Abstract
The transplantation of human pancreatic islets is a therapeutic possibility for a subset of type 1 diabetic patients who experience severe hypoglycemia. Pre- and post-transplantation loss in islet viability and function, however, is a major efficacy-limiting impediment. To investigate the effects of inflammation and hypoxia, the main obstacles hampering the survival and function of isolated, cultured, and transplanted islets, we conducted a comprehensive metabolomics evaluation of human islets in parallel with dynamic glucose-stimulated insulin release (GSIR) perifusion studies for functional evaluation. Metabolomics profiling of media and cell samples identified a total of 241 and 361 biochemicals, respectively. Metabolites that were altered in highly significant manner in both included, for example, kynurenine, kynurenate, citrulline, and mannitol/sorbitol under inflammation (all elevated) plus lactate (elevated) and N-formylmethionine (depressed) for hypoxia. Dynamic GSIR experiments, which capture both first- and second-phase insulin release, found severely depressed insulin-secretion under hypoxia, whereas elevated baseline and stimulated insulin-secretion was measured for islet exposed to the inflammatory cytokine cocktail (IL-1β, IFN-γ, and TNF-α). Because of the uniquely large changes observed in kynurenine and kynurenate, they might serve as potential biomarkers of islet inflammation, and IDO on the corresponding pathway could be a worthwhile therapeutic target to dampen inflammatory effects.
Keywords: cytokines, human islets, hyperglycemia, hypoxia, insulin secretion, metabolomics, perifusion
Graphical abstract
Introduction
The transplantation of human islet cells is the only current cell-based therapy that has been proven to provide insulin independence in patients with type 1 diabetes (T1D). With long-term safety and efficacy demonstrated in T1D patients complicated by severe hypoglycemia, this therapeutic approach is likely to become an approved clinical treatment in the US1–4. While this is one of the safest and least invasive transplant procedures and with considerable progress made in the cell processing procedure5 and resulting clinical outcomes4, efficacy is still limited due to loss of islet viability and function during pre- and post-transplantation periods6–8. Throughout this process, including the cold ischemia period in the cadaveric donor, the islet isolation procedure itself, the culture before transplantation, and the period immediately after transplantation, islets are exposed to various unfavorable environmental conditions, whereby hypoxia and inflammation have been identified as the main ones.
Islets, which are highly sensitive to low oxygen conditions, are subjected to hypoxia even before isolation due to organ ischemia and loss of physiological oxygen delivery, and prolonged ischemic times increase the corresponding damage9, 10. Inflammatory conditions can also be initiated at this early stage, as islets are isolated from cadaveric donors and are exposed to the corresponding unfavorable conditions. The enzymatic and mechanical stresses employed during the isolation process further exacerbate these two conditions11. The isolation and purification process of human islets is a five to seven hour multi-step procedure needed to extract the islets that represent only about 1–2% of the total pancreatic tissue volume. The islet preparation is then kept in culture for 24 to 72 hours to allow for the required quality controls and for the initiation of immunosuppressive therapy in the recipient. Following transplantation, islets are exposed to the elevated blood glucose levels of the recipients, which is known to be detrimental (glucotoxicity)12, 13. Furthermore, immediately post-transplantation, there is a significant loss of islet mass and function due to the inflammatory response mounted by the recipient14 as well as the hypoxia resulting from the oxygen diffusion limitations15 due to insufficient vascularization16 and lack of active nutrient transport. In avascular islets, hypoxia resulting from oxygen diffusion limitations is a major factor limiting viability and functionality. For example, when isolated islets are cultured under normoxic conditions (atmospheric oxygen, 21% O2), larger islets tend to show central necrosis, which becomes more severe after exposure to hypoxic culture conditions17–20. Until revascularization, which occurs only after several days, transplanted islets have to rely on passive nutrient diffusion and have to survive in a tissue environment of only about 5% oxygen21 (typical tissue oxygen levels; the average oxygen concentration in arterial and venous blood being 130 and 54 µM22 corresponding to ~12% and 5%) – a significant hurdle to overcome.
In light of these stress factors, we sought to investigate the effects of inflammation and hypoxia under basal and hyperglycemic conditions on isolated human islets. We conducted a comprehensive metabolomic evaluation of changes in their biochemical composition, in parallel with dynamic glucose-stimulated insulin release (GSIR) perifusion studies for functional evaluation. Our goal was to understand how an environment of inflammation and/or hypoxia during ex vivo culture of isolated human pancreatic islets affects global metabolism and response to glucose. A further goal was to attempt to identify biomarkers of islet health/potency that could be predictive for long-term survival in vitro and in vivo, by comparing islets under culture conditions that mimic detrimental conditions (e.g., hypoxia and inflammation). Limited previous studies on metabolomics of diabetes onset, pancreatic islets, and β-cells or cell lines have been conducted23–26, as recently summarized27. An important limitation of these studies has been the need for several million cells for LC-MS type analyses (e.g., >3 million)27, meaning a need of several thousand islets per conditions (as an average islet contains around 1,000–2,000 cells). Improved analytics, however, has overcome this obstacle. The method employed herein requires ~100,000 cells (i.e., ~100 islets) and 0.5 mL media per sample. This approach not only decreases the burden of procurement of limited human islets, it permits parallel experiments of various experimental conditions using a single human donor, while also ensuring sufficient islets for functional GSIR studies.
Materials and Methods
Human Pancreatic Islets Isolation and Culture
Human islet samples used for the present comparison were from isolations performed at the Human Islet Cell Processing Facility at the Diabetes Research Institute (University of Miami, Miller School of Medicine, Miami, FL, USA). The islet isolation protocol, as part of the Clinical Pancreatic Islet Transplantation Study, was approved by the Institutional Review Board (IRB) of the University of Miami and the FDA. Human pancreases were procured from deceased multi-organ donors for whom consent was obtained by accredited Organ Procurement Organizations (OPOs) from the donor’s families or next of kin. Islets were isolated by using a modification of the automated method28, as described before28–30. This study was conducted according to the principles expressed in the Declaration of Helsinki.
Islets isolated from ten cadaveric donors were used for the study. The donor characteristics of ten human pancreases used for this study are shown in Table S1 of the Supporting Information. Islets from these human pancreases were isolated at the Current Good Manufacturing Practice (cGMP) Human Islet Cell Processing Facility of the Diabetes Research Institute at the Miller School of Medicine, University of Miami. Islet yield and purity were determined by dithizone staining, and islets were counted and scored using a standard algorithm for the calculation of 150 µm diameter islet equivalent (IEQ) number31, 32. Islets were cultured in CMRL-1066 medium (Gibco) supplemented with 2% human albumin, at 22°C in 5% CO2 humidified incubator overnight prior experimental conditions. For the present study, human islet samples from each of the 10 isolations were incubated for 24 hours under standard, hypoxic (3% O2)33, 34, or inflammatory conditions (cytokine cocktail)35, 36, followed by an additional 24 h under the same conditions with either basal (5.5 mM) or high (16 mM) glucose37, 38. Each islet sample was divided into six 1,000 IEQ aliquots that were cultured in 3 mL culture media in six-well plates. At the end of the 24 + 24 h culture, cell and media samples were collected, stored at −80°C, and sent to Metabolon (Durham, NC) for analysis. The cytokine cocktail used contained 50 U/mL (0.25 ng/mL) interleukin-1β (IL-1β), 1000 U/mL (21.5 ng/mL) tumor necrosis factor-α (TNF-α), and 1000 U/mL (50 ng/mL) interferon-γ (IFN-γ) (R&D Systems, Inc. Minneapolis, MN). From four isolations, additional samples cultured under similar conditions were kept for the next day and used for glucose stimulated insulin secretion studies. All islet cells were cultured in CMRL-based culture medium.
Dynamic Glucose-Stimulated Insulin Release (GSIR)
A subset of islets (n = 4; low glucose samples only) were also subjected to dynamic GSIR perifusion studies. GSIR perifusion experiments were performed as described before39 using a custom built apparatus that allows parallel perifusion in up to eight channels with programmable influx (PERI-04, Biorep, Inc., Miami, FL). Briefly, one hundred islets were handpicked and loaded in Perspex microcolumns, between two layers of acrylamide-based microbeads slurry (Bio-Gel P-4, Bio-Rad Laboratories, Hercules, CA). Perifusing buffer containing 125 mM NaCl, 5.9 mM KCl, 1.28 mM CaCl2, 1.2 mM MgCl2, 25 mM HEPES, 0.1% bovine serum albumin at 37°C with selected glucose (low = 3 mM; high = 11 mM) or KCl (25 mM) concentrations was circulated through the columns at a rate of 100 µL/min. After 45–60 min of washing with the low glucose solution for stabilization, islets were stimulated with the following sequence: 5 min of low glucose, 20 min of high glucose, 15 min of low glucose, 10 min of KCl, and 10 min of low glucose. Serial samples (100 µL) were collected every minute from the outflow tubing of the columns in an automatic fraction collector designed for a multi-well plate format. The sample container harboring the islets and the perifusion solutions were kept at 37°C in a built-in temperature controlled chamber. The perifusate in the collecting plate was kept at <4°C to preserve the integrity of the analytes. Insulin concentrations were determined with a commercially available ELISA kit (Mercodia Inc., Winston Salem, NC). Values obtained with the human kit are in mU/L; they were converted to µg/L using 1 µg/L = 23 mU/L per the guidelines of the manufacturer.
