegative with an intact a-wave and a subnormal b-wave. Our 26 index patients included 16 males and 10 females. It is possible that some of the males have the X-linked form of complete stationary night blindness; however, they have not been evaluated for mutations in the NYX gene (25, 26) that causes this form of night blindness. An X-linked inheritance pattern for many participating males could not be convincingly established through a family history. The 10 female patients in this study had no known affected relatives. We excluded patients with congenital stationary night blindness who had been found previously to have mutations in the genes encoding rhodopsin (RHO), the α subunit of rod transducin (GNAT1), or rhodopsin kinase (RHOK) (27-29); however, not all index patients had been evaluated for mutations in these genes.
Three of the 26 index patients had allelic mutations in GRM6 that were interpreted as likely to be pathogenic. None of these mutations was found among control individuals (n = 178-189), and each is predicted to encode an absent or likely nonfunctional protein, as described below.
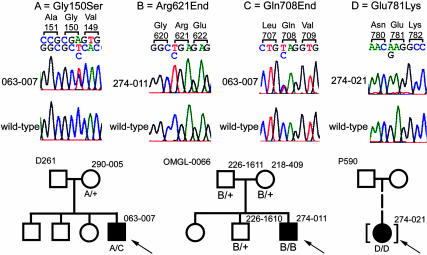
Patient 274-011 was a male homozygote with the nonsense mutation Arg-621 → End (c.1861C>T; CGA to TGA) in exon 8 (of a total of 10 exons). The corresponding mutant RNA would likely be subject to nonsense-mediated decay so that little or no protein would be expressed. Even if the mutant protein were expressed, it would lack the terminal 257 residues including six of the seven transmembrane domains and the intracellular G-protein binding region (30, 31). Genetic analysis of the patient's family showed that both parents were heterozygotes, as was an unaffected brother (Fig. 1). The patient's mother had retinitis pigmentosa. Her disease was probably of an autosomal recessive type because she had no relatives with this condition; the responsible gene remains unknown. A previously reported analysis of this family showed that the patient, his mother, and his unaffected brother were also heterozygous carriers of a missense change of unknown pathogenic potential in the SLC24A1 gene encoding the rod Na-Ca+K-exchanger (32).
Fig. 1.
The top portion of this figure shows the DNA sequence chromatograms of the four mutations identified in the GRM6 gene in patients with night blindness. For each mutation displayed, the top chromatogram is the mutant sequence, and the bottom is wild-type sequence. The sequence listed above the chromatograms corresponds with the mutant sequence, with the wild-type base listed below. The wild-type codons are shown above the sequence. The sequence and chromatograms for the mutation Gly-150 → Ser are shown in the antisense direction. The bottom portion of the figure shows the analysis of these mutations in the available relatives of the patients. The number to the top left of each pedigree is the family identifier. The genotype of each patient tested is listed below the patient symbol. The probands are marked with arrows.
Patient 063-007 was a compound heterozygote male with the nonsense mutation Gln-708 → End (c.2122C>T; CAG to TAG) in exon 8 and the missense mutation Gly-150 → Ser (c.448G>A; GGC to AGC) in exon 1. He had no affected relatives. The mutations were determined to be on separate alleles because an analysis of the patient's unaffected mother, the only available family member, revealed that she was a heterozygote with only the Gly-150 → Ser mutation (Fig. 1). The nonsense mutation Gln-708 → End is interpreted as a null allele because its transcribed RNA is likely subject to nonsense-mediated decay. Even if the mutant mRNA were translated, it would encode a protein lacking three of the seven transmembrane domains and much of the G-protein binding region. The missense mutation Gly-150 → Ser likely encodes a defective receptor because it affects a Gly residue that is present at this position in all metabotropic glutamate receptors and in the closely related parathyroid calcium-sensing receptor CASR (see Fig. 5, which is published as supporting information on the PNAS web site) (31). This Gly residue is three residues away from a Ser that, through a water-mediated interaction, forms part of the binding site for the ligand, glutamate (33, 34). Further evidence for the importance of Gly-150 comes from a previously reported missense mutation (Gly-143 → Glu) affecting the homologous residue in the parathyroid calcium-sensing receptor CASR (35). The Gly-143 → Glu mutation in CASR has been interpreted as a null allele because it causes semidominant familial hypercalciuric hypercalcemia, a disease in which haploinsufficiency for CASR causes hypercalcemia and associated problems in calcium homeostasis (36).
