Abstract
Background
Infliximab (IFX) is effective in the treatment of inflammatory bowel disease; however, the effect is often not durable. It is unknown if proactive therapeutic concentration monitoring (TCM) of IFX improves outcomes.
Methods
This is a retrospective observational study examining the use of proactive TCM and titration of IFX to a target concentration for patients with inflammatory bowel disease in clinical remission at a tertiary care center. The primary aim was to describe the clinical course of patients who had proactive TCM. A secondary analysis was done to assess if this strategy was superior to the standard of care.
Results
Forty-eight patients were identified as having proactive TCM. Fifteen percent had an initial undetectable trough concentration. Twenty-five percent (12 of 48) of patients escalated IFX after the first proactive TCM while 15% (7 of 48) of patients de-escalated IFX therapy over the study period. A control group of 78 patients was identified. Patients who had proactive TCM had a greater probability of remaining on IFX than controls (hazard ratio, 0.3; 95% confidence interval, 0.1–0.6; log rank test; P = 0.0006). The probability of remaining on IFX was greatest for patients who achieved a trough concentration >5 μg/mL (hazard ratio, 0.03; 95% confidence interval, 0.01–0.1; P < 0.0001 versus trough <5 μg/mL). Fewer patients in the proactive TCM group stopped IFX (10% versus 31%, P = 0.009).
Conclusions
In this pilot observational study, proactive TCM of IFX frequently identified patients with low or undetectable trough concentrations and resulted in a greater probability of remaining on IFX.
Keywords: inflammatory bowel disease, infliximab, therapeutic drug monitoring, infliximab concentration, optimized monotherapy
Infliximab (IFX) is an anti–tumor necrosis factor antibody used for the treatment of numerous immune-mediated inflammatory disorders, including Crohn’s disease (CD) and ulcerative colitis (UC). It is efficacious for the induction and maintenance of remission in patients with moderate to severe inflammatory bowel disease (IBD).1–5 Unfortunately, the durability of IFX is disappointing due to secondary loss of response (LOR) and adverse events such as infusion reactions (IRs). In the ACCENT I trial, less than 40% of patients with an initial response to IFX remained in clinical remission at 54 weeks.1 Others have calculated an annual risk of LOR to IFX of 13% per patient-year.6
Part of the inability to maintain long-term remission is thought to be from formation of antibody to IFX (ATI). ATI is associated with an increased risk of IR and LOR to IFX.7,8 Undetectable or low trough concentrations are associated with an increased risk of immunogenicity and anti-drug antibody formation with both IFX and adalimumab.9,10 More so, higher IFX trough concentrations are associated with improved clinical outcomes.11 In patients with UC, a trough concentration of >3 μg/mL after induction predicted a sustained response to IFX.12,13 Similar findings have been noted in patients with CD where detectable trough concentrations correlated with clinical remission and mucosal healing.14,15
Proactive therapeutic concentration monitoring (TCM) is the measurement of a drug concentration at a prespecified time point followed by titration of the drug to a target rage. TCM is the standard of care in other clinical scenarios, such as solid organ transplant,16 use of cyclosporine or tacrolimus in UC,17,18 and use of certain antibiotics.19,20 In these settings, TCM decreases toxicity and improve outcomes.16–20
We postulate that proactive TCM of IFX with titration of IFX to a target concentration will improve long-term outcomes in patients with IBD. The primary aim of this pilot observational study was to describe the outcomes of proactive TCM of IFX in a cohort of patients who achieved clinical remission on IFX. Outcomes of interest included initial and subsequent IFX trough levels, dosing changes including dose escalation and de-escalation, and outcomes of patients on IFX monotherapy. The secondary aims were to assess if proactive TCM was associated with a longer duration of IFX compared with a control group and to assess reasons for cessation of IFX.
MATERIALS AND METHODS
Patient Identification
For the primary outcome, we performed a chart review of patients receiving IFX for IBD at the Beth-Israel Deaconess Medical Center (Boston, MA). Starting in 2009, one IBD attending physician (A.S.C.) began proactive TCM of IFX with the aim of titrating IFX to a target concentration. All patients who had an IFX level sent by this physician were identified. Patients were excluded if (1) the IFX infusions were not administered at the hospital’s infusion center, (2) the IFX concentration was drawn from cord blood, (3) there was no follow-up visit after the IFX concentration was drawn, (4) the IFX concentration was not documented in a gastroenterology clinic note, or (5) patient failed to receive at least one maintenance infusion of IFX. For a patient to be considered as having had proactive TCM of IFX, the patient must have had an IFX trough concentration while in clinical remission and not done for a reactive purpose (i.e., for symptoms concerning for IBD or concern for an IFX-mediated side effect).