Metabolomic Analysis
Small molecules were extracted in a methanol solution containing process assessment standards as described previously40. The resulting clarified supernatant extract was divided into five aliquots, one for each of the four individual LC/MS analyses and one spare, briefly evaporated to remove the organic solvent and stored overnight under nitrogen before preparation for analysis. The global biochemical profiling analysis was comprised of four unique arms consisting of reverse phase chromatography positive ionization methods optimized for hydrophilic compounds (LC/MS Pos Polar) and hydrophobic compounds (LC/MS Pos Lipid), reverse phase chromatography with negative ionization conditions (LC/MS Neg), as well as a HILIC chromatography method coupled to negative (LC/MS Polar)41. All of the methods alternated between full scan MS and data-dependent MSn scans. The scan range varied slightly between methods but generally covered 70–1000 m/z.
Experimentally detected metabolites were identified by matching the ion chromatographic retention index, accurate mass, and mass spectral fragmentation signatures with a reference library consisting of over 3,200 entries created from authentic standard metabolites under the identical analytical procedure as the experimental samples42. In-house peak detection and integration software was used. The data output from the software was a list of m/z ratios, fragmentation spectra, retention indices, and area under the curve (AUC) values. All proposed identifications were then manually reviewed and hand curated by an analyst who approved or rejected each identification based on the criteria above42.
Statistical Analysis
Two types of statistical analyses were performed: (1) significance tests and (2) classification analysis. Standard statistical analyses were performed in ArrayStudio on log-transformed data. For analyses not standard in ArrayStudio, the programs R (http://cran.r-project.org/) or JMP were used. Following normalization to DNA for cells, log transformation and imputation of missing values, if any, with the minimum observed value for each compound, ANOVA contrasts with a repeated measures component were used as significance test to identify biochemicals that differed significantly (p < 0.05) between experimental groups. An estimate of the false discovery rate (q-value) was calculated to take into account the multiple comparisons that normally occur in metabolomic-based studies. Classification analyses used included principal components analysis (PCA), hierarchical clustering, and random forest. For the scaled intensity graphics, each biochemical in original scale is rescaled to set the median equal to 1. To visualize interaction networks and biological pathways of the significant metabolites we utilized Metabolon’s Metabolync plugin to Cytoscape (http://cytoscape.org), an open source software platform43.
Results
Metabolomics Data Overview
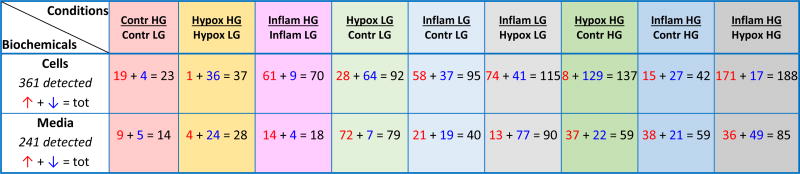
Human islets from ten different donors were cultured in parallel for 24 h under standard, hypoxic (3% O2), and inflammatory conditions (cytokine cocktail of IL-1β, IFN-γ, and TNF-α), followed by addition of either basal (5.5 mM) or high (16 mM) glucose to each of these for another 24 h (experimental schematic summarized in Supplementary Figure S1). Cell and media (supernatant) samples (10×3×2×2 = 120) were collected and subjected to a detailed metabolomics analysis, as described in the Methods section. Following data acquisition and curation, a total of 241 and 361 compounds of known identity (named biochemicals) were identified for media and cells, respectively. Analysis by two-way ANOVA with repeated measures identified biochemicals exhibiting significant interaction and main effects for experimental parameters of condition (hypoxia, inflammation) and the addition of high glucose. Table 1 provides a summary comparison of the biochemicals that exhibited statistically significant changes (p < 0.05) for these pairwise comparisons. For example, high glucose alone (16 vs. 5.5. mM for the second 24 h of the study) caused a significant change in 23 and 14 metabolite levels in the cells and media, respectively. With respect to the control samples under basal (5.5 mM glucose) culture conditions, hypoxia significantly altered 92 cellular and 79 media soluble compounds. Conversely, exposure to the inflammatory cocktail significantly altered 95 cellular and 40 media soluble compounds, when cultured under the same basal glucose levels. Compared to the control samples under high glucose conditions, the corresponding numbers are 137 & 42 cellular compounds and 59 & 59 media soluble compounds for hypoxia & inflammatory conditions, respectively (Table 1).
Table 1.
Summary of statistically significantly altered biochemicals (p < 0.05, ANOVA contrast) from the total of 361 and 241 named biochemicals detected in cells and media, respectively. For each pairwise comparison, the number of compounds with significantly different levels is indicated and the breakdown shows the numbers for those with
 and
and
 levels in red and blue, respectively.
levels in red and blue, respectively.
Notation: LG, low (basal) glucose; HG, high glucose.
Overall Data Segregation: Principal Component, Hierarchical Cluster, and Random Forest Analyses
Principal component analysis (PCA) is a mathematical procedure that allows differences across a large set of variables to be represented as a smaller set of ‘principal’ variables44. Transformation is done so that the first principal component (PC1) has the largest possible variance (i.e., accounts for as much variability as possible), and then each additional component has the highest remaining variance possible, while being orthogonal to all preceding components. By reducing dimensionality, this transformation allows one to visualize how individual samples within a group cluster. In this study, PCA was used to assess whether the treatment groups could be segregated based on differences in their overall metabolic signature. The first three principal components account for a good portion of the variability in the data (35.0 + 21.5 + 6.5 = 63.0% and 24.3 + 17.3 + 8.9 = 50.5% in cells and media, respectively), but there was relatively poor separation of control and treatment groups (Supplementary Figure S2). Nevertheless, the hypoxic samples did exhibit a modest shift along the component 2 and 3 axes (PC2, PC3), particularly for the cell samples.
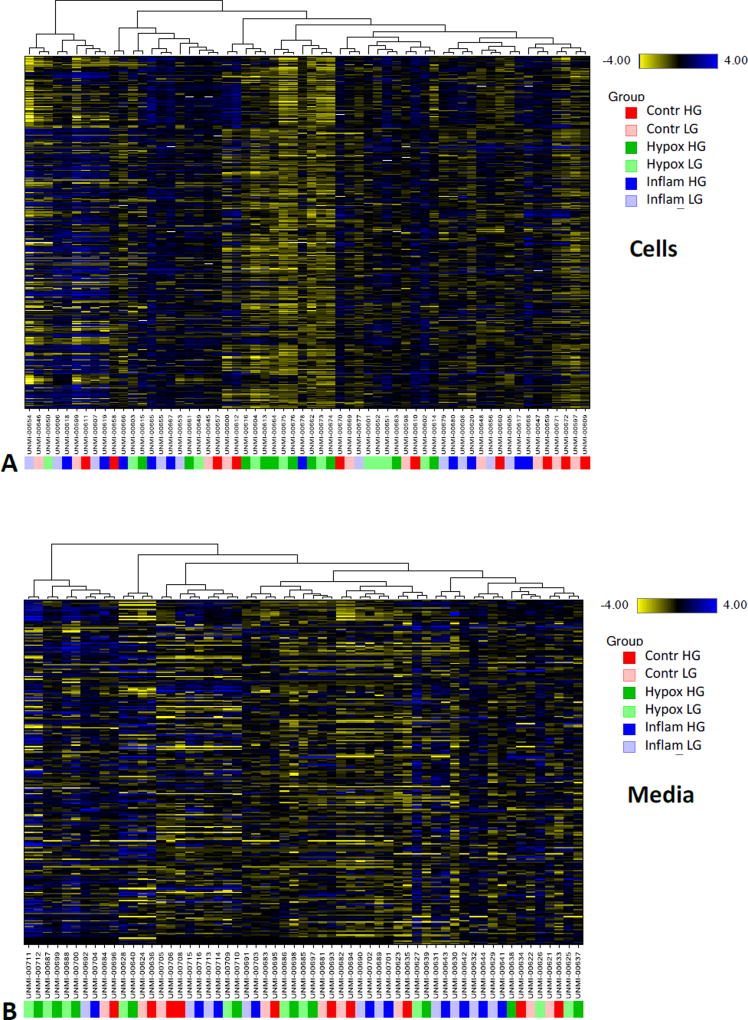
To provide a different perspective into potential group segregation, hierarchical cluster analysis (HCA) was also performed. Results revealed that both cellular and media groups segregated primarily by sample identity, rather than treatment conditions; indicating that individual differences in metabolic profiles were more powerful classifiers than treatment condition (Figure 1). Nevertheless, hypoxia exposed cell samples showed a tendency to cluster as highlighted by the accumulation of the corresponding green color-coded samples around the middle of the graph for cells in Figure 1. Collectively, these high-level views of the data set indicate that overall global metabolic profiles were similar between the study groups. Despite the relatively muted shift in global metabolic tissue profiles, there were several changes of individual biochemicals that provided insight into injury-associated tissue alterations as discussed below.