Patient 274-021 was a female homozygote with the missense mutation Glu-781 → Lys (c.2341G>A; GAG to AAG). The patient had been adopted, and no family history was available. This residue is in the third intracellular loop between transmembrane domains V and VI in a stretch of three residues that are perfectly conserved among metabotropic glutamate receptors and the related CASRs (Fig. 5) (31). The mutation changes a negatively charged Glu residue normally at position 781 into a positively charged Lys residue.
We additionally identified 39 other changes in GRM6 (see Table 2, which is published as supporting information on the PNAS web site) interpreted as nonpathogenic or of uncertain pathogenicity. Fifteen of these were missense changes. The missense changes Glu-222 → Lys and Gly-229 → Glu were found in only one patient (063-011); we interpreted the changes to be in cis because the patient and his unaffected sister were heterozygotes for both changes, whereas neither change was present in their mother. A second mutation affecting the other allele in patient 063-011 was not found. The patient was a high myope with refractive errors of approximately -20 diopters spherical equivalent, a finding that was very different from the three patients with GRM6 mutations affecting both alleles (see below). Whether a phenotype is associated with the Glu-222 → Lys+Gly-229 → Glu allele remains uncertain. Two other missense changes, Val-243 → Phe and Ser-583 → Phe, were found in one patient each, both heterozygotes, and not in any control individuals. We found no mutation of the other GRM6 allele in these patients. There were three missense changes with minor allele frequencies of >1% that were interpreted as polymorphisms (Pro-59 → Gln, Met-712 → Val, and Ala-807 → Val). There were eight rare missense variants found in controls and, in some cases, also in patients (Ala-7 → Thr, Arg-28 → His, Arg-118 → Ser, Ile-245 → Val, Arg-578 → Cys, Pro-589 → Leu, Arg-653 → His, and Leu-698 → Val). Except for Gly-229 → Glu, none of the missense rare variants or missense polymorphisms affected residues that were conserved among the members of the mGluR protein family and the related family of calcium-sensing proteins whose sequences are available in the GenBank database (Fig. 5). Finally, 24 isocoding rare variants or polymorphisms were found among the patients and/or controls (Table 2), including 15 synonymous changes in codons, 8 changes in introns (not affecting canonical splice acceptor or donor sites), and one change in the 3′ untranslated region.
Clinical Findings in Patients with GRM6 Mutations. Some of the clinical findings in one of the three patients with GRM6 mutations (274-011) have been reported previously (18). Here, we summarize and expand on these initial findings and describe those found in the other two patients. All three patients reported the inability to see in dim light from early childhood; patient 063-007 specifically stated that he had never seen stars in the night sky. Best corrected visual acuities are given in Table 1. The patients were nearly emmetropic or moderately myopic (Table 1). Contrast sensitivity was evaluated in patients 274-011 and 063-007 and was found to be 1.68 and 1.65, respectively; both values are in the normal range for age (37). Color vision was normal in all patients as documented with the Farnsworth Panel D-15 or 100-Hue tests, the Ishihara color plates, and/or a Nagel anomaloscope. Patient 063-007 had horizontal nystagmus that was accentuated on end-gaze; the other two patients had no nystagmus.
Table 1. Clinical characteristics of patients.
| Patient | Sex | Age, yrs | Refractive error,* diopters | Visual acuity OD; OS† | Dark-adapted threshold elevation, logunits |
|---|---|---|---|---|---|
| 274-011 | Male | 30 | −0.19 | 20/15; 20/15 | 2.5-3 |
| 37 | −0.25 | 20/20; 20/20 | Not done | ||
| 063-007 | Male | 17 | −2.75 | 20/60; 20/60 | 2 |
| 31 | −4.62 | 20/40; 20/40 | 2.5-3 | ||
| 274-021 | Female | 14 | −5.38 | 20/25; 20/30 | 2 |
Spherical equivalent averaged between the two eyes.
OD, right eye; OS, left eye.
When the mGluR6 receptor is pharmacologically blocked in a nonhuman primate, there is a delay in the reaction time to light increments but not to light decrements (38). To test for a comparable phenomenon, we presented patient 063-007 with simultaneously appearing white and black letters on a gray background. The patient reported that the two letters became visible simultaneously. If the appearance of the white or black letter in the pair was delayed by 0.5 sec, the patient correctly identified the sequence of their appearance. The other two patients were not tested in this manner.