For patients who had proactive TCM, the initial the goal was to titrate to a detectable level, but in 2010, the target range changed to 5 to 10 μg/mL. Typically, for an undetectable trough concentration, the dose of IFX was increased to 7.5 mg/kg with the following infusion at 6 weeks, subsequently returning to every 8 week infusions. For a detectable trough concentration that was <5 μg/mL, the dose of IFX was typically increased by 50 or 100 mg. For a trough concentration >10 μg/mL on 2 occasions, the dose was decreased, or if the patient was already at 5 mg/mL, the interval between infusions was increased. No changes were made for a trough in the target range of 5 to 10 μg/mL.
Control Group, Variables, and Outcomes
For the secondary outcome, a control group was established from the hospital’s infusion center of patients who achieved clinical remission on IFX but did not have proactive TCM of IFX. Patients from the IBD center who were on IFX were identified and their charts reviewed to ensure they achieved clinical remission on IFX.
The control group was treated with the current standard of care but did not undergo proactive TCM of IFX. In both groups, reactive testing was performed as indicated for LOR or a concern for an antibody-mediated side effect. Dose escalation of IFX in the control group was performed for patients with LOR at the discretion of the treating physician. Dose escalations were performed according to the current standard of care and did not exceed IFX 10 mg/kg every 4 weeks.
A trough concentration was defined as any IFX concentration performed within 7 days of the next infusion. For both the proactive TCM and the control groups, clinical remission was defined as lack of symptoms attributable to underlying IBD based on the treating gastroenterologist’s documentation. The duration of IFX was measured in weeks based on infusion center records. When IFX was stopped, the charts were reviewed for the reason for cessation of therapy.
Infliximab Concentration Testing
Prometheus Laboratories (San Diego, CA) processed all IFX and ATI concentrations. The period of the study overlapped with the use of 2 methods of IFX and ATI detection. Initially, testing was performed through solid phase enzyme-linked immunosorbent assay (ELISA). In July 2012, testing was changed to a non-radiolabeled liquid phase mobility shift assay (high performance liquid chromatography), which allows for the detection of antibody in the presence of detectable IFX concentrations. Additionally, the high performance liquid chromatography test had a lower limit of detection for IFX (1 versus 1.4 μg/mL from ELISA).
Statistical Methods
Categorical data were compared by chi-square test or Fisher’s exact test, and continuous data were compared by t test or Wilcoxon test as appropriate. Duration of IFX was expressed as a time-to-event curve, with patients censored at their last documented clinical encounter that they were receiving IFX or on August 1, 2013. Patients lost to follow-up were analyzed in a last observation carried forward fashion. Patients were a priori stratified by “TCM” or “no TCM” status, trough concentration achieved, and use of combination therapy. To explore for potential confounding, a proportional hazard model (Cox) was constructed including all of the baseline characteristics.
Sensitivity analysis was conducted to reduce potential selection bias from patients on IFX before the start of proactive TCM. The sensitivity analysis was done by examining a subset of patients who started maintenance IFX after January 1, 2009. Log rank test was used to assess for significance. For the primary outcome a P value of <0.05 was used for significance.
Cox regression analysis was done to assess for confounding. All baseline characteristics were initially included in the model. Variables were eliminated from the model if they were nonsignificant by the Wald test (P < 0.1). After a variable was eliminated, the parameter estimate for TCM was assessed. If the parameter estimate changed by more than 10%, then the eliminated variable was added back into the model as a potential confounder. All P values reported for proportional hazard regression were based on the effect likelihood ratio test.