Figure 1.
Hierarchical clustering of the present metabolomics data in cell (A) and media samples (B) obtained from human islets (n = 10) cultured under different conditions as indicated (control, hypoxia, and inflammatory cytokines with low or high glucose). Within the clusters, blueish coloring represent elevated metabolite levels, while yellowish coloring represent decreased metabolite levels. Samples are color-coded as indicated by the figure key on the right side.
Random forest analysis (RFA), a statistical tool that utilizes a supervised classification technique based on an ensemble of decision trees45, was also performed to aid in the identification of such biomarkers that can allow separation among experimental groups. Despite the relatively poor group separation in PCA and HCA, RFA identified biochemicals that were able to classify the media samples with a surprising degree of accuracy (Supplementary Figure S3, left): the predictive accuracy was 83% for all samples (compared to a 16.7% random chance expected for a six-group comparison). In these samples, glucose and glycolysis-associated biochemicals (e.g., maltose, fructose, and succinate) were important differentiators, as were choline, phosphocholine (choline phosphate), cytidine, and uridine. RFA was less accurate in classifying cell samples with an overall predictive accuracy of only 35%, and, for the control and hypoxic groups, this analysis failed to generate results better than random chance. However, when RFA misclassified cell samples, it tended to identify correctly the treatment condition, but not the low or high glucose level within that treatment group. The predictive accuracy of RFA for cells exposed to inflammatory conditions was much better than random chance across the six-group comparison – indicating that inflammation produced a metabolic phenotype distinct from the control or hypoxic conditions.
Glucose Mediated Changes
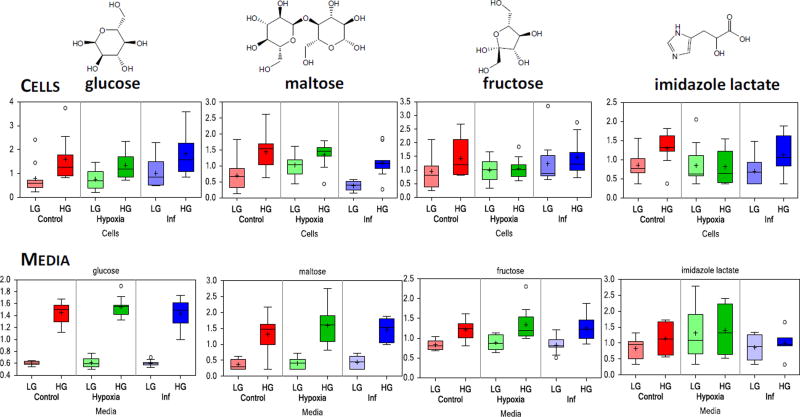
Comparison of the metabolomic profiles of the samples cultured in high glucose (HG, 16 mM) for the second 24 with those maintained at basal (LG, 5.5 mM) level found a number of biochemicals with significantly altered levels; however, this subset was a relatively small fraction of the overall metabolites identified (5–20%; Table 1). High glucose imparted a significant change in 23, 37, and 70 metabolite levels in cells and 14, 28, and 18 in media under control, hypoxic, and inflammatory conditions, respectively. As expected, measured glucose levels in high glucose (HG) media exhibited an approximate three-fold elevation when compared to low (LG) glucose samples (Figure 2, bottom left). Metabolites that showed significant changes in both cells and media include: maltose, fructose, and imidazole lactate (Figure 2). Exposure of islets to HG resulted in increased levels of maltose and maltotriose under all conditions (control, hypoxia, and inflammation). Since these markers report on glycogen metabolism, this suggests that some features of glucose processing by the islets after glucose stimulation, such as glycogen synthesis, remained intact despite islet stress. In addition, elevation of imidazole lactate, a metabolite involved in histidine degradation, under HG conditions was also observed. Metabolic sub-families that were most strongly affected by exposure to high glucose are summarized in Supplementary Figure S4, using metabolic pathway enrichment (PE) values for all pairwise comparisons (i.e., HG vs. LG under control, inflammatory cytokine, and hypoxic conditions; data for media samples shown).
Figure 2.
Box plot data illustrating some of the metabolites significantly altered in both cell and media samples by culture in high glucose conditions (16 vs. 5.5 mM for 24 h). Data (scaled intensity) are shown as median surrounded by a box indicating upper and lower quartiles and bars of minimum and maximum of distribution (mean indicated by a plus symbol, extreme values shown as circles), and are color-coded per condition (control in red, hypoxia in green, and inflammation in blue). LG and HG denote standard (low) and high glucose cultures, respectively during the second 24 h. For glucose-induced changes, compare the darker HG and lighter LG columns within each condition. Values are normalized in terms of raw area counts with each biochemical rescaled to set the median equal to 1.
Inflammation Mediated Changes
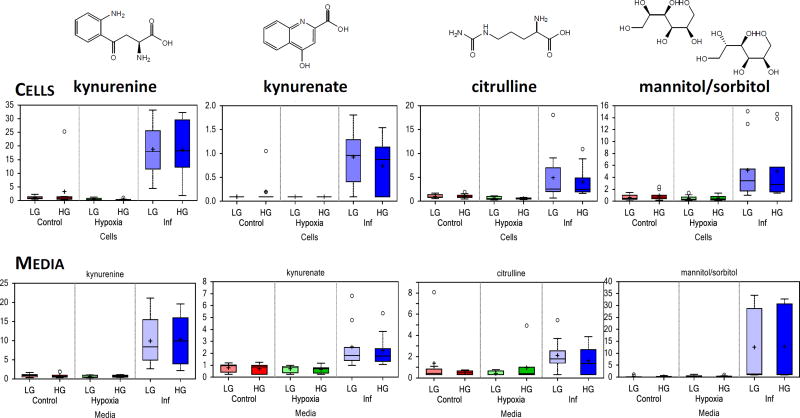
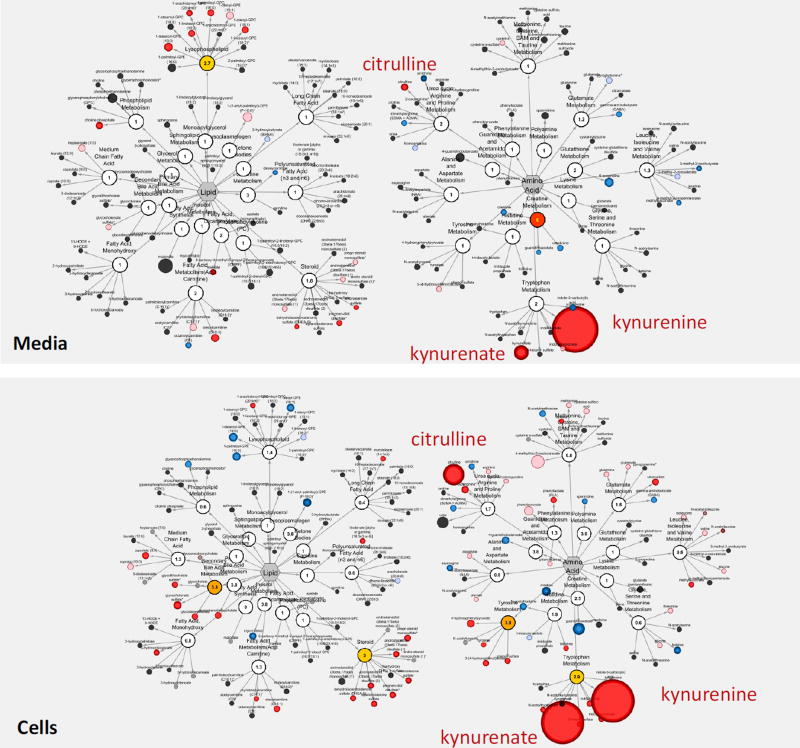
To investigate the effects of inflammatory stimuli on human islets, changes in their metabolic profiles due to culture in the presence of a cytokine cocktail (IL-β, TNF-α, and IFN-γ; 24 + 24 h) was examined. Compared to the corresponding controls, a total of 95 and 40 metabolites were found to be differentially expressed in cells and in media, respectively under low glucose, while 42 cellular and 59 media metabolites were altered under high glucose (Table 1). Several of these, such as kynurenine, kynurenate, citrulline, and mannitol/sorbitol, were consistently and significantly altered (Figure 3); exhibiting the largest fold-changes of the present study. Some of the main metabolic pathways affected are illustrated using the Metabolic Pathway Classification Network view of the metabolomic data for both media and cell samples in Figure 4. The metabolic sub-families that were most strongly affected are also summarized in Supplementary Figure S5 using metabolic PE values.