After prolonged dark adaptation (i.e., >30-45 min), all three patients could perceive spots of light only if the spots were ≈100-1,000 times (2-3 log units) more intense than what can be perceived by normal controls. This 2- to 3-log-unit threshold elevation was close to what would be expected for an individual functioning with the cone system alone. An evaluation of the time course of dark adaptation in a region of the retina 7° below fixation in patient 063-007 did not reveal a rod-cone break, suggesting that the rod photoreceptor mechanism provided little or no additional light sensitivity beyond that of the cone mechanism. However, evaluation of patients 274-011 (18) and 063-007 with a Tübinger perimeter showed that at positions in the visual field 10-45° nasally or temporally from fixation, rods mediated the threshold for a short-wavelength stimulus.
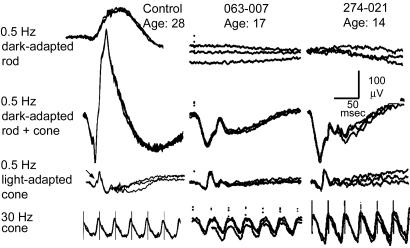
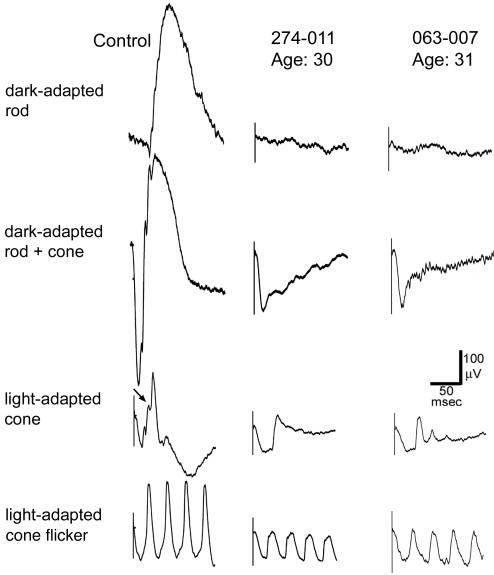
ERG responses to single, brief (10 μsec) dim light flashes that would normally selectively stimulate rod photoreceptors were not detectable (i.e., amplitudes <10 μV) (Figs. 2 and 3). Dark-adapted responses recorded at MEEI to single brief bright flashes that would normally stimulate both rods and cones showed a-waves that were normal in amplitude and timing (patients 063-007 and 274-021; Fig. 2). With brighter single flashes recorded at UIC (patients 274-011 and 063-007; Fig. 4), the rod-plus-cone a-waves were reduced in amplitude (176 μV; normal range, 282-537 μV; mean, 417 μV) and had delayed implicit times. With either flash intensity, the b-waves were substantially reduced in amplitude, resulting in the ERGs having electronegative waveforms. In the light-adapted cone response to a single light flash, oscillatory potentials that are normally present on the upward slope of the b-wave were absent (Figs. 2 and 3); the lack of these oscillatory potentials is typical of patients with the Schubert-Bornschein type of stationary night blindness (39). Single-flash cone responses recorded at UIC had flattened a-waves (Fig. 3). Responses to flickering light that isolated cone responses were normal in amplitude and timing (Fig. 2) or were moderately subnormal in amplitude with slightly delayed timing (Fig. 3).
Fig. 2.
ERGs of a normal control and patients 063-007 at age 17 and 274-021 at age 14 recorded at the Berman-Gund Laboratory at MEEI. The top row shows the dark-adapted rod responses to 0.5-Hz blue light flashes; the second row shows the dark-adapted rod-plus-cone responses to 0.5-Hz bright white light flashes; the third row shows the light-adapted cone responses to 0.5-Hz flashes; and the bottom row shows the cone responses to 30-Hz white light without a background light. In each tracing, two or three sweeps are super-imposed to illustrate reproducibility. In the 0.5-Hz cone response in the normal control (third tracing from the top), an oblique arrow points to an oscillation on the upward slope of the b-wave that is not observed in the patients. (Scale bars: 50 msec horizontally and 100 μV vertically for all tracings.)
Fig. 3.