RESULTS
Demographic Data
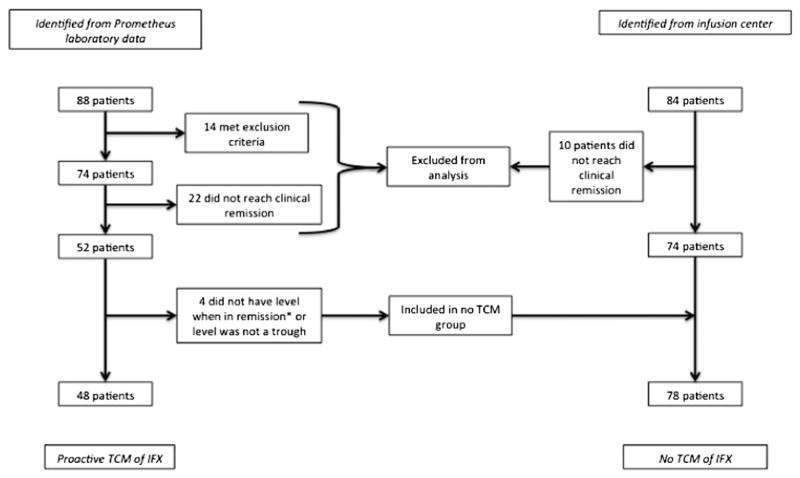
A total of 192 IFX concentrations were obtained from 88 patients. Forty-eight patients met the criteria for proactive TCM of IFX (Fig. 1 and Table 1).
FIGURE 1.
Flow chart detailing patient selection and categorization as “proactive TCM” or “no TCM.” *Did achieve clinical remission before IFX concentration testing. *Patients achieved remission on IFX but not at the time a trough level was checked.
TABLE 1.
Patient Characteristics
| Characteristic | Proactive TCM, n (%) | No TCM, n (%) | P |
|---|---|---|---|
| Number of patients | 48 | 78 | |
| Gender | 0.2 | ||
| Male | 33 (69) | 45 (58) | |
| Female | 15 (31) | 33 (42) | |
| Median age at IFX initiation (IQR) | 35 (29–42.5) | 34.9 (26.2–49.7) | 0.9 |
| Disease | 0.2 | ||
| CD | 38 (79) | 52 (67) | |
| UC | 10 (21) | 24 (31) | |
| IBD—unclassified | 0 | 2 (3) | |
| Median age at diagnosis (IQR) | 23.5 (19.3–28) | 25 (19.5–36.5) 0.3 | |
| Disease location | |||
| CD | 0.1 | ||
| Ileocolonic | 24 (63.2) | 26 (50) | |
| Ileum | 9 (23.7) | 10 (19.2) | |
| Colon | 5 (13.2) | 18 (34.6) | |
| Perianal | 16 (42) | 19 (36.5) | 0.7 |
| UC | 0.8 | ||
| Extensive/pancolitis | 6 (60) | 13 (54.2) | |
| Left sided | 4 (40) | 11 (45.8) | |
| Previous IBD surgery | 19 (40) | 19 (25) | 0.08 |
| Tobacco status | 0.6 | ||
| Current | 5 (10) | 7 (9) | |
| Former | 12 (25) | 14 (18) | |
| Never | 31 (56) | 57 (73) | |
| Combination therapy | 21 (44) | 31 (40) | 0.7 |
IQR, interquartile range (25–75).
Infliximab Concentration and Dosing Changes
Data regarding the initial IFX concentration and dosing changes in the proactive TCM group are presented in Table 2. The median duration of IFX before proactive TCM was 43 weeks (interquartile range, 32–72). Only 29% (14 of 48) of patients in the TCM group were in the target therapeutic range based on initial testing. Changes to IFX after TCM were common with 35% (17 of 48) undergoing a change in IFX dosing. Most patients underwent dose escalation (12 of 48); however 10% (5 of 48) de-escalated (either stopped or decreased the dose of IFX) therapy after initial TCM.
TABLE 2.
Characteristics of Infliximab Trough Concentrations Among Patients with Proactive TCM
| First Proactive Trough Concentration, n (%)a | Subsequent Proactive Trough Concentration, n (%)a | |
|---|---|---|
| Number of trough concentrations obtained | 48 | 40 |
| Tests performed per assay | ||
| ELISA | 37 (77) | 7 (18) |
| HPLC | 11 (23) | 33 (82) |
| Median first IFX trough concentration (IQR) | 5.9 ug/ml (2.8–9.9) | 7.6 ug/ml (4.3–12.3) |
| IFX undetectable | 7 ug/ml (15) | 1 (3) |
| IFX trough concentration <3 ug/ml | 13 (27) | 4 (10) |
| IFX trough concentration <5 ug/ml | 23 (48) | 13 (33) |
| IFX trough concentration = 5–10 ug/ml | 14 (29) | 14 (35) |
| IFX trough concentration >10 ug/ml | 11 (23) | 15 (38) |
| Change | 17 (35) | 10 (25) |
| Escalation of therapy | 12 (71) | 8 (80) |
| Decrease of therapy | 3 (18) | 2 (20) |
| Stopped therapy | 2 (12) | — |
Reactive IFX concentrations were excluded.