Figure 3.
Box plot data illustrating some of the metabolite significantly altered in both cell and media samples by culture in the presence of inflammatory cytokines (cocktail of IL-1β, IFN-γ, and TNF-α for 24 + 24 h). Notation is the same as in Figure 2. For inflammation-induced changes, compare blue vs. red columns (lighter and darker shades for changes under LG and HG conditions, respectively).
Figure 4.
Effect of inflammatory cytokines (IL-1β, IFN-γ, and TNF-α) in standard culture (5.5 mM) on human islet metabolism shown using MetaboLync Pathway Classification Network for both media (top) and cell (bottom) samples for the Lipid and Amino Acid super-families. Within each pathway, the size of the circle correlates with the magnitude of change, and the color indicates significant change vs. control (p < 0.05; red increased, blue decreased).
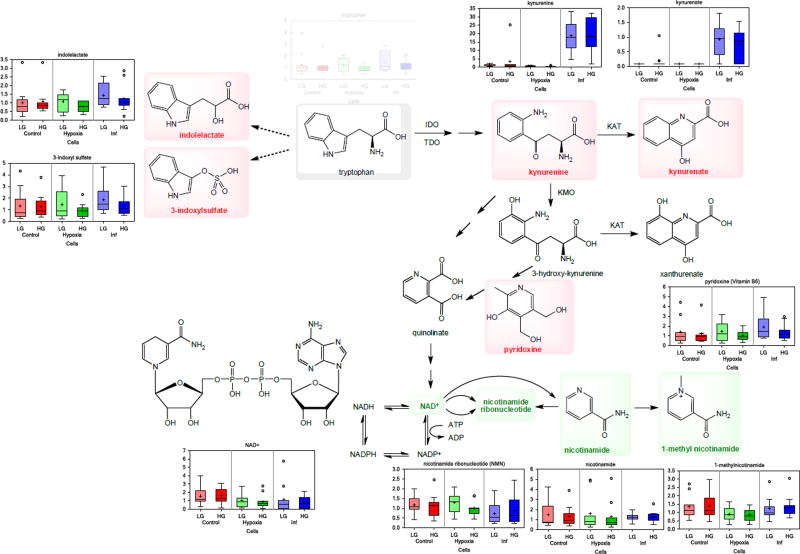
Culturing islets with these inflammatory cytokines led to the large accumulation of kynurenine and kynurenate in both cell and media samples (Figure 3, Figure 4). These metabolites are part of the tryptophan metabolic pathway and are downstream products of indoleamine-2,3-dioxygenase (IDO), whose expression is known to be induced by inflammatory cytokines including IFN-γ and TNF-α, including in human islets46. Notably, these changes occur with associated increases in the detected tryptophan intermediates 3-indoxyl sulfate and indolelactate, and the concomitant loss of several nicotinamide intermediates, including nicotinamide ribonucleotide (NMN), NAD+, and 1-methylnicotinamide in islet cells (Figure 5). Tryptophan levels, however, were not depleted in either the cell or the media samples. Largely parallel results were observed in media samples; hence, collectively, these results indicate that inflammatory conditions perturb tryptophan metabolism in islets to favor the indole and kynurenine axes, while reducing nicotinamide availability. Among other metabolites strongly affected, citrulline was also significantly increased under the inflammatory stimulus in both cells and media (Figure 3). Citrulline, a component of the urea cycle, is also produced during nitric oxide production by nitric oxide synthases, of which the inducible NOS (iNOS) isoform is potently induced by IL-147. Additionally, in the polyol pathway, mannitol/sorbitol also increased in both. Note that mannitol and sorbitol cannot be distinguished by the MS-based technique used here.
Figure 5.
Brief overview of the tryptophan metabolic pathway with box plots included showing metabolites detected here and significantly altered by culture with inflammatory cytokines (blue rectangles). Metabolites significantly increased are shown in bold red, those significantly decreased in bold green color; the notation of the box plots is the same as used before in Figure 2 or Figure 3. The large changes in kynurenine and kynurenate are associated with significant increases in tryptophan intermediates 3-indoxyl sulfate and indolelactate as well as with concomitant loss of several nicotinamide intermediates suggesting that inflammatory conditions perturb tryptophan metabolism to favor the indole and kynurenine axes.
Hypoxia Mediated Changes
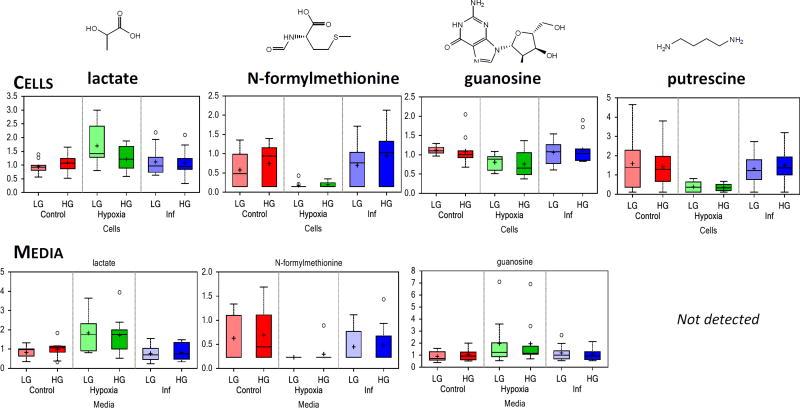
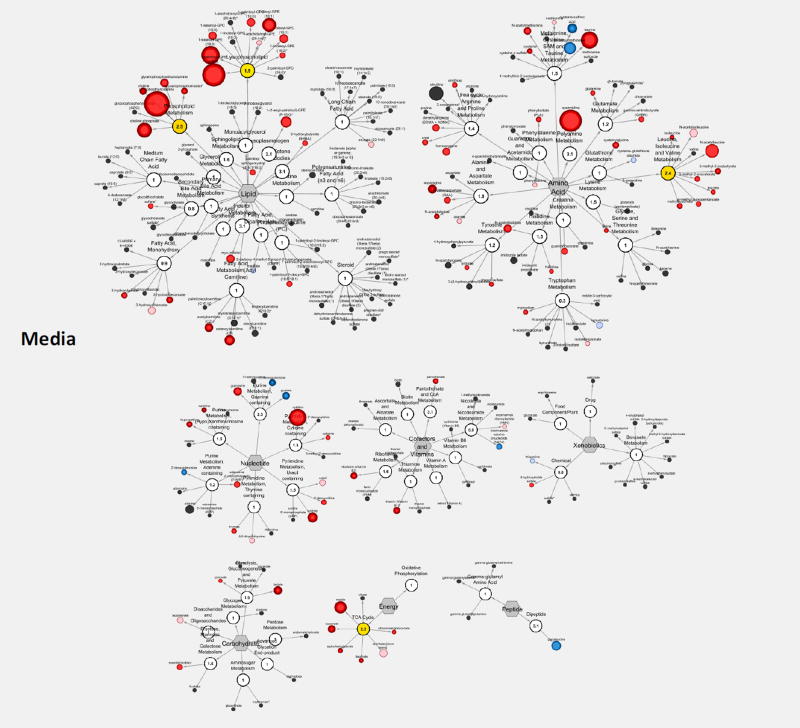
Relative to control conditions, hypoxia (3% O2) caused a considerable portion of the identified metabolites to be significantly differentially expressed: 92 and 79 in cells and media, respectively with low glucose, and 137 and 59 with high glucose (Table 1). Metabolites most significantly altered included lactate, N-formylmethionine, guanosine, and putrescine among others (Figure 6). Some of the affected metabolites and metabolic sub-families are shown in Figure 7, and those most strongly affected are summarized in Supplementary Figure S6 using metabolic PE values.
Figure 6.
Box plot data illustrating some of the metabolite significantly altered in both cell and media samples by culture under hypoxic conditions (3% O2 for 24 + 24 h). Notation is the same as in Figure 2. For hypoxia-induced changes, compare green vs. red columns (lighter and darker shades for changes under LG and HG conditions, respectively).
Figure 7.
Effect of hypoxia in standard culture (5.5 mM) on human islet metabolism shown using MetaboLync Pathway Visualization for media samples. Within each pathway, the size of the circle correlates with the magnitude of change, and the color indicates significant change vs. control (p < 0.05; red increased, blue decreased).