ERGs of a normal control (Left) and patients 274-011 at age 30 (Center) and 063-007 at age 31 (Right) recorded at the UIC. Left shows normal ERG recordings for comparison. The types of recordings from top to bottom are the same as those in Fig. 2, except that the light stimuli are brighter (see Materials and Methods), the flash frequencies are greater (see labels at left), and the 32-Hz cone ERGs (bottom row) were recorded with a background light. In the light-adapted cone response in the normal control (third tracing from the top), an oblique arrow points to one of the two oscillations on the upward slope of the b-wave that is not observed in the patients. (Scale bars: 50 msec horizontally and 100 μV vertically for all tracings.)
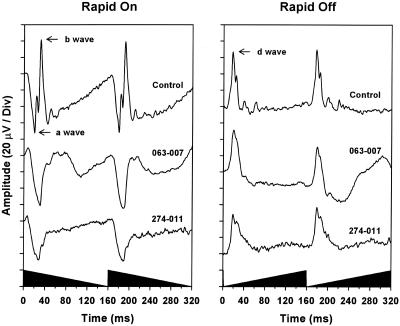
Fig. 4.
ERGs in response to sawtooth light stimuli. ERGs of a normal control (top traces) and patients 063-007 (middle traces) and 274-011 (bottom traces) in response to rapid-on (Left) and rapid-off (Right) sawtooth stimuli at a frequency of 6.25 Hz. The a-, b-, and d-waves of the control subject are indicated by arrows. The shaded region on the x axis illustrates the stimulus waveform.
Patients 274-011 and 063-007 were reevaluated ≈8 and 13 years after their initial visits, respectively. Neither reported a worsening of the symptom of night blindness over the time interval. Patient 274-011 developed sparse midperipheral bone spicule-shaped pigment clumps primarily in the nasal retina. There was also a defect in his temporal visual field that was not apparent on his initial examination. His ERGs showed 16% and 22% reductions, respectively, in the photopic single-flash and 32-Hz flicker responses compared with his initial visit; these decreases are within the range of intervisit variation observed with this technique. Patient 063-007 was found at his second visit to have improved visual acuity (20/40 vs. 20/60; Table 1). His ERG amplitudes (Fig. 3) were less than those obtained at age 17, which might be explained, at least in part, by the difference in recording procedures used for the measurements.
ERGs to elicit cone ON and OFF responses were recorded in two patients (274-011 and 063-007). Responses to rapid-on sawtooth stimuli (i.e., each “sawtooth” cycle had an abrupt onset of maximum light intensity followed by a gradual, linear reduction down to the constant background light at the end of the cycle) showed prominent and normal a-waves but substantially reduced b-waves in the ON responses, with the b-wave being more severely reduced in patient 274-011 than in patient 063-007 (Fig. 4). ERG recordings to the complementary rapid-off sawtooth stimuli showed nearly normal OFF responses with clearly evident d-waves (Fig. 4).
Discussion
The diagnosis of Schubert-Bornschein stationary night blindness (40) is applied to night-blind individuals who have dark-adapted, brief-flash ERGs with normal to near-normal a-waves but severely reduced b-waves leading to negative ERG waveforms (39). The majority of such patients are males, and the inheritance is often X-linked. The disease is subdivided into two categories, termed “incomplete” and “complete” (41), that respectively feature a modest or minimal residual rod ERG b-wave that can be detected with suitable techniques. The incomplete and complete X-linked forms are caused by mutations in the CACNA1F and NYX genes, respectively (25, 42, 43). Our patients have a form of night blindness with an electronegative ERG that is likely caused by recessive mutations in the GRM6 gene on chromosome 5q35 (17). It is likely that one or more other genes also cause forms of autosomal recessive night blindness, because we found GRM6 mutations in only 1 of 10 female patients who, because of their gender, are unlikely to express X-linked mutations. Mice with recessive loss-of-function mutations in the homologous grm6 gene have comparable ERGs and thus serve as an animal model that preceded the recognition of this newly defined human disease (15).
At his most recent visit at age 37, patient 274-011 exhibited previously unobserved visual field defects and retinal pigment deposits seen by funduscopy, both of which suggest a retinal degenerative change. These signs of retinal degeneration might possibly be related to his additionally being a carrier of a mutation in an as-yet-unidentified retinitis pigmentosa gene (his mother has retinitis pigmentosa as described above), and thus his findings might not necessarily portend a degenerative disease associated with GRM6 mutations alone. ERG amplitudes of patient 063-007 were slightly lower at age 31 than age 17 in recordings performed at MEEI (data not shown). No follow-up is yet available for patient 274-021, who is still a teenager. In short, the small number of patients identified with GRM6 mutations and the limited follow-up information available makes it difficult to predict the long-term course of their retinal disease.