ELISA, solid phase enzyme-linked immunosorbent assay; HPLC, high performance liquid chromatography; IQR, interquartile range (25–75).
Although dose escalations were common, patients did not have the dose doubled, as is the practice for reactive dose escalation. The median dose escalation following proactive TCM was 100 mg (range, 50–250 mg). Even with these smaller adjustments, more patients were in the therapeutic window of 5 to 10 μg/mL on subsequent testing. Additionally, a total of 15% (7 of 48) of the TCM group de-escalated (decreased or stopped) IFX therapy at some point over the study period in response to TCM. The overall median duration of IFX therapy in patients with practice TCM was 144 weeks (range, 36–685 wk).
Two patients who underwent proactive TCM stopped IFX related to routine drug concentration monitoring. One was in clinical remission on IFX following a diverting ileostomy. IFX trough concentration testing demonstrated an undetectable trough concentration and ATI level >100 U/mL and IFX was stopped. Another was in remission on IFX but developed psoriasis. IFX concentration done with the intent to titrate the dose demonstrated an undetectable trough and an ATI concentration of 6.8 U/mL. The patient elected to stop IFX given the psoriasis and ATI. Three other patients stopped IFX for reasons unrelated to drug monitoring. One developed flat low-grade dysplasia and had a colectomy, another developed drug-induced lupus with a therapeutic dose of IFX, and the last developed a delayed IR with ATI on reactive IFX testing. Notably, the last patient was targeted to a detectable level and not a range of 5 to 10 μg/mL (i.e., underwent TCM before 2010). This patient’s trough concentration of 2.2 μg/mL was considered adequate at the time and no dose escalation was done. Subsequent reactive testing in the setting of delayed IR demonstrated ATI.
Among patients who had TCM, 75% (36 of 48) achieved a trough concentration of 5 μg/mL or higher. In that subset of patients, no one developed ATI or an IR. The only patient in this group to stop IFX did so after a colectomy for flat low-grade dysplasia. Of the eight 8 who failed to achieve a trough of >3 μg/mL, 6 stopped IFX: 5 for IRs (acute and delayed) with ATI and 1 for drug-induced lupus.
Optimized Monotherapy
Among patients who had proactive TCM, 31 patients were on IFX monotherapy at the end of the study period and achieved an IFX trough of >3 μg/mL. Eight patients were initially on combination therapy with an immunomodulator (7 on a thiopurine and 1 on methotrexate) while the remaining patients were on monotherapy for the entire duration of treatment. Of the patients initially on combination therapy, 7 had an IFX concentration after the immunomodulator was discontinued; 4 had subtherapeutic IFX concentrations requiring escalation of IFX. All 31 patients receiving optimized monotherapy remained on IFX at the end of the study period. The median duration of IFX of this group was 175 weeks (range, 53.1–685.9 wk).