Lactate, a classic marker of hypoxia, spiked in both cells and media indicating that islets transitioned to anaerobic glycolysis to produce ATP, resulting in lactate production via the action of lactate dehydrogenase to re-oxidize NADH to NAD+. Note that while, β-cells are known to have low levels of lactate dehydrogenase to make glucose sensing more sensitive48, 49, here, we investigated whole islets. N-formylmethionine, a marker of mitochondrial protein turnover, was dramatically decreased, as were more general markers of protein turnover, i.e., the N-acetyl amino acids. Relative to control islets, monoacylglycerols and diacylglycerols (DAGs) were largely elevated in hypoxic islets (data not shown).
Effects on Islet Morphology and Function (GSIR)
While the hypoxic conditions used in this study severely affected islet structure, leading to fragmented islets with noticeable loss in insulin content and viability, culture with the present cytokine cocktail (IL-1β 50 U/mL, TNF-α 1000 U/mL, and IFN-γ 1000 U/mL for 48 h) had a relatively modest effect on islet morphology. This is illustrated in Supplementary Figure S7, for a representative set of islets stained with diphenylthiocarbazone (dithizone, DTZ), which stains islets red by chelating the zinc in the insulin granules of their β-cells.
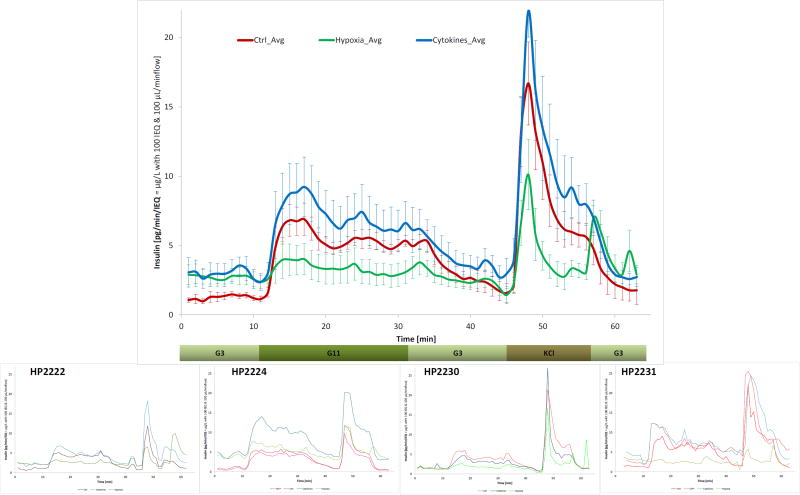
In parallel with the metabolomics studies, dynamic glucose-stimulated insulin release (GSIR) perifusion was also performed on a subset of islets (n = 4) to evaluate the effects of hypoxia and cytokines on the insulin secretion of human islets. With the improvement in equipment and analytical techniques, GSIR studies, which were introduced in the early 1970s50, can now provide robust quantitative characterization of insulin release kinetics, including both first- and second-phase response, under fully controllable experimental conditions of varying external concentrations of glucose and other segretagogues39, 51–54. GSIR is routinely used to assess islet quality and function55. In this study, perifusions were performed using an automated perifusion machine (PERI4-02, Biorep) that allows parallel assays for up to eight chambers and our standard protocol of low-high-low (3 → 11 → 3 mM) glucose39, 56. As shown in Figure 8, hypoxia severely depressed the ability of islets to secrete insulin in response to a HG challenge, whereas the inflammatory conditions used elevated both baseline and stimulated insulin secretions (per unit islet volume; i.e., islet equivalent, IEQ), indicating stressed islets. The stimulation index (SI), calculated as the ratio of average insulin secretions per unit time at high vs. low glucose, was 4.1 for the control islets, in strong agreement with published results for human islets57. Hypoxic islets showed an elevated baseline secretion and dramatically reduced ability to respond to high glucose challenge (SI of 1.3). Surprisingly, islets under inflammatory conditions exhibited a similar stimulation curve although with an overall elevated insulin secretion resulting in a somewhat reduced stimulation index (SI of 2.3) (Figure 8).
Figure 8.
Dynamic glucose-stimulated insulin release (GSIR) perifusion data for islets following different incubation conditions from the present study. All data here are from islets cultured with basal glucose for the entire 48 h. The main figure shows the average secreted insulin collected every minute for a low (3 mM) → high (11 mM) → low (3 mM) glucose challenge followed by a KCl step. Data are for n = 4 different islet samples; results of the individual perifusions are shown in the small insets.
Discussion
Although islet transplantation is a promising therapeutic option for patients with T1D1–4, there is a need to minimize islet cell damage during isolation, pre-transplant culture, and immediately following transplantation. A main goal of the present study was to understand how an environment of inflammation or hypoxia during ex vivo culture of isolated human pancreatic islets affects global metabolic and functional response to glucose. Another important goal was to identify potential biomarkers for islet health/potency, as well as for inflammation or hypoxia induced damage.
Culture conditions used here were selected based on previous publications looking at proteomic or metabolic changes in islets or corresponding cell lines. These studies indicated that exposure to stress for at least 24–48 h is needed to cause significant observable changes. Accordingly, here, islets were cultured for 24 h in basal glucose (5.5 mM) under standard, hypoxic (3% O2)33, 34, or inflammatory conditions (cytokine cocktail of IL-1β, IFN-γ, and TNF-α)35, 36, followed by an additional 24 h under either basal or high (16 mM) glucose (Supplementary Figure S1). We investigated the effect of high glucose because prolonged exposure to hyperglycemia is known to impair insulin secretion and induce pancreatic β-cell death58, and transplanted islets in T1D recipients are likely to be exposed to these conditions. We observed the significant elevation of several metabolites, both in the cell and the media samples, following high glucose exposure. Elevation of some of these metabolites (e.g., fructose) under high glucose has also been observed in previous studies using the β-cell line 832/1359, 60. Furthermore, imidazole lactate, a metabolite involved in the histidine metabolism, was also upregulated in high glucose conditions. Interestingly, GABA concentrations were decreased in cell samples following high glucose conditions. While this is in agreement with observations made by Pizarro-Delgado and co-workers for rat islets61, these trends are contrary to those of Wallace and co-workers, who observed increased GABA levels in β-cell lines25. Overall, only a limited number of the first- and second-phase related metabolite changes summarized in Table 1 of a recent review by Gooding and co-workers27, which were observed within a couple of hours post-glucose challenge, were confirmed here after 24 h high glucose exposure. Further, the adenylosuccinate (S-AMP) increase and inosine monophosphate (IMP) decrease reported by Gooding and co-workers in the insulinoma cell line 832/13 with 2 h incubation62 were not detected in our study (S-AMP, not detected; IMP increased, but not significantly in cells, not detected in media). These discrepancies may be due to the longer high glucose exposure times used here, as well as to the use of human islet versus insulinoma cell lines.
The characterization of the damage caused to pancreatic islets by an inflammatory milieu is of obvious interest. To evaluate this, we employed a cytokine cocktail of IL-1β (50 U/mL, 0.25 ng/mL), IFN-γ (1,000 U/mL, 50 ng/mL), and TNF-α (1,000 U/mL, 21.5 ng/mL) previously described in the literature35, 36. All concentrations were well above the corresponding median effective concentrations (EC50s), being about 20, 100, and 200-fold higher, respectively; hence, a strong inflammatory response was produced, as indicated by the considerable changes in the metabolomics signature (Table 1, Figure 4, Figure 5, Figure 6), even if viability (Supplementary Figure S7) and GSIR (Figure 8) were only moderately affected. Modified cytokine cocktails have been employed by other groups, e.g., IL-1β (2 ng/mL), IFN-γ (100 ng/mL), and TNF-α (100 ng/mL)63. One of our most notable observations here is the large, but consistent, perturbations induced by the inflammatory milieu in the tryptophan pathway,in particular in kynurenine and kynurenate levels (Figure 5). The tryptophan/kynurenine pathway is the main route of tryptophan degradation, and it generates several metabolites of various activity. Infections are known to activate this pathway, which appears to serve both as a direct defense mechanism and as a means of modulating the immune response64, 65. Notably, kynurenate is a broad-spectrum, non-selective glutamate receptor antagonist that is known to have neuroprotective effects, and kynurenate analogs are being studied for their neuroprotective effects66 – of particular interest here as pancreatic β-cells share a large number of similarities with neuronal cells. IDO activation is likely a self-protecting mechanism in several autoimmune disorders66. On the other hand, several of the metabolites other than kynurenate are toxic, and it is quite likely that the anti-inflammatory effects of IDO activation ultimately acts as a double-edged sword66, 67. Along these lines, IDO activation may protect islets from cytotoxic damage in the short term, while chronic exposure to various tryptophan metabolites can lead to β-cell attrition46. Because of the unique and pronounced metabolic signature of kynurenine and kynurenate in our study, these agents might serve as useful biomarkers of inflammation in human islets. Our results also suggest IDO as a worthwhile therapeutic target to dampen the effects of inflammation in pancreatic islets.