The mGluR6 receptors of ON bipolar cells can be blocked pharmacologically by 2-amino-4-phosphonobutyrate (APB) (44). Studies of the visual function of monkeys after the intraocular administration of APB indicated that their dark-adapted visual thresholds are elevated 1.5-2 log units (38); this result would correspond to the symptom of night blindness observed in our patients with GRM6 mutations. APB produced only mild defects in monkeys' visual acuity, color vision, and stereopsis (38). The most noteworthy abnormality was an ≈250-msec delay in monkeys' conditioned responses to visual stimuli that involved light increments such as the sudden appearance of a bright spot of light against a background. There was no delay in responses to light decrements such as dark spots appearing against the same background (38). These findings bring to mind a previous study of our patient 274-011 that found a much smaller delay (≈20 msec) in responses of the occipital cortex to light increments, but not to light decrements, as measured by visual evoked responses (18). However, upon presenting a simultaneously appearing pair of white and black letters to another of our patients (063-007), there was no relative delay in the perception of either the white letter (i.e., a luminance increment) or the black letter (a luminance decrement). This apparent discrepancy with one of the results from tests of APB-treated monkeys (38) highlights the difficulty in interpreting those results because APB is now known to affect many other retinal cell types besides ON bipolars [see the discussion by Wong et al. (45) for a concise tabulation of relevant works].
The symptom of night blindness cannot be entirely explained by the defect in signal transmission between rods and their ON bipolar cells, because rods have alternative electrical connections to the inner retina that bypass the ON bipolar cells (46). In the area centralis of the cat retina, anatomic studies have shown that each cone has electrical gap junctions with ≈48 neighboring rods (47). Rod signals can be transmitted to cones through these gap junctions and thence to cone bipolar cells and ganglion cells (48-50). This pathway also is active in primates, but it is effective at transmitting rod signals only at mesopic intensities (51). This characteristic may account for the observation, based on studies of two of our patients (274-011 and 063-007) with chromatic stimuli, that rods mediated the dark-adapted threshold for a short-wavelength test stimulus; the stimulus intensity at threshold was in the mesopic range.
Besides the well established neural pathway from rods through cones to the inner retina, there may be yet other mechanisms by which rods can signal the inner retina that bypass rod ON bipolar cells and that account for the residual rod-mediated sensitivity in the GRM6 patients. Indirect evidence for an additional pathway comes from transgenic mice without cones (52). These mice have rod-generated signals in ON and OFF ganglion cells even after pharmacologic blocking of mGluR6 receptors of rod ON bipolar cells and blocking the connections between AII amacrine cells to cone ON and OFF bipolar cells (52). A separate anatomical study found that in the rabbit retina some OFF bipolar cells with normal dendrites to cones also extend some of their dendrites to rods and that these dendrites might be functional because they had the glutamate receptor GluR2 (53).
The limited degradation of visual function in response to the loss of the mGluR6-mediated signaling to ON bipolar cells exemplifies the resiliency of the architecture and signal processing of the retina. The resiliency could be partly explained if there are other glutamate receptors on cone ON bipolar cells besides mGluR6. In fish, ON bipolar cells respond to glutamate from cones mainly through excitatory amino acid transporters, with mGluR6 receptors playing an apparently minor role (45, 54). The ON responses to sawtooth ERGs in our patients are severely reduced (Fig. 4), but they still have small residual b-waves; perhaps these b-waves are mediated by alternative signaling pathways connecting cones and ON bipolar cells analogous to those in fish.