Proactive TCM Compared with Standard of Care
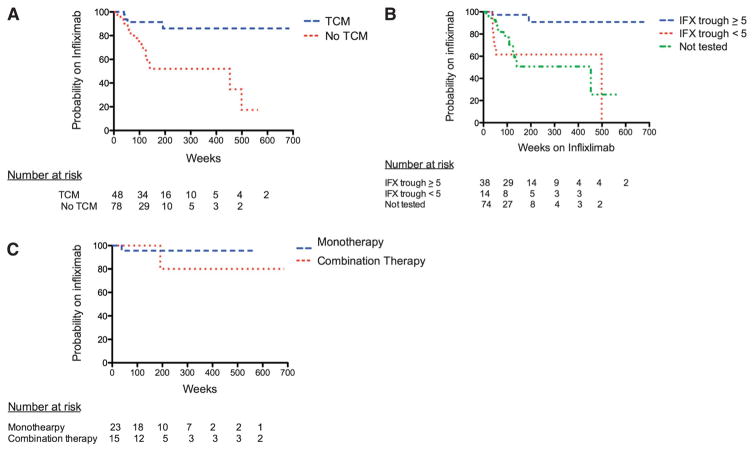
Seventy-eight patients were identified as a standard of care control group without proactive TCM (Fig. 1). No significant differences were noted between the 2 groups (Table 1). Patients who had proactive TCM of IFX had a greater probability of remaining on IFX compared with patients who did not have TCM (hazard ratio [HR], 0.3; 95% confidence interval [CI], 0.1–0.6; log rank test P = 0.0006; Fig. 2A). The probability of being on IFX therapy at 5 years in the proactive TCM group was 86% compared with 52% in the group that did not have TCM. Patients who achieved an IFX trough concentration of ≥5 μg/mL had a greater probability of remaining on IFX than those who did not (HR, 0.1; 95% CI, 0.02–0.4; log rank test P = 0.0005; Fig. 2B) or those who had no TCM (HR, 0.1; 95% CI, 0.02–0.3; log rank test P = 0.0002; Fig. 2B). Patients with a trough concentration of <5 μg/mL did not have a significantly different IFX duration compared with the group without proactive TCM (HR, 1.08; 95% CI, 0.4–2.6; log rank test P = 0.9; Fig. 2B). Overall, similar results were noted for patients who achieved a trough concentration of ≥3 μg/mL. However, patients with a trough concentration of <3 μg/mL did have a significantly lower probability of remaining on IFX than those in the group that did not have TCM (HR, 3.4; 95% CI, 1.2–8.6; log rank test P = 0.009; Fig., Supplemental Digital Content 1, http://links.lww.com/IBD/A548).
FIGURE 2.
Duration of IFX in weeks based on a priori subgroups. A, Probability of continuing on IFX in the proactive TCM versus control groups (HR, 0.3; 95% CI, 0.1–0.6; log rank test; P = 0.0006). B, Probability of continuing IFX based on trough concentration. Log rank test for IFX trough ≥5 μg/mL (at any point in therapy) versus never achieving an IFX trough <5 mg/mL, P < 0.0001 (HR, 0.03; 95% CI, 0.001–0.1). Log rank test for IFX trough ≥5 μg/mL versus no trough testing, P < 0.0001 (HR, 0.2; 95% CI, 0.07–0.4). Log rank test for IFX trough <5 μg/mL (at any point in therapy) versus no trough testing, P = 0.6 (HR, 1.3; 95% CI, 0.5–3.3). C, Probability of continuing IFX between monotherapy with IFX and combination therapy. All had a trough concentration ≥5 μg/mL (HR, 0.6; 95% CI, 0.03–09.9; log rank test; P = 0.7).
Results of univariate analysis and multiple Cox regression analysis are shown in Tables, Supplemental Digital Content 2 and 3, http://links.lww.com/IBD/A549 and http://links.lww.com/IBD/A550, respectively. Three variables were included in the final model regression model: proactive TCM of IFX, disease, and provider. In the final regression model, only pro-active TCM of IFX remained significantly associated with remaining on IFX (HR, 0.07; 95% CI, 0.01–0.4; likelihood ratio P = 0.01).
Among patients who achieved a trough concentration of ≥5 μg/mL, there was no difference in the probability of remaining on IFX between patients on monotherapy or combination therapy (HR, 0.6; 95% CI, 0.03–9.9; log rank test P = 0.7; Fig. 2C). Similarly, among all patients in the cohort, there was no difference in the probability of remaining on IFX between patients on mono-therapy or combination therapy (HR, 1.3; 95% CI, 0.6–2.8; log rank test P = 0.5; Fig., Supplemental Digital Content 4, http://links.lww.com/IBD/A551). Sensitivity analysis was performed examining the subset of patients who started IFX maintenance therapy after January 1, 2009. The significance of the results between the proactive TCM group and the control group did not change (HR, 0.3; 95% CI, 0.1–0.7; log rank test P = 0.003; Fig., Supplemental Digital Content 5, http://links.lww.com/IBD/A552).