There are relatively limited studies published so far on kynurenine and related compounds in islets, but strong upregulation of IDO accompanied by increase in kynurenine has been shown in pancreatic islet, in particular by IFN-γ and somewhat less so by IL-1β or TNF-α46. In general agreement with our observations, a recent study64 has shown that (a) IDO1 and kynurenine 3-monoxygenase (KMO) expression are potently activated by proinflammatory cytokines (IFN-γ, IL-1β) and glucolipotoxicity respectively and are done so more prominently in β-cells than in non β-cells /note that HG here did not affect kynurenines/; (b) islet kynurenine/kynurenate production ratio is enhanced following IFN-γ and glucolipotoxicity; and (c) acute exposure to kynurenine potentiates GSIR in normal islets.
We also observed a significant increase in citrulline, a product of the nitric oxide synthase reaction, following culture with the cytokine cocktail. A somewhat similar cocktail of cytokines (IL-1β, 1 ng/mL and IFN-γ, 100 U/mL for 18 h) has been shown to not cause apoptosis, but possibly preferentially induce a programmed necrotic cell death68. The effects of this cocktail on the metabolic profiles of 832/13 cells was also investigated by Collier and co-workers and found to strongly induce citrulline, as well as pyruvate, malate, and inosine68.
Another somewhat unexpected observation here was the increase in GSIR following 48 h of exposure to inflammatory conditions, although some previous publications indicate this trend. For example, short term exposure of β-cells to low concentrations (e.g., 0.1 – 20 ng/mL) of IL-1β has been shown to improve insulin secretion69. This has also been found in human islets, but depending on the body-mass index (BMI) of the subject70. An incretin-like effect, i.e., that postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation has been shown recently by Dror and co-workers71. These may likely support our observed functional results.
Finally, as mentioned, hypoxia is also a major factor limiting viability and functionality in avascular islets as islets have to rely on passive transport for nutrient delivery and, among all nutrients, diffusion limitations are most severe for oxygen. This is well-illustrated by our model15, 39 calculated values shown in Supplementary Figure S8. While tissue oxygen concentrations are around 5%21, 22, most of the islet tissue will be exposed to lower oxygen concentrations, hence 3% is a reasonable choice and one that has been used by others33, 34. For hypoxic islets, levels of N-formylmethionine, an amino acid used for initiation of mitochondrial protein synthesis72, were significantly decreased, indicating β-cell death and stress73, 74. Further, polyamine metabolites were decreased, albeit only in the media, as a result of the oxidative stress75. The effects of (severe) hypoxia (1% O2) for different durations (2, 4, 6, 12, and 24 h) in INS-1 cells were investigated relatively recently by NMR metabolomics by Tian and co-workers76. Cell viability decreased considerably after 12 to 24 h of hypoxia (by approximately 15% and 40% at 5% and 1% O2, respectively) even if these were single cell cultures and not islet spheroids, where hypoxia in the core is likely to be much more severe15. Tian et al. observed decreased creatine-containing compounds and increased taurine-containing compounds in pancreatic β-cells at the early (2–6 h) and late (12–24 h) stage hypoxia. Herein, human islets exposed to less severe hypoxia exhibited similar trends for both cellular and soluble media components, as decreased cellular creatine (by 0.77 and 0.66 in LG and HG), but unchanged media creatine (1.10 and 0.84 – not significant) and increased taurine levels in media (3.88 and 3.26-fold), but decreased in islets (0.68 and 0.57) were observed. Tian et al. also reported decreasing glycerophosphocholine levels during early stage hypoxia. This study validated this for islet cell samples (0.71 and 0.58 for glycerophosphorylcholine, GPC, in LG and HG), but not in media.
Conclusions
In summary, our results provide, for the first time, a detailed metabolomics insight into the pathways altered by hypoxic and inflammatory conditions in whole human islets under both basal and hyperglycemic conditions. Since these are the main challenges hampering the survival and function of isolated, cultured, and transplanted islets, trends observed in this study should help in deciphering their impact of these factors on normal islet physiology and lead to tailored interventions that can improve islet isolation, culture, and transplant procedures for research and clinical purposes. One of the most notable observations was the consistently large changes induced by an inflammatory milieu in kynurenine and kynurenate, suggesting that they might serve as biomarkers of islet inflammation and that IDO on the corresponding metabolic pathway could be a therapeutic target to modulate the effects of inflammation in islets.
Supplementary Material
Supplementary Table S1. Summary of the donor characteristics for the human islet samples.
Supplementary Figure S1. Schematic design of the present study.
Supplementary Figure S2. Principal component analysis of the present metabolomics data.
Supplementary Figure S3. Random forest analysis of the present metabolomics data.
Supplementary Figure S4. Metabolic Pathway Enrichment for high versus basal glucose.
Supplementary Figure S5. Metabolic Pathway Enrichment for the effect of inflammatory cytokines.
Supplementary Figure S6. Metabolic Pathway Enrichment for the effect of hypoxia.
Supplementary Figure S7. Comparison of DTZ stained islets.
Supplementary Figure S8. Comparison of model-calculated oxygen and glucose concentrations.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (1UC4DK104208).
Footnotes
Author Contributions
Conceived and designed the experiments: CR, MGC, PB, CLS, ATG. Performed the experiments: ATG, MGC. Analyzed and interpreted the data: KLP, GAM, MGC, PB. Contributed reagents/materials/analysis tools: CR, CLS, KLP, GAM, PB. Drafted the manuscript: KLP, GAM, MGC. Wrote the paper: PB. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat. Rev. Immunol. 2004;4(4):259–268. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 2.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am. J. Transplant. 2008;8(10):1990–1997. doi: 10.1111/j.1600-6143.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- 3.Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, Ricordi C, Robien MA, Senior PA, Shapiro AM, Stock PG, Turgeon NA Clinical Islet Transplantation, C. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230–1240. doi: 10.2337/dc15-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 2017;13(5):268–277. doi: 10.1038/nrendo.2016.178. [DOI] [PubMed] [Google Scholar]
- 5.Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, Czarniecki CW, Barbaro B, Bridges ND, Cano J, Clarke WR, Eggerman TL, Hunsicker LG, Kaufman DB, Khan A, Lafontant DE, Linetsky E, Luo X, Markmann JF, Naji A, Korsgren O, Oberholzer J, Turgeon NA, Brandhorst D, Friberg AS, Lei J, Wang LJ, Wilhelm JJ, Willits J, Zhang X, Hering BJ, Posselt AM, Stock PG, Shapiro AM. National Institutes of Health-Sponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a complex cellular product at eight processing facilities. Diabetes. 2016;65(11):3418–3428. doi: 10.2337/db16-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noguchi H, Naziruddin B, Jackson A, Shimoda M, Ikemoto T, Fujita Y, Chujo D, Takita M, Peng H, Sugimoto K, Itoh T, Kobayashi N, Onaca N, Levy MF, Matsumoto S. Fresh islets are more effective for islet transplantation than cultured islets. Cell Transplant. 2012;21(2–3):517–523. doi: 10.3727/096368911X605439. [DOI] [PubMed] [Google Scholar]
- 7.Ihm SH, Matsumoto I, Zhang HJ, Ansite JD, Hering BJ. Effect of short-term culture on functional and stress-related parameters in isolated human islets. Transpl Int. 2009;22(2):207–216. doi: 10.1111/j.1432-2277.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- 8.Olsson R, Carlsson PO. Better vascular engraftment and function in pancreatic islets transplanted without prior culture. Diabetologia. 2005;48(3):469–476. doi: 10.1007/s00125-004-1650-x. [DOI] [PubMed] [Google Scholar]
- 9.Itoh T, Sugimoto K, Takita M, Shimoda M, Chujo D, SoRelle JA, Naziruddin B, Levy MF, Matsumoto S. Low temperature condition prevents hypoxia-induced islet cell damage and HMGB1 release in a mouse model. Cell Transplant. 2012;21(7):1361–1370. doi: 10.3727/096368912X637514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau J, Henriksnas J, Svensson J, Carlsson PO. Oxygenation of islets and its role in transplantation. Curr. Opin. Organ Transplant. 2009;14(6):688–693. doi: 10.1097/MOT.0b013e32833239ff. [DOI] [PubMed] [Google Scholar]