Some of the clinical findings in our patients, including the normal to moderately subnormal visual acuity, the severely reduced ERG b-waves in response to brief flashes, and the defect in ERG ON responses, parallel those found in patients with the complete form of X-linked night blindness caused by NYX mutations (41, 55, 56). However, two of our patients with GRM6 mutations had greater reductions of a-wave amplitudes than are typically seen in the complete form of X-linked night blindness (18); further investigations of additional cases are necessary to determine whether this finding would clinically distinguish patients with GRM6 vs. NYX mutations. Like the GRM6 patients, the NYX patients have residual rod responses that are possibly mediated through the gap junctions with cones (57). The similarities in the GRM6 and NYX phenotypes support the speculation that the NYX gene product, a membrane-bound extracellular protein whose exact function is currently obscure (58), is involved in ON bipolar cell signaling. A distinctive electrical defect is found in patients with the incomplete form of X-linked stationary night blindness due to mutations in the CACNA1F gene; their ERGs suggest abnormalities in both the ON and OFF bipolar pathways (59). Further clinical evaluations of patients with defects in GRM6, NYX, CACNA1F, and yet-to-be-identified genes encoding proteins important in inner retinal neural pathways should help to elucidate the signal processing of the human retina.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants EY08683, EY00169, EY14104, and EY08301, and grants from the Foundation Fighting Blindness (Owings Mills, MD).
Author contributions: T.P.D., E.L.B., G.A.F., M.A.S., and K.R.A. designed research; T.P.D., T.L.M., E.L.B., G.A.F., M.A.S., K.R.A., D.J.D., and A.S.R. performed research; T.P.D., T.L.M., E.L.B., G.A.F., M.A.S., and K.R.A. analyzed data; and T.P.D., T.L.M., E.L.B., G.A.F., M.A.S., and K.R.A. wrote the paper.
Abbreviations: APB, 2-amino-4-phosphonobutyrate; ERG, electroretinogram; MEEI, Massachusetts Eye and Ear Infirmary; UIC, University of Illinois at Chicago.
References
- 1.Wässle, H. & Boycott, B. B. (1991) Physiol. Rev. 71, 447-480. [DOI] [PubMed] [Google Scholar]
- 2.Sieving, P. A. (1993) Trans. Am. Ophthalmol. Soc. 91, 701-773. [PMC free article] [PubMed] [Google Scholar]
- 3.Penn, R. D. & Hagins, W. A. (1969) Nature 223, 201-205. [DOI] [PubMed] [Google Scholar]
- 4.Stockton, R. A. & Slaughter, M. M. (1989) J. Gen. Physiol. 93, 101-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush, R. A. & Sieving, P. A. (1994) Invest. Ophthalmol. Visual Sci. 35, 635-645. [PubMed] [Google Scholar]
- 6.Kawasaki, K., Tsuchida, Y. & Jacobson, J. H. (1971) Am. J. Ophthalmol. 72, 367-365. [DOI] [PubMed] [Google Scholar]
- 7.Sieving, P. A., Murayama, K. & Naarendorp, F. (1994) Visual Neurosci. 11, 519-532. [DOI] [PubMed] [Google Scholar]
- 8.Howarth, C. I. (1961) J. Optical Soc. Am. 51, 345-352. [DOI] [PubMed] [Google Scholar]
- 9.Granit, R. (1947) Sensory Mechanisms of the Retina (Oxford Univ. Press, London).
- 10.Brown, K. T. (1968) Vision Res. 8, 633-677. [DOI] [PubMed] [Google Scholar]
- 11.Dowling, J. E. & Ripps, H. (1973) Nature 242, 101-103. [DOI] [PubMed] [Google Scholar]
- 12.Ripps, H. (1982) Invest. Ophthalmol. Visual Sci. 23, 588-609. [PubMed] [Google Scholar]