Fewer patients in the proactive TCM group stopped IFX compared with the control group (10% versus 31%, respectively, P = 0.009). No patients in the proactive TCM group stopped IFX due to ongoing clinical symptoms or acute IR, whereas 15 patients (19%) in the control group stopped IFX for recurrent clinical symptoms and 6 (7.7%) for acute IR (Table 3). Among patients in the control group without proactive TCM who stopped IFX for recurrent IBD symptoms, 73% (11 of 15) underwent a reactive escalation of IFX therapy before stopping IFX; the majority (73%) underwent a dose increase to 10 mg/kg of IFX while the remainder had the IFX interval shortened. Two patients did not undergo dose escalation because reactive testing demonstrated therapeutic IFX levels.
TABLE 3.
Reasons Why IFX Was Stopped
| Proactive TCM | No TCM | |
|---|---|---|
| Recurrent IBD symptoms | 0 | 15 |
| Adverse events | ||
| Pneumonia | 0 | 1 |
| Drug-induced lupus | 1 | 0 |
| Psoriasis | 1 | 0 |
| High antibody concentration | 1 | 0 |
| IRs | ||
| Acute IR | 0 | 6 |
| Delayed IR | 1 | 0 |
| Other (unrelated to IFX)a | 1 | 2 |
Includes: unable to afford co-payment, surgery for adhesive small bowl obstruction, and colectomy for flat low-grade dysplasia.
DISCUSSION
In this pilot observational study, proactive TCM of IFX trough concentration was associated with a greater probability of remaining on IFX. We found that patients with proactive TCM had a probability of remaining on IFX of greater than 80% for our study period, whereas those who achieved a trough concentration of ≥5 μg/mL had a probability of remaining on IFX of greater than 90%. No patient who underwent TCM of IFX and titration to a target range stopped IFX due to recurrent clinical symptoms or an acute IR. However, in our control group, who did not undergo proactive TCM, 19% stopped due to recurrent clinical symptoms (the majority of whom underwent a reactive dose escalation) and 8% stopped due to acute IR. The secondary LOR and acute IR in the group that did not undergo proactive TCM raise the suspicion that these patients developed ATI. In the proactive TCM arm, 15% (7 of 48) of patients had an undetectable first IFX trough concentration that may be associated with increased immunogenicity and ultimately LOR or IR.7–9,21 Thus, despite receiving maintenance therapy, this group is likely at a high risk of eventually forming ATIs due to a mechanism similar to that caused by intermittent dosing. We hypothesize that the improved duration of IFX in the proactive TCM group was due to fewer undetectable and subtherapeutic trough concentrations and subsequently less ATI formation.
A recent prospective randomized clinical trial evaluated IFX concentration monitoring for patients with IBD (TAXIT).22 In TAXIT, all patients underwent IFX dose optimization based on a single proactive monitoring measurement to a trough level of 3 to 7 μg/mL. After dose optimization there was a significant increase in patients in clinical remission for CD (64.4% before optimization versus 91.7% after optimization, P = 0.02) and a greater proportion of UC patients in clinical remission (88.1% before optimization versus 95.8% after optimization, P = 1). After all patients were optimized to a therapeutic drug level, one arm underwent continued proactive TCM while the other did not. At 1 year, the rates of clinical remission were similar in both groups while the group that underwent drug concentration monitoring required rescue therapy less frequently (5.5% versus 17.3%; P = 0.004). Our study differs from TAXIT in 2 important ways. First our control population did not undergo an initial dose optimization based on a proactive monitoring measurement. It is possible that dose optimization based on a single proactive monitoring measurement is sufficient for most patients for 1 year. Additionally, we were able to follow patients for greater than 1 year. Our control group had a similar probability of being on IFX at 1 year, although after 1 year the probability favored proactive TCM. Although important information was gained from TAXIT, our results demonstrate that there may be a long-term benefit to continued proactive IFX concentration monitoring.
Target Trough IFX Concentration
The optimal trough concentration of IFX remains unclear. Previous studies have suggested that a concentration of 3 μg/mL predicts a sustained response.12–15 We found that patients who achieved a trough concentration of ≥3 or ≥5 μg/mL had a significantly longer duration of IFX compared with those who did not. No patient who achieved a trough concentration of ≥5 μg/mL developed ATI, an IR, or stopped IFX due to recurrent IBD symptoms. Although a trough ≥3 μg/mL is likely effective, we hypothesize that in a real-world setting, patients who have a trough concentration of ≥5 μg/mL are more likely to avoid developing undetectable trough concentrations before subsequent doses. Additionally, it seems that small dose adjustments (50–100 mg increments of IFX) can move the IFX trough concentration into the target range for patients in clinical remission.