- 11.Lakey JRT, Helms LMH, Kin T, Korbutt GS, Rajotte RV, Shapiro AMJ, Warnock GL. Serine-protease inhibition during islet isolation increases islet yield from human pancreases with prolonged ischemia. Transplantation. 2001;72(4):565–570. doi: 10.1097/00007890-200108270-00003. [DOI] [PubMed] [Google Scholar]
- 12.Eizirik DL, Korbutt GS, Hellerstrom C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J. Clin. Invest. 1992;90(4):1263–1268. doi: 10.1172/JCI115989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, Usellini L, Nano R, Bonini P, Bertuzzi F, Marlier LN, Davalli AM, Carandente O, Pontiroli AE, Melino G, Marchetti P, Lauro R, Sesti G, Folli F. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50(6):1290–1301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- 14.Emamaullee JA, Shapiro AM. Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant. 2007;16(1):1–8. doi: 10.3727/000000007783464461. [DOI] [PubMed] [Google Scholar]
- 15.Buchwald P. A local glucose-and oxygen concentration-based insulin secretion model for pancreatic islets. Theor. Biol. Med. Model. 2011;8:20. doi: 10.1186/1742-4682-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes. 2011;60(9):2350–2353. doi: 10.2337/db09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasir B, Aiello LP, Yoon KH, Quickel RR, Bonner-Weir S, Weir GC. Hypoxia induces vascular endothelial growth factor gene and protein expression in cultured rat islet cells. Diabetes. 1998;47(12):1894–1903. doi: 10.2337/diabetes.47.12.1894. [DOI] [PubMed] [Google Scholar]
- 18.Giuliani M, Moritz W, Bodmer E, Dindo D, Kugelmeier P, Lehmann R, Gassmann M, Groscurth P, Weber M. Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell Transplant. 2005;14(1):67–76. doi: 10.3727/000000005783983287. [DOI] [PubMed] [Google Scholar]
- 19.MacGregor RR, Williams SJ, Tong PY, Kover K, Moore WV, Stehno-Bittel L. Small rat islets are superior to large islets in in vitro function and in transplantation outcomes. Am. J. Physiol. Endocrinol. Metab. 2006;290(5):E771–E779. doi: 10.1152/ajpendo.00097.2005. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu H, Kang D, Medrano L, Barriga A, Mendez D, Rawson J, Omori K, Ferreri K, Tai YC, Kandeel F, Mullen Y. Isolated human islets require hyperoxia to maintain islet mass, metabolism, and function. Biochem. Biophys. Res. Commun. 2016;470(3):534–538. doi: 10.1016/j.bbrc.2016.01.110. [DOI] [PubMed] [Google Scholar]
- 21.Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50(3):489–495. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]
- 22.Fournier RL. Basic Transport Phenomena in Biomedical Engineering. 3. CRC Press; Boca Raton, FL: 2011. p. 449. [Google Scholar]
- 23.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sysi-Aho M, Ermolov A, Gopalacharyulu PV, Tripathi A, Seppanen-Laakso T, Maukonen J, Mattila I, Ruohonen ST, Vahatalo L, Yetukuri L, Harkonen T, Lindfors E, Nikkila J, Ilonen J, Simell O, Saarela M, Knip M, Kaski S, Savontaus E, Oresic M. Metabolic regulation in progression to autoimmune diabetes. PLoS Comput. Biol. 2011;7(10):e1002257. doi: 10.1371/journal.pcbi.1002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace M, Whelan H, Brennan L. Metabolomic analysis of pancreatic beta cells following exposure to high glucose. Biochim. Biophys. Acta. 2013;1830(3):2583–2590. doi: 10.1016/j.bbagen.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Wang S, Puhl MD, Jiang X, Hyrc KL, Laciny E, Wallendorf MJ, Pappan KL, Coyle JT, Wice BM. Global biochemical profiling identifies beta-hydroxypyruvate as a potential mediator of type 2 diabetes in mice and humans. Diabetes. 2015;64(4):1383–1394. doi: 10.2337/db14-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gooding JR, Jensen MV, Newgard CB. Metabolomics applied to the pancreatic islet. Arch. Biochem. Biophys. 2016;589:120–130. doi: 10.1016/j.abb.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 29.Pileggi A, Ricordi C, Kenyon NS, Froud T, Baidal DA, Kahn A, Selvaggi G, Alejandro R. Twenty years of clinical islet transplantation at the Diabetes Research Institute - University of Miami. Clin. Transpl. 2004:177–204. [PubMed] [Google Scholar]
- 30.Khan A, Pileggi A, Ricordi C. Islet cell products: processing, quality controls and implications for bioartificial applications. In: Hallé JP, de Vos P, Rosenberg L, editors. The Bioartificial Pancreas and other Biohybrid Therapies. Research Signpost; Trivandrum, India: 2009. pp. 383–402. [Google Scholar]
- 31.Ricordi C, Gray DWR, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, Lake SP, London NJM, Socci C, Alejandro R, Zeng Y, Scharp DW, Viviani G, Falqui L, Tzakis A, Bretzel RG, Federlin K, Pozza G, James RFL, Rajotte RV, Di Carlo V, Morris PJ, Sutherland DER, Starzl TE, Mintz DH, Lacy PE. Islet isolation assessment in man and large animals. Acta Diabetol. Lat. 1990;27(3):185–195. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 32.Buchwald P, Wang X, Khan A, Bernal A, Fraker C, Inverardi L, Ricordi C. Quantitative assessment of islet cell products: estimating the accuracy of the existing protocol and accounting for islet size distribution. Cell Transplant. 2009;18(10–11):1223–1235. doi: 10.3727/096368909X476968. [DOI] [PubMed] [Google Scholar]
- 33.Lai Y, Brandhorst H, Hossain H, Bierhaus A, Chen C, Bretzel RG, Linn T. Activation of NFκB dependent apoptotic pathway in pancreatic islet cells by hypoxia. Islets. 2009;1(1):19–25. doi: 10.4161/isl.1.1.8530. [DOI] [PubMed] [Google Scholar]
- 34.Lablanche S, Cottet-Rousselle C, Argaud L, Laporte C, Lamarche F, Richard MJ, Berney T, Benhamou PY, Fontaine E. Respective effects of oxygen and energy substrate deprivation on beta cell viability. Biochim. Biophys. Acta. 2015;1847(6–7):629–39. doi: 10.1016/j.bbabio.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Brozzi F, Nardelli TR, Lopes M, Millard I, Barthson J, Igoillo-Esteve M, Grieco FA, Villate O, Oliveira JM, Casimir M, Bugliani M, Engin F, Hotamisligil GS, Marchetti P, Eizirik DL. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia. 2015;58(10):2307–2316. doi: 10.1007/s00125-015-3669-6. [DOI] [PubMed] [Google Scholar]
- 36.Eizirik DL, Sammeth M, Bouckenooghe T, Bottu G, Sisino G, Igoillo-Esteve M, Ortis F, Santin I, Colli ML, Barthson J, Bouwens L, Hughes L, Gregory L, Lunter G, Marselli L, Marchetti P, McCarthy MI, Cnop M. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 2012;8(3):e1002552. doi: 10.1371/journal.pgen.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrimpe-Rutledge AC, Fontes G, Gritsenko MA, Norbeck AD, Anderson DJ, Waters KM, Adkins JN, Smith RD, Poitout V, Metz TO. Discovery of novel glucose-regulated proteins in isolated human pancreatic islets using LC-MS/MS-based proteomics. J. Proteome Res. 2012;11(7):3520–3532. doi: 10.1021/pr3002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waanders LF, Chwalek K, Monetti M, Kumar C, Lammert E, Mann M. Quantitative proteomic analysis of single pancreatic islets. Proc. Natl. Acad. Sci. USA. 2009;106(45):18902–18907. doi: 10.1073/pnas.0908351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buchwald P, Cechin SR, Weaver JD, Stabler CL. Experimental evaluation and computational modeling of the effects of encapsulation on the time-profile of glucose-stimulated insulin release of pancreatic islets. Biomed. Eng. Online. 2015;14:28. doi: 10.1186/s12938-015-0021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009;81(16):6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 41.Evans AM, Bridgewater BR, Liu Q, Mitchell MW, Robinson RJ, Dai H, Stewart SJ, DeHaven CD, Miller LAD. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics. 2014;4:132. [Google Scholar]
- 42.DeHaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J. Cheminform. 2010;2(1):9. doi: 10.1186/1758-2946-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shannon P, M A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–5204. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jolliffe IT. Principal Component Analysis. Springer; New York: 1986. p. 271. [Google Scholar]
- 45.Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. [Google Scholar]
- 46.Sarkar SA, Wong R, Hackl SI, Moua O, Gill RG, Wiseman A, Davidson HW, Hutton JC. Induction of indoleamine 2,3-dioxygenase by interferon-gamma in human islets. Diabetes. 2007;56(1):72–79. doi: 10.2337/db06-0617. [DOI] [PubMed] [Google Scholar]