- 13.Slaughter, M. M. & Miller, R. F. (1985) J. Neurosci. 5, 224-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakajima, Y., Iwakabe, H., Akazawa, C., Nawa, H., Shigemoto, R., Mizuno, N. & Nakanishi, S. (1993) J. Biol. Chem. 268, 11868-11873. [PubMed] [Google Scholar]
- 15.Masu, M., Iwakabe, H., Tagawa, Y., Miyoshi, T., Yamashita, M., Fukuda, Y., Sasaki, H., Hiroi, K., Nakamura, Y., Shigemoto, R., et al. (1995) Cell 80, 757-765. [DOI] [PubMed] [Google Scholar]
- 16.Iwakabe, H., Katsuura, G., Ishibashi, C. & Nakanishi, S. (1997) Neuropharmacology 36, 135-143. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto, T., Inazawa, J., Okamoto, N., Tagawa, Y., Bessho, Y., Honda, Y. & Nakanishi, S. (1997) Eur. J. Neurosci. 9, 1226-1235. [DOI] [PubMed] [Google Scholar]
- 18.Barnes, C. S., Alexander, K. R. & Fishman, G. A. (2002) Ophthalmology 109, 575-583. [DOI] [PubMed] [Google Scholar]
- 19.Berson, E. L., Rosner, B., Sandberg, M. A. & Dryja, T. P. (1991) Arch. Ophthalmol. 109, 92-101. [DOI] [PubMed] [Google Scholar]
- 20.Peachey, N. S., Fishman, G. A., Derlacki, D. J. & Alexander, K. R. (1988) Ophthalmology 95, 677-685. [DOI] [PubMed] [Google Scholar]
- 21.Sandberg, M. A., Sullivan, P. L. & Berson, E. L. (1981) Invest. Ophthalmol. Visual Sci. 21, 765-769. [PubMed] [Google Scholar]
- 22.Andréasson, S. O. L., Sandberg, M. A. & Berson, E. L. (1988) Am. J. Ophthalmol. 105, 500-503. [DOI] [PubMed] [Google Scholar]
- 23.Birch, D. G. & Sandberg, M. A. (1997) Doc. Ophthalmol. 92, 269-280. [DOI] [PubMed] [Google Scholar]
- 24.Pelli, D. G., Robson, J. G. & Wilkins, A. J. (1988) Clin. Vision Sci. 2, 187-199. [Google Scholar]
- 25.Bech-Hansen, N. T., Naylor, M. J., Maybaum, T. A., Sparkes, R. L., Koop, B., Birch, D. G., Bergen, A. A. B., Prinsen, C. F. M., Polomeno, R. C., Gal, A., et al. (2000) Nat. Genet. 26, 319-323. [DOI] [PubMed] [Google Scholar]
- 26.Pusch, C. M., Zeitz, C., Brandau, O., Pesch, K., Achatz, H., Feil, S., Scharfe, C., Maurer, J., Jacobi, F. K., Pinckers, A., et al. (2000) Nat. Genet. 26, 324-327. [DOI] [PubMed] [Google Scholar]
- 27.Dryja, T. P., Berson, E. L., Rao, V. R. & Oprian, D. D. (1993) Nat. Genet. 4, 280-283. [DOI] [PubMed] [Google Scholar]
- 28.Dryja, T. P., Hahn, L. B., Reboul, T. & Arnaud, B. (1996) Nat. Genet. 13, 358-360. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto, S., Sippel, K. C., Berson, E. L. & Dryja, T. P. (1997) Nat. Genet. 15, 175-178. [DOI] [PubMed] [Google Scholar]
- 30.Laurie, D. J., Schoeffter, P., Widerhold, K. H. & Sommer, B. (1997) Neuropharmacology 36, 145-152. [DOI] [PubMed] [Google Scholar]
- 31.Pin, J. P. & Duvoisin, R. (2004) Neuropharmacology 34, 1-26. [DOI] [PubMed] [Google Scholar]
- 32.Sharon, D., Yamamoto, H., McGee, T. L., Rabe, V., Szerencsei, R. T., Winkfein, R. J., Prinsen, C. F. M., Barnes, C. S., Andreasson, S., Fishman, G. A., et al. (2002) Invest. Ophthalmol. Visual Sci. 43, 1971-1979. [PubMed] [Google Scholar]
- 33.Kunishima, N., Shimada, Y., Tsuji, Y., Sato, T., Yamamoto, M., Kumasaka, T., Nakanishi, S., Jingami, H. & Morikawa, K. (2000) Nature 407, 971-977. [DOI] [PubMed] [Google Scholar]
- 34.Jingami, H., Nakanishi, S. & Morikawa, K. (2003) Curr. Opin. Neurobiol. 13, 271-278. [DOI] [PubMed] [Google Scholar]