The upper border of the trough concentration is less clear. There is likely little benefit from a trough concentration of >10 μg/mL. It is possible that supratherapeutic concentrations of IFX predispose to adverse events (e.g., infections, malignancy). Additionally, IFX is expensive. Given the lack of clear benefit, the theoretical potential for increased adverse events, and the unequivocal increased cost, de-escalating therapy for select patients is another benefit of proactive TCM. In our cohort, 15% ultimately underwent IFX dose de-escalation in response to proactive TCM. This effect is likely to become more pronounced over time as more patients achieve remission on IFX. Furthermore, some patients may have high concentrations of ATI likely rendering IFX ineffective; this occurred in 2% (1 of 48) of our patients. Early identification of high concentrations of ATI can spare the patient from the potential development of an adverse event and the cost of futile IFX therapy.
Confounding the ability to choose a target range is the variability of IFX and ATI concentrations between different assays. Our study overlapped between 2 types of assays, ELISA and high performance liquid chromatography. There may be up to a 2 μg/mL difference between these 2 assays.23 Thus, although we demonstrate that the proactive TCM is associated with a prolonged duration of IFX, we cannot clearly define the optimal cut off for a trough concentration. Given the variance of the assays, a target range of at least 5 μg/mL likely ensured that all patients had a detectable trough concentration.
Optimized Monotherapy
Recently, Cornillie et al24 noted in a post hoc analysis of ACCENT I that patients with immunomodulator use in addition to IFX had higher IFX concentrations at week 14 (approximately 2 μg/mL higher). They suggest that the benefit of combination therapy seen in other trials may have been related to the actual IFX trough concentration and not a synergistic effect of combination therapy. In support of this hypothesis, we identified a subset of patients on “optimized monotherapy” who never stopped IFX and included a number of patients who successfully deescalated from combination therapy. Although current data suggest that combination therapy is superior to monotherapy with IFX in biologic and immunomodulator-naive patients,5 our data suggest that optimizing IFX to a trough concentration of ≥5 μg/mL may provide an alternative treatment strategy to combination therapy. If ultimately confirmed, this observation could be quite important because many physicians and patients are reluctant to use combination therapy due to the adverse events associated with thiopurines.25 However, the cost of this approach may be higher because a higher IFX dose may be required to achieve a target level off of combination therapy.
Limitations
Our study is inherently limited by the retrospective nature of the design. Although the electronic medical record system is robust, exact data on clinical remission are based on the provider’s documented assessment. Despite this, it is unlikely that a patient failing IFX would continue on therapy, making duration of IFX a relatively unbiased surrogate end point. Another limitation is that only one provider performed proactive TCM of IFX. However, other patients from this provider were included in the control group and the provider was not associated with the probability of remaining on IFX on COX regression. Additionally, the benefit seen within the proactive TCM group was for those with an IFX concentration of >5 while those with a low IFX level were similar to the control group. Due to the nature of the study, we cannot exclude the possibility that there is residual confounding accounting for the benefits associated with proactive TCM of IFX. However, the duration of IFX among the proactive TCM group is superior to other real-word published data on the duration of IFX.26 Whereas, our control group has similar outcomes to historical data.1,6,26 Another limitation is that the study period spanned 2 different techniques for measuring IFX concentrations, which can result in different IFX concentrations (up to 2 μg/mL).23 Thus, further assessment is needed to determine the optimal trough concentration. Finally, optimizing IFX therapy involves the cost of drug concentration monitoring, which can be prohibitive. However, if drug concentration monitoring improves outcomes on IFX, this may decrease the overall costs of health care. Additionally, given the potential for de-escalation or cessation of IFX, the overall cost–benefit ratio may favor prospective monitoring of IFX concentrations.
In conclusion, in this pilot observational trial, proactive TCM of IFX for patients in clinical remission frequently identified patients with low or undetectable trough concentrations. Dose escalations following proactive TCM were common, although dose de-escalations were also performed. Additionally, proactive TCM of IFX and titration to a target range was associated with an increased duration of IFX therapy.
Supplementary Material
Acknowledgments
Supported by a National Institutes of Health training grant (5T32DK007760-14 to B.P.V.).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ibdjournal.org).