- 47.McDaniel ML, Kwon G, Hill JR, Marshall CA, Corbett JA. Cytokines and nitric oxide in islet inflammation and diabetes. Proc. Soc. Exp. Biol. Med. 1996;211(1):24–32. doi: 10.3181/00379727-211-43950d. [DOI] [PubMed] [Google Scholar]
- 48.Sekine N, Cirulli V, Regazzi R, Brown LJ, Gine E, Tamarit-Rodriguez J, Girotti M, Marie S, MacDonald MJ, Wollheim CB, Rutter GA. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J. Biol. Chem. 1994;269(7):4895–4902. [PubMed] [Google Scholar]
- 49.Alcazar O, Tiedge M, Lenzen S. Importance of lactate dehydrogenase for the regulation of glycolytic flux and insulin secretion in insulin-producing cells. Biochem. J. 2000;352(Pt 2):373–380. [PMC free article] [PubMed] [Google Scholar]
- 50.Lacy PE, Walker MM, Fink CJ. Perifusion of isolated rat islets in vitro. Participation of the microtubular system in the biphasic release of insulin. Diabetes. 1972;21(10):987–998. doi: 10.2337/diab.21.10.987. [DOI] [PubMed] [Google Scholar]
- 51.Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42(1):12–21. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 52.Sweet IR, Khalil G, Wallen AR, Steedman M, Schenkman KA, Reems JA, Kahn SE, Callis JB. Continuous measurement of oxygen consumption by pancreatic islets. Diabetes Technol. Therap. 2002;4(5):661–672. doi: 10.1089/152091502320798303. [DOI] [PubMed] [Google Scholar]
- 53.Cabrera O, Jacques-Silva MC, Berman DM, Fachado A, Echeverri F, Poo RE, Khan A, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A. Automated, high-throughput assays for evaluation of human pancreatic islet function. Cell. Transplant. 2008;16(10):1039–1048. [PMC free article] [PubMed] [Google Scholar]
- 54.Bocca N, Pileggi A, Molano RD, Marzorati S, Wu W, Bodor N, Ricordi C, Buchwald P. Soft corticosteroids for local immunosuppression: exploring the possibility for the use of loteprednol etabonate in islet transplantation. Pharmazie. 2008;63(3):226–232. [PubMed] [Google Scholar]
- 55.Kayton NS, Poffenberger G, Henske J, Dai C, Thompson C, Aramandla R, Shostak A, Nicholson W, Brissova M, Bush WS, Powers AC. Human islet preparations distributed for research exhibit a variety of insulin-secretory profiles. Am. J. Physiol. Endocrinol. Metab. 2015;308(7):E592–E602. doi: 10.1152/ajpendo.00437.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchwald P, Cechin SR. Glucose-stimulated insulin secretion in isolated pancreatic islets: Multiphysics FEM model calculations compared to results of perifusion experiments with human islets. J. Biomed. Sci. Eng. 2013;6(5A):26–35. [Google Scholar]
- 57.Henquin JC, Dufrane D, Kerr-Conte J, Nenquin M. Dynamics of glucose-induced insulin secretion in normal human islets. Am. J. Physiol. Endocrinol. Metab. 2015;309(7):E640–E650. doi: 10.1152/ajpendo.00251.2015. [DOI] [PubMed] [Google Scholar]
- 58.Hribal ML, Perego L, Lovari S, Andreozzi F, Menghini R, Perego C, Finzi G, Usellini L, Placidi C, Capella C, Guzzi V, Lauro D, Bertuzzi F, Davalli A, Pozza G, Pontiroli A, Federici M, Lauro R, Brunetti A, Folli F, Sesti G. Chronic hyperglycemia impairs insulin secretion by affecting insulin receptor expression, splicing, and signaling in RIN beta cell line and human islets of Langerhans. FASEB J. 2003;17(10):1340–1342. doi: 10.1096/fj.02-0685fje. [DOI] [PubMed] [Google Scholar]
- 59.Huang M, Joseph JW. Metabolomic analysis of pancreatic beta-cell insulin release in response to glucose. Islets. 2012;4(3):210–222. doi: 10.4161/isl.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lorenz MA, El Azzouny MA, Kennedy RT, Burant CF. Metabolome response to glucose in the beta-cell line INS-1 832/13. J. Biol. Chem. 2013;288(15):10923–10935. doi: 10.1074/jbc.M112.414961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pizarro-Delgado J, Braun M, Hernandez-Fisac I, Martin-Del-Rio R, Tamarit-Rodriguez J. Glucose promotion of GABA metabolism contributes to the stimulation of insulin secretion in beta-cells. Biochem. J. 2010;431(3):381–389. doi: 10.1042/BJ20100714. [DOI] [PubMed] [Google Scholar]
- 62.Gooding JR, Jensen MV, Dai X, Wenner BR, Lu D, Arumugam R, Ferdaoussi M, MacDonald PE, Newgard CB. Adenylosuccinate is an insulin secretagogue derived from glucose-induced purine metabolism. Cell Rep. 2015;13(1):157–167. doi: 10.1016/j.celrep.2015.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grunnet LG, Aikin R, Tonnesen MF, Paraskevas S, Blaabjerg L, Storling J, Rosenberg L, Billestrup N, Maysinger D, Mandrup-Poulsen T. Proinflammatory cytokines activate the intrinsic apoptotic pathway in β-cells. Diabetes. 2009;58(8):1807–1815. doi: 10.2337/db08-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu JJ, Raynal S, Bailbe D, Gausseres B, Carbonne C, Autier V, Movassat J, Kergoat M, Portha B. Expression of the kynurenine pathway enzymes in the pancreatic islet cells. Activation by cytokines and glucolipotoxicity. Biochim. Biophys. Acta. 2015;1852(5):980–991. doi: 10.1016/j.bbadis.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol. Cell Biol. 2003;81(4):247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 66.Vécsei L, Szalárdy L, Fülöp F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat. Rev. Drug Discov. 2013;12(1):64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 67.Kwidzinski E, Bechmann I. IDO expression in the brain: a double-edged sword. J. Mol. Med. 2007;85(12):1351–1359. doi: 10.1007/s00109-007-0229-7. [DOI] [PubMed] [Google Scholar]
- 68.Collier JJ, Burke SJ, Eisenhauer ME, Lu D, Sapp RC, Frydman CJ, Campagna SR. Pancreatic beta-cell death in response to pro-inflammatory cytokines is distinct from genuine apoptosis. PloS One. 2011;6(7):e22485. doi: 10.1371/journal.pone.0022485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arous C, Ferreira PG, Dermitzakis ET, Halban PA. Short term exposure of beta cells to low concentrations of interleukin-1beta improves insulin secretion through focal adhesion and actin remodeling and regulation of gene expression. J. Biol. Chem. 2015;290(10):6653–6669. doi: 10.1074/jbc.M114.611111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hajmrle C, Smith N, Spigelman AF, Dai X, Senior L, Bautista A, Ferdaoussi M, MacDonald PE. Interleukin-1 signaling contributes to acute islet compensation. JCI Insight. 2016;1(4):e86055. doi: 10.1172/jci.insight.86055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dror E, Dalmas E, Meier DT, Wueest S, Thevenet J, Thienel C, Timper K, Nordmann TM, Traub S, Schulze F, Item F, Vallois D, Pattou F, Kerr-Conte J, Lavallard V, Berney T, Thorens B, Konrad D, Boni-Schnetzler M, Donath MY. Postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 2017;18(3):283–292. doi: 10.1038/ni.3659. [DOI] [PubMed] [Google Scholar]
- 72.Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol. Rev. 1983;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic beta-cell death. Trends Endocrinol. Metab. 2011;22(7):266–274. doi: 10.1016/j.tem.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kennedy J, Katsuta H, Jung MH, Marselli L, Goldfine AB, Balis UJ, Sgroi D, Bonner-Weir S, Weir GC. Protective unfolded protein response in human pancreatic beta cells transplanted into mice. PloS One. 2010;5(6):e11211. doi: 10.1371/journal.pone.0011211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorlach A, Dimova EY, Petry A, Martinez-Ruiz A, Hernansanz-Agustin P, Rolo AP, Palmeira CM, Kietzmann T. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 2015;6:372–385. doi: 10.1016/j.redox.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian L, Kim HS, Kim H, Jin X, Jung HS, Park KS, Cho KW, Park S, Moon WK. Changes in metabolic markers in insulin-producing beta-cells during hypoxia-induced cell death as studied by NMR metabolomics. J. Proteome Res. 2013;12(8):3738–3745. doi: 10.1021/pr400315e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Summary of the donor characteristics for the human islet samples.
Supplementary Figure S1. Schematic design of the present study.
Supplementary Figure S2. Principal component analysis of the present metabolomics data.
Supplementary Figure S3. Random forest analysis of the present metabolomics data.
Supplementary Figure S4. Metabolic Pathway Enrichment for high versus basal glucose.
Supplementary Figure S5. Metabolic Pathway Enrichment for the effect of inflammatory cytokines.
Supplementary Figure S6. Metabolic Pathway Enrichment for the effect of hypoxia.
Supplementary Figure S7. Comparison of DTZ stained islets.
Supplementary Figure S8. Comparison of model-calculated oxygen and glucose concentrations.