- 35.Chou, Y.-H. W., Pollak, M. R., Brandi, M. L., Toss, G., Arnqvist, H., Atkinson, A. B., Papapoulos, S. E., Marx, S., Brown, E. M., Seidman, J. G., et al. (1995) Am. J. Hum. Genet. 56, 1075-1079. [PMC free article] [PubMed] [Google Scholar]
- 36.Ho, C., Conner, D. A., Pollak, M. R., Ladd, D. J., Kifor, O., Warren, H. B., Brown, E. M., Seidman, J. G. & Seidman, C. E. (1995) Nature 11, 389-394. [DOI] [PubMed] [Google Scholar]
- 37.Mäntyjärvi, M. & Laitinen, T. (2001) J. Cataract Refractive Surg. 27, 261-266. [DOI] [PubMed] [Google Scholar]
- 38.Schiller, P. H., Sandell, J. H. & Maunsell, J. H. R. (1986) Nature 322, 824-825. [DOI] [PubMed] [Google Scholar]
- 39.Hill, D. A., Arbel, K. F. & Berson, E. L. (1974) Am. J. Ophthalmol. 78, 127-136. [DOI] [PubMed] [Google Scholar]
- 40.Schubert, G. & Bornschein, H. (1952) Ophthalmologica 123, 396-413. [DOI] [PubMed] [Google Scholar]
- 41.Miyake, Y., Yagasaki, K., Horiguchi, M., Kawase, Y. & Kanda, T. (1986) Arch. Ophthalmol. 104, 1013-1020. [DOI] [PubMed] [Google Scholar]
- 42.Strom, T. M., Nyakatura, G., Apfelstedt-Sylla, E., Hellebrand, H., Lorenz, B., Weber, B. H. F., Wutz, K., Gutwillinger, N., Rüther, K., Drescher, B., et al. (1998) Nat. Genet. 19, 260-263. [DOI] [PubMed] [Google Scholar]
- 43.Bech-Hansen, N. T., Naylor, M. J., Maybaum, T. A., Pearce, W. G., Koop, B., Fishman, G. A., Mets, M., Musarella, M. A. & Boycott, K. M. (1998) Nat. Genet. 19, 264-267. [DOI] [PubMed] [Google Scholar]
- 44.Slaughter, M. M. & Miller, R. F. (1981) Science 211, 182-185. [DOI] [PubMed] [Google Scholar]
- 45.Wong, K. Y., Adolph, A. R. & Dowling, J. E. (2005) J. Neurophys. 93, 84-93. [DOI] [PubMed] [Google Scholar]
- 46.Raviola, E. & Gilula, N. B. (1973) Proc. Natl. Acad. Sci. USA 70, 1677-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith, R. G., Freed, M. A. & Sterling, P. (1986) J. Neurosci. 6, 3505-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson, R. (1977) J. Comp. Neurol. 172, 109-135. [DOI] [PubMed] [Google Scholar]
- 49.Nelson, R., Kolb, H. & Freed, M. A. (1993) J. Comp. Neurol. 329, 68-84. [DOI] [PubMed] [Google Scholar]
- 50.DeVries, S. H. & Baylor, D. A. (1995) Proc. Natl. Acad. Sci. USA 92, 10658-10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneeweis, D. M. & Schnapf, J. L. (1995) Science 268, 1053-1056. [DOI] [PubMed] [Google Scholar]
- 52.Soucy, E., Wang, Y. S., Nirenberg, S., Nathans, J. & Meister, M. (1998) Neuron 21, 481-493. [DOI] [PubMed] [Google Scholar]
- 53.Li, W., Keung, J. W. & Massey, S. C. (2004) J. Comp. Neurol. 474, 1-12. [DOI] [PubMed] [Google Scholar]
- 54.Wong, K. Y., Cohen, E. D. & Dowling, J. E. (2005) J. Neurophys. 93, 94-107. [DOI] [PubMed] [Google Scholar]
- 55.Miyake, Y., Yagasaki, K., Horiguchi, M. & Kawase, Y. (1987) Jpn. J. Ophthalmol. 31, 81-87. [PubMed] [Google Scholar]
- 56.Khan, N. W., Kondo, M., Hiriyanna, K. T., Jamison, J. A., Bush, R. A. & Sieving, P. A. (2005) J. Neurophys. 93, 481-492. [DOI] [PubMed] [Google Scholar]
- 57.Scholl, H. P. N., Langrová, H., Pusch, C. M., Wissinger, B., Zrenner, E. & Apfelstedt-Sylla, E. (2001) Invest. Ophthalmol. Visual Sci. 42, 2728-2736. [PubMed] [Google Scholar]
- 58.Zeitz, C., Scherthan, H., Freier, S., Feil, S., Suckow, V., Schweiger, S. & Berger, W. (2003) Invest. Ophthalmol. Visual Sci. 44, 4184-4191. [DOI] [PubMed] [Google Scholar]
- 59.Miyake, Y. (2002) Nippon Ganka Gakkai Zasshi 106, 737-755. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.