A. C. Moss has participated in advisory boards for Prometheus, Janssen, and Abbvie and has consulted for Pfizer and AQI diagnostics. W. J. Sandborn has consulted for Prometheus and Janssen and has research support from Janssen. A. S. Cheifetz has participated in advisory boards for Prometheus, Janssen, and Abbvie and consulted for Pfizer. The remaining authors have no conflicts of interest to disclose.
References
- 1.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2005;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 2.Behm BW, Bickston SJ. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008:CD006893. doi: 10.1002/14651858.CD006893. [DOI] [PubMed] [Google Scholar]
- 3.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 4.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 5.Colombel J-F, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 6.Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–767. doi: 10.1038/ajg.2008.88. [DOI] [PubMed] [Google Scholar]
- 7.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 8.Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2012;108:40–47. doi: 10.1038/ajg.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology. 2009;137:1628–1640. doi: 10.1053/j.gastro.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 10.Radstake TRDJ, Svenson M, Eijsbouts AM, et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1739–1745. doi: 10.1136/ard.2008.092833. [DOI] [PubMed] [Google Scholar]
- 11.Yamada A, Sono K, Hosoe N, et al. Monitoring functional serum antitumor necrosis factor antibody level in Crohn’s disease patients who maintained and those who lost response to anti-TNF. Inflamm Bowel Dis. 2010;16:1898–1904. doi: 10.1002/ibd.21259. [DOI] [PubMed] [Google Scholar]
- 12.Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn’s disease. J Crohns Colitis. 2013;7:736–743. doi: 10.1016/j.crohns.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2009;59:49–54. doi: 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 14.Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–1254. doi: 10.1016/j.cgh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Paul S, Del Tedesco E, Marotte H, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2568–2576. doi: 10.1097/MIB.0b013e3182a77b41. [DOI] [PubMed] [Google Scholar]
- 16.Monchaud C, Marquet P. Pharmacokinetic optimization of immunosuppressive therapy in thoracic transplantation: part I. Clin Pharmacokinet. 2009;48:419–462. doi: 10.2165/11317230-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Assche G, D’Haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125:1025–1031. doi: 10.1016/s0016-5085(03)01214-9. [DOI] [PubMed] [Google Scholar]
- 18.Ziring DA, Wu SS, Mow WS, et al. Oral tacrolimus for steroid-dependent and steroid-resistant ulcerative colitis in children. J Pediatr Gastroenterol Nutr. 2007;45:306–311. doi: 10.1097/MPG.0b013e31805b82e4. [DOI] [PubMed] [Google Scholar]
- 19.Zelenitsky S, Rubinstein E, Ariano R, et al. Vancomycin pharmacodynamics and survival in patients with methicillin-resistant Staphylococcus aureus-associated septic shock. Int J Antimicrob Agents. 2013;41:255–260. doi: 10.1016/j.ijantimicag.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Hansen M, Christrup LL, Jarløv JO, et al. Gentamicin dosing in critically ill patients. Acta Anaesthesiol Scand. 2001;45:734–740. doi: 10.1034/j.1399-6576.2001.045006734.x. [DOI] [PubMed] [Google Scholar]
- 21.Colombel J-F, Feagan BG, Sandborn WJ, et al. Therapeutic drug monitoring of biologics for inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:349–358. doi: 10.1002/ibd.21831. [DOI] [PubMed] [Google Scholar]
- 22.Casteele NV, Gils A, Ballet V, et al. Randomizsed Controlled Trial of Drug Level Versus Clinically Based Dosing of Infliximab Maintenance Therapy in IBD: Final Results of the Taxit Study. Paper presented at: The 21st United European Gastroenterology Week; October 12–16, 2013; Berlin, Germany. [Google Scholar]
- 23.Steenholdt C, Ainsworth MA, Tovey M, et al. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohn’s disease. Ther Drug Monit. 2013;35:530–538. doi: 10.1097/FTD.0b013e31828d23c3. [DOI] [PubMed] [Google Scholar]
- 24.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. doi: 10.1136/gutjnl-2012-304094. [published online ahead of print March 4, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stallmach A, Hagel S, Bruns T. Adverse effects of biologics used for treating IBD. Best Pract Res Clin Gastroenterol. 2010;24:167–182. doi: 10.1016/j.bpg.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58:492–500. doi: 10.1136/gut.2008.155812